- 1Department of Biomedical Engineering, Stony Brook University, Stony Brook, NY, USA
- 2Department of Medicine, University of North Carolina, Chapel Hill, NC, USA
Despite association with low bone density and skeletal fractures, marrow adipose tissue (MAT) remains poorly understood. The marrow adipocyte originates from the mesenchymal stem cell (MSC) pool that also gives rise to osteoblasts, chondrocytes, and myocytes, among other cell types. To date, the presence of MAT has been attributed to preferential biasing of MSC into the adipocyte rather than osteoblast lineage, thus negatively impacting bone formation. Here, we focus on understanding the physiology of MAT in the setting of exercise, dietary interventions, and pharmacologic agents that alter fat metabolism. The beneficial effect of exercise on musculoskeletal strength is known: exercise induces bone formation, encourages growth of skeletally supportive tissues, inhibits bone resorption, and alters skeletal architecture through direct and indirect effects on a multiplicity of cells involved in skeletal adaptation. MAT is less well studied due to the lack of reproducible quantification techniques. In recent work, osmium-based 3D quantification shows a robust response of MAT to both dietary and exercise intervention in that MAT is elevated in response to high-fat diet and can be suppressed following daily exercise. Exercise-induced bone formation correlates with suppression of MAT, such that exercise effects might be due to either calorie expenditure from this depot or from mechanical biasing of MSC lineage away from fat and toward bone, or a combination thereof. Following treatment with the anti-diabetes drug rosiglitazone – a PPARγ-agonist known to increase MAT and fracture risk – mice demonstrate a fivefold higher femur MAT volume compared to the controls. In addition to preventing MAT accumulation in control mice, exercise intervention significantly lowers MAT accumulation in rosiglitazone-treated mice. Importantly, exercise induction of trabecular bone volume is unhindered by rosiglitazone. Thus, despite rosiglitazone augmentation of MAT, exercise significantly suppresses MAT volume and induces bone formation. That exercise can both suppress MAT volume and increase bone quantity, notwithstanding the skeletal harm induced by rosiglitazone, underscores exercise as a powerful regulator of bone remodeling, encouraging marrow stem cells toward the osteogenic lineage to fulfill an adaptive need for bone formation. Thus, exercise represents an effective strategy to mitigate the deleterious effects of overeating and iatrogenic etiologies on bone and fat.
Marrow Adipose Tissue
Increased marrow adipose tissue (MAT) is associated with states of impaired bone formation (1, 2) and dysfunctional hematopoiesis (3–5), although its physiological role remains unclear. In humans, pathologists have noted that MAT increases in healthy subjects with age, beginning in the distal long bones and accruing proximally such that by age 25, approximately 70% of the marrow space is filled with MAT (4). In addition to physiologic MAT, which accrues with aging, this fat depot – housed within bone – is abundant in states of low bone density: osteoporosis (6), anorexia nervosa (7), skeletal unloading (8, 9), and anti-diabetes therapies (10), conditions that are also associated with skeletal fractures. Adipocytes within the marrow originate from the mesenchymal stem cell (MSC) pool that also gives rise to osteoblasts, chondrocytes, and myocytes, among other cell types (11, 12). Recent work suggests that increased marrow fat can also be demonstrated in the setting of preserved or increased bone density (high-fat feeding or obesity) (13–15) and, thus, challenges the premise that the relationship between MAT and bone volume is reciprocal. The MAT/bone relationship is further complicated by the identification of a new population of Grem1+ MSC (16), a phenotype capable of differentiating into osteoblasts and chondrocytes, but not adipocytes: the Grem1+ population differs from the LepR+ MSC, which do generate marrow adipocytes. Whether senile marrow invasion with adipocytes represents a later predominance of a LepR+ MSC population is unknown but complicates considerations as to the physiologic and/or pathologic role of MAT.
In the case of diet-induced obesity, marrow fat also increases compared to normal weight controls, but whether this contributes to bone fragility is unclear (17). Nevertheless, if the burden of fat across the marrow space is inevitable, then perhaps what’s more worthy of an investigation is the quality of the MAT being generated, possibly representing a direct reflection of the health of the surrounding bone. Importantly, the unsaturation index of MAT increases with aging, and thus, this feature of MAT may shed light on its physiology; nonetheless, unsaturation index of MAT is unaffected by physical activity (18). While subcutaneous white fat depots store excess energy and provide a clear evolutionary advantage during times of scarcity (19), MAT’s purpose remains indeterminate, harboring characteristics of both white and brown fat (20). WAT serves as a source of adipokines and inflammatory markers that have both positive (e.g., adiponectin) (21) and negative (22) effects on metabolic and cardiovascular endpoints. Visceral abdominal fat is a distinct depot of WAT that is proportionally associated with negative metabolic and cardiovascular morbidity (23), regenerates cortisol (24), and has been linked to reduced bone formation (25, 26). WAT substantially differs from brown adipose tissue (BAT), as defined by a panel of proteins that support BAT’s thermogenic role (27). MAT, by virtue of its specific marrow location and its adipocyte origin from at least LepR+ marrow MSC, is clearly demarcated from non-bone fat depots by higher expression of bone transcription factors (28) and likely represents a unique fat phenotype (29). Recently, MAT was noted to produce a greater proportion of adiponectin – an adipokine associated with improved metabolism – than WAT (30), suggesting an endocrine function for MAT as distinct depot, akin, but different from that of WAT. Moreover, deficiency of histone deacetylase 3 (Hdac3), known to play a major role in skeletal development and lipid metabolism, increases MAT volume, implicating this important transcriptional regulator in MAT development (31). Potentially, MAT might serve multiple functions, reflecting those of both white and brown fat, storing lipid in preexisting adipocytes, secreting adipokines, and generating heat. Exercise universally affects the metabolism of both WAT (32) and more recently BAT (33, 34); thus, exercise intervention can be harnessed as a powerful tool to query the poorly understood physiology of MAT.
Measurement and Quantification of Marrow Adipose Tissue
In order to quantify and characterize the effects of exercise on MAT, various analytic methods were considered. Until recently, qualitative measurements of MAT have relied on bone histology (35, 36), which is subject to site selection bias and cannot adequately quantify the volume of fat in the marrow. Nevertheless, histological techniques and fixation make possible in situ visualization of MAT, quantification of adipocyte size, and MAT’s association with the surrounding endosteum, milieu of cells, and secreted factors (37–39).
