
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 13 April 2017
Sec. Neuroendocrine Science
Volume 8 - 2017 | https://doi.org/10.3389/fendo.2017.00075
This article is part of the Research Topic Comparative Studies of Energy Homeostasis in Vertebrates View all 14 articles
The arcuate nucleus is generally conserved across vertebrate taxa in its neuroanatomy and neuropeptide expression. Gene expression of agouti-related protein (AGRP), neuropeptide Y (NPY), pro-opiomelanocortin (POMC), and cocaine- and amphetamine-regulated transcript (CART) has been established in the arcuate nucleus of several bird species and co-localization demonstrated for AGRP and NPY. The proteins encoded by these genes exert comparable effects on food intake in birds after central administration to those seen in other vertebrates, with AGRP and NPY being orexigenic and CART and α-melanocyte-stimulating hormone anorexigenic. We have focused on the measurement of arcuate nucleus AGRP and POMC expression in several avian models in relation to the regulation of energy balance, incubation, stress, and growth. AGRP mRNA and POMC mRNA are, respectively, up- and downregulated after energy deprivation and restriction. This suggests that coordinated changes in the activity of AGRP and POMC neurons help to drive the homeostatic response to replace depleted energy stores in birds as in other vertebrates. While AGRP and POMC expression are generally positively and negatively correlated with food intake, respectively, we review here situations in some avian models in which AGRP gene expression is dissociated from the level of food intake and may have an influence on growth independent of changes in appetite. This suggests the possibility that the central melanocortin system exerts more pleiotropic functions in birds. While the neuroanatomical arrangement of AGRP and POMC neurons and the sensitivity of their activity to nutritional state appear generally conserved with other vertebrates, detailed knowledge is lacking of the key nutritional feedback signals acting on the avian arcuate nucleus and there appear to be significant differences between birds and mammals. In particular, recently identified avian leptin genes show differences between bird species in their tissue expression patterns and appear less closely linked in their expression to nutritional state. It is presently uncertain how the regulation of the central melanocortin system in birds is brought about in the situation of the apparently reduced importance of leptin and ghrelin compared to mammals.
Neural circuitry in the arcuate nucleus of the hypothalamus is well established in mammals as being particularly important for the regulation of energy balance (1, 2). Two neuronal cell types are involved. One synthesizes both agouti-related protein (AGRP) and neuropeptide Y (NPY), and the other produces α-melanocyte-stimulating hormone (α-MSH) and other peptides from the pro-opiomelanocortin (POMC) precursor together with cocaine- and amphetamine-regulated transcript (CART). AGRP and α-MSH peptides secreted from arcuate nucleus neurons bind to melanocortin receptors in the brain and collectively comprise the components of the central melanocortin system. AGRP/NPY neurons exert anabolic effects on food intake and body mass while POMC/CART neurons are catabolic. Evidence from non-mammalian vertebrate taxa suggests that the neuronal circuitry has been evolutionarily conserved both in its neuroanatomical location and in the genes the cells express. For example, AGRP, NPY, POMC, and CART mRNAs have all been localized in several teleost fish species in the lateral tuberal nucleus (NLT), the equivalent of the mammalian arcuate nucleus (3–6). Comparable observations have been made in birds, where the arcuate nucleus has historically been named the infundibular nucleus. Immunoreactive cell bodies and mRNA have been localized in the arcuate nucleus in several bird species for NPY, AGRP, and POMC (7–12). Furthermore, the co-expression of AGRP and NPY mRNA characteristic of laboratory rodents has been demonstrated in individual arcuate nucleus neurons in the Japanese quail (Coturnix coturnix japonica) (13). Less is known about CART in birds and its co-expression with POMC has yet to be formally demonstrated. However, immunoreactive CART neuronal cell bodies have been identified in the arcuate nucleus of the zebra finch (Taeniopygia guttata) (14).
We consider in this review the extent to which the evolutionary neuroanatomical conservation of the arcuate nucleus neurons implicated in energy balance in birds is conserved at the functional level, with an emphasis on our recent studies on the regulation of AGRP and POMC expression in several avian models.
It is well established in laboratory rodents that neuropeptide gene expression in the arcuate nucleus AGRP/NPY and POMC/CART neurons is sensitive to nutritional state (physiologically reported in mammals by variation in plasma leptin concentrations) as part of a counter-regulatory response to loss of body energy stores during situations of negative energy balance such as fasting or food restriction (2). Comparable findings have been obtained in birds in response to both experimental food deprivation and chronic restriction. For example, AGRP mRNA was increased by food deprivation for 24 h in adult Japanese quail, a response also consistently observed after food deprivation for 24–48 h in domestic broiler chicks (11, 15–18). AGRP expression returned to baseline levels after 24 h refeeding (15–18). We have extended these observations to investigate the effects of chronic food restriction in growing broiler breeder hens. Food restriction of the parent hens of broiler chickens during the growth phase is a common practice in the poultry industry in order to mitigate poor reproductive performance when birds are fed ad libitum (19). We observed that AGRP mRNA was strongly increased in 12-week-old hens that had been maintained on an industrial food restriction regime for 6 weeks and gene expression returned to baseline after 2 days’ refeeding (20). A further comparison within the same experiment indicated that the level of AGRP mRNA was sensitive to feeding history: in two groups of hens sampled at the same body mass, AGRP gene expression was significantly higher in a group that had been maintained at an intermediate level of food restriction for 6 weeks compared to a group that had been maintained on commercial food restriction for 4 weeks before being released onto 2 weeks of ad libitum feeding. These results suggested that AGRP gene expression in broiler breeder hens is at least as sensitive to chronic food restriction as in mammals such as sheep, Siberian hamsters (Phodopus sungorus), rats, and golden spiny mice (Acomys russatus) (21–24). Furthermore, its expression is more closely linked to feeding state than reported in some rat studies of chronic food restriction where either no change was observed in AGRP mRNA, or where arcuate nucleus neuropeptide mRNAs had not returned to baseline levels after 4 weeks’ refeeding (25, 26).
It would be predicted from mammalian studies that food deprivation and restriction would exert an opposite, inhibitory, effect on POMC mRNA levels compared to AGRP mRNA because POMC gene expression induces a catabolic effect on energy balance (2). However, in birds, some studies have reported no change in POMC mRNA level after 24–48 h food deprivation in Japanese quail and broiler chicks (11, 17) while significantly decreased POMC expression has been observed in other broiler chick studies (15, 16, 18). For chronic food restriction, we detected no difference in POMC expression after 6 weeks’ restriction in our study of 12-week-old broiler breeder hens mentioned above, but another investigation reported a significant decrease after 7 days’ restriction in both 3-week-old broiler and layer chicks (20, 27). The greater variability in detecting altered POMC mRNA and the reduced magnitude of the change in POMC expression compared to that for AGRP in response to food deprivation or restriction may reflect a greater relative importance of increased AGRP expression in the counter-regulatory response to lost energy stores or, alternatively, a possibly greater role for regulatory control at the level of POMC-derived peptide secretion as has been reported in mammals (28).
