- 1Department of Epidemiology, College of Public Health, University of Iowa, Iowa City, IA, United States
- 2Department of Biostatistics, College of Public Health, University of Iowa, Iowa City, IA, United States
Background: Findings from previous studies examining the association between gestational diabetes mellitus (GDM) and subsequent risk of cardiovascular disease (CVD) have been inconsistent and inconclusive. We aimed to examine the associations of a previous history of GDM with risk of CVD and status of cardiovascular risk factors in a nationwide population-based study in the United States.
Methods: This study included 8,127 parous women aged 20 years or older in the 2007–2014 cycles of the National Health and Nutrition Examination Survey in the United States. The exposure was self-reported diagnostic history of GDM and the outcomes were self-reported diagnostic history of CVD and measurements of cardiovascular risk factors, including blood pressure and blood lipids. Regression models with sample weights were used to examine the associations of GDM with CVD and cardiovascular risk factors.
Results: Among women with a history of both GDM and CVD, CVD was diagnosed on average 22.9 years after the diagnosis of GDM. After adjustment for demographic, socioeconomic, and lifestyle factors, a history of GDM was associated with 63% higher odds of CVD [odds ratio (OR) 1.63, 95% confidence interval (CI) 1.02, 2.62, p-value = 0.04]. Further adjustment for body mass index (BMI) modestly attenuated the association (OR 1.52, 95% CI 0.95, 2.44, p-value = 0.08). A history of GDM was significantly associated with lower serum level of HDL-cholesterol (adjusted β-coefficient −3.33, 95% CI −5.17, −1.50, p-value ≤ 0.001), but not associated with total cholesterol, LDL-cholesterol, triglycerides, or systolic or diastolic blood pressure. Similarly, the association between a history of GDM and HDL cholesterol was attenuated after additional adjustment for BMI (adjusted β-coefficient −1.68, 95% CI −3.38, 0.03, p-value = 0.54).
Conclusion: Women with a previous history of GDM have significantly higher risk for developing CVD and lower serum level of HDL cholesterol, compared to women without a history of GDM. The associations may be explained, at least partly, by BMI.
Introduction
Gestational diabetes mellitus (GDM), a form of glucose intolerance during pregnancy, is a common pregnancy complication affecting approximately 7% (ranging from 1 to 14%) of all pregnancies in the US (1). GDM not only increases short-term risk of adverse pregnancy and birth outcomes but also increases long-term risk of various health outcomes for mothers later in life. A plethora of research has demonstrated that women with a history of GDM are more likely to develop type 2 diabetes. Several studies also suggest that GDM is associated with atherosclerosis (2), metabolic syndrome (3, 4), endothelial and cardiac dysfunction (3), and other intermediate cardiovascular morbidities (5). Based on existing evidence, the American Heart Association has proposed GDM as a major cardiovascular risk factor (6).
Cardiovascular disease (CVD) is the leading cause of death globally (7). Early identification and modification of risk factors has been shown to reduce mortality and morbidity in people with diagnosed or undiagnosed CVD (8). Evidence is still limited on the association between GDM and subsequent risk of overt CVD. Moreover, findings from available studies have been inconsistent and inconclusive (9–12). For instance, Carr et al. found a significantly increased risk of CVD after GDM in women with a family history of type 2 diabetes (9). However, Savitz et al. reported that GDM was significantly associated with the risk of subsequent type 1 and type 2 diabetes, but not CVD outcomes (11). These may be partly due to different settings (population-based vs. hospital-based), population characteristics (e.g., age, race/ethnicity) of participants, and ways of assessment of CVD (self-reports vs. examination) in those studies. Further, some studies examining the association between GDM and CVD appear to be a lack of diversity among participants. These underlie the need for further investigations among the general population with multiple ethnic groups to depict this association (13).
