- 1Department of Medicine–Endocrinology, Diabetes, and Clinical Nutrition, OHSU Northwest Pituitary Center, Oregon Health & Science University, Portland, OR, USA
- 2Department of Neurological Surgery, OHSU Northwest Pituitary Center, Oregon Health & Science University, Portland, OR, USA
Subcutaneous (SC) injection of pasireotide, a somatostatin analog, is approved for the treatment of adults with Cushing’s disease (CD) for whom pituitary surgery was unsuccessful or is not an option. We highlight the symptomatic and biochemical improvement of six patients with recurrent CD treated with pasireotide SC at a single center for at least 1 year. Patients were treated either through commercial use (n = 5) or through the Phase 3 trial (n = 1; http://ClinicalTrials.gov identifier, NCT00434148; study number, B2305). Most patients (n = 5) were female, and the mean age at diagnosis was 35.8 years. All patients demonstrated biochemical control at 1 year of treatment. Three of the five real-world patients followed for more than 1 year remain on pasireotide SC and are controlled. Two patients discontinued pasireotide SC; one patient because of persistently elevated urinary-free cortisol levels and gallstones, and the other because of treatment for an unrelated brain tumor. Symptomatic improvement varied, but all patients demonstrated weight loss. Nausea and mild, transient injection-site reactions were the most frequently reported adverse events. Although glycated hemoglobin (HbA1c) increased after treatment initiation, four of five patients maintained HbA1c levels ≤7.0% while receiving pasireotide SC and concomitant individualized diabetes medication, if necessary. In patients who discontinued pasireotide SC, HbA1c levels decreased within 6 weeks. This report documents real-world use of pasireotide SC and indicates its effectiveness as a long-term treatment option for patients with CD. Although hyperglycemia was observed in most patients, it was managed with appropriate monitoring and treatment and was reversible upon discontinuation of pasireotide SC.
Background
Cushing’s disease (CD) has an estimated incidence of 6.2–7.6 per million person-years in the United States (1) and is the most common cause of Cushing’s syndrome, accounting for nearly 70% of cases (2). CD is characterized by an elevation in cortisol levels caused by an adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma (3, 4). Failure to accurately identify and treat patients with the disease could lead to an increased risk of mortality and worsened morbidity compared with the control population. Diagnosis of CD may be challenging because many of the signs and symptoms of hypercortisolism are non-specific, and similar to common health conditions such as hypertension, impaired glucose tolerance (diabetes), obesity, and osteoporosis (3, 4).
While transsphenoidal surgical resection of the corticotroph tumor is the recommended first-line treatment for patients with CD (3–6), lifelong recurrence rates are 23–33% after initial remission (5). Results from a meta-analysis of 74 studies published between 1976 and 2014 showed that mean remission rates in patients with pituitary microadenomas and macroadenomas were 82.1% (median, 85.7%; range, 48.7–100%) and 62.3% (median, 64.1%; range, 30.8–100%), respectively (6). Mean recurrence rates reported in patients with microadenomas and macroadenomas were 11.7% (median, 10.9%; range, 0–36.4%) and 18.8% (median, 13.9%; range 0–59%), respectively (6). In addition, surgical failures, including disease persistence and recurrence after surgery, are more common in patients with macroadenomas (mean, 48.8%; median, 52.2%; range, 0–71.4%) than in patients with microadenomas (mean, 25%; median, 21.2%; range, 0–55.5%) (6). Thus, in cases of postsurgical disease recurrence or those in which surgery is not appropriate, medical therapy plays an important role in the long-term management of CD (5–7).
Pasireotide subcutaneous (SC) injection is a somatostatin analog approved for the treatment of adults with CD for whom pituitary surgery was unsuccessful or is not an option (7). In a Phase 3 trial in patients with CD (http://ClinicalTrials.gov identifier, NCT00434148; study number B2305), treatment with pasireotide SC was associated with normalization of urinary-free cortisol (UFC) levels at 6 months in 15% of patients treated with a 0.6-mg twice-daily (bid) dose and 26% of patients treated with a 0.9-mg bid dose (8). The B2305 study also demonstrated significant improvements in the signs and symptoms of CD and reductions in pituitary tumor volume. A separate analysis of the data from this Phase 3 trial showed marked improvements in the signs and symptoms of CD during 12 months of pasireotide treatment while UFC levels decreased (9).
Overall, there are few reports of long-term treatment with pasireotide SC. Data obtained in the open-ended, open-label extension trial of the B2305 study showed that the mean decrease in the UFC level after 12 months of treatment (57.3%) was maintained through 24 months (62.1%), suggesting that the efficacy and clinical benefit of pasireotide SC are sustained over a longer period of time (10). A single-center study in Italy reported that treatment with pasireotide led to a substantial reduction in tumor size within 6–12 months of initiation in a subset of eight patients from the B2305 study (11). In a 5-year follow-up of 16 patients who remained on treatment, UFC reductions and clinical improvements were found to be sustained (12).
Data on patients with CD in a non-research setting are limited. This article describes the symptomatic and biochemical responses of six patients with recurrent CD treated with pasireotide SC for at least 1 year at a single center, five of whom were treated in a real-world setting.
Introduction
Five patients were treated with pasireotide SC for at least 1 year as part of standard clinical care at the Oregon Health & Science University, Northwest Pituitary Center, Portland, OR, USA. Chart review was performed to assess patient baseline characteristics, treatment course, symptomatic and biochemical responses, adverse events (AEs), and the tolerability of pasireotide SC over time. UFC assays were performed at a single diagnostic laboratory using liquid chromatography-tandem mass spectrometry (LC-MS/MS). All glycated hemoglobin (HbA1c) analyses were performed using an immunochemical assay; salivary cortisol assays were performed at the same laboratory using enzyme-linked immunosorbent methodology. One additional patient at this center was part of the B2305 study group, which was a Phase 3, double-blind, randomized, multicenter trial that evaluated the control of UFC by pasireotide SC in patients with confirmed persistent or recurrent CD after surgery, or those with newly diagnosed CD who were not candidates for surgery (http://ClinicalTrials.gov identifier, NCT00434148). The primary endpoint was normalization of UFC level. Other endpoints included changes in serum and salivary cortisol levels over time, changes in clinical signs and symptoms, quality of life, and safety. Patients were randomly assigned to receive pasireotide SC at doses of 0.6 or 0.9 mg bid. This study design has been previously described in detail by Colao et al. (8). The Oregon Health & Science University institutional review board (IRB) approved both the prospective and the retrospective study, which included these patients and data analysis for this study. Written informed consent from individual patients in the retrospective study was waived per IRB protocol. Written informed consent was obtained from the patient in the prospective clinical trial.
Investigation
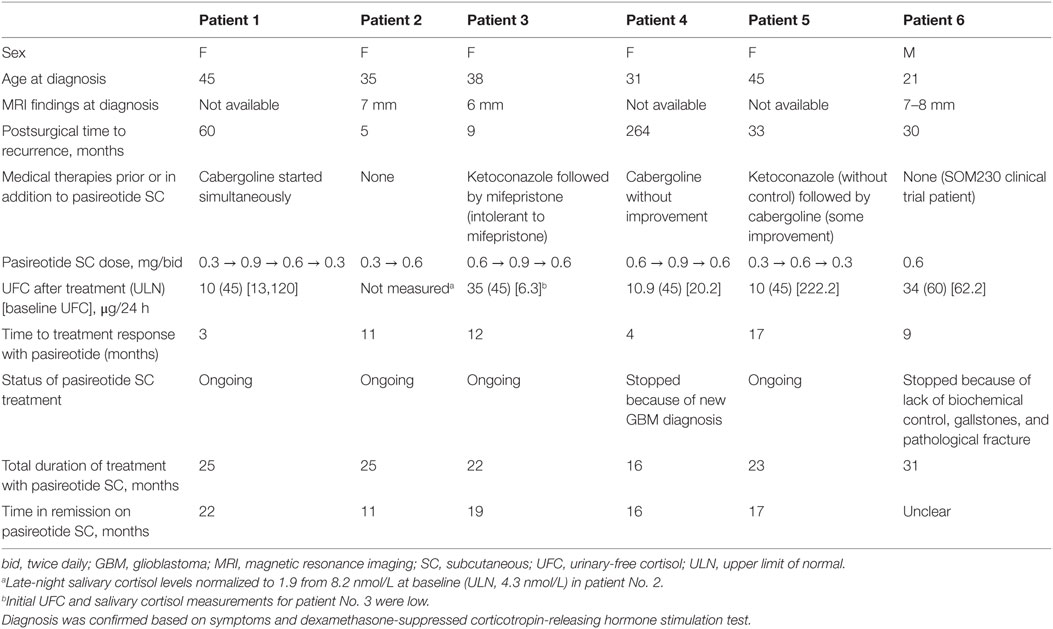
The mean age of the six study patients at diagnosis was 35.8 years, and most patients were female (n = 5; Table 1). The mean disease duration was 12 years (range, 3–25 years). The most common symptoms reported by patients included abnormal weight gain, fatigue, cushingoid face, and ecchymosis. All patients underwent surgery, and the median time to recurrence after surgery was approximately 3 years (range, 5 months–22 years). All patients had at least 1 elevated salivary cortisol value at the time of recurrence.
Treatment
Three patients were initially treated with pasireotide SC 0.6 mg bid, and three patients received an initial dose of 0.3 mg bid. Dosing was increased to 0.9 mg bid in three patients; dosing in these patients was subsequently reduced to 0.6 mg bid. The dose was further reduced to 0.3 mg bid in two patients. Two patients were naive to medical treatment (one of these patients was included in the B2305 study group), and the remaining four patients initiated pasireotide SC due to failure of or intolerance to other medical therapies (e.g., ketoconazole, mifepristone, cabergoline; Table 1). Pasireotide SC was used in combination with cabergoline (1.0–1.5 mg twice weekly) in two of the real-world setting patients. The mean duration of treatment in all cases was 23.6 months (range, 16–31 months). The average time to treatment response was 4 months.
Outcome and Follow-up
All patients demonstrated biochemical and clinical control at 1 year of treatment (e.g., Figure 1). Biochemical control was defined as normalization of either UFC or salivary cortisol based on current clinical guidelines and expert opinion publications. Four patients remain on pasireotide SC, three of whom are controlled. The remaining patient, who continues on 0.3 mg bid pasireotide SC therapy, had elevated salivary cortisol values shortly after 1 year of therapy, for which the dose was titrated up. Patients were monitored for 24-h UFC levels, serum cortisol, and ACTH, starting 1 month after the initiation of treatment and continuing every 3 months thereafter until cortisol levels normalized. The drug dose was titrated (with pasireotide SC uptitrated or downtitrated by 0.3 mg bid at each step, except for patient No. 1, for whom the pasireotide SC dose was initially uptitrated by 0.6 mg bid) to maintain cortisol levels and vital signs within normal ranges and avoid symptoms of adrenal insufficiency.
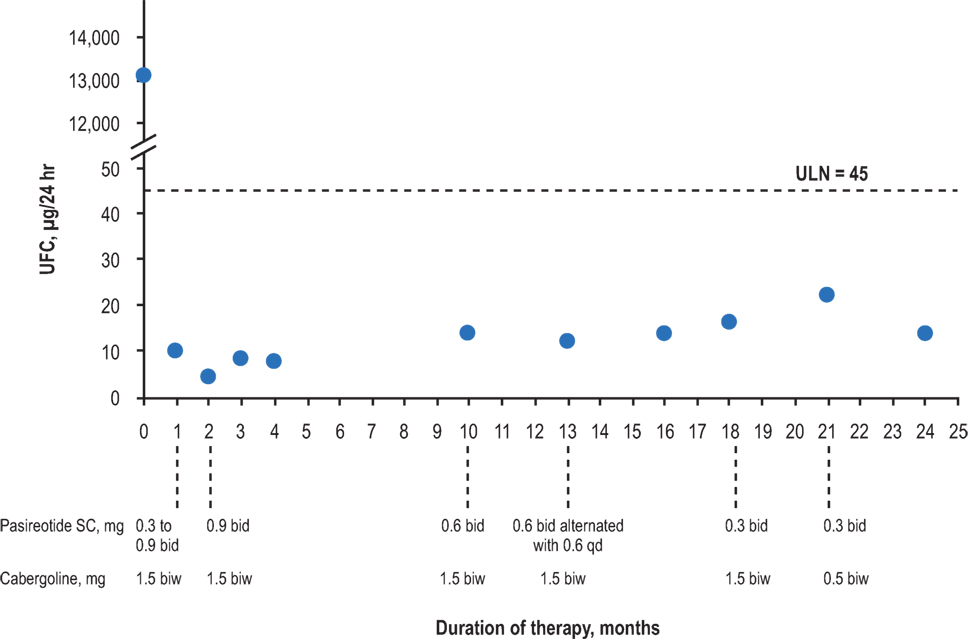
Figure 1. Urinary-free cortisol levels of patient No. 1 over the duration of treatment. bid, twice daily; biw, twice weekly; qd, once daily; UFC, urinary-free cortisol; ULN, upper limit of normal.
The patient treated in the B2305 study setting (patient No. 6) was controlled at the end of the study. However, patient No. 6 ultimately had recurrent UFC level elevations and gallstones that required discontinuation of pasireotide SC after 31 months of treatment. Another patient (patient No. 4) discontinued pasireotide SC treatment due to an unrelated brain tumor diagnosis and began high-dose dexamethasone treatment for that condition. The mean duration of treatment for patients continuing on the drug was 15 months (range, 12–17 months), with a mean time to normalization of UFC or late-night salivary cortisol levels of 6 months (range, 3–11 months). Specific symptomatic improvements varied across the six patients, but all patients demonstrated weight loss.
All patients reported nausea, and three patients experienced injection-site reactions. Both AEs were considered to be mild in severity and transient. Nausea resolved with administration of injections after meals. Injection-site reactions were self-limiting; however, pretreatment with ibuprofen and the administration of ice to the site prior to injection were prescribed as initial treatment. The average baseline HbA1c level was 5.9% (range, 4.8–7.5%), with increases ranging from 0.6 to 3.1% (Figure 2). Four out of the five real-world patients exhibited HbA1c ≤7.0% at the last visit while receiving pasireotide SC. One of these four patients (patient No. 5) had a normal HbA1c reading throughout the treatment and did not require antidiabetic medication. Patient No. 2 was diagnosed with diabetes mellitus before initiating treatment with pasireotide. Hyperglycemia was managed in all other cases by the provision of concomitant and individualized medical treatment, as necessary. Patient No. 1 received naglitinide 60 mg three times daily (tid), initially with 0.9 mg of pasireotide SC; naglitinide treatment was discontinued after approximately 8 months and the pasireotide SC dose was reduced to 0.6 mg. Similar discontinuation of diabetic medication was observed in patient No. 4, who received glimepiride 2 mg and sitagliptin 50 mg daily, initially with 0.6 mg of pasireotide SC. Patient No. 4’s HbA1c increased from 5.8 to 8.9% after initiating pasireotide treatment. This patient was then administered antidiabetic treatment with glimepiride 2 mg daily and sitagliptin 50 mg daily, which reduced HbA1c levels to 6.5%. Pasireotide SC was increased after 2 months to 0.9 mg. After 18 months, the pasireotide SC dose was reduced to 0.6 mg; after 26 months, the diabetic medications were discontinued. HbA1c remained stable at 6.5% after discontinuation of antiglycemics. Patient No. 3 received metformin 500 mg bid and glimepiride 2 mg daily with pasireotide SC 0.6 mg. Metformin was increased to 1,000 mg bid to normalize HbA1c levels after 28 months of treatment. Patient No. 2 received glargine 36 U daily and aspart 12 U tid, initially with 0.3 mg pasireotide SC. After 8 months, metformin 1,000 mg bid was initiated and glargine dosing was increased to 48–50 U daily. At month 15, the pasireotide SC dose was increased to 0.6 mg and sitagliptin 100 mg was taken daily; HbA1c levels remained >7%, including after glargine was reduced to 26 U daily.
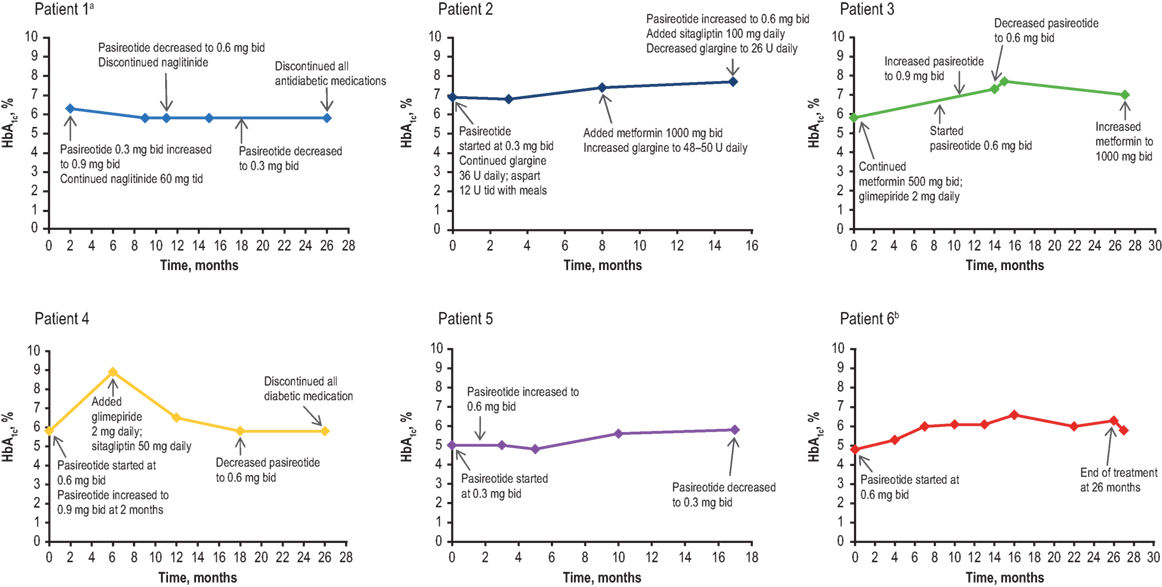
Figure 2. HbA1c levels of patients over the duration of treatment. bid, twice daily; HbA1c, glycated hemoglobin; tid, three times daily. aBaseline data not available. bMetformin 500 mg bid was prescribed before the initial HbA1c result and was stopped after 11 days when HbA1c results showed low values.
The B2305 study patient (patient No. 6) was initially treated with metformin 500 mg bid, which was reduced in 1 week to 500 mg daily, but treatment was discontinued 3 weeks after initial treatment when HbA1c levels were observed to be low. All patients received counseling regarding diet and exercise. In general, increases in HbA1c levels were reversible upon cessation of pasireotide SC.
Discussion
This cross-sectional case series review provides the first real-world, long-term treatment evidence of the efficacy and safety of pasireotide SC as a viable medical treatment option for patients with recurrent CD. This chart review showed biochemical control and overall symptomatic improvement exhibited by all patients that varied over the course of long-term treatment. It also supports previous studies in the literature that highlight the importance of an early diagnosis of recurrence, thus improving the chances of control; elevated late-night salivary cortisol levels are an early biochemical marker of recurrent CD (5, 13, 14). Most patients experienced hyperglycemia, which was controlled by prescribed concomitant antidiabetic medication and periodic monitoring of the associated changes. Two cases of pasireotide SC discontinuation were neither immediate, nor directly related to treatment.
This case series provides helpful examples of the successful management of AEs in a clinical setting. Similar to other somatostatin analogs and previous clinical studies, the most common AEs experienced by patients were gastrointestinal and were managed by dietary modifications and the intermittent use of antidiarrheal agents, such as loperamide. Results from a mechanistic study conducted in healthy volunteers showed that pasireotide, independent of dosing, caused significant decreases in insulin levels without changes in hepatic or peripheral insulin sensitivity (15). In all of the cases presented here, hyperglycemia was promptly diagnosed during the early stages of treatment based on previous studies and guidelines for CD treatment that emphasize the monitoring of blood glucose and HbA1c levels (16). Blood glucose levels were adequately controlled in accordance with the recommendations provided in the literature, which include following dietary restrictions, and individualized medical treatment regimens (16). Hyperglycemic effects were reported to be reversed within 6 weeks to 4 months of drug discontinuation, similar to other cases in the literature (17). Thus, these case studies highlight in a real-world setting the overall safety, tolerability, and manageability associated with pasireotide SC treatment that has been demonstrated in clinical trials.
These cases provide some indication of how pasireotide SC might perform in a broader patient population. They also provide an expanded understanding of drug interactions and outcomes under different scenarios, which is outside of the scope of clinical trial protocols. However, this study has certain limitations that should be considered when analyzing the impact of the outcomes. The analysis is retrospective and based on a limited number of patients compared with the large sample sizes of clinical trials. Another limitation to be noted is that biochemical control in patient No. 1 and patient No. 5 was achieved with a combination of pasireotide and cabergoline. Patient No. 1 began treatment with cabergoline 3 weeks before initiating pasireotide, and this combination therapy was continued. Thus, the concomitant treatment may have influenced UFC measurements and/or normalization. In addition, the data were obtained from a single center, whose treatment practices may not be reflective of those practiced by all clinics that treat patients with CD. Overall, these data help bridge the gap in knowledge between outcomes from well-defined and regulated clinical trials and actual clinical practice, resulting in broader understanding by physicians/prescribers of the potential medical impact of pasireotide SC.
Concluding Remarks
This study addresses an unmet need for data on treatment patterns with pasireotide SC in a real-world setting in patients with recurrent CD. Safety outcomes and manageability were consistent with previous observations in clinical studies. In addition, this is the first study to describe successful hyperglycemia management with concomitant medication and adequate monitoring in a real-world practice. Correlations between dose adjustments in diabetic medications and pasireotide SC were also documented in this study. The evidence generated through these cases may assist in optimizing patient access and influencing treatment decisions by physicians, such as selecting patients who will benefit the most from this treatment.
Ethics Statement
The study has been carried out in accordance with guidelines recommended by and it was approved by the OHSU IRB. The patient in the prospective clinical trial signed informed consent and the requirement for the other patients informed consent has been waived by the OHSU IRB.
Author Contributions
CY, SH, and MF contributed to design the study, participated in data interpretation and drafting, and revised the manuscript. JW, AI, and JC participated in data interpretation and drafting and revised the manuscript. All authors read and approved the final version of the manuscript for publication.
Conflict of Interest Statement
CY has served as a consultant for Chiasma, Ipsen, Novartis, and Pfizer. SH is a coinvestigator in clinical trials sponsored by Chiasma, Cortendo, and Novartis, with research support provided to Oregon Health & Science University. MF has been a principal investigator in clinical trials sponsored by Chiasma, Cortendo, Ipsen, Novartis, and Pfizer, with research support provided to Oregon Health & Science University. She has also served as an ad hoc consultant for Chiasma, Cortendo, Novartis, and Pfizer. AI has a fellowship partially funded by DePuy Synthes. JW and JC have nothing to disclose.
Acknowledgments
Medical editorial assistance was provided by MedThink SciCom (Raleigh, NC, USA). Support for editorial assistance and the open access fee were provided by Novartis Pharmaceuticals Corporation.
Funding
No funding was obtained to support this study.
References
1. Broder MS, Neary MP, Chang E, Cherepanov D, Ludlam WH. Incidence of Cushing’s syndrome and Cushing’s disease in commercially-insured patients <65 years old in the United States. Pituitary (2015) 18:283–9. doi: 10.1007/s11102-014-0569-6
2. Arafah BM, Nasrallah MP. Pituitary tumors: pathophysiology, clinical manifestations and management. Endocr Relat Cancer (2001) 8:287–305. doi:10.1677/erc.0.0080287
3. Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab (2008) 93:2454–62. doi:10.1210/jc.2007-2734
4. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet (2015) 386:913–27. doi:10.1016/S0140-6736(14)61375-1
5. Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2015) 100:2807–31. doi:10.1210/jc.2015-1818
6. Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of Cushing’s disease. Endocr Rev (2015) 36:385–486. doi:10.1210/er.2013-1048
7. Signifor (Pasireotide) [Package Insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation (2012).
8. Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med (2012) 366:914–24. doi:10.1056/NEJMoa1105743
9. Pivonello R, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing’s disease: results from a Phase III study. Clin Endocrinol (Oxf) (2014) 81:408–17. doi:10.1111/cen.12431
10. Schopohl J, Gu F, Rubens R, Van Gaal L, Bertherat J, Ligueros-Saylan M, et al. Pasireotide can induce sustained decreases in urinary cortisol and provide clinical benefit in patients with Cushing’s disease: results from an open-ended, open-label extension trial. Pituitary (2015) 18:604–12. doi:10.1007/s11102-014-0618-1
11. Simeoli C, Auriemma RS, Tortora F, De Leo M, Iacuaniello D, Cozzolino A, et al. The treatment with pasireotide in Cushing’s disease: effects of long-term treatment on tumor mass in the experience of a single center. Endocrine (2015) 50:725–40. doi:10.1007/s12020-015-0557-2
12. Petersenn S, Salgado LR, Schopohl J, Portocarrero-Ortiz L, Amaldi G, Lacroix A, et al. Pasireotide maintained reduction in urinary free cortisol and improvements in clinical signs in patients with Cushing’s disease remaining on treatment for 60 months. Endocrine Society’s 98th Annual Meeting and Expo. Boston, MA (2016).
13. Carroll TB, Javorsky BR, Findling JW. Postsurgical recurrent Cushing’s disease: clinical benefit of early intervention in patients with normal urinary free cortisol. Endocr Pract (2016) 22:1216–23. doi:10.4158/EP161380.OR
14. Amlashi FG, Swearingen B, Faje AT, Nachtigall LB, Miller KK, Klibanski A, et al. Accuracy of late-night salivary cortisol in evaluating postoperative remission and recurrence in Cushing’s disease. J Clin Endocrinol Metab (2015) 100:3770–7. doi:10.1210/jc.2015-2107
15. Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab (2013) 98:3446–53. doi:10.1210/jc.2013-1771
16. Colao A, De Block C, Gaztambide MS, Kumar S, Seufert J, Casanueva FF. Managing hyperglycemia in patients with Cushing’s disease treated with pasireotide: medical expert recommendations. Pituitary (2014) 17:180–6. doi:10.1007/s11102-013-0483-3
Keywords: pasireotide, real-world clinical experience, hyperglycemia, Cushing’s disease, recurrent Cushing’s disease
Citation: Yedinak CG, Hopkins S, Williams J, Ibrahim A, Cetas JS and Fleseriu M (2017) Medical Therapy with Pasireotide in Recurrent Cushing’s Disease: Experience of Patients Treated for At Least 1 Year at a Single Center. Front. Endocrinol. 8:35. doi: 10.3389/fendo.2017.00035
Received: 12 December 2016; Accepted: 10 February 2017;
Published: 27 February 2017
Edited by:
Marek Bolanowski, Wrocław Medical University, PolandReviewed by:
Przemyslaw Witek, Military Institute of Medicine, PolandLeandro Kasuki, Instituto Estadual do Cérebro Paulo Niemeyer, Brazil
Copyright: © 2017 Yedinak, Hopkins, Williams, Ibrahim, Cetas and Fleseriu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Fleseriu, Zmxlc2VyaXVAb2hzdS5lZHU=
 Chris G. Yedinak
Chris G. Yedinak Sarah Hopkins1,2
Sarah Hopkins1,2 Jessica Williams
Jessica Williams Justin Schultz Cetas
Justin Schultz Cetas Maria Fleseriu
Maria Fleseriu