The significance of altered AGRP and POMC gene expression in response to manipulation of energy status is beginning to be understood in laboratory rodents. For example, the respective increased and decreased AGRP and POMC mRNA following a fast appears to be matched by parallel changes at the level of increased release of AGRP peptide and decreased release of α- and γ-MSH peptides (29–31) and by, respectively, increased and decreased firing of AGRP and POMC neurons (32, 33). Furthermore, optogenetic approaches have demonstrated that feeding behavior can be directly induced or inhibited by light activation of AGRP and POMC neurons, respectively (34). In contrast, most studies of the central melanocortin system in birds have focused on mRNA measurements as an indicator of the activity of the neurons. However, a few investigations have demonstrated the presence of central melanocortin system peptides in the avian arcuate nucleus. For example, AGRP immunoreactivity was identified in the Khaki Campbell duck (Anas platyrhynchos) arcuate nucleus (12), and evidence for altered synthesis of AGRP peptide is available for the ring dove (Streptopelia risoria), where increased numbers of AGRP-immunoreactive cell bodies were observed in the medio-basal hypothalamus following a 48-h fast and also during the post-hatching phase of the reproductive cycle when the parent birds are in negative energy balance as a result of the demands of feeding offspring (10, 35). Relatively little is known in birds about the processing of POMC-derived peptides in the hypothalamus and their relative importance for energy balance regulation. However, immunoreactive neuronal cell bodies for α-MSH, β-endorphin, and N-terminal POMC (pro-γ-MSH) were detected in the arcuate nucleus of broiler chickens (7), and co-localization of POMC mRNA and α-MSH peptide was observed in individual arcuate nucleus neurons in Japanese quail (11).
Given the general lack of information in birds about AGRP and POMC signaling above the mRNA level, the significance of changes in gene expression in relation to energy status is generally inferred from the mammalian literature and from the behavioral effects of the encoded peptides. There is behavioral evidence that domestic pigeons and Japanese quail eat more in a refeeding period after food deprivation compared to control birds that had been allowed to feed freely over the experimental fasting phase (13, 36). This increased food intake combined with knowledge about the effects of energy deprivation on AGRP and POMC gene expression is consistent with the idea that fasting stimulates food intake by increased secretion of AGRP peptide and, in some situations, reduced secretion of POMC-derived peptides such as α-MSH.
The avian central melanocortin system peptides appear to exert their effects by acting on melanocortin receptors in the hypothalamus as in mammals. Characterization of the pharmacological properties of the five chicken melanocortin receptor subtypes in vitro revealed a relatively greater affinity for ACTH-derived peptides than for α-MSH compared to their mammalian orthologs (37). This might suggest a more significant role in birds for ACTH as a central melanocortin receptor ligand. Studies have not been performed to localize or quantify ACTH peptide in the avian hypothalamus, but its synthesis and secretion have been reported in the hypothalamus of laboratory rats (28), and an inhibitory effect on feeding of the centrally administered peptide has been observed in rats, domestic pigeons (Columba livia), and broiler chicks (38–40). Another POMC peptide, β-endorphin, has been localized in the chicken arcuate nucleus, as noted above (7). It stimulates food intake after central injection of the ostrich and mammalian peptide, respectively, in the domestic pigeon and the chicken (41, 42). Further investigation is needed to explore the context and significance of β-endorphin secretion from POMC/CART neurons.
Pharmacological studies have been performed in birds on the melanocortin 4 receptor (MC4R), the melanocortin receptor most strongly associated with the regulation of food intake in mammals. The synthetic compounds HS014 and HS024 were identified in vitro as selective antagonists and melanotan II (MTII) as a potent agonist in the chicken (37). The predicted stimulatory effects on food intake for the antagonist, and inhibitory effects for the agonist, have been confirmed for HS014 and MTII in ring doves and in broiler and layer chickens following central or peripheral injection (27, 35, 43). Several studies have investigated the effect of central injection of MSH peptides on food intake in domestic chicks. Inhibitory effects of α-, β-, and γ2-MSH have been observed (44–47) with reports of differential sensitivity between broiler and layer and high and low-growth lines (48, 49). However, while the amino acid sequence of α-MSH is conserved across vertebrates, the chicken sequences for β- and γ2-MSH differ between birds and mammals and appear to have a less potent effect on food intake in chicks when administered centrally compared to mammalian versions (43). This suggests a more prominent role for α-MSH among the avian POMC-derived peptides. As in mammals, there is evidence for competition between the α-MSH agonist and the AGRP antagonist at central melanocortin receptors because central injection of AGRP dose-dependently attenuated the inhibitory effect on feeding of α-MSH in domestic chicks (45). Central injections of AGRP alone stimulated food intake, as expected, in the ring dove but with an apparently lower potency than observed in laboratory rodents, which may reflect the use of heterologous human AGRP fragments in the ring dove experiment (35). Layer chicks were observed to be more sensitive in their feeding response to central AGRP injection than broiler chicks (45).
While pharmacological and behavioral studies suggest that the actions of AGRP- and POMC-derived peptides, particularly α-MSH, are conserved between birds and mammals, knowledge is lacking in birds about the site of action of the peptides and the central melanocortin receptor subtypes that naturally mediate their signaling effects: the projections of AGRP/NPY and POMC/CART neurons have not been studied, and the central melanocortin receptors involved have not been precisely localized. Both MC4R and melanocortin 3 receptor (MC3R) mRNA have been quantified in the chicken hypothalamus in real-time PCR studies (15, 17, 50, 51) and the melanocortin 5 receptor more generally in the brain (50, 52). Increased MC4R gene expression has been observed after 48 h food deprivation in broiler chicks (15, 17), which may be in response to decreased agonistic drive. Little is known about the possible role of the MC3R in the regulation of energy balance in birds, but its hypothalamic expression was higher in chickens lines selected for low compared to high body weight (50, 51).
While the dynamic coordinated changes in arcuate nucleus AGRP and POMC gene expression in response to energy shortage are well established in mammals and conserved in birds, less is known about the occurrence and influence of altered basal expression of these genes. Uniquely avian models are available to test the hypothesis that variation in the levels of AGRP and POMC mRNA measured in individuals with free access to food promotes the expression of natural seasonal changes in feeding behavior and metabolism. We have recently investigated this in a chicken strain that exhibits natural incubation behavior. Hens incubate their eggs over a 3-week period that is associated with a suite of behavioral and physiological changes known as “broodiness” (53). Hens spend an increasing amount of time sitting on their eggs and cease laying. Linked to this is the expression of natural anorexia when birds reduce their food intake and reduce body mass over the incubation period (54). Experimental food deprivation and refeeding in junglefowl hens suggested that the level around which body mass is defended (or set point) is reduced during incubation, and the concept was extended to other vertebrate species of natural “animal anorexias” that are expressed in phases of life history such as hibernation or territorial defense where the time available to feed is limited (55, 56).
We drew on these studies to investigate how AGRP and POMC gene expression changes during the incubation phase in hens (57). One possible outcome was that there would be no change in gene expression because in birds with free access to food, body mass is at its appropriate defended level despite the fact that body mass is lower: altered AGRP and POMC expression would only be expected in response to perturbation such as food deprivation or restriction. Alternatively, it was possible that changes in basal gene expression play a role in promoting the loss of body mass. In this case, increased POMC expression would be expected, combined with unaltered, or reduced, AGRP expression. We controlled for possible confounding effects of, respectively, enlarged and regressed ovaries in laying and incubating hens by pair-feeding two groups of laying hens to the amount of food eaten voluntarily by incubating birds (57). This resulted in ovarian regression in those groups that matched that shown in the incubating hens. One of the pair-fed groups was allowed to refeed for 5 days so that food intake and body mass stabilized to reveal the natural ad libitum food intake level in birds with regressed ovaries. In birds sampled 21 days after the onset of incubation, POMC gene expression was increased to a level on the border of statistical significance in the incubating hens compared to the two control groups, consistent with the idea that this is linked to an increased anorexic drive. However, unexpectedly, AGRP mRNA was higher in both incubating and pair-fed birds, compared to the re-fed control group. This finding is interesting in suggesting that increased AGRP gene expression (and the assumed associated increase in AGRP peptide signaling) does not necessarily result in increased food intake. This situation was not unique to incubation. A related experiment arose from our observation that hens transferred from single housing in a cage to housing in a pen showed reduced food intake in their new environment presumably because they perceived the transfer as stressful (57). Measurements of AGRP and POMC gene expression 6 days after the housing transfer showed results comparable to the incubation experiment, this time with significantly increased POMC mRNA combined with increased AGRP expression. This result is again consistent with increased POMC expression contributing to anorexic drive that leads to the reduced food intake. The fact that AGRP gene expression is increased during incubation and after housing transfer suggests that its sensitivity to reduced energy availability is maintained despite an apparent change in the defended set point for body mass during incubation. The normal stimulatory effects of AGRP expression on food intake may be overridden by a relatively greater inhibitory influence of the increased POMC expression and, in the case of incubation, a possible inhibitory influence on feeding of hypothalamic vasoactive intestinal polypeptide, the expression of which is causatively linked to incubation behavior in birds (53). The fact that AGRP gene expression is increased in these situations may be of adaptive significance in promoting more rapid restoration of energy stores when incubation ends and as part of the recovery from the effects of a stressor. For incubation, it is also possible that increased AGRP mRNA is linked to altered daily patterns of behavior during incubation when expression of ingestive behavior is confined to two daily recesses from nest sitting but during which feeding may be relatively intense (53).
Another opportunity provided by avian models to investigate the possibility of seasonal regulation of AGRP and POMC gene expression is represented by species that show increased appetite and fat deposition as preparation for migratory flight (58). Laboratory studies in captive birds have revealed that the increased appetite (hyperphagia) and fat deposition that occur before migration are stimulated by changes in daylength and appear to involve changes in the level around which body mass is regulated as has been suggested for incubation (59). Seasonal changes in reproductive physiology mediated by photoperiod in birds and seasonal mammals have been linked to release of thyroid-stimulating hormone from the pars tuberalis of the pituitary gland. This results in conversion within the medio-basal hypothalamus of thyroxine into triiodothyronine (T3) that promotes release of gonadotropin-releasing hormone from neuron terminals in the median eminence (60). It is possible that locally increased tissue concentrations of T3 mediate seasonal cycles in appetite and fat deposition in addition to reproduction. This is suggested by a mammalian study of Siberian hamsters that received hypothalamic implants of T3. This procedure on short day animals induced changes in body mass characteristic of exposure to long photoperiods (61). Furthermore, there is evidence in domestic chicks that experimentally increased T3 stimulates hypothalamic AGRP gene expression both in vivo and in vitro (62). However, there is limited evidence in hamsters for an important role for the arcuate nucleus and its neuropeptides in driving seasonal cycles in food intake and body mass (63). We are currently investigating whether the situation is similar in birds by quantifying AGRP gene expression in Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii) after photostimulation. This will test whether increased AGRP gene expression is associated with seasonally increased food intake in this migratory species.
In addition to a possible role of basal changes in gene expression in regulating reproductive and seasonal changes in food intake and body weight, our recent studies suggest that AGRP and POMC expression may be related to growth. In our chronic food restriction experiment on broiler breeder hens (20) reviewed in relation to energy homeostasis above, we noted that AGRP gene expression was inversely related to body mass in the groups maintained on ad libitum feeding at the time of sampling (Figure 1). Birds were the same age at the time they were killed and had all been maintained on commercial restricted feeding for the first 6 weeks. The highest body mass was attained in the birds fed ad libitum over the next 6 weeks and the lowest in birds that were only allowed to refeed for 2 days. AGRP mRNA remained significantly elevated in birds fed ad libitum for 2 weeks compared to 6 weeks. Thus, the level of AGRP expression could be regarded as a measure of the growth potential of the different experimental groups, with the highest expression in birds that were furthest away from the natural growth trajectory. We have further evidence for this at the genetic level. When we compared AGRP gene expression between males and females in 12-week-old broiler breeder chickens that had been re-fed for 2 days after food restriction, expression was significantly higher in males and this difference was replicated in ad libitum-fed fully mature chickens of another genetic strain (64). The higher AGRP expression in males is consistent with the fact that they grow faster and attain a higher mature body mass. It would therefore be predicted that AGRP mRNA would be higher in fully fed birds in chicken strains that grow more rapidly. We obtained evidence for this from trait linkage analysis of birds from a broiler-layer cross that differed in growth rate (65). We identified a genotype that explained 19% of the difference in body mass between the lines that was associated with lower global tissue expression of the cholecystokinin A receptor (CCKAR) in the high-growth haplotype. We demonstrated that the high-growth birds were less sensitive to the inhibitory effects on food intake of intraperitoneal cholecystokinin (CCK) injection and that they showed significantly higher expression of AGRP (but no difference in POMC mRNA). This suggests that the tone of CCK signaling influences AGRP expression. It could be predicted that increased AGRP expression is causative in generating higher growth by stimulating food intake. However, we have been unable to find a consistent difference in daily food intake between the lines. This demonstrates again, as we observed for incubation, that high AGRP gene expression can be dissociated from increased feeding. Any effects of AGRP on growth rate must therefore be independent of food intake. The mechanisms involved are currently unclear and point to a need in birds to investigate the effects of central melanocortin system signaling on metabolic aspects of energy balance regulation distinct from feeding behavior.
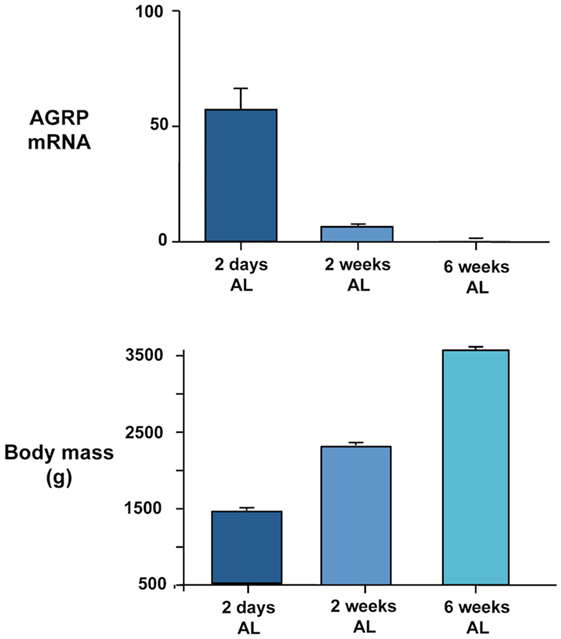
Figure 1. Inverse relationship between AGRP gene expression quantified in dissected basal hypothalamus by real-time PCR (upper panel) and body mass (lower panel) in female broiler breeder chickens (Ross 308 line). Birds were maintained on a commercial food restriction program from hatch before being transferred to ad libitum (AL) feeding at 2 days, 2 weeks, and 6 weeks before the birds were killed at 12 weeks of age. Further experimental details are provided in Ref. (20).
While our observations linking AGRP expression to growth in birds are preliminary and require further investigation, they serve to highlight a possible involvement of the central melanocortin system in growth regulation that has received limited attention in vertebrates compared to its more general effects on energy homeostasis. Experimental inactivation or natural mutation of the MC4R in laboratory mice and humans is associated with increased linear growth (66, 67). The mechanisms underlying this effect are not fully understood but appear to be independent of growth hormone secretion and involve hyperinsulinemia (68, 69). More direct evidence for a link between the central melanocortin system and growth has been obtained from teleost fish for which it has been reported that transgenic overexpression of the natural melanocortin receptor antagonist agouti-signaling protein reversed the pattern of sexually dimorphic growth in zebrafish (Danio rerio) (70) and that AGRP and POMC neurons project directly to the pituitary gland (71). Transgenic overexpression of AGRP itself in zebrafish led to both obesity and increased linear growth, while suppression of AGRP expression reduced larval growth rate and was mediated through the MC4R (71, 72). Thus it seems that AGRP exerts more pleiotropic effects on hormonal axes through the pituitary gland in teleosts compared to mammals and more investigation is needed to determine whether this applies to non-mammalian vertebrates more generally including birds.
Less is known in birds than mammals about the regulatory feedback signals to the arcuate nucleus that mediate the physiological changes in AGRP and POMC gene expression reviewed above. This is due in part to uncertainty over the status of leptin in birds, with leptin being a key regulator of the central melanocortin system in mammals (2). Leptin genes have recently been identified in several avian species after a 20-year search (73–77). However, they have low (about 30%) amino acid sequence identity with mammalian leptins, with variable tissue expression patterns between species and limited expression in adipose tissue (78). Mammalian leptins inhibit food intake in birds after central or peripheral injection but peripheral infusion of “chicken leptin” (later shown to be mouse leptin) had no effect on AGRP and POMC gene expression in young broilers (79). Thus, unlike the situation in mammals, there is currently no direct evidence to support an action of leptin on AGRP and POMC gene expression, but further investigation is needed that takes into account the new information on avian leptin.
A pronounced difference between birds and mammals is also apparent for the effects of ghrelin on food intake. There is commonality in that, as in mammals, ghrelin is expressed in the avian stomach (proventriculus) and its gene expression and circulating protein are increased by fasting and decreased by refeeding in layer chickens and Japanese quail (80). Also, in free-living garden warblers (Sylvia borin) sampled at a stopover site during spring migration, plasma ghrelin concentrations were positively associated with higher fat scores (81). However, unlike the situation in mammals, central and peripheral injections of mammalian and chicken ghrelin decrease, rather than stimulate, feeding (80, 81). There is evidence in mammals for a regulatory influence of ghrelin on AGRP/NPY neurons (82). Expression of the ghrelin receptor (GHSR) has been detected in the domestic chick hypothalamus (83), but its localization there, including in the arcuate nucleus, is unknown. The inhibitory effect of centrally administered ghrelin on food intake appeared to be mediated via corticotropin-releasing factor rather than through AGRP/NPY neurons because ghrelin injection did not influence NPY gene expression (84).
At the local tissue level, there is suggestive evidence for a link in birds between arcuate nucleus neuropeptide gene expression and hypothalamic energy sensing as there is in mammals. Immunoreactivity for the energy sensor AMP-activated protein kinase (AMPK) was observed in the chicken arcuate nucleus and food deprivation for 48 h increased phosphorylated AMPKα in parallel with AGRP mRNA (17, 85). In broiler chicks, 24 h food deprivation led to increased expression of the AMPK subunit mRNAs AMPKα2, AMPKβ1, AMPKβ2, and AMPKγ1 along with, respectively, increased and decreased AGRP and POMC mRNAs (16). When domestic broiler chicks were fed with the AMPK inhibitor α-lipoic acid (α-LPA), hypothalamic AMPKα1 mRNA was decreased along with food intake, confirming α-LPA’s inhibitory effect (86). However, the pattern of expression of AGRP and POMC was opposite to that predicted for a regulatory effect of AMPK. The same study (86) also confirmed the expression in the chick hypothalamus of hypoxia-inducible factor-1α, a nuclear transcription factor that influences POMC gene expression in mammals in response to local hypoxia (87). More generally, microarray and real-time PCR analysis of gene expression in the hypothalamus of broiler chicks food-deprived for 48 h suggested that fasting induces metabolic switching (16, 18). Genes associated with fatty acid oxidation and inhibition of glycolysis were upregulated, and those linked to fatty acid synthesis and transport downregulated, in parallel with increased AGRP and reduced POMC gene expression. The suggestion of metabolic switching was supported by central injection of compounds influencing glycolysis and fatty acid oxidation (18). Injection of α-LPA decreased food intake, which is consistent with the finding of the dietary administration study above and with α-LPA stimulating glycolysis through inhibition of pyruvate dehydrogenase kinase isoform 4 (18). In contrast, injection of the glycolytic inhibitor 2-deoxyglucose stimulated food intake. The possible importance of the metabolic sensor sirtuin 1 in mediating the metabolic switching was suggested by the fact that administration of its inhibitor NADH and activator NAD+, respectively, decreased and increased food intake (18). However, the effect of these manipulations on neuropeptide gene expression was not reported so that it is not clear to what extent the changes in food intake observed are directly attributable to regulatory effects on AGRP/NPY and POMC/CART neurons. Thus, overall, although suggestive, evidence for a direct effect of metabolic sensing on AGRP and POMC gene expression is lacking and further investigation is needed.
Signals that may influence central melanocortin system gene expression within the hypothalamus have been explored by microarray and pathway analysis in newly hatched broiler chicks that were food deprived for up to 48 h and re-fed (15). Functional interactions, supported by hypothalamic cell culture experiments, were identified within a network of six genes encoding the neuropeptide relaxin-3, the neuropeptide receptors NPY5R and somatostatin receptor 5, and the β2 adrenergic and metabotropic glutamate receptor 8 neurotransmitter receptors together with POMC, which appeared to play a central role. POMC expression was downregulated by fasting while the other genes were upregulated.
For metabolic hormones, evidence is needed for co-expression of hormone receptors in individual AGRP/NPY and POMC/CART neurons. So far, this has been established only for insulin. Before the discovery of leptin, insulin was favored in mammals as a long-term regulator of energy balance because fasting, or basal, concentrations report body fat content, the hormone is transported into the brain, and insulin receptors are present on AGRP/NPY neurons (2). It is less clear whether it plays a similar role in the long-term regulation of energy balance of birds: although circulating insulin concentrations are correlated with food intake in chickens, they did not differ under fasting conditions between selected lines of fat and lean birds (88). However, central injection of insulin decreased food intake in layer chicks and the effect was blocked by coadministration of the MC4R antagonist HS014 (89, 90). A direct effect of insulin on arcuate nucleus neurons was suggested by the demonstration of co-localization of insulin receptor immunoreactivity with that of NPY, and with α-MSH in individual neurons (91). Insulin stimulates POMC gene expression and inhibits that of AGRP and NPY in the brain of laboratory rodents (92), and this regulatory influence appears to have been conserved to some extent in birds. Thus, in the chick studies, central insulin injections consistently stimulated POMC expression, but a decrease in NPY mRNA was observed in one study and not another, and no decrease in AGRP mRNA was detected (89, 90). Overall, however, the results suggest an involvement of insulin in the response of POMC/CART and possibly AGRP/NPY neurons to fasting.
Other metabolic hormones that have a regulatory influence on AGRP/NPY and POMC/CART neurons in mammals are corticosterone and thyroid hormones. Circulating corticosterone is increased by fasting in birds and mammals and, respectively, increases and decreases AGRP and POMC gene expression in laboratory rodents (93, 94). There is evidence in domestic chicks for a suppressive effect of corticosterone on POMC expression (62, 95). However, there is more variability in the response of AGRP gene expression to centrally or peripherally administered corticosterone or to the glucocorticoid receptor agonist dexamethasone (62, 95–97). Thyroid hormones have already been mentioned in the context of preparation for migration above. There is evidence in both birds and mammals for a stimulatory effect of T3 on AGRP gene expression (63, 98). Thus, overall, there is some commonality in the regulatory influences of corticosterone and T3 on AGRP/NPY and POMC/CART neurons between birds and mammals.
We identified a possible link between signaling by the gut peptide CCK and AGRP expression in the context of growth as reviewed above (65). It is unclear whether the increased AGRP expression we observed in high-growth haplotype chickens is a secondary consequence of reduced CCKAR expression and experimental manipulations of CCK signaling in other chicken strains are needed. In mammals, there is evidence for an involvement of the central melanocortin system, both in the hindbrain and the hypothalamus, in the inhibitory effects of CCK on feeding because MC4R knockout mice show a reduced sensitivity (99, 100).
In addition to feedback effects by metabolic hormones, we have recently investigated the possibility for a sensitivity of AGRP gene expression to signals arising from gut fullness. This has been in the applied context of attempting to improve the welfare of broiler breeder chickens that experience prolonged hunger during the industrial practice of food restriction when birds receive a limited ration of food per day. As an alternative, it is possible to use alternative diets containing a high proportion of food that is normally high in fiber and of low energy density (101). This could potentially mitigate some of the undesirable effects of constant hunger by providing more total food and therefore more opportunity for the expression of natural foraging and ingestive behaviors. However, it is uncertain whether birds fed on such diets still experience a “metabolic hunger.” To address this, we provided restricted-fed 12-week-old broiler breeder birds with ad libitum access to food for 2 days compared to birds re-fed a diet over the same time period that was diluted with the non-nutritive bulking agent ispaghula husk, and to birds that remained on a restricted diet (64). We measured significantly increased AGRP expression and decreased POMC expression compared to fully fed controls in both food-restricted birds and in those receiving the dietary bulking agent. This suggests that AGRP and POMC gene expression is insensitive to mechanosensory signals relating to gut fullness. We are performing further experiments to confirm this using other more industrially relevant diet formulations.
The striking neuroanatomical conservation among vertebrates of the arcuate nucleus AGRP/NPY and POMC/CART neurons appears to be accompanied in birds by functional conservation in these cells of a coordinated signaling response to energy deprivation whereby AGRP/NPY neurons are stimulated and POMC/CART neurons are inhibited, which promotes replacement of lost energy stores when food becomes available. However, the picture is somewhat incomplete in birds compared to mammals with investigations tending to be skewed toward the measurement of gene expression rather than being focused on secretion of the peptides. Information is lacking on the connections of the neurons, both within and outside the hypothalamus, and little is known about the metabolic and energetic effects of the arcuate nucleus peptides distinct from their effects on food intake. Knowledge in birds has also been limited by a lack of availability of transgenic methods to assess the effects on energy balance of experimental genetic activation and inactivation, although such methods now appear to be close to routine application (102).
The use of unique avian models to explore aspects of the regulation of the central melanocortin system has highlighted possible differences from mammals and emphasized adaptations that may be representative of those in other non-mammalian vertebrates. These include changes in basal expression of AGRP and POMC in relation to seasonal changes in food intake, energy balance, and reproduction that tend not to be apparent in mammalian models (63). There are also situations in birds in which AGRP gene expression is dissociated from the pattern of food intake, particularly in relation to sexually dimorphic growth, which widens the perspective of investigations into the functions of the melanocortin system in birds and other vertebrates beyond the regulation of appetite.
The apparent functional conservation of changes in central melanocortin system gene expression in response to energy shortage is somewhat puzzling from the evidence available on how the system is regulated by feedback signals. Circulating leptin is acknowledged to play a prominent regulatory role on the system in mammals whereas in birds its sites of synthesis are variable and less focused on adipose tissue, and it appears to act more as an autocrine/paracrine signaling factor than as a circulating hormone (78). The recent discovery of avian leptin genes together with the knowledge that the pattern of expression is more representative of other non-mammals than mammals offers the opportunity for the regulation of the avian melanocortin system to be viewed from a new perspective. More commonality between birds and mammals is evident in the regulatory effects of other metabolic hormones such as insulin, corticosterone, and T3. However, it is presently unclear whether these hormones exert the main regulatory effects on the system with diminished input from leptin and ghrelin, or whether other regulatory mechanisms are present that are either ancestral and representative of other non-mammalian vertebrates rather than mammals or are unique and linked to the general adaptations that have evolved in birds to support flight.
TB wrote the article with substantial intellectual and editorial input from ID on its content and structure. Both authors approved the work for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Our work has been supported by DEFRA Grant AW1141 “Quantifying the subjective state of feed-restricted broiler chickens using behavioural and neurochemical measures” and by BBSRC Grant BB/L000571/1 “Investigating how the type and quantity of food affect foraging behaviour and the neural circuits controlling feeding in broiler breeder chickens.” The Roslin Institute is funded by the BBSRC through Institute Strategic Grant funding.
1. Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol (1998) 402:435–41. doi:10.1002/(SICI)1096-9861(19981228)402:4<435::AID-CNE1>3.3.CO;2-D
2. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature (2000) 404:661–71. doi:10.1038/35007534
3. Cerdá-Reverter JM, Anglade I, Martínez-Rodríguez G, Mazurais D, Muñoz-Cueto JA, Carrillo M, et al. Characterization of neuropeptide Y expression in the brain of a perciform fish, the sea bass (Dicentrarchus labrax). J Chem Neuroanat (2000) 19:197–210. doi:10.1016/S0891-0618(00)00063-6
4. Schiöth HB, Cerdá-Reverter JM, Peter RE. The central melanocortin system regulates food intake in goldfish. Regul Pept (2003) 115:101–13. doi:10.1016/S0167-0115(03)00144-7
5. Song Y, Golling G, Thacker TL, Cone RD. Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine (2003) 22:257–65. doi:10.1385/ENDO:22:3:257
6. Le HT, Angotzi AR, Ebbesson LO, Karlsen Ø, Rønnestad I. The ontogeny and brain distribution dynamics of the appetite regulators NPY, CART and pOX in larval Atlantic cod (Gadus morhua L). PLoS One (2016) 11:e0153743. doi:10.1371/journal.pone.0153743
7. Gerets HH, Peeters K, Arckens L, Vandesande F, Berghman LR. Sequence and distribution of pro-opiomelanocortin in the pituitary and brain of the chicken (Gallus gallus). J Comp Neurol (2000) 417:250–62. doi:10.1002/(SICI)1096-9861(20000207)417:2<250::AID-CNE9>3.0.CO;2-Z
8. Kameda Y, Miura M, Nishimaki T. Localization of neuropeptide Y mRNA and peptide in the chicken hypothalamus and their alterations after food deprivation, dehydration and castration. J Comp Neurol (2001) 436:376–88. doi:10.1002/cne.1074
9. Strader AD, Buntin JD. Neuropeptide-Y: a possible mediator of prolactin-induced feeding and regulator of energy balance in the ring dove (Streptopelia risoria). J Neuroendocrinol (2001) 13:386–92. doi:10.1046/j.1365-2826.2001.00642.x
10. Strader AD, Buntin JD. Changes in agouti-related peptide during the ring dove breeding cycle in relation to prolactin and parental hyperphagia. J Neuroendocrinol (2003) 15:1046–53. doi:10.1046/j.1365-2826.2003.01092.x
11. Phillips-Singh D, Li Q, Takeuchi S, Ohkubo T, Sharp PJ, Boswell T. Fasting differentially regulates expression of agouti-related peptide, pro-opiomelanocortin, prepro-orexin, and vasoactive intestinal polypeptide mRNAs in the hypothalamus of Japanese quail. Cell Tissue Res (2003) 313:217–25. doi:10.1007/s00441-003-0755-8
12. Mirabella N, Esposito V, Squillacioti C, De Luca A, Paino G. Expression of agouti-related protein (AgRP) in the hypothalamus and adrenal gland of the duck (Anas platyrhynchos). Anat Embryol (2004) 209:137–41. doi:10.1007/s00429-004-0431-0
13. Boswell T, Li Q, Takeuchi S. Neurons expressing neuropeptide Y mRNA in the infundibular nucleus of Japanese quail are activated by fasting and co-express agouti-related protein mRNA. Brain Res Mol Brain Res (2002) 100:31–42. doi:10.1016/S0169-328X(02)00145-6
14. Singh O, Kumar S, Singh U, Kumar V, Lechan RM, Singru PS. Cocaine- and amphetamine-regulated transcript peptide (CART) in the brain of zebra finch, Taeniopygia guttata: organization, interaction with neuropeptide Y, and response to changes in energy status. J Comp Neurol (2016) 524:3014–41. doi:10.1002/cne.24004
15. Higgins SE, Ellestad LE, Trakooljul N, McCarthy F, Saliba J, Cogburn LA, et al. Transcriptional and pathway analysis in the hypothalamus of newly hatched chicks during fasting and delayed feeding. BMC Genomics (2010) 11:162. doi:10.1186/1471-2164-11-162
16. Lei L, Lixian Z. Effect of 24 h fasting on gene expression of AMPK, appetite regulation peptides and lipometabolism related factors in the hypothalamus of broiler chicks. Asian-Australas J Anim Sci (2012) 25:1300–8. doi:10.5713/ajas.2012.12153
17. Song Z, Liu L, Yue Y, Jiao H, Lin H, Sheikhahmadi A, et al. Fasting alters protein expression of AMP-activated protein kinase in the hypothalamus of broiler chicks (Gallus gallus domesticus). Gen Comp Endocrinol (2012) 178:546–55. doi:10.1016/j.ygcen.2012.06.026
18. Fang X-L, Zhu X-T, Chen S-F, Zhang Z-Q, Zeng Q-J, Deng L, et al. Differential gene expression pattern in hypothalamus of chickens during fasting-induced metabolic reprogramming: functions of glucose and lipid metabolism in the feed intake of chickens. Poult Sci (2014) 93:2841–54. doi:10.3382/ps.2014-04047
19. Mench JA. Broiler breeders: feed restriction and welfare. Worlds Poult Sci J (2002) 58:23–9. doi:10.1079/WPS20020004
20. Dunn IC, Wilson PW, Smulders TV, Sandilands V, D’Eath RB, Boswell T. Hypothalamic agouti-related protein expression is affected by both acute and chronic experience of food restriction and re-feeding in chickens. J Neuroendocrinol (2013) 25:920–8. doi:10.1111/jne.12088
21. Henry BA, Rao A, Ikenasio BA, Mountjoy KG, Tilbrook AJ, Clarke IJ. Differential expression of cocaine- and amphetamine-regulated transcript and agouti related-protein in chronically food-restricted sheep. Brain Res (2001) 918:40–50. doi:10.1016/S0006-8993(01)02918-3
22. Gutman R, Hacmon-Keren R, Choshniak I, Kronfeld-Schor N. Effect of food availability and leptin on the physiology and hypothalamic gene expression of the golden spiny mouse: a desert rodent that does not hoard food. Am J Physiol Regul Integr Comp Physiol (2008) 295:R2015–23. doi:10.1152/ajpregu.00105.2008
23. Mercer JG, Moar KM, Logie TJ, Findlay PA, Adam CL, Morgan PJ. Seasonally inappropriate body weight induced by food restriction: effect on hypothalamic gene expression in male Siberian hamsters. Endocrinology (2001) 142:4173–81. doi:10.1210/endo.142.10.8454
24. Bertile F, Oudart H, Criscuolo F, Le Maho Y, Raclot T. Hypothalamic gene expression in long-term fasted rats: relationship with body fat. Biochem Biophys Res Commun (2003) 303:1106–13. doi:10.1016/S0006-291X(03)00481-9
25. Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol (2003) 285:R1030–6. doi:10.1152/ajpregu.00734.2002
26. Kinzig KP, Hargrave SL, Tao EE. Central and peripheral effects of chronic food restriction and weight restoration in the rat. Am J Physiol Endocrinol Metab (2009) 296:E282–90. doi:10.1152/ajpendo.90523.2008
27. Hen G, Yosefi G, Simchaev V, Shinder D, Hruby VJ, Friedman-Einat M. The melanocortin circuit in obese and lean strains of chicks. J Endocrinol (2006) 190:527–35. doi:10.1677/joe.1.06783
28. Pritchard LE, Oliver RL, McLoughlin JD, Birtles S, Lawrence CB, Turnbull AV, et al. Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology (2003) 144:760–6. doi:10.1210/en.2002-220866
29. Li J-Y, Finniss S, Yang Y-K, Zeng Q, Qu S-Y, Barsh G, et al. Agouti-related protein-like immunoreactivity: characterization of release from hypothalamic tissue and presence in serum. Endocrinology (2000) 141:1942–50. doi:10.1210/en.141.6.1942
30. Perello M, Stuart RC, Nillni EA. Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. Am J Physiol Endocrinol Metab (2007) 292:E1348–57. doi:10.1152/ajpendo.00466.2006
31. Breen TL, Conwell IM, Wardlaw SL. Effects of fasting, leptin, and insulin on AGRP and POMC peptide release in the hypothalamus. Brain Res (2005) 1032:141–8. doi:10.1016/j.brainres.2004.11.008
32. Yang Y, Arasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell (2011) 146:992–1003. doi:10.1016/j.cell.2011.07.039
33. Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife (2015) 4:e071122. doi:10.7554/eLife.07122
34. Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci (2011) 14:351–5. doi:10.1038/nn.2739
35. Strader AD, Schiöth HB, Buntin JD. The role of the melanocortin system and the melanocortin-4 receptor in ring dove (Streptopelia risoria) feeding behavior. Brain Res (2003) 960:112–21. doi:10.1016/S0006-8993(02)03799-X
36. Ziegler HP. Feeding behavior in the pigeon: a neurobehavioral analysis. In: Goodman IJ, Schein MW, editors. Birds: Brain and Behavior. New York: Academic Press (1974). p. 101–32.
37. Ling MK, Hotta E, Kilianova Z, Haitina T, Ringholm A, Johanson L, et al. The melanocortin receptor subtypes in chicken have high preference to ACTH-derived peptides. Br J Pharmacol (2004) 143:626–37. doi:10.1038/sj.bjp.0705900
38. Schulz C, Paulus K, Lobmann R, Dallman M, Lehnert H. Endogenous ACTH, not only α-melanocyte-stimulating hormone, reduces food intake mediated by hypothalamic mechanisms. Am J Physiol Endocrinol Metab (2010) 298:E237–44. doi:10.1152/ajpendo.00408.2009
39. Deviche P, Delius JD. Short-term modulation of domestic pigeon (Columbia livia L.) behaviour induced by intraventricular administration of ACTH. Z Tierpsychol (1981) 55:335–42. doi:10.1111/j.1439-0310.1981.tb01276.x
40. Shipp SL, Yi J, Dridi S, Gilbert ER, Cline MA. The central anorexigenic mechanism of adrenocorticotropic hormone involves the caudal hypothalamus in chicks. Neuropeptides (2015) 53:29–35. doi:10.1016/j.npep.2015.07.005
41. Deviche P, Schepers G. Intracerebroventricular injection of ostrich β-endorphin to satiated pigeons induces hyperphagia but not hyperdipsia. Peptides (1984) 5:691–4. doi:10.1016/0196-9781(84)90008-1
42. McCormack JF, Denbow DM. Feeding, drinking and temperature responses to intracerebroventricular beta-endorphin in the domestic fowl. Peptides (1988) 9:709–15. doi:10.1016/0196-9781(88)90110-6
43. Saneyasu T, Honda K, Kamisoyama H, Nakayama Y, Ikegami K, Hasegawa S. Alpha-melanocyte stimulating hormone plays an important role in the regulation of food intake by the central melanocortin system in chicks. Peptides (2011) 32:996–1000. doi:10.1016/j.peptides.2011.03.006
44. Kawakami S-I, Bungo T, Ando R, Ohgushi A, Shimojo M, Masuda Y, et al. Central injection of α-melanocyte-stimulating hormone inhibits fasting- and neuropeptide Y-induced feeding in neonatal chicks. Eur J Pharmacol (2000) 398:361–4. doi:10.1016/S0014-2999(00)00344-7
45. Tachibana T, Sugahara K, Ohgushi A, Ando R, Kawakami S, Yoshimatsu T, et al. Intracerebroventricular injection of agouti-related protein attenuates the anorexigenic effect of alpha-melanocyte stimulating hormone in neonatal chicks. Neurosci Lett (2001) 305:131–4. doi:10.1016/S0304-3940(01)01827-4
46. Cline MA, Smith ML. Central alpha-melanocyte stimulating hormone attenuates behavioral effects of neuropeptide Y in chicks. Physiol Behav (2007) 91:588–92. doi:10.1016/j.physbeh.2007.03.021
47. Smith ML, Kohart NA, Newmyer BA, Cline MA. Gamma(2)-melanocyte stimulating hormone decreases food intake in chicks. Neurosci Lett (2009) 465:210–3. doi:10.1016/j.neulet.2009.08.021
48. Cline MA, Nandar W, Bowden C, Hein PP, Denbow DM, Siegel PB. Differential feeding responses to central alpha-melanocyte stimulating hormone in genetically low and high body weight selected lines of chickens. Life Sci (2008) 83:208–13. doi:10.1016/j.lfs.2008.06.003
49. Honda K, Saneyasu T, Hasegawa S, Kamisoyama H. A comparative study of the central effects of melanocortin peptides on food intake in broiler and layer chicks. Peptides (2012) 37:13–7. doi:10.1016/j.peptides.2012.06.015
50. Ka S, Lindberg J, Strömstedt L, Fitzsimmons C, Lindqvist N, Lundeberg J, et al. Extremely different behaviours in high and low body weight lines of chicken are associated with differential expression of genes involved in neuronal plasticity. J Neuroendocrinol (2009) 21:208–16. doi:10.1111/j.1365-2826.2009.01819.x
51. Yi J, Gilbert ER, Siegel PB, Cline MA. Fed and fasted chicks from lines divergently selected for low or high body weight have differential hypothalamic appetite-associated factor mRNA expression profiles. Behav Brain Res (2015) 286:58–63. doi:10.1016/j.bbr.2015.02.008
52. Takeuchi S, Takahashi S. Melanocortin receptor genes in the chicken – tissue distributions. Gen Comp Endocrinol (1998) 112:220–31. doi:10.1006/gcen.1998.7167
53. Sharp PJ. Broodiness and broody control. In: Hocking PM, editor. Biology of Breeding Poultry. Wallingford, UK: CABI Publishing (2009). p. 181–205.
54. Savory CJ. Changes in food intake and body weight of bantam hens during breeding. Appl Anim Ethol (1979) 5:283–8. doi:10.1016/0304-3762(79)90062-2
55. Sherry DF, Mrosovsky N, Hogan JA. Weight loss and anorexia during incubation in birds. J Comp Physiol Psychol (1980) 94:89–98. doi:10.1037/h0077647
56. Mrosovsky N, Sherry DF. Animal anorexias. Science (1980) 207:837–42. doi:10.1126/science.6928327
57. Dunn IC, Wilson PW, D’Eath RB, Boswell T. Hypothalamic agouti-related peptide mRNA is elevated during natural and stress-induced anorexia. J Neuroendocrinol (2015) 27:681–91. doi:10.1111/jne.12295
58. Cornelius JM, Boswell T, Jenni-Eiermann S, Breuner CW, Ramenofsky M. Contributions of endocrinology to the migration life history of birds. Gen Comp Endocrinol (2013) 190:47–60. doi:10.1016/j.ygcen.2013.03.027
59. King JR, Barker S, Farner DS. A comparison of energy reserves during autumnal and vernal migratory periods in the white crowned sparrow, Zonotrichia leucophrys gambelii. Ecology (1963) 44:513–21. doi:10.2307/1932530
60. Nishiwaki-Ohkawa T, Yoshimura T. Molecular basis for regulating seasonal reproduction in vertebrates. J Endocrinol (2016) 229:R117–27. doi:10.1530/JOE-16-0066
61. Murphy M, Ebling FJP. The role of hypothalamic tri-iodothyronine availability in seasonal regulation of energy balance and body weight. J Thyroid Res (2011) 2011:387562. doi:10.4061/2011/387562
62. Byerly MS, Simon J, Lebihan-Duval E, Duclos MJ, Cogburn LA, Porter TE. Effects of BDNF, T3, and corticosterone on expression of the hypothalamic obesity gene network in vivo and in vitro. Am J Physiol (2009) 296:R1180–9. doi:10.1152/ajpregu.90813.2008
63. Ebling FJP. On the value of seasonal mammals for identifying mechanisms underlying the control of food intake and body weight. Horm Behav (2014) 66:56–65. doi:10.1016/j.yhbeh.2014.03.009
64. Caughey S, Wilson P, Mukhtar N, D’Eath R, Dunn I, Boswell T. Sex differences in basal hypothalamic anorectic and orexigenic gene expression after re-feeding with normal and non-nutritive diets. In: Bédécarrats G, editor. ISAE 2016: Program and Abstracts of the 11th International Symposium on Avian Endocrinology, Oct 11-14. Niagara, Canada (2016). 58 p.
65. Dunn IC, Meddle SL, Wilson PW, Wardle CA, Law AS, Bishop VR, et al. Decreased expression of the satiety signal receptor CCKAR is responsible for increased growth and body weight during the domestication of chickens. Am J Physiol (2013) 304:E909–21. doi:10.1152/ajpendo.00580.2012
66. Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell (1997) 88:131–41. doi:10.1016/S0092-8674(00)81865-6
67. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med (2003) 348:1085–95. doi:10.1056/NEJMoa022050
68. Martinelli CE, Keogh JM, Greenfield JR, Henning E, van der Klaauw AA, Blackwood A, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab (2011) 96:E181–8. doi:10.1210/jc.2010-1369
69. Tan HY, Steyn FJ, Huang L, Cowley M, Veldhuis JD, Chen C. Hyperphagia in male melanocortin 4 receptor deficient mice promotes growth independently of growth hormone. J Physiol (2016) 594:7309–26. doi:10.1113/JP272770
70. Guillot R, Cortés R, Navarro S, Mischitelli M, García-Herranz V, Sánchez E, et al. Behind melanocortin antagonist overexpression in the zebrafish brain: a behavioral and transcriptomic approach. Horm Behav (2016) 82:87–100. doi:10.1016/j.yhbeh.2016.04.011
71. Zhang C, Forlano PM, Cone RD. AgRP and POMC neurons are hypophysiotropic and coordinately regulate multiple endocrine axes in a larval teleost. Cell Metab (2012) 15:256–64. doi:10.1016/j.cmet.2011.12.014
72. Song Y, Cone RD. Creation of a genetic model of obesity in a teleost. FASEB J (2007) 21:2042–9. doi:10.1096/fj.06-7503com
73. Prokop JW, Schmidt C, Gasper D, Duff RJ, Milsted A, Ohkubo T, et al. Discovery of the elusive leptin in birds: identification of several ‘missing links’ in the evolution of leptin and its receptor. PLoS One (2014) 9:e92751. doi:10.1371/journal.pone.0092751
74. Friedman-Einat M, Cogburn LA, Yosefi S, Hen G, Shinder D, Shirak A, et al. Discovery and characterization of the first genuine avian leptin gene in the rock dove (Columba livia). Endocrinology (2014) 155:3376–84. doi:10.1210/en.2014-1273
75. Huang G, Li J, Wang H, Lan X, Wang Y. Discovery of a novel functional leptin protein (LEP) in zebra finches: evidence for the existence of an authentic avian leptin gene predominantly expressed in the brain and pituitary. Endocrinology (2014) 155:3389–96. doi:10.1210/en.2014-1084
76. Friedman-Einat M, Seroussi E. Quack leptin. BMC Genomics (2014) 15:551. doi:10.1186/1471-2164-15-551
77. Seroussi E, Cinnamon Y, Yosefi S, Genin O, Smith JG, Rafati N, et al. Identification of the long-sought leptin in chicken and duck: expression pattern of the highly GC-rich avian leptin fits an autocrine/paracrine rather than endocrine function. Endocrinology (2016) 157:737–51. doi:10.1210/en.2015-1634
78. Boswell T, Dunn IC. Regulation of the avian central melanocortin system and the role of leptin. Gen Comp Endocrinol (2015) 221:278–83. doi:10.1016/j.ygcen.2014.12.009
79. Dridi S, Swennen Q, Decuypere E, Buyse J. Mode of leptin action in chicken hypothalamus. Brain Res (2005) 1047:214–23. doi:10.1016/j.brainres.2005.04.034
80. Kaiya H, Kangawa K, Miyazato M. Update on ghrelin biology in birds. Gen Comp Endocrinol (2013) 190:170–5. doi:10.1016/j.ygcen.2013.04.014
81. Goymann W, Lupi S, Kaiya H, Cardinale M, Fusani L. Ghrelin affects stopover decisions and food intake in a long-distance migrant. Proc Natl Acad Sci U S A (2017) 114:1946–51. doi:10.1073/pnas.1619565114
82. Wang Q, Liu C, Uchida A, Chuang J-C, Walker A, Liu T, et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab (2014) 3:64–72. doi:10.1016/j.molmet.2013.10.001
83. Nie Q, Fang M, Xie L, Peng X, Xu H, Luo C, et al. Molecular characterization of the ghrelin and ghrelin receptor genes and effects on fat deposition in chicken and duck. J Biomed Biotechnol (2009) 2009:567120. doi:10.1155/2009/567120
84. Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, et al. Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept (2005) 15:201–8. doi:10.1016/j.regpep.2004.09.003
85. Proszkowiec-Weglarz M, Richards MP, Ramachandran R, McMurtry JP. Characterization of the AMP-activated protein kinase pathway in chickens. Comp Biochem Physiol B (2006) 143:92–106. doi:10.1016/j.cbpb.2005.10.009
86. Wang Y, Song Z, Everaert N, De Ketelaere B, Willemsen H, Decuypere E, et al. The anorectic effects of alpha-lipoicacid are mediated by central AMPK and are not due to taste aversion in chicken (Gallus gallus). Physiol Behav (2014) 132:66–72. doi:10.1016/j.physbeh.2014.04.047
87. Zhang H, Zhang G, Gonzalez FJ, Park SM, Cai D. Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol (2011) 9:e1001112. doi:10.1371/journal.pbio.1001112
88. Simon J. Chicken as a useful species for the comprehension of insulin action. Crit Rev Poult Biol (1989) 2:121–48.
89. Honda K, Kamisoyama H, Saneyasu T, Sugahara K, Hasegawa S. Central administration of insulin suppresses food intake in chicks. Neurosci Lett (2007) 423:153–7. doi:10.1016/j.neulet.2007.07.004
90. Shiraishi J, Yanagita K, Fujita M, Bungo T. Central insulin suppresses feeding behavior via melanocortins in chicks. Domest Anim Endocrinol (2008) 34:223–8. doi:10.1016/j.domaniend.2007.05.002
91. Shiraishi J, Tanizawa H, Fujita M, Kawakami S, Bungo T. Localization of hypothalamic insulin receptor in neonatal chicks: evidence for insulinergic system control of feeding behavior. Neurosci Lett (2011) 491:177–80. doi:10.1016/j.neulet.2011.01.031
92. Porte D Jr, Baskin DG, Schwartz MW. Leptin and insulin action in the central nervous system. Nutr Rev (2002) 60:S20–9. doi:10.1301/002966402320634797
93. Harvey S, Klandorf H. Reduced adrenocortical function and increased thyroid function in fasted and refed chickens. J Endocrinol (1983) 98:129–35. doi:10.1677/joe.0.0980129
94. Makimura H, Mizuno T, Isoda F, Beasley J, Silverstein J, Mobbs C. Role of glucocorticoids in mediating effects of fasting and diabetes on hypothalamic gene expression. BMC Physiol (2003) 3:5. doi:10.1186/1472-6793-3-4
95. Liu L, Song Z, Sheikhahmadi A, Jiao H, Lin H. Effect of corticosterone on gene expression of feed intake regulatory peptides in laying hens. Comp Biochem Physiol B (2012) 162:81–7. doi:10.1016/j.cbpb.2012.04.005
96. Liu L, Song Z, Jiao H, Lin H. Glucocorticoids increase NPY gene expression via hypothalamic AMPK signaling in broiler chicks. Endocrinology (2014) 155:2190–8. doi:10.1210/en.2013-1632
97. Liu L, Xu S, Wang X, Jiao H, Zhao J, Lin H. Effect of dexamethasone on hypothalamic expression of appetite-related genes in chickens under different diet and feeding conditions. J Anim Sci Biotechnol (2016) 7:23. doi:10.1186/s40104-016-0084-x
98. Herwig A, Campbell G, Mayer CD, Boelen A, Anderson RA, Ross AW, et al. A thyroid hormone challenge in hypothyroid rats identifies T3 regulated genes in the hypothalamus and in models with altered energy balance and glucose homeostasis. Thyroid (2014) 24:1575–93. doi:10.1089/thy.2014.0169
99. Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci (2004) 7:335–6. doi:10.1038/nn1214
100. Blevins JE, Morton GJ, Williams DL, Caldwell DW, Bastian LS, Wisse BE, et al. Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8. Am J Physiol (2009) 296:R476–84. doi:10.1152/ajpregu.90544.2008
101. D’Eath RB, Tolkamp BJ, Kyriazakis I, Lawrence AB. ‘Freedom from hunger’ and preventing obesity: the animal welfare implications of reducing food quantity or quality. Anim Behav (2009) 77:275–88. doi:10.1016/j.anbehav.2008.10.028
Keywords: melanocortin, AGRP, pro-opiomelanocortin, melanocortin 4 receptor, hypothalamus, birds, leptin
Citation: Boswell T and Dunn IC (2017) Regulation of Agouti-Related Protein and Pro-Opiomelanocortin Gene Expression in the Avian Arcuate Nucleus. Front. Endocrinol. 8:75. doi: 10.3389/fendo.2017.00075
Received: 30 January 2017; Accepted: 27 March 2017;
Published: 13 April 2017
Edited by:
Maximilian Michel, University of Michigan, USAReviewed by:
Kazuhisa Honda, Kobe University, JapanCopyright: © 2017 Boswell and Dunn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy Boswell, dGltb3RoeS5ib3N3ZWxsQG5jbC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.