Recent advances in cell surface and intracellular marker identification and single-cell microfluidic analyses have led to greater resolution and high-throughput ex vivo quantification. Flow cytometric quantification can be used to purify adipocytes from the stromal vascular fraction of most fat depots (40). Early research with such machinery cited adipocytes as too large (50–200 mm) and fragile for cytometer-based purification, as their cytoskeleton lacks rigidity, rendering them susceptible to lysis; however, recent advances have been made to mitigate this (41). One may distinguish discrete adipocyte subpopulations from other cells by utilizing internal lipid content and surface biomarker identification. Filtration of the marrow (pore size = 150 μm) permits adequate flowthrough for adipocytes and smaller cellular contents. Subsequent centrifugation of the suspension aids in isolating adipocytes. Maintaining laminar flow and optimal temperatures when sorting has led to greater viability and precision. However, accumulation of lipid and protein content can adhere to the sheath tubing, thereby clogging the instrumentation (42). High-binding affinity of protein to antibodies bound by fluorescent probes used in FACS has made the identification of MAT and the cells that cohabitate the marrow increasingly specific, though these measurements provide little information on adipocyte location within the marrow microenvironment.
To improve our understanding of MAT, novel imaging techniques have recently been developed as a means to visualize and quantify MAT, in situ. Although proton magnetic resonance spectroscopy (1H-MRS) has been used with success to quantify vertebral MAT in humans (43), it is more difficult to employ in laboratory animals (44). Magnetic resonance imaging (MRI) provides MAT assessment in the vertebral skeleton (45) in conjunction with μCT-based marrow density measurements (46). A volumetric method to identify, quantify, and localize MAT in rodent bone has been recently developed, requiring osmium staining of bones and μCT imaging (47), followed by image analysis of osmium-bound lipid volume (in cubic millimeter) relative to bone volume (see Figure 1) (13, 48). Briefly, femurs stripped of connective tissue are decalcified, immersed in osmium tetroxide, and placed in potassium dichromate (49). Bones are scanned using μCT imaging (resolution 10 μm × 10 μm × 10 μm). Image processing consists of rigid image coalignment (Figures 1B,D, Slicer) (50), regional masking, allowing consistent regional measurements and superimposed visualizations. Additional bone masks are established in a semiautomatic contouring of the femur. As osmium is significantly more dense than bone [Hounsfield units (HU) ~ 700–2000], HU thresholds are set to capture low osmium from 2000 to 3000 HU, mid osmium from 3000 to 4000 HU (Figures 1E,F, green), and high osmium from 4000 to 5000 HU (Figures 1E,F, blue), and quantified accordingly (cubic millimeter). The lowest threshold is set above dense cortical bone (2000 HU) (51), and, thus, the contribution of potentially mislabeled cortical bone to the osmium volume is negligible (51, 52). Following quantification, the femur is subdivided into anatomical regions wherein regional osmium volume is normalized to bone volume. Aligned bone images are then averaged across all images as a reference for visualization. Average images are also computed for each group to obtain color-coded visualizations of the osmium densities to allow additional visual comparison of MAT between groups (Figure 2). This technique provides reproducible quantification and visualization of MAT, enabling the ability to quantify changes in MAT with diet, exercise, and agents that constrain precursor lineage allocation.
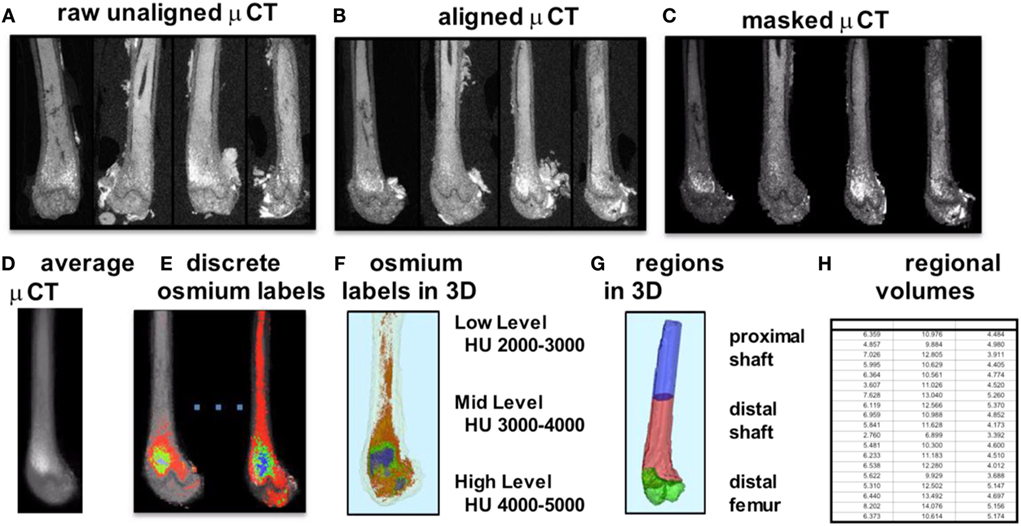
Figure 1. Overview of method for visualization and quantification of marrow adipose tissue (MAT). Osmium-stained femorae are visualized via μCT. Femorae (A) are rigidly aligned (B). Bone masks (C) are averaged (D). Osmium within the bone mask is quantified as volumetric (cubic millimeter) measurements of low (red), mid (green), and high (blue) osmium-containing regions in the femur and (E) overlaid on μCT images for viewing. 3D rendering of osmium regions (F) with same coloring as (E), colors slightly offset due to transparent bone mask. In (G), the femur is subdivided into three anatomical regions of interest. (H) is a pictorial representation of a data spreadsheet containing regional osmium measurements as osmium volume normalized to bone volume (in %).