Thus, we sought to determine the risk of developing CVD among women with a history of GDM compared with those without such a history using data from the National Health and Nutrition Examination Survey (NHANES), a nationally representative and diverse population of the United States. Additionally, we compared cardiovascular risk factors between women with and without a history of GDM. We hypothesized that women with a history of GDM would have greater risk of developing overt CVD and unfavorable cardiovascular risk factors compared to women without a history of GDM.
Materials and Methods
Study Population
The study population consisted of parous women from the 2007 to 2014 cycles of the NHANES. Briefly, the NHANES, conducted by the Centers for Disease Control and Prevention (CDC), is a large-scale, ongoing, nationally representative health survey of the non-institutionalized US population. NHANES survey data are released every 2 years, with each 2-year cycle consisting of approximately 10,000 participants (14). The surveys comprise population-based, cross-sectional surveys that aim to capture data on diet, nutritional status, general health, disease history, and health behaviors (14). The surveys use multistage, probability clusters to develop a population sample that is nationally representative of the US based on age, sex, and race/ethnicity. In doing so, NHANES proportionally oversamples certain subpopulations of the US in comparison to others to better target the health interests of these subpopulations (15). From 2007 to 2010, NHANES cycles oversampled Hispanic persons, non-Hispanic black persons, low-income non-Hispanic white, and other persons at or below 130% of the federal poverty level (16). The oversampled subpopulations changed slightly in the 2011–2012 and 2013–2014 cycles, with the addition of the low-income non-Hispanic non-Black Asian subgroup, which replaced the non-Hispanic white subgroup in the 2007–2010 cycles (17). NHANES data along with documents on the survey methods and other information are publicly available on the NHANES online website (18). All subjects gave written informed consent in accordance with the Declaration of Helsinki. The study protocol was approved by the NCHS Research Ethics Review Board.
For this analysis, we included female participants, aged 20 years or older, with a prior history of pregnancy. Individuals who reported having a diagnosis of CVD or diabetes present before or during the same year as their diagnosis of GDM were excluded. Finally, we included 8,127 women in this study. The University of Iowa Institutional Review Board has approved this study.
Exposure Measurement
During the one-time interview through home visit, women were asked “Have you ever been pregnant?” Parous women were further asked, “During your pregnancy, were you ever told by a doctor or other health professional that you had diabetes, sugar diabetes or gestational diabetes?” and “How old were you when you were first told you had diabetes during a pregnancy?” Similarly, the participants were also asked about their diagnostic history and timing for overt diabetes. Based on their response to these questions, parous women were classified as having or not having a history of GDM. Participants who had overt diabetes before the diagnosis of GDM were excluded from the analysis.
Covariate Assessment
Information on age, race/ethnicity, annual household income, smoking status, and physical activity were obtained during interviews (18). Information on diet was obtained through two 24-h dietary recalls and total energy intake and alcohol intake was calculated using food composition database. Measurements of height, weight, and waist circumference were performed following a standardized protocol, and body mass index (BMI) was computed as weight in kilograms divided by the square of height in meters.
Outcome Measurement
The primary outcome was CVD, which was self-reported by participants in NHANES during the interview through the following questions: “Has a doctor or other health professional ever told you that you … had 1) congestive heart failure? 2) coronary heart disease? 3) angina/angina pectoris? 4) heart attack? 5) stroke?” and “How old were you when you were first told you had 1) congestive heart failure? 2) coronary heart disease? 3) angina/angina pectoris? 4) heart attack? 5) stroke?” Accordingly, we classified women as developing CVD if they reported having a diagnosis of one or more of these diseases and were classified as not having CVD if they did not report any of these diseases.
Secondary outcomes were CVD risk factors, including blood pressure and blood lipids. Blood pressure, including systolic blood pressure and diastolic blood pressure, was directly measured using standardized protocols. Total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol levels in serum samples were measured using enzymatic methods. Low-density lipoprotein (LDL) cholesterol was calculated from measured values of total cholesterol, triglycerides, and HDL cholesterol via the Friedewald’s formula (19): [LDL cholesterol] = [total cholesterol] − [HDL cholesterol] − [triglycerides/5].
