- 1Laboratory of Cell Biology, INTA, University of Chile, Santiago, Chile
- 2Maine Medical Center, Portland, ME, USA
The function of marrow adipocytes and their origin has not been defined although considerable research has centered on their presence in certain conditions, such as osteoporosis. Less work has focused on the qualitative aspects of marrow fat. Bone marrow serum is composed of multiple nutrients that almost certainly relate to functional aspects of the niche. Previous studies using non-invasive techniques have shown that osteoporotic individuals have more marrow fat and that the ratio of saturated: unsaturated fatty acid is high. We recently reported that bone marrow sera from osteoporotic patients with fracture showed a switch toward decreased content of total saturated versus unsaturated fatty acids, compared to patients without fracture highlighting a dynamic relationship between the composition of fatty acids in the bone microenvironment and the metabolic requirements of cells. The relative distribution of fatty acids differed considerably from that in the serum providing further evidence that energy utilization is high and that marrow adipocytes may contribute to this pool. Whether these lipids can affect osteoblast function in a positive or negative manner is still not certain but will require further investigation.
The Bone Marrow Microenviroment
In adults, the rigid bone structure of the cortex encloses a dynamic and flexible cell organization sustaining continuous bone and bone marrow stroma remodeling, as well as blood formation. These processes rest on preserving specific adult stem cells (SCs) that are characterized by their capacity for self-renewal and multilineage differentiation. These unique properties of SCs are not only cell-autonomous in vivo, but also controlled by their surrounding microenvironment, which is currently called the SC niche. The original Schofield’s proposition for hematopoietic stem cells (HSCs) in the bone marrow emphasized the input from other marrow cells types to maintain SCs behavior and prevent maturation (1). Such cell- depending interactions provide a specialized microenvironment (“niche”) that allows cell lodging while maintaining self-renewal of SCs; loss of such an association would lead to cell differentiation. At present, the niche concept has been assumed to explain the behavior of SCs in several tissue types while evolving to take account of specific cell types, anatomical sites, soluble molecules, signaling cascades and gradients, as well as physical factors, such as weight bearing, shear stress, oxygen tension, and temperature (2–5). Among the most studied somatic SCs in mammals are the HSC and mesenchymal stromal/stem cells (MSCs) that integrate a unique niche in the bone marrow made of heterotypic SC pairs (6). Therefore, the bone marrow niche concept comprises a complex microenvironment, providing spatial and temporal coordinated signals to support SC function; balanced inputs from the niche can sustain homeostatic SC self-renewal and differentiation, but under pathological conditions they could constrain SCs functioning.
Mesenchymal Stem Cells or Marrow Stromal Cells
In the bone marrow, MSCs mainly localize lining blood vessels, in particular on the sinusoids, the characteristic vessels of bone marrow microcirculation (7, 8), often in close association with HSCs (6, 9, 10). Then, the sinusoidal wall brings together both HSCs and MSCs, framing and sustaining a mutual niche (6, 11). Such perivascular localization has also been demonstrated for MSCs in several tissues through the body, which support the proposition that MSCs are fundamental to the healing of many tissues. MSCs in the bone marrow are ontogenically distinct populations taking over various biological functions, such as niches for HSCs (6, 12, 13), progenitor cells for bone formation during bone remodeling or repair (14), for cartilage and adipocyte formation, and vascular support (15–17).
Ex vivo, hMSCs are relatively easy to obtain from a small bone marrow aspirate and they can simply be isolated from other marrow nucleated cells by their adherence to plastic dishes. Subsequently, these cells can be expanded under in vitro conditions in which they display varying proliferation and differentiation potential; however, as cells are expanded consecutively under standard conditions, they lose some of these capacities. Clonal analysis demonstrated that MSCs obtained from the bone marrow are a heterogeneous mixture of cells that differ in their stage of lineage commitment and extent of differentiation (18–21). Therefore, MSCs populations in the bone marrow or those that are isolated and maintained in culture are not homogeneous, but rather assemble progenitor cells with diverse biological properties, such that not all MSCs are alike.