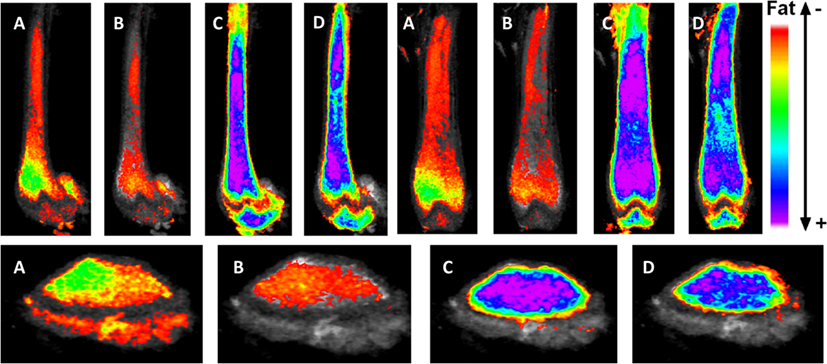
Figure 2. Exercise suppresses marrow adipose tissue accumulation, despite PPARγ agonist treatment. Visualization of osmium (lipid-binder) stain by μCT in sagittal (top left), coronal (top right), and axial (bottom) planes in the femur of C57BL/6 mice. Visualization is performed by superimposing and averaging the images of each femur (n = 5 per group) and colored labeling of osmium according to Hounsfield unit (HU) density. The four experimentals are as follows: control (A), rosiglitazone (B), control-exercise (C), and rosiglitazone-exercise (D).
Exercise Regulation of Marrow Adipose Tissue in the Setting of High-Fat Feeding
Marrow adipose tissue volume has recently been demonstrated to increase during short-term, high-fat feeding in rodents relative to the total bone volume (13), rising more rapidly than calorie induction of visceral fat depot size (53). A positive association between obesity and MAT was noted in rodents (15) and humans (43). In fact, obese individuals generally present with higher bone density (54–62) and are unlikely to experience classical osteoporotic fragility fractures (63). Because MAT has been associated with states of low bone density and increased fracture risk (6, 8, 64), the impact of fat in the marrow likely varies depending on its etiology. As obesity associates with increased bone density and lower fracture, MAT in obesity could represent a distinct fat depot that supports skeletal anabolism.
The Nurse’s Health Study showed that exercise affords a morbidity and mortality benefit that is independent of weight loss or calorie expenditure (65). One well-known effect of exercise is to improve bone strength, limiting the impact of postmenopausal osteoporosis (66). Exploring the salutary effects of exercise on bone and MAT in the setting of HFD, small animal models have been employed to address the degree of responsivity under said conditions. In one such experiment, 6 weeks of daily voluntary running exercise suppresses MAT accumulation in mice fed both a regular and HFD (13), in contrast to their non-exercised counterparts. While HFD does not perturb trabecular bone parameters as compared to control, significant gains in trabecular bone volume and trabecular thickness are noted in tibiae of exercised animals. Cortical bone parameters are unaltered by HFD and exercise in this short-term study. Thus, the trabecular compartment might be more receptive to both metabolic disarray and to mechanical signals from exercise than cortical bone, especially when challenged with elevated MAT via HFD.
Exercise Regulation of Marrow Adipose Tissue in the Setting of Rosiglitazone
It is accepted that elevated MAT due to PPARγ-agonist treatment in mice (48, 49, 67) and humans (68, 69) is due to PPARγ-induction of adipogenesis from marrow MSC. In vitro, rosiglitazone (PPARγ-agonist)-treated MSCs resist mechanically stimulated adipogenesis when treated with a dynamic load (70). In vivo, it was previously hypothesized that exercise might not overcome pathologically induced MAT in the setting of rosiglitazone; however, interestingly, exercise suppresses MAT even in the face of powerful adipogenic biasing (via MSC) in rosiglitazone-treated mice (48). While rosiglitazone significantly elevates cortical porosity in proximal tibiae, bone quantity is unaffected in these young mice. Exercise associates with increased trabecular bone volume fraction and trabecular thickness in control and rosiglitazone-treated mice (48). This further highlights the positive impact of exercise and mechanical signals on healthy bone as well as bone challenged by pharmacologic agents shown to facilitate marrow adiposity as well as cortical porosities.
Exercise and Mechanical Regulation of Bone Quality and Quantity
Exercise and Musculoskeletal Mass
Of the many health benefits attributed to exercise – improved neurological endpoints (71, 72), cardiovascular health (73), reduced inflammation (74–77), and decreased risk for chronic disease development, perhaps, most immediately and visually apparent is its ability to augment musculoskeletal mass (78–81). Exercise is known to encourage anabolic responses in musculoskeletal tissue (i.e., bone, muscle, ligament, and tendon) (82) as a consequence of successive bouts of musculoskeletal-loading. Indeed, skeletal tissue is known to adapt to meet loading demands (83), altering its bone remodeling strategy to sustain maximum loads. Prominent and varied forces are exerted across the appendicular and axial skeleton (84) through exercise: the musculoskeletal construct, thereby, acts as a conduit for transducing muscle contractive perturbations at the bone surface at both low frequencies (e.g., bicep flexion) and high frequencies (type II fast-twitch muscle). At the cellular level, these responses are mediated by a wide spectrum of mechanical stimuli of both high- and low-magnitude stresses, information that is internalized by cells through cytoskeletal and transmembrane-bound integrins linking the extracellular environment with the genetic machinery encased within the nucleus (85–90). Thus, these mechanical factors transcribe osteo-, chondro-, or myogenic (91) growth factors (RunX2) while deterring pathways conducive to adipogenesis (PPARγ) (70, 92–95). We now know that these signals propagate across the Wnt/β–Catenin transduction pathways (96), upregulating expression of genes that drive osteogenic (RunX2) and chondrogenic (SOX9) growth, while also being positioned across other complex signaling pathways involving other signals of interest, such as MAPK, pRB, FGFs, and TGF-β. The ability of mechanical stimuli to regulate musculoskeletal mass is likely multifactorial, occurring through repression of fat generation as well as bone resorptive pathways (97), while at the same time stimulating musculoskeletal anabolism. Additionally, exercise has been shown to improve resistance to fracture, specifically through the separation of exercise into shorter regimens (98).