Statistical Analysis
All statistical analyses accounted for the complex, multistage, stratified, and cluster-sampling design (including oversampling of certain subpopulations) of NHANES by using sample weights, strata, and primary sampling units embedded in the NHANES data. Comparisons of baseline characteristics among women with and without a history of GDM were performed using t test for continuous variables and the chi-square test for categorical variables.
We used multivariable logistic regression to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of CVD risk according to history of GDM. We used multivariable linear regression to estimate the β-coefficient and 95% CIs for the associations of history of GDM with blood lipids and blood pressure levels. In multivariable models, we adjusted for age, race/ethnicity, education, family income to poverty ratio, smoking status, alcohol intake, physical activity, total energy intake, and BMI.
Excess adiposity is a strong risk factor for both GDM (20) and CVD (21). Of note, 46.2% of GDM cases was attributable to overweight or obesity (20). Because there was evidence indicating a possible effect modification on the association between GDM and CVD by BMI (22), we performed stratified analyses according to obesity status. In addition, we performed stratified analyses according to hypertension and diabetes status. We conducted interaction tests via multiplicative interaction terms in the multivariable models. For the analysis of the secondary outcomes (i.e., blood pressure and blood lipids), we excluded participants who developed CVD and who were currently on medication for hypertension or dyslipidemia. We have checked model assumptions for all the analyses. All analyses were performed using survey procedures of SAS 9.4.
Results
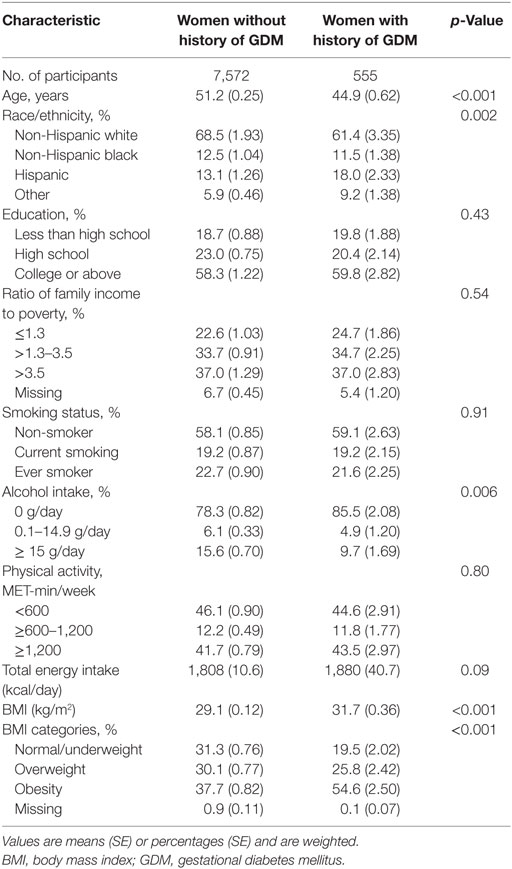
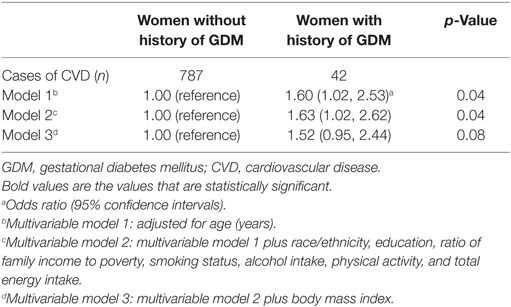
We identified 787 women who developed CVD among 7,572 women without a history of GDM, and 42 women developed CVD among 555 women with a history of GDM. In the analytical population, women with a history of GDM were more likely to be younger, non-white, more obese, and drink less alcohol (Table 1). Among women with a history of both GDM and CVD, CVD was diagnosed on average 22.9 (SE = 1.8) years after the diagnosis of GDM.