In theory, the lineage fate of MSCs appears to be determined during cell “commitment,” at very early stages of cell differentiation. During this almost unknown period, both intrinsic (genetic) and environmental (local and/or systemic) conditions interplay to outline the cell’s fate toward one of the possible lineages. Factors, such as age (22), culture condition (23), microenvironment (24), mechanical strain (25), and some pathologies (26, 27), appear to affect the intrinsic activity of MSCs.
Multilineage differentiation capacity has been related to wide range gene expression at intermediary levels, which along cell commitment and differentiation shifts to a selective mode of restricted genes expressed at high level (21, 28–30). Several fundamental signaling pathways participate in regulating the lineage commitment of MSCs, including transforming growth factor-beta (TGFβ)/bone morphogenic protein (BMP) signaling, wingless type MMTV integration site (Wnt) signaling, Hedgehogs (Hh), Notch, and fibroblast growth factors (FGFs) (31, 32).
Relationship Between the Osteo/Adipogenic Processes the Obesity Theory of Osteoporosis
The formation, maintenance, and repair of bone tissue depend on fine-tuned interlinks in the activities of cells derived from the two SC types housed in the bone marrow interstice, above described. HSCs along the myeloid differentiation lineage generate osteoclasts (33), whereas osteoblasts derive from MSCs, which are also progenitor cells for adipocytes (34). Bone diseases, such as osteopetrosis, osteopenia, and osteoporosis, show imbalance between bone formation and resorption (33).
Since MSCs can generate several cell types, the control of lineage commitment of MSCs appears critical. Alteration in such control has been observed in some bone diseases resulting in abnormal bone remodeling, which show divergent commitment of MSCs to adipocytes and osteoblasts. For instance, increased marrow fat content has been demonstrated in osteoporosis patients, the most common bone remodeling disorder (35, 36). Also, this alteration of the osteo/adipogenic processes is observed in other bone loss conditions, such as aging, immobilization, microgravity, ovariectomy, diabetes, and glucocorticoid or thiazolidinedione treatments, highlighting the harmful consequence of marrow adipogenesis in osteogenic disorders (37–39).
Osteoblasts and adipocytes originate from a common precursor, MSCs; preserving bone tissue requires adequate osteoblastic differentiation while minimizing adipogenesis. Commitment and differentiation of MSCs into a specific phenotype in vivo is postulated to be controlled by hormonal and local factors (paracrine/autocrine) regulating the expression and/or activity of master differentiation genes (32, 40). Research on MSCs differentiation in vitro showed that activation of PPARγ2 and C/EBPs match to the master transcription factors for adipogenic differentiation (41–43), while Runx2 and osterix are required for osteogenic differentiation (44). PPARγ2 positively regulates adipocyte differentiation while acting as a dominant negative regulator of osteogenic differentiation (45, 46). By contrast, an increase in bone mass density was observed in PPARγ2-deficient mice model (47). On the other hand, Runx2 expression by MSCs inhibits their differentiation into adipocytes, as shown by experiments in Runx2/calvarial cells, which spontaneously differentiate into adipocytes (48). However, due to the heterogeneity of the earliest MSC, cell expression of only adipogenic or osteogenic markers is unlikely. Indeed, osterix positive marrow adipocytes have been shown in lineage tracing studies, and marrow adipocytes can be traced to peroxiredoxin 1 (Rosen, personal communication). Moreover, some Runx2-positive cells isolated in high marrow adiposity states also have large lipid droplets and express perilipin (49).
Thus, in most cases, there is a reciprocal relationship in the regulation of these differentiation processes whose alteration would facilitate adipose accretion in the bone marrow, at the expense of osteoblast formation, decreasing bone mass (50–52). Such altered conditions would prevail in the bone marrow of osteoporotic patients and other bone loss conditions, disrupting the activities of MSCs and their microenvironment (37, 40, 50). This proposition has been termed the obesity theory of osteoporosis.
According to this proposition, observations in iliac crest biopsies from elderly women show considerable accumulation of adipocytes; i.e., 70–80% of the total marrow volume in osteoporotic patients, compared to that of healthy elderly women. More recently, observations done by magnetic resonance imaging (MRI) allowed similar conclusion in patients with low bone density (53–55). However, the origin of marrow adipocytes and their true nature has been more difficult to discern.
Human Bone Marrow Fat
In newborn mammals, there is scarce marrow fat; however, adipocyte number increases with age such that in humans older than 30 years, most of the femoral cavity is occupied by adipose tissue (56). The function of human marrow fat was largely overlooked; at first, it was considered “filler” for the void left by trabecular bone during aging or after radiation treatments. Early observations from the 1970s highlighted the different characteristics between adipocytes within the red and yellow marrow, suggesting that marrow adipocytes may have a region-specific lipid composition (57, 58). In humans, adipocytes accumulate within the yellow marrow at or slightly before birth, regardless of prematurity, and accelerate between 4 and 8 weeks of age (59, 60). Early adipose tissue is established in distal skeletal regions, including the hands, feet, and distal tibia. This is often called constitutive marrow fat. After attaining some bulk, distal marrow adipose tissue histologically resembles peripheral white adipose tissue, and is relatively devoid of active hematopoiesis. During adulthood, marrow fat accretion continues in areas of red, hematopoietic marrow (61), which show histologically single adipocytes interspersed with sites of active hematopoiesis. These regions appear to be spatially distinct in rats and mice; but in human, both types of adipose tissue may allocate in the same skeletal region (62).
In a recent study in mice, Scheller et al. (62) related region-specific fat differences in development to functional differences, such as regulation, adipocyte size, lipid composition, gene expression, and genetic determinants of marrow adipocytes, proposing that distal fat areas embody constitutive marrow adipose tissue (cMAT), while proximal adipose tissue contains regulated marrow adipose tissue (rMAT). Although cMAT showed histologic similarities with white extra-medullar adipose tissue (WAT), the lipid composition, cold regulation, and gene expression of WAT is more related to that of rMAT, concluding that lipid metabolism in WAT and rMAT adipocytes may be similar. Of note, cMAT showed increased unsaturation index compared to both, rMAT and WAT. Scheller’s study and those of others highlight that not all marrow adipocytes are equivalent, sustaining connotation for the marrow niche and its relationship to skeletal and whole-body metabolism.
Bone marrow fat has a role in systemic and local energy metabolism (63–66). When dysfunctional, it may disrupt the complex relationships in the bone marrow microenvironment, constraining functioning of both HSCs and MSCs, and thereby affecting their progeny (67, 68).
Bone marrow adipocytes have endocrine, autocrine, and paracrine effects; the activity of these cells on neighboring marrow result from the production of adipokines, steroids, cytokines, and free fatty acids (51, 64, 69–71), which could sustain or suppress the hematopoietic and osteogenic processes (10, 50, 51, 63, 64, 72).
The modification of bone marrow adipose tissue, either in size or function, may have similar consequences to that of extra medullary fat, which by unbalanced production of signaling products underlay in several human diseases, including obesity, lipodystrophy, atherogenesis, diabetes, and inflammation.
Marrow Fat Composition and Osteoporosis
Current information on bone marrow fat points to diverse adipocytes that have development-dependent skeletal distribution, showing distinct properties, such as lipid composition, gene expression, and probably subjected to different peripheral and local regulation. Underlying the relationship between such bone marrow fat and bone function is a balance in the number and quality of adipose cells; hence, the interest in characterizing bone marrow fat and searching for the quality of human marrow fat, for instance, in a bone remodeling disorder like osteoporosis.
Skeletal and marrow cells utilize fatty acids as substrates for their energy needs (73), although the contribution of marrow adipocytes to such requirements is most unknown; as it is the role of fatty acids stored in the marrow adipocytes in bone remodeling and hematopoiesis. Moreover, the lipid composition in the interstitial compartment surrounding bone marrow cells, its physiological significance, its variation under a bone stress situation, or its relationship with systemic lipids are undefined.
Studies on the composition of bone marrow fat suggested that it could have clinical relevance, in addition to marrow fat size and volume. Such conclusion is derived from in situ non-invasive MRI-based analysis of bone marrow, in patients with chronic diseases, such as osteoporosis (54), type 2 diabetes mellitus (74), or diabetic and non-diabetic women with prevalent fragility fractures (75). These studies demonstrated significantly lower unsaturation of bone marrow lipids in patients than corresponding controls. Moreover, Patsch and colleagues showed that low unsaturation and high saturation levels of lipids was accentuated in diabetic patients with prevalent fractures (75).
Physiological levels of molecules in the human bone marrow milieu are practically unknown; measurement has been particularly difficult not only because of tissue seclusion, but because of the complicated anatomy and blood perfusion of bone requiring invasive bone marrow sampling. Thus, knowledge on the availability to bone marrow cells of metabolites or regulatory compounds is scarce, limited to some pathologic condition or estimated from measurements in plasma (69, 76–78). A soluble fraction, named “bone marrow serum (BMS),” obtained after spinning human bone marrow aspirates allows measurement of physiologically significant concentrations in the bone cell microenvironment, which are dissimilar from circulating plasma or whole blood (70, 79, 80).
In such fraction, we tested the hypothesis that BMS reflects the uniqueness of the marrow compartment in respect to the sequestration and utilization of fatty acids, by studying the composition of total soluble fatty acids in BMS and plasma samples from control and osteoporotic women with and without hip fracture (81). Results revealed a specific pattern of fatty acid composition in the bone marrow milieu that was characterized by higher saturated and decreased unsaturated fatty acids, compared to that of the circulation. The proportion of fatty acids in the BMS varied in the range of lipids in plasma, but its relative levels of unsaturated and saturated fatty acids compares well to those observed in the human marrow fat tissue (62, 75, 82), implying a distinct lipid content in BMS that replicates relevant fatty acids derived from the activity of adipocytes and other marrow cells, rather than those provided by blood plasma. The lipid content in BMS of all women studied suggests an active exchange of lipids between marrow cells; thus, the surplus of saturated fatty acids in BMS could result from palmitic and stearic acids supplied mainly by adipocytes, while marrow cells apparently preserve their pool of unsaturated fatty acids, except for oleic acid that is higher in BMS than in plasma (81).
By contrast, when the content of fatty acids in BMS and blood plasma was evaluated according to women’s bone mass density, no consistent differences in fatty acid composition were apparent in BMS, nor in plasma, which is analogous to former observations on the composition of bone fat tissue (53, 54, 74). Notwithstanding, there was a trend toward higher saturation and lower unsaturation in BMS fatty acids as compared to plasma. The analysis of the relative proportion of individual fatty acids suggests an active distribution of unsaturated fatty acids.
The former conclusion is supported by further analysis of data on the composition of fatty acids in both BMS and plasma in the osteoporosis group, in relation to the presence or not of a hip fracture (Figure 1). A switch toward decreased content of total saturated versus unsaturated fatty acids was observed in BMS of women with fractures, emphasizing a dynamic relationship between the composition of fatty acid in the bone microenvironment and the metabolic requirements of cells. In women with fractures, stearic acid content significantly decreased concomitant with increased oleic acid content, implying a substrate to product relationship to fulfill specific requirements for unsaturated fatty acids. Moreover, increased activity of cyclooxygenase (COX), COX-2 could be proposed from the total removal of polyunsaturated eicosatrienoic and arachidonic fatty acids (81).
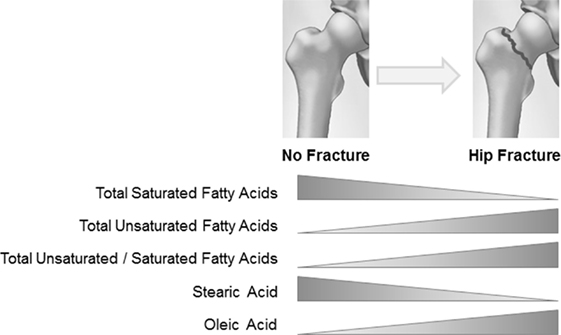
Figure 1. Relationship between hip fracture and fatty acid composition in the bone marrow serum of osteoporotic women.
It is difficult to define the origin (adipocyte or other marrow cell production) and type of lipids that change the fatty acid content of the BMS fraction after fracture, in part because the measurement was limited to the total fatty acid pool in the soluble fraction of the bone marrow. After a hip fracture bone cell metabolism may change in order to provide extra energy for damage repair by osteoblasts, or to suppress excessive inflammation. Interestingly, the lipid fraction in BMS of fractured women appears adjust to such conditions, thus diminished level of saturated fatty acid could result from increased supply of energetic substrate to marrow cells (bone, fat, and hematopoietic), while an increased exchange of oleic and polyunsaturated fatty acids could be related to their regulatory functions.
In conclusion, in this report, we summarize evidence on the role that adipocytes may play in the bone marrow in health and disease. Bone marrow adipose tissue appears as distinct from other extra marrow fat tissues, heterogeneous in its development, and allocated in subpopulations that seem to have different physiological properties. The regulation of the number and quality adipocytes is a necessity to preserve a functional bone marrow microenvironment to sustain SCs. In a bone disease like osteoporosis, there is increased differentiation of the precursor MSCs toward adipocytes, altering the local marrow microenvironment but leading to bone loss. In addition to fat mass, the quality of lipids could also participate in disrupting local regulation; hence, the interest in knowing the fatty acid composition of marrow adipose tissue. The measurement of the fat composition in BMS portrays a dynamic exchange of fatty acids in this fraction implying the connotation of locally produced lipids. The fatty acid composition in the BMS is enriched in saturated fatty acids and decreased in unsaturated fatty acids, as compared to blood plasma. Therefore, it is clear that qualitative aspects of marrow adiposity provide significant insights into the metabolism of the bone marrow niche, and may offer clues as to the pathophysiology of bone diseases, such as osteoporosis.
Ethics Statement
This study was approved by the INTA, University of Chile IRB.
Author Contributions
JR did the research and helped prepare the manuscript. AP did the background work. CF also performed some of the experiments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by a grant from the Fondo Nacional de Ciencia y Tecnología (FONDECYT·#1160214) and NIDDK R24 092759.
References
1. Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells (1978) 4:7–25.
2. Eliasson P, Rehn M, Hammar P, Larsson P, Sirenko O, Flippin LA, et al. Hypoxia mediates low cell-cycle activity and increases the proportion of longterm-reconstituting hematopoietic stem cells during in vitro culture. Exp Hematol (2010) 38:301–10. doi: 10.1016/j.exphem.2010.01.005
3. Kulkeaw K, Ishitani T, Kanemaru T, Fucharoen S, Sugiyama D. Cold exposure down-regulates zebrafish hematopoiesis. Biochem Biophys Res Commun (2010) 394:859–64. doi:10.1016/j.bbrc.2010.01.047
4. Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, et al. Biomechanical forces promote embryonic haematopoiesis. Nature (2009) 459:1131–5. doi:10.1038/nature08073
5. Guzmán A, García C, Marín AP, Tortajada A, Ruiz MT, Fernández de Henestrosa AR, et al. Formation of micronucleated erythrocytes in mouse bone-marrow under conditions of hypothermia is not associated with stimulation of erythropoiesis. Mutat Res (2008) 656:8–13. doi:10.1016/j.mrgentox.2008.06.016
6. Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature (2010) 466:829–34. doi:10.1038/nature09262
7. Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell (2005) 121:1109–21. doi:10.1016/j.cell.2005.05.026
8. Kiel MJ, Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity (2006) 25:862–4. doi:10.1016/j.immuni.2006.11.005
9. Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell (2007) 131:324–36. doi:10.1016/j.cell.2007.08.025
10. Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity (2010) 33:387–99. doi:10.1016/j.immuni.2010.08.017
11. Bianco P. Minireview: the stem cell next door: skeletal and hematopoietic stem cell “niches” in bone. Endocrinology (2011) 152:2957–62. doi:10.1210/en.2011-0217
12. Isern J, García-García A, Martín AM, Arranz L, Martín-Pérez D, Torroja C, et al. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function formation. Elife (2014) 2014(3):e03696. doi:10.7554/eLife.03696
13. Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med (2011) 208(3):421–8. doi:10.1084/jem.20110132
14. Blair HC, Zaidi M, Schlesinger PH. Mechanisms balancing skeletal matrix synthesis and degradation. Biochem J (2002) 364:329–41. doi:10.1042/bj20020165
15. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science (1999) 284:143–7. doi:10.1126/science.284.5411.143
16. Hegner B, Weber M, Dragun D, Schulze-Lohoff E. Differential regulation of smooth muscle markers in human bone marrow-derived mesenchymal stem cells. J Hypertens (2005) 23:1191–202. doi:10.1097/01.hjh.0000170382.31085.5d
17. Tao H, Han Z, Han Z-C, Li Z. Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int (2016) 2016:1314709. doi:10.1155/2016/1314709
18. Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci (2000) 113:1161–6.
19. Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res (1997) 12:1335–47. doi:10.1359/jbmr.1997.12.9.1335
20. Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol (1999) 107:275–81. doi:10.1046/j.1365-2141.1999.01715.x
21. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med (2004) 8:301–16. doi:10.1111/j.1582-4934.2004.tb00320.x
22. Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, et al. Age-related intrinsic changes in human bone marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell (2008) 7:335–43. doi:10.1111/j.1474-9726.2008.00377.x
23. Kultere B, Friedl G, Jandrositz A, Sánchez-Cabo F, Prokesch A, Paar C, et al. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast diff erentiation. BMC Genomics (2007) 8:70. doi:10.1186/1471-2164-8-70
24. Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol (2010) 222:268–77. doi:10.1002/jcp.21940
25. McBride SH, Falls T, Knothe Tate ML. Modulation of stem cell shape and fate B: mechanical modulation of cell shape and gene expression. Tissue Eng Part A (2008) 14:1573–80. doi:10.1089/ten.tea.2008.0113
26. Seebach C, Henrich D, Tewksbury R, Wilhelm K, Marzi I. Number and proliferative capacity of human mesenchymal stem cells are modulated positively in multiple trauma patients and negatively in atrophic nonunions. Calcif Tissue Int (2007) 80:294–300. doi:10.1007/s00223-007-9020-6
27. Hofer EL, Labovsky V, La Russa V, Vallone VF, Honegger AE, Belloc CG, et al. Mesenchymal stromal cells, colony-forming unit fibroblasts, from bone marrow of untreated advanced breast and lung cancer patients suppress fi broblast colony formation from healthy marrow. Stem Cells Dev (2010) 19:359–70. doi:10.1089/scd.2008.0375
28. Song L, Webb NE, Song Y, Tuan RS. Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells (2006) 24:1707–18. doi:10.1634/stemcells.2005-0604
29. Zipori D. The stem state: Plasticity is essential, whereas self-renewal and hierarchy are optional. Stem Cells (2005) 23:719–26. doi:10.1634/stemcells.2005-0030
30. Zipori D. The stem state: mesenchymal plasticity as a paradigm. Curr Stem Cell Res Ther (2006) 1:95–102. doi:10.2174/157488806775269133
31. Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ (2016) 23(7):1128–39. doi:10.1038/cdd.2015.168
32. Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci (2009) 66:236–53. doi:10.1007/s00018-008-8429-z
33. Teitelbaum SL. Bone resorption by osteoclasts. Science (2000) 289:1504–8. doi:10.1126/science.289.5484.1504
35. Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and replacement of cell populations of marrow by adipose tissue – a quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res (1971) 80:147–54. doi:10.1097/00003086-197110000-00021
36. Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology (2001) 2:165–71. doi:10.1023/A:1011513223894
37. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPARg 2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell (2004) 3:379–89. doi:10.1111/j.1474-9728.2004.00127.x
38. Zayzafon M, Gathings WE, McDonald JM. Modeled microgravity inhibit osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology (2004) 145:2421–32. doi:10.1210/en.2003-1156
39. Forsen L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trondelag Health Survey. Diabetologia (1999) 42:920–5. doi:10.1007/s001250051248
40. Nuttall M, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol (2004) 4:290–4. doi:10.1016/j.coph.2004.03.002
41. Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev (2008) 22:2941–52. doi:10.1101/gad.1709008
42. Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev (1991) 5:1538–52. doi:10.1101/gad.5.9.1538
43. Kim J, Ko J. A novel PPARgamma2 modulator sLZIP controls the balance between adipogenesis and osteogenesis during mesenchymal stem cell differentiation. Cell Death Differ (2014) 21:1642–55. doi:10.1038/cdd.2014.80
44. Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem (2006) 99:1233–9. doi:10.1002/jcb.20958
45. Lecka-Czernik B, GubriJ I, Moerman EA, Kajkenova O, Lipschitz DA, Manolagas SC, et al. Inhibition of Osf2/Cbaf1 expression and terminal osteoblast differentiation by PPAR-gamma2. J Cell Biochem (1999) 74:357–71. doi:10.1002/(SICI)1097-4644(19990901)74:3<357::AID-JCB5>3.0.CO;2-7
46. Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS, Park SW, et al. Activation of peroxisome proliferator-activated receptor-γ inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem (2003) 278:23270–7. doi:10.1074/jbc.M211610200
47. Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chans S, et al. Enhanced bone formation in lipodystrophic PPARγhyp/hyp mice relocates haematopoiesis to the spleen. EMBO Rep (2004) 5:1007–12. doi:10.1038/sj.embor.7400254
48. Kobayashi H, Gao Y, Ueta C, Yamaguchi A, Komori T. Multiline-age diff erentiation of Cbfa1-defi cient calvarial cells in vitro. Biochem Biophys Res Commun (2000) 273:630–6. doi:10.1006/bbrc.2000.2981
49. McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN, et al. Hdac3 deficiency increases marrow adiposity and induces lipid storage and glucocorticoid metabolism in osteochondroprogenitor cells. J Bone Miner Res (2016) 31(1):116–28. doi:10.1002/jbmr.2602
50. Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol (2006) 2:35–43. doi:10.1038/ncprheum0070
51. Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional and pathological implications. Crit Rev Eukaryot Gene Expr (2009) 19:109–24. doi:10.1615/CritRevEukarGeneExpr.v19.i2.20
52. Rodríguez JP, Astudillo P, Rios S, Pino AM. Involvement of adipogenic potential of human bone marrow mesenchymal stem cells (MSCs) in osteoporosis. Curr Stem Cell Res Ther (2008) 3:208–18. doi:10.2174/157488808785740325
53. Griffith JF, Yeung DKW, Ahuja AT, Choy CWY, Mei WY, Lam SSL, et al. A study of bone marrow and subcutaneous fatty acid composition in subjects of varying bone mineral density. Bone (2009) 44:1092–6. doi:10.1016/j.bone.2009.02.022
54. Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging (2005) 22(2):279–85. doi:10.1002/jmri.20367
55. Blake GM, Griffith JF, Yeung DK, Leung PC, Fogelman I. Effect of increasing vertebral marrow fat content on BMD measurement, T-Score status and fracture risk prediction by DXA. Bone (2009) 44:495–501. doi:10.1016/j.bone.2008.11.003
56. Moore SG, Dawson KL. Red and yellow marrow in the femur: age related changes in appearance at MR imaging. Radiology (1990) 175(1):219–23. doi:10.1148/radiology.175.1.2315484
57. Tavassoli M. Ultrastructural development of bone marrow adipose cell. Acta Anat (1976) 94:65–77. doi:10.1159/000144545
58. Tavassoli M. Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med (1976) 100:16–8.
59. Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci (2014) 1311:14–30. doi:10.1111/nyas.12327
60. Emery JL, Follett GF. Regression of bone-marrow haemopoiesis from the terminal digits in the foetus and infant. Br J Haematol (1964) 10:485–9. doi:10.1111/j.1365-2141.1964.tb00725.x
61. Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol (1985) 14:10–9. doi:10.1007/BF00361188
62. Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun (2015) 6:7808. doi:10.1038/ncomms8808
63. Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone (2011) 50(2):546–52. doi:10.1016/j.bone.2011.06.016
64. Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone (2012) 50(2):534–9. doi:10.1016/j.bone.2011.06.032
65. Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab (2011) 96(3):782–6. doi:10.1210/jc.2010-1922
66. Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J Clin Endocrinol Metab (2010) 95(6):2977–82. doi:10.1210/jc.2009-2336
67. Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature (2009) 460:259–63. doi:10.1038/nature08099
68. Clabaut A, Delplace S, Chauveau C, Hardouin P, Broux O. Human osteoblasts derived from mesenchymal stem cells express adipogenic markers upon coculture with bone marrow adipocytes. Differentiation (2010) 80:40–5.
69. Lee WY, Kang MI, Oh ES, Oh KW, Han JH, Cha BY, et al. The role of cytokines in the changes in bone turnover following bone marrow transplantation. Osteoporos Int (2002) 13:62–8. doi:10.1007/s198-002-8339-5
70. Pino AM, Ríos S, Astudillo P, Fernández M, Figueroa P, Seitz G, et al. Concentration of adipogenic and pro inflammatory cytokines in the bone marrow supernatant fluid of osteoporotic women. J Bone Min Res (2010) 25:492–8. doi:10.1359/jbmr.090802
71. Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab (2000) 11(8):327–32. doi:10.1016/S1043-2760(00)00301-5
72. Kawai M, de Paula FJ, Rosen CJ. New insights into osteoporosis: the bone-fat connection. J Intern Med (2012) 272(4):317–29. doi:10.1111/j.1365-2796.2012.02564.x
73. Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, et al. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone (2008) 43(2):230–7. doi:10.1016/j.bone.2008.03.022
74. Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging (2012) 35(1):117–24. doi:10.1002/jmri.22757
75. Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Min Res (2013) 28(8):1721–8. doi:10.1002/jbmr.1950
76. Wiig H, Berggreen E, Borge BA, Iversen PO. Demonstration of altered signaling responses in bone marrow extracellular fluid during increased hematopoiesis in rats using a centrifugation method. Am J Physiol Heart Circ Physiol (2004) 286:H2028–34. doi:10.1152/ajpheart.00934.2003
77. Iversen PO, Wiig H. Tumor necrosis factor A and adiponectin in bone marrow interstitial fluid from patients with acute myeloid leukemia inhibit normal hematopoiesis. Clin Cancer Res (2005) 11:6793–9. doi:10.1158/1078-0432.CCR-05-1033
78. Khosla S, Peterson JM, Egan K, Jones JD, Riggs BL. Circulating cytokine levels in osteoporotic and normal women. J Clin Endocrinol Metab (1994) 79:707–11. doi:10.1210/jcem.79.3.8077350
79. Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med (2012) 18(7):1095–101. doi:10.1038/nm.2793
80. Li X, Shet K, Rodriguez JP, Pino AM, Kurhanewicz J, Schwartz A, et al. Unsaturation level decreased in bone marrow lipids of postmenopausal women with low bone density using high resolution HRMAS NMR. Presented at: 34th Annual Conference of American Society of Bone Mineral Research (ASBMR); Oct 9–12. Minneapolis, MN, USA (2012).
81. Miranda M, Pino AM, Fuenzalida K, Rosen CJ, Seitz G, Rodríguez JP. Characterization of fatty acid composition in bone marrow fluid from postmenopausal women: modification after hip fracture. J Cell Biochem (2016) 117(10):2370–6. doi:10.1002/jcb.25534
Keywords: lipids, fatty acids, unsaturated, bone marrow cells, bone marrow examination, adipocytes
Citation: Pino AM, Miranda M, Figueroa C, Rodríguez JP and Rosen CJ (2016) Qualitative Aspects of Bone Marrow Adiposity in Osteoporosis. Front. Endocrinol. 7:139. doi: 10.3389/fendo.2016.00139
Received: 04 August 2016; Accepted: 10 October 2016;
Published: 25 October 2016
Edited by:
Xinhua Qu, Shanghai Ninth People’s Hospital, ChinaReviewed by:
Jan Tuckermann, University of Ulm, GermanyJawed Akhtar Siddiqui, University of Nebraska Medical Center, USA
Copyright: © 2016 Pino, Miranda, Figueroa, Rodríguez and Rosen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clifford J. Rosen, Y2pyb2ZlbkBnbWFpbC5jb20=
 Ana María Pino1
Ana María Pino1 Clifford J. Rosen
Clifford J. Rosen