Skeletal Unloading
The importance of mechanical information on bone adaptation can also be exemplified by observing the response of musculoskeletal tissue and the underlying cellular dynamics in the absence of mechanical cues (99–101). Whereas exercise delivers large quanta of mechanical information, as a function of disuse (chronic bed rest, microgravity, or reduced physical activity) (102, 103), regulation of musculoskeletal tissue homeostasis is compromised, instead elevating conditions wherein muscle, tendon, and ligament (and fat) undergo catalysis and rapid resorption of bone. Together, these tissue-level responses heighten the occurrence of osteoporotic bone and degree of fracture risk: these outcomes are in direct response to lapses in mechanical input (104–107). Whether chronic skeletal unloading encourages a specific MAT phenotype remains unclear, yet, studies have definitively shown that extended bed rest drives an increased marrow adipogenesis (108). In the absence of mechanical cues, PPARγ and receptor activator of nuclear factor-Kappa-B ligand (RANKL), which promotes osteoclast-mediated bone resorption, are both elevated, indicating an effect that could be stemmed upon reintroduction of mechanical stimuli.
Mechanical Effect on Bone and Fat Precursors
Both in vitro and in vivo studies have demonstrated that MSCs and early, non-committed progenitors exhibit unquestionable responsivity to mechanical loading (93, 109). Osteocytes (88, 110), osteoblasts (111, 112), and pre-osteoblast MC3T3 cells (113) in the marrow are other known mechanosensitive cells and contribute to the complex transduction of mechanical information driving osteogenic gene expression. While mechanical signals can inhibit osteoclastogenesis and subsequent bone resorption through direct effects on osteocyte and MSC expression of RANKL, it is known that mechanical effects on bone remodeling also involve regulation of MSC differentiation toward osteogenesis (93, 114, 115) facilitated by Wnt/β–Catenin signaling and uncommitted precursors. This is, in part, due to the plasticity of stem cells to differentiate specifically toward one mesenchymal lineage over another as dictated by environmental cues, such as exogenous mechanical stimuli (93), local substrate rigidity (113, 116, 117), and regional cytokine signaling gradients (11, 118). These factors drive MSC and other resident marrow cells toward fulfilling their role in musculoskeletal homeostasis by promoting formation of bone and other critical tissues that support skeletal health in lieu of engaging pathways conducive to adipogenesis. Exercise not only encourages MSC proliferation but downstream lineages are also influenced as well: lipid droplets and adipocyte cell diameters are reduced while driving osteogenic potential through upregulated alkaline phosphatase activity (119). In vivo studies show decreased adipocytes and increased pre-osteoblasts in the marrow of running rats (38) and climbing mice (120).
Rodent studies highlight increased bone formation rates in response to exercise and mechanical signals via dynamic histomorphometry (104), including running exercise (121–124). These responses persist through incorporation of non-exercise mechanical loading interventions [low-magnitude mechanical signals (LMMS)], which have been demonstrated to increase bone formation rates in loaded tibiae of mice (125, 126). In consideration of the phenotypic differences in lineage subtypes across niches, the bone marrow-derived MSC has recently been suggested to have unique, focal-specific properties (127). Therefore, it is important to weigh the contribution of precursor cells and other progenitors in the presence of mature adipocytes or the marrow, when considering the effect mechanical stimuli may have on their interaction with the surrounding milieu.
Low-Magnitude Mechanical Signals Effect on Bone and Marrow Adiposity
While physical activity presents an ideal strategy to introduce exogenous low-frequency, high-magnitude mechanical cues to musculoskeletal tissue, this approach is impractical for those patients with compromised bone microarchitecture (i.e., osteoporosis, osteopenia) (105, 109, 128–130) or muscle instability (131–133), populations that could benefit the most from their effects. Alternatively, mechanical signals delivered to the skeleton in the form of high-frequency, LMMS (fast-twitch muscles controlling balance and posture) can be introduced outside of the context of physical activity (134). For instance, low strain (<100 μs) displacements contribute more toward maintaining musculoskeletal health than higher magnitude strains and can be delivered whole-body using platforms to oscillate in the high-frequency domain (20–100 Hz), while maintaining a low-magnitude (i.e., sub-gravitational) acceleration. In doing so, these platforms partially reintroduce the spectral content of muscle contraction (105, 135), thereby exerting a beneficial quotient of exercise without risking fracture to bone resulting from extreme loads prevalent in exercise. Moreover, when separated into multiple administrations, the effects of both exercise and LMMS are amplified (95), encouraging enhancement in the responsiveness of MSC. Importantly, in humans, the non-pharmacologic therapy LMMS prevent bone loss due to Crohn’s disease (136), in children recovering from various cancers (137), and in other disabling conditions where bone losses are apparent (138, 139).
Exercise and the Browning of Marrow Adipose Tissue
Upon initiation of exercise, there is an increase in uptake and oxidation of lipids in skeletal muscle (140). When exercise intensity increases, fuel selection shifts toward an increase in carbohydrate and decrease in fat utilization. In contrast, endurance training is associated with a shift toward an enhanced lipid utilization (140). BAT, initially observed in hibernating mammals and human infants, dissipates energy in the form of heat through non-shivering thermogenesis (141). Inducible brown fat depots – beige fat – have been discovered within the white adipose tissue of adult humans (142). On exposure to cold or β-adrenergic stimulation, these beige/brite fat cells express high levels of mitochondrial uncoupling protein UCP1 and fat globules become multilocular (143), characteristics of the brown fat phenotype. Irisin, a muscle-derived hormone induced by exercise, also activates UCP1 expression and browning of white adipose tissue (33): coactivator PPAR-γ coactivator-1 α (PGC1-α) stimulates irisin, and transgenic mice overexpressing PGC1-α exhibit increased energy expenditure despite no changes in food intake or activity (33). Overall, there is evidence that fat depots can alter phenotype to serve functional demands. Since exercise browns white adipose depots (33), it is conceivable that exercise might result in analogous browning of MAT. Indeed, running exercise increased UCP1 in bone mRNA (48). UCP1 is localized in the mitochondrial inner membrane of mammalian BAT and is, therefore, a specific marker for BAT (144). This increase in UCP1 with exercise may indicate a brown phenotype within MAT adipocytes; however, this requires further confirmation. Interestingly, exercise-induced increases in UCP1 expression may correlate with increases in irisin (33), although irisin’s role in exercise physiology remains unclear (145). Finally, recent work suggests that irisin may have direct effects on bone in addition to its known effect on adipocytes, and thus irisin’s role in skeletal health remains an area of active investigation (146–148).