Compared to women without a history of GDM, women with a history of GDM were more likely to develop CVD, with multivariable-adjusted ORs (95% CIs) of CVD as 1.63 (1.02, 2.62). However, the associations were attenuated and became non-significant after additional adjustment for BMI (Table 2). In a stratified analysis by obesity status, we observed a stronger association between GDM and CVD in obese women [2.16 (1.34, 3.49)], compared to non-obese women [1.58 (1.01, 2.50)] (Table S1 in Supplementary Material). The association did not vary by hypertension status or diabetes status.
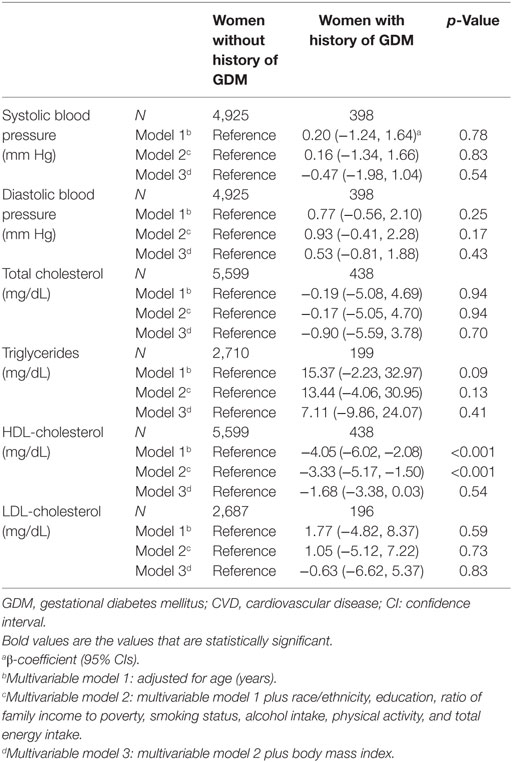
In terms of cardiovascular risk factors, women with a history of GDM had lower levels of HDL cholesterol than those without a history of GDM, with adjusted β-coefficient (95% CIs) as −3.33 (−5.17, −1.50) (Table 3). The association did not vary by hypertension status or diabetes status. The association between history of GDM and triglyceride levels differed by hypertension status. Specifically, a history of GDM was not associated with triglyceride levels among women without hypertension; however, among women with hypertension, a history of GDM was associated with higher level of triglycerides, with β-coefficient (95% CIs) as 47.72 (4.78, 90.67) (Table S2 in Supplementary Material). A history of GDM was not significantly associated with total cholesterol, LDL cholesterol, systolic blood pressure, or diastolic blood pressure.
Discussion
This study suggests that women with a history of GDM are at greater risk for developing CVD later in life than women without a history of GDM. In addition, women with prior GDM have lower levels of HDL cholesterol and higher levels of triglycerides, compared to women without a history of GDM.
Previous studies on the association of GDM and CVD risk in the general population are sparse. Those studies were conducted in Canada (12, 23, 24), Sweden (22), and France (25). Our results, using data from a nationwide population-based study in the United States were generally consistent with those previous studies. In addition, the magnitude of risk between GDM and CVD presented in this study appears to be similar to that of previous, mostly hospital-based, studies among US women (3, 9, 11, 26). One study in a UK population found a significant association between GDM and calculated CVD risk (based on the Framingham score) in the age-adjusted model, but following further adjustment, the association became non-significant (10).
The associations between GDM and CVD risk factors in this study are consistent with some but not all of previous studies. Zajdenverg et al. found very similar findings with no significant cardiovascular risk factor differences between women with and without a history of GDM but also observed lower levels of HDL cholesterol among women with a history of GDM (27). In some instances, no significant associations between GDM and CVD risk factors have been observed, including no differences in HDL cholesterol (28). Alternatively, other studies have found significant differences in CVD risk factors, with women who have a history of GDM tending to be more obese, have higher blood pressure, and higher triglyceride levels (10, 29, 30).