Conclusion
Marrow adipose tissue, housed within bone and interspersed with hematopoietic elements, remains a poorly understood fat depot, likely due to its anatomic location, rendering it inaccessible and thus challenging to quantify. Clinicians are particularly interested in the physiology of this fat depot due to its association with low bone density states and pharmacologic agents that increase fracture risk. As more robust volumetric imaging and quantification tools emerge [e.g., osmium-μCT Ref. (13, 48)], precise determinations can be made regarding MAT physiology and relationship to bone health. Interestingly, these methodologies have pointed to an increase in MAT in the setting of high-fat feeding (13), without an impact on bone quantity. Additionally, we are now able to visualize the effect of pharmacologic PPARγ activation with rosiglitazone on bone and to appreciate the significant encroachment of marrow fat (Figure 2) as well as to quantify these dramatic findings. Interestingly, both regimented exercise and LMMS serve to counter the effects of obesity and potent pharmacologic agents on bone remodeling. Further, in evaluating the response of MAT to mechanical stimuli, we highlight a positive effect toward normalizing bone parameters and, in doing so, constraining expansion of MAT across the marrow. It is possible that exercise serves to “brown” MAT as indicated in Ref. (48); however, further work is needed to establish the metabolic purpose of MAT in the setting of exercise. It remains unclear whether exercise-induced bone formation biases MSCs away from adipogenesis in order to recruit osteoblasts or whether alternative mechanisms are involved. In vitro, MSCs are highly responsive to mechanical signals during differentiation; indeed, mechanical loading slows adipogenesis (38, 92, 93, 149–151). Conversely, MSCs under microgravity conditions decrease osteogenic differentiation in favor of fat formation, an event accompanied by elevated nuclear expression of PPARγ (152). Thus, in the setting of mechanical input or exercise, bone formation is increased and marrow fat is suppressed, highlighting a likely mechanistic relationship between MAT and bone in the setting of mechanical stimulation. Other pathways are likely involved in the exercise regulation of MAT: lipogenesis, lipid uptake, skeletal anabolism, regulation of hematopoiesis in the bone marrow, and regulation of adipokines and cytokines. The elucidation of these pathways and their role in MAT/bone regulation in the setting of exercise remains an area of active investigation.
Author Contributions
MS and GP wrote this review together as a team. Each author contributed approximately 50% to the writing of this review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
Funding for this work was provided by NIAMS: AR062097 and NIDDK: P30DK056350.
References
1. Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol (2002) 55(9):693–8. doi:10.1136/jcp.55.9.693
2. Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging (2005) 22(2):279–85. doi:10.1002/jmri.20367
3. Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res (1971) 80:147–54. doi:10.1097/00003086-197110000-00021
4. Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol (1985) 14(1):10–9. doi:10.1007/BF00361188
5. Duque G. As a matter of fat: new perspectives on the understanding of age-related bone loss. IBMS Bonekey (2007) 4(4):129–40. doi:10.1138/20070257
6. Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ, et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab (2012) 97(8):2782–91. doi:10.1210/jc.2012-1477
7. Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, et al. Marrow fat and bone – new perspectives. J Clin Endocrinol Metab (2013) 98(3):935–45. doi:10.1210/jc.2012-3634
8. Wronski TJ, Morey ER. Skeletal abnormalities in rats induced by simulated weightlessness. Metab Bone Dis Relat Res (1982) 4(1):69–75. doi:10.1016/0221-8747(82)90011-X
9. Ahdjoudj S, Lasmoles F, Holy X, Zerath E, Marie PJ. Transforming growth factor beta2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma. J Bone Miner Res (2002) 17(4):668–77. doi:10.1359/jbmr.2002.17.4.668
10. Rubin MR, Manavalan JS, Agarwal S, McMahon DJ, Nino A, Fitzpatrick LA, et al. Effects of rosiglitazone vs metformin on circulating osteoclast and osteogenic precursor cells in postmenopausal women with type 2 diabetes mellitus. J Clin Endocrinol Metab (2014) 99(10):E1933–42. doi:10.1210/jc.2013-3666
11. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science (1999) 284(5411):143–7. doi:10.1126/science.284.5411.143
12. Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci (2009) 66(2):236–53. doi:10.1007/s00018-008-8429-z
13. Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone (2014) 64C:39–46. doi:10.1016/j.bone.2014.03.044
14. Doucette CR, Horowitz MC, Berry R, MacDougald OA, Anunciado-Koza R, Koza RA, et al. A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J Cell Physiol (2015) 230(9):2032–7. doi:10.1002/jcp.24954
15. Lecka-Czernik B, Stechschulte LA, Czernik PJ, Dowling AR. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol Cell Endocrinol (2015) 410:35–41. doi:10.1016/j.mce.2015.01.001
16. Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell (2015) 160(1–2):269–84. doi:10.1016/j.cell.2014.11.042
17. Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol (2014) 10(12):737–48. doi:10.1038/nrendo.2014.169
18. Huovinen V, Viljakainen H, Hakkarainen A, Saukkonen T, Toiviainen-Salo S, Lundbom N, et al. Bone marrow fat unsaturation in young adults is not affected by present or childhood obesity, but increases with age: a pilot study. Metabolism (2015) 64(11):1574–81. doi:10.1016/j.metabol.2015.08.014
19. Bellisari A. Evolutionary origins of obesity. Obes Rev (2008) 9(2):165–80. doi:10.1111/j.1467-789X.2007.00392.x
20. Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone (2012) 50(2):546–52. doi:10.1016/j.bone.2011.06.016
21. Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab (2013) 2(3):133–41. doi:10.1016/j.molmet.2013.04.001
22. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol (2006) 6(10):772–83. doi:10.1038/nri1937
23. Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf) (2012) 205(2):194–208. doi:10.1111/j.1748-1716.2012.02409.x
24. Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science (2001) 294(5549):2166–70. doi:10.1126/science.1066285
25. Bredella MA, Lin E, Gerweck AV, Landa MG, Thomas BJ, Torriani M, et al. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab (2012) 97(11):4115–22. doi:10.1210/jc.2012-2246
26. Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab (2013) 98(6):2562–72. doi:10.1210/jc.2013-1047
27. Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev (2013) 27(3):234–50. doi:10.1101/gad.211649.112
28. Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev (2013) 9(1):32–43. doi:10.1007/s12015-012-9365-8
29. Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem (2006) 98(2):251–66. doi:10.1002/jcb.20777
30. Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab (2014) 20(2):368–75. doi:10.1016/j.cmet.2014.06.003
31. McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN, et al. Hdac3 deficiency increases marrow adiposity and induces lipid storage and glucocorticoid metabolism in osteochondroprogenitor cells. J Bone Miner Res (2016) 31(1):116–28. doi:10.1002/jbmr.2602
32. Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF, et al. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab (2009) 297(2):E495–504. doi:10.1152/ajpendo.90424.2008
33. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature (2012) 481(7382):463–8. doi:10.1038/nature10777
34. Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes (2015) 64(7):2361–8. doi:10.2337/db15-0227
35. Bielohuby M, Matsuura M, Herbach N, Kienzle E, Slawik M, Hoeflich A, et al. Short-term exposure to low-carbohydrate, high-fat diets induces low bone mineral density and reduces bone formation in rats. J Bone Miner Res (2010) 25(2):275–84. doi:10.1359/jbmr.090813
36. Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res (2013) 28(4):865–74. doi:10.1002/jbmr.1807
37. Rosen CJ, Ackert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, et al. Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone (2004) 35(5):1046–58. doi:10.1016/j.bone.2004.07.008
38. David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology (2007) 148(5):2553–62. doi:10.1210/en.2006-1704
39. Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature (2009) 460(7252):259–63. doi:10.1038/nature08099
40. Majka SM, Miller HL, Sullivan T, Erickson PF, Kong R, Weiser-Evans M, et al. Adipose lineage specification of bone marrow-derived myeloid cells. Adipocyte (2012) 1(4):215–29. doi:10.4161/adip.21496
41. Majka SM, Miller HL, Helm KM, Acosta AS, Childs CR, Kong R, et al. Analysis and isolation of adipocytes by flow cytometry. Methods Enzymol (2014) 537:281–96. doi:10.1016/B978-0-12-411619-1.00015-X
42. Bernstein RL, Hyun WC, Davis JH, Fulwyler MJ, Pershadsingh HA. Flow cytometric analysis of mature adipocytes. Cytometry (1989) 10(4):469–74. doi:10.1002/cyto.990100416
43. Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) (2011) 19(1):49–53. doi:10.1038/oby.2010.106
44. de Paula FJ, Dick-de-Paula I, Bornstein S, Rostama B, Le P, Lotinun S, et al. VDR haploinsufficiency impacts body composition and skeletal acquisition in a gender-specific manner. Calcif Tissue Int (2011) 89(3):179–91. doi:10.1007/s00223-011-9505-1
45. Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, et al. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res (2012) 27(9):1864–71. doi:10.1002/jbmr.1640
46. Rantalainen T, Nikander R, Heinonen A, Cervinka T, Sievanen H, Daly RM. Differential effects of exercise on tibial shaft marrow density in young female athletes. J Clin Endocrinol Metab (2013) 98(5):2037–44. doi:10.1210/jc.2012-3748
47. Scheller EL, Troiano N, Vanhoutan JN, Bouxsein MA, Fretz JA, Xi Y, et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol (2014) 537:123–39. doi:10.1016/B978-0-12-411619-1.00007-0
48. Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, et al. Exercise regulation of marrow fat in the setting of PPARgamma agonist treatment in female C57BL/6 mice. Endocrinology (2015) 156(8):2753–61. doi:10.1210/en.2015-1213
49. Liu L, Aronson J, Huang S, Lu Y, Czernik P, Rahman S, et al. Rosiglitazone inhibits bone regeneration and causes significant accumulation of fat at sites of new bone formation. Calcif Tissue Int (2012) 91(2):139–48. doi:10.1007/s00223-012-9623-4
50. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging (2012) 30(9):1323–41. doi:10.1016/j.mri.2012.05.001
51. Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am (2011) 93(11):1057–63. doi:10.2106/JBJS.J.00160
52. Aamodt A, Kvistad KA, Andersen E, Lund-Larsen J, Eine J, Benum P, et al. Determination of Hounsfield value for CT-based design of custom femoral stems. J Bone Joint Surg Br (1999) 81(1):143–7. doi:10.1302/0301-620X.81B1.8880
53. Reid IR, Ames R, Evans MC, Sharpe S, Gamble G, France JT, et al. Determinants of total body and regional bone mineral density in normal postmenopausal women – a key role for fat mass. J Clin Endocrinol Metab (1992) 75(1):45–51. doi:10.1210/jcem.75.1.1619030
54. Dawson-Hughes B, Shipp C, Sadowski L, Dallal G. Bone density of the radius, spine, and hip in relation to percent of ideal body weight in postmenopausal women. Calcif Tissue Int (1987) 40(6):310–4. doi:10.1007/BF02556691
55. Ribot C, Tremollieres F, Pouilles JM, Bonneu M, Germain F, Louvet JP. Obesity and postmenopausal bone loss: the influence of obesity on vertebral density and bone turnover in postmenopausal women. Bone (1987) 8(6):327–31. doi:10.1016/8756-3282(87)90062-7
56. Liel Y, Edwards J, Shary J, Spicer KM, Gordon L, Bell NH. The effects of race and body habitus on bone mineral density of the radius, hip, and spine in premenopausal women. J Clin Endocrinol Metab (1988) 66(6):1247–50. doi:10.1210/jcem-66-6-1247
57. Kin K, Kushida K, Yamazaki K, Okamoto S, Inoue T. Bone mineral density of the spine in normal Japanese subjects using dual-energy X-ray absorptiometry: effect of obesity and menopausal status. Calcif Tissue Int (1991) 49(2):101–6. doi:10.1007/BF02565129
58. Harris S, Dallal GE, Dawson-Hughes B. Influence of body weight on rates of change in bone density of the spine, hip, and radius in postmenopausal women. Calcif Tissue Int (1992) 50(1):19–23. doi:10.1007/BF00297292
59. Tremollieres FA, Pouilles JM, Ribot C. Vertebral postmenopausal bone loss is reduced in overweight women: a longitudinal study in 155 early postmenopausal women. J Clin Endocrinol Metab (1993) 77(3):683–6. doi:10.1210/jcem.77.3.8370689
60. May H, Murphy S, Khaw KT. Age-associated bone loss in men and women and its relationship to weight. Age Ageing (1994) 23(3):235–40. doi:10.1093/ageing/23.3.235
61. Khosla S, Atkinson EJ, Riggs BL, Melton LJ III. Relationship between body composition and bone mass in women. J Bone Miner Res (1996) 11(6):857–63. doi:10.1002/jbmr.5650110618
62. Evans AL, Paggiosi MA, Eastell R, Walsh JS. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res (2015) 30(5):920–8. doi:10.1002/jbmr.2407
63. De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int (2005) 16(11):1330–8. doi:10.1007/s00198-005-1863-y
64. Devlin MJ. Why does starvation make bones fat? Am J Hum Biol (2011) 23(5):577–85. doi:10.1002/ajhb.21202
65. Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med (2004) 351(26):2694–703. doi:10.1056/NEJMoa042135
66. Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ Jr. Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann Intern Med (1988) 108(6):824–8. doi:10.7326/0003-4819-108-6-824
67. Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology (2002) 143(6):2376–84. doi:10.1210/en.143.6.2376
68. Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology (2009) 150(3):1330–40. doi:10.1210/en.2008-0936
69. Grey A, Beckley V, Doyle A, Fenwick S, Horne A, Gamble G, et al. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. Eur J Endocrinol (2012) 166(6):1087–91. doi:10.1530/EJE-11-1075
70. Case N, Thomas J, Xie Z, Sen B, Styner M, Rowe D, et al. Mechanical input restrains PPARgamma2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone (2013) 52(1):454–64. doi:10.1016/j.bone.2012.08.122
71. Taubert M, Villringer A, Lehmann N. Endurance exercise as an “endogenous” neuro-enhancement strategy to facilitate motor learning. Front Hum Neurosci (2015) 9:692. doi:10.3389/fnhum.2015.00692
72. Klein C, Jonas W, Iggena D, Empl L, Rivalan M, Wiedmer P, et al. Exercise prevents high-fat diet-induced impairment of flexible memory expression in the water maze and modulates adult hippocampal neurogenesis in mice. Neurobiol Learn Mem (2016) 131:26–35. doi:10.1016/j.nlm.2016.03.002
73. Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation (2003) 107(1):e2–5. doi:10.1161/01.CIR.0000048890.59383.8D
74. Bjorholt PG, Hoyeraal HM, Munthe E, Pahle J, Kogstad O, Sydnes OA, et al. Physical activity in the treatment of inflammatory rheumatic disorders. Scand J Soc Med Suppl (1982) 29:235–9.
75. Woods JA, Davis JM. Exercise, monocyte/macrophage function, and cancer. Med Sci Sports Exerc (1994) 26(2):147–56. doi:10.1249/00005768-199402000-00004
76. You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med (2013) 43(4):243–56. doi:10.1007/s40279-013-0023-3
77. Khoo J, Dhamodaran S, Chen DD, Yap SY, Chen RY, Tian HH. Exercise-induced weight loss is more effective than dieting for improving adipokine profile, insulin resistance, and inflammation in obese men. Int J Sport Nutr Exerc Metab (2015) 25(6):566–75. doi:10.1123/ijsnem.2015-0025
78. Woo SL, Gomez MA, Amiel D, Ritter MA, Gelberman RH, Akeson WH. The effects of exercise on the biomechanical and biochemical properties of swine digital flexor tendons. J Biomech Eng (1981) 103(1):51–6. doi:10.1115/1.3138246
79. Turner CH, Robling AG. Mechanisms by which exercise improves bone strength. J Bone Miner Metab (2005) 23(Suppl):16–22. doi:10.1007/BF03026318
80. Wallace IJ, Kwaczala AT, Judex S, Demes B, Carlson KJ. Physical activity engendering loads from diverse directions augments the growing skeleton. J Musculoskelet Neuronal Interact (2013) 13(3):283–8.
81. Hinton PS, Nigh P, Thyfault J. Effectiveness of resistance training or jumping-exercise to increase bone mineral density in men with low bone mass: a 12-month randomized, clinical trial. Bone (2015) 79:203–12. doi:10.1016/j.bone.2015.06.008
82. Judex S, Gross TS, Zernicke RF. Strain gradients correlate with sites of exercise-induced bone-forming surfaces in the adult skeleton. J Bone Miner Res (1997) 12(10):1737–45. doi:10.1359/jbmr.1997.12.10.1737
83. Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec (1987) 219(1):1–9. doi:10.1002/ar.1092190104
84. Rubin C, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int (1985) 37:411–7. doi:10.1007/BF02553711
85. Tjandrawinata RR, Vincent VL, Hughes-Fulford M. Vibrational force alters mRNA expression in osteoblasts. FASEB J (1997) 11(6):493–7.
86. Ko KS, McCulloch CA. Intercellular mechanotransduction: cellular circuits that coordinate tissue responses to mechanical loading. Biochem Biophys Res Commun (2001) 285(5):1077–83. doi:10.1006/bbrc.2001.5177
87. Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene (2006) 367:1–16. doi:10.1016/j.gene.2005.10.028
88. Thompson WR, Uzer G, Brobst K, Xie Z, Sen B, Yen S, et al. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Sci Rep (2015) 5:11049. doi:10.1038/srep11049
89. Uzer G, Thompson WR, Sen B, Xie Z, Yen SS, Miller S, et al. Cell mechanosensitivity to extremely low magnitude signals is enabled by a LINCed nucleus. Stem Cells (2015) 33(6):2063–76. doi:10.1002/stem.2004
90. Uzer G, Fuchs RK, Rubin J, Thompson WR. Concise review: plasma and nuclear membranes convey mechanical information to regulate mesenchymal stem cell lineage. Stem Cells (2016) 34(6):1455–63. doi:10.1002/stem.2342
91. Murfee WL, Hammett LA, Evans C, Xie L, Squire M, Rubin C, et al. High-frequency, low-magnitude vibrations suppress the number of blood vessels per muscle fiber in mouse soleus muscle. J Appl Physiol (1985) (2005) 98(6):2376–80. doi:10.1152/japplphysiol.01135.2004
92. Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology (2008) 149(12):6065–75. doi:10.1210/en.2008-0687
93. Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res (2009) 24(1):50–61. doi:10.1359/jbmr.080817
94. Styner M, Sen B, Xie Z, Case N, Rubin J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J Cell Biochem (2010) 111(4):1042–50. doi:10.1002/jcb.22793
95. Sen B, Xie Z, Case N, Styner M, Rubin CT, Rubin J. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech (2011) 44(4):593–9. doi:10.1016/j.jbiomech.2010.11.022
96. Case N, Rubin J. Beta-catenin – a supporting role in the skeleton. J Cell Biochem (2010) 110(3):545–53. doi:10.1002/jcb.22574
97. Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int (2000) 67(1):10–8. doi:10.1007/s00223001089
98. Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res (2005) 20(5):809–16. doi:10.1359/JBMR.041222
99. Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol (2010) 6(1):50–9. doi:10.1038/nrrheum.2009.239
100. Gupta S, Vijayaraghavan S, Uzer G, Judex S. Multiple exposures to unloading decrease bone’s responsivity but compound skeletal losses in C57BL/6 mice. Am J Physiol Regul Integr Comp Physiol (2012) 303(2):R159–67. doi:10.1152/ajpregu.00499.2011
101. Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, et al. Osteocyte apoptosis caused by hindlimb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J Bone Miner Res (2016) 31(7):1356–65. doi:10.1002/jbmr.2807
102. Globus RK, Bikle DD, Morey-Holton E. The temporal response of bone to unloading. Endocrinology (1986) 118(2):733–42. doi:10.1210/endo-118-2-733
103. Bikle DD, Sakata T, Halloran BP. The impact of skeletal unloading on bone formation. Gravit Space Biol Bull (2003) 16(2):45–54.
104. Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J (2001) 15(12):2225–9. doi:10.1096/fj.01-0166com
105. Rubin C, Turner AS, Muller R, Mittra E, McLeod K, Lin W, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res (2002) 17(2):349–57. doi:10.1359/jbmr.2002.17.2.349
106. Judex S, Garman R, Squire M, Busa B, Donahue LR, Rubin C. Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res (2004) 19(4):607–13. doi:10.1359/JBMR.040110
107. Squire M, Brazin A, Keng Y, Judex S. Baseline bone morphometry and cellular activity modulate the degree of bone loss in the appendicular skeleton during disuse. Bone (2008) 42(2):341–9. doi:10.1016/j.bone.2007.09.052
108. Trudel G, Payne M, Madler B, Ramachandran N, Lecompte M, Wade C, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol (2009) 107(2):540–8. doi:10.1152/japplphysiol.91530.2008
109. Pagnotti GM, Adler BJ, Green DE, Chan ME, Frechette DM, Shroyer KR, et al. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone (2012) 51(3):570–7. doi:10.1016/j.bone.2012.05.004
110. You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone (2008) 42(1):172–9. doi:10.1016/j.bone.2007.09.047
111. Tanaka SM, Li J, Duncan RL, Yokota H, Burr DB, Turner CH. Effects of broad frequency vibration on cultured osteoblasts. J Biomech (2003) 36(1):73–80. doi:10.1016/S0021-9290(02)00245-2
112. Garman R, Rubin C, Judex S. Small oscillatory accelerations, independent of matrix deformations, increase osteoblast activity and enhance bone morphology. PLoS One (2007) 2(7):e653. doi:10.1371/journal.pone.0000653
113. Khatiwala CB, Peyton SR, Metzke M, Putnam AJ. The regulation of osteogenesis by ECM rigidity in MC3T3-E1 cells requires MAPK activation. J Cell Physiol (2007) 211(3):661–72. doi:10.1002/jcp.20974
114. Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, et al. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone (2006) 39(5):1059–66. doi:10.1016/j.bone.2006.05.012
115. Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res (2007) 22(11):1720–31. doi:10.1359/jbmr.070721
116. Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, et al. Cell differentiation by mechanical stress. FASEB J (2002) 16(2):270–2. doi:10.1096/fj.01-0656fje
117. Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, et al. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials (2013) 34(8):1942–53. doi:10.1016/j.biomaterials.2012.11.012
118. Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther (2006) 1(3):365–9. doi:10.2174/157488806778226821
119. Maredziak M, Smieszek A, Chrzastek K, Basinska K, Marycz K. Physical activity increases the total number of bone-marrow-derived mesenchymal stem cells, enhances their osteogenic potential, and inhibits their adipogenic properties. Stem Cells Int (2015) 2015:379093. doi:10.1155/2015/379093
120. Menuki K, Mori T, Sakai A, Sakuma M, Okimoto N, Shimizu Y, et al. Climbing exercise enhances osteoblast differentiation and inhibits adipogenic differentiation with high expression of PTH/PTHrP receptor in bone marrow cells. Bone (2008) 43(3):613–20. doi:10.1016/j.bone.2008.04.022
121. Yeh JK, Liu CC, Aloia JF. Effects of exercise and immobilization on bone formation and resorption in young rats. Am J Physiol (1993) 264(2 Pt 1):E182–9.
122. Huang TH, Lin SC, Chang FL, Hsieh SS, Liu SH, Yang RS. Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. J Appl Physiol (1985) (2003) 95(1):300–7. doi:10.1152/japplphysiol.01076.2002
123. Iwamoto J, Shimamura C, Takeda T, Abe H, Ichimura S, Sato Y, et al. Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats. J Bone Miner Metab (2004) 22(1):26–31. doi:10.1007/s00774-003-0443-5
124. Wallace JM, Ron MS, Kohn DH. Short-term exercise in mice increases tibial post-yield mechanical properties while two weeks of latency following exercise increases tissue-level strength. Calcif Tissue Int (2009) 84(4):297–304. doi:10.1007/s00223-009-9228-8
125. Judex S, Boyd S, Qin YX, Turner S, Ye K, Muller R, et al. Adaptations of trabecular bone to low magnitude vibrations result in more uniform stress and strain under load. Ann Biomed Eng (2003) 31(1):12–20. doi:10.1114/1.1535414
126. Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone (2003) 33(6):946–55. doi:10.1016/j.bone.2003.07.009
127. Wallace IJ, Pagnotti GM, Rubin-Sigler J, Naeher M, Copes LE, Judex S, et al. Focal enhancement of the skeleton to exercise correlates with responsivity of bone marrow mesenchymal stem cells rather than peak external forces. J