The underlying mechanisms linking GDM to CVD remain to be elucidated. Obesity is a shared risk factor of GDM and CVD. In this study, we observed a significant association between GDM and CVD among both obese and non-obese women, although the association seemed stronger among obese women compared to non-obese women. Previous studies have shown that altered lipid metabolism, impaired endothelial function, and vascular inflammation may be implicated in the pathogenesis of CVD after GDM (31, 32). Our results indicate that low levels of HDL cholesterol may contribute to the increased risk of CVD in women with prior GDM. Low HDL cholesterol is an established and independent risk factor for coronary artery disease (33) and atherosclerotic CVD (34). In our study, women with a history of GDM reported comparable physical activity levels to women without a history of GDM, but had higher values for BMI and total energy intake. BMI has been inversely associated with HDL cholesterol levels (35). The results in our study also suggested that the association between GDM and low HDL cholesterol levels might be partly explained by BMI. It has been proposed that insulin resistance can cause irregularities in the shapes and sizes of HDL cholesterols, which is an abnormality known to be inversely related to plasma triglyceride levels (35), suggesting that low HDL cholesterol could be secondary to elevated plasma triglyceride levels and increased BMI. It is worth noting that low HDL cholesterol is a more significant risk factor for CVD in women than it is for men (34), which could increase CVD morbidity in women with prior GDM.
The major strength of this population-based study is the use of a nationally representative sample, which facilitate generalization of the findings to the general population in the United States. In addition, with the detailed data collected in the NHANES, we were able to control potential confounding effects from a variety of demographic, socioeconomic, anthropometric, and lifestyle factors. This study has some limitations. First, a history of GDM and CVD diagnosis were both self-reported in NHANES, potentially leading to misclassification of GDM and CVD outcomes. However, previous validation studies have shown high agreement between self-reported and medical record data for both GDM (36) and CVD (37, 38) in US women. Self-report is also applied in NHANES for diet, physical activity, and smoking status. It has been observed that total energy intake was underreported by overweight individuals in previous cycles of NHANES (14), therefore, the USDA’s automated multi-pass method has been employed to reduce misreporting in NHANES 24-h recalls (39). Second, although the sample size was relatively large, we did not have sufficient power to estimate the risk for each of the CVD outcomes. Finally, temporal relationship and reverse causation are common causes for concern in many observational studies, especially in a cross-sectional study setting. However, it is unlikely that reverse causation between GDM and CVD affected our results because the average length of time between a pregnancy complicated with GDM and diagnosis of CVD in our study was 22.9 years. In addition, we excluded cases that reported having a diagnosis of CVD prior to their GDM.
Our findings have important clinical and public health implications. The majority of previous studies involving high-risk populations of CVD focused on older adults. Women who are diagnosed with GDM during pregnancy represent younger, high-risk but usually overlooked population of CVD. For women, the routine screening of GDM during pregnancy offers a unique lifetime opportunity at a young age to reveal their risk of cardiometabolic disorders later in life. Based on the findings of increased risk of CVD after GDM, targeted interventions may be implemented to mitigate the risk at a young age in women with GDM, which could have benefits including but not limited to improved cardiovascular health.
Conclusion
In a nationwide population-based study in the United States, we show that women with a previous history of GDM have significantly higher risk for developing CVD and lower serum level of HDL cholesterol, compared to women without a history of GDM. These associations may be explained, at least partly, by BMI.
Ethics Statement
All subjects gave written informed consent in accordance with the Declaration of Helsinki. The study protocol was approved by the NCHS Research Ethics Review Board.
Author Contributions
DS drafted and revised the manuscript and was responsible for manuscript submission. YS performed data analysis, wrote statistical methods, interpreted results, and critically revised the manuscript. LS and JO interpreted results and critically revised the manuscript. WB conceived the study idea, directed statistical analyses, interpreted results, and critically revised the manuscript. All authors reviewed and approved the final manuscript for submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, ND, declared a shared affiliation, though no other collaboration, with the authors to the handling editor, who ensured that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
We acknowledge the NHANES participants and staff for providing the data. Dr. Lixi Yu contributed to initial SAS programming and data analysis.
Funding
This research was supported by the Robert W. Hansen Diabetes Fund from the Fraternal Order of Eagles Charity Foundation.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fendo.2017.00144/full#supplementary-material.
References
1. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care (2004) 27(Suppl 1):S88–90. doi:10.2337/diacare.27.2007.S88
2. Li JW, He SY, Liu P, Luo L, Zhao L, Xiao YB. Association of gestational diabetes mellitus (GDM) with subclinical atherosclerosis: a systemic review and meta-analysis. BMC Cardiovasc Disord (2014) 14:132. doi:10.1186/1471-2261-14-132
3. Bentley-Lewis R. Late cardiovascular consequences of gestational diabetes mellitus. Semin Reprod Med (2009) 27(4):322–9. doi:10.1055/s-0029-1225260
4. Xu Y, Shen S, Sun L, Yang H, Jin B, Cao X. Metabolic syndrome risk after gestational diabetes: a systematic review and meta-analysis. PLoS One (2014) 9(1):e87863. doi:10.1371/journal.pone.0087863
5. Sullivan SD, Umans JG, Ratner R. Gestational diabetes: implications for cardiovascular health. Curr Diab Rep (2012) 12(1):43–52. doi:10.1007/s11892-011-0238-3
6. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women – 2011 update: a guideline from the American Heart Association. J Am Coll Cardiol (2011) 57(12):1404–23. doi:10.1016/j.jacc.2011.02.005
7. WHO. (2016). Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
8. WHO. Prevention of Cardiovascular Disease: Guidelines for Assessment and Management of Total Cardiovascular Risk. Geneva, Switzerland: WHO Press (2007).
9. Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care (2006) 29(9):2078–83. doi:10.2337/dc05-2482
10. Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation (2012) 125(11):1367–80. doi:10.1161/CIRCULATIONAHA.111.044784
11. Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol (2014) 180(1):41–4. doi:10.1093/aje/kwu118
12. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care (2008) 31(8):1668–9. doi:10.2337/dc08-0706
13. Marcinkevage JA, Narayan KM. Gestational diabetes mellitus: taking it to heart. Prim Care Diabetes (2011) 5(2):81–8. doi:10.1016/j.pcd.2010.10.002
14. Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr (2016) 7(1):121–34. doi:10.3945/an.115.009258
15. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1 (2013) 56:1–37.
16. Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, et al. National health and nutrition examination survey: sample design, 2007–2010. Vital Health Stat 2 (2013) 160:1–23.
17. Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat 2 (2014) 162:1–33.
18. National Center for Health Statistics, Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey. (2017). Available from: https://wwwn.cdc.gov/nchs/nhanes/default.aspx
19. Friedewald W, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem (1972) 18:499–502.
20. Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health (2010) 100(6):1047–52. doi:10.2105/AJPH.2009.172890
21. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol (2009) 53(21):1925–32. doi:10.1016/j.jacc.2008.12.068
22. Fadl H, Magnuson A, Ostlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG (2014) 121(12):1530–6. doi:10.1111/1471-0528.12754
23. Kaul P, Savu A, Nerenberg KA, Donovan LE, Chik CL, Ryan EA, et al. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: a population-level analysis. Diabet Med (2015) 32(2):164–73. doi:10.1111/dme.12635
24. Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ (2009) 181(6–7):371–6. doi:10.1503/cmaj.090569
25. Goueslard K, Cottenet J, Mariet AS, Giroud M, Cottin Y, Petit JM, et al. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol (2016) 15:15. doi:10.1186/s12933-016-0338-0
26. Freibert SM, Mannino DM, Bush H, Crofford LJ. The association of adverse pregnancy events and cardiovascular disease in women 50 years of age and older. J Womens Health (Larchmt) (2011) 20(2):287–93. doi:10.1089/jwh.2010.2097
27. Zajdenverg L, Rodacki M, Faria JP, Pires ML, Oliveira JE, Halfoun VL. Precocious markers of cardiovascular risk and vascular damage in apparently healthy women with previous gestational diabetes. Diabetol Metab Syndr (2014) 6:63. doi:10.1186/1758-5996-6-63
28. Kim C, Cheng YJ, Beckles GL. Cardiovascular disease risk profiles in women with histories of gestational diabetes but without current diabetes. Obstet Gynecol (2008) 112(4):875–83. doi:10.1097/AOG.0b013e31818638b5
29. Madarasz E, Tamas G, Tabak AG, Kerenyi Z. Carbohydrate metabolism and cardiovascular risk factors 4 years after a pregnancy complicated by gestational diabetes. Diabetes Res Clin Pract (2009) 85(2):197–202. doi:10.1016/j.diabres.2009.05.001
30. Pallardo LF, Herranz L, Martin-Vaquero P, Garcia-Ingelmo T, Grande C, Janez M. Impaired fasting glucose and impaired glucose tolerance in women with prior gestational diabetes are associated with a different cardiovascular profile. Diabetes Care (2003) 26(8):2318–22. doi:10.2337/diacare.26.8.2318
31. Brewster S, Zinman B, Retnakaran R, Floras JS. Cardiometabolic consequences of gestational dysglycemia. J Am Coll Cardiol (2013) 62(8):677–84. doi:10.1016/j.jacc.2013.01.080
32. Harreiter J, Dovjak G, Kautzky-Willer A. Gestational diabetes mellitus and cardiovascular risk after pregnancy. Womens Health (Lond) (2014) 10(1):91–108. doi:10.2217/whe.13.69
33. Perez-Mendez O, Pacheco HG, Martinez-Sanchez C, Franco M. HDL-cholesterol in coronary artery disease risk: function or structure? Clin Chim Acta (2014) 429:111–22. doi:10.1016/j.cca.2013.12.001
34. LaRosa JC. Lipids and cardiovascular disease: do the findings and therapy apply equally to men and women? Womens Health Issues (1992) 2(2):102–11; discussion 11–3. doi:10.1016/S1049-3867(05)80278-6
35. Barter PJ. The causes and consequences of low levels of high density lipoproteins in patients with diabetes. Diabetes Metab J (2011) 35(2):101–6. doi:10.4093/dmj.2011.35.2.101
36. Hosler AS, Nayak SG, Radigan AM. Agreement between self-report and birth certificate for gestational diabetes mellitus: New York State PRAMS. Matern Child Health J (2010) 14(5):786–9. doi:10.1007/s10995-009-0529-3
37. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol (2004) 57(10):1096–103. doi:10.1016/j.jclinepi.2004.04.005
38. Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol (2004) 160(12):1152–8. doi:10.1093/aje/
Keywords: gestational diabetes mellitus, cardiovascular disease, cardiovascular risk factors, pregnancy complications, blood lipids
Citation: Shostrom DCV, Sun Y, Oleson JJ, Snetselaar LG and Bao W (2017) History of Gestational Diabetes Mellitus in Relation to Cardiovascular Disease and Cardiovascular Risk Factors in US Women. Front. Endocrinol. 8:144. doi: 10.3389/fendo.2017.00144
Received: 24 January 2017; Accepted: 07 June 2017;
Published: 26 June 2017
Edited by:
Mohamed Abu-Farha, Dasman Diabetes Institute, KuwaitReviewed by:
Trevor S. Ferguson, University of the West Indies, MonacoNirav Dhanesha, University of Iowa, United States
Ludmila Brunerova, Charles University, Czechia
Copyright: © 2017 Shostrom, Sun, Oleson, Snetselaar and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Bao, d2VpLWJhb0B1aW93YS5lZHU=
†These authors have contributed equally to this work.
 Derrick C. V. Shostrom
Derrick C. V. Shostrom Yangbo Sun
Yangbo Sun Jacob J. Oleson2
Jacob J. Oleson2 Wei Bao
Wei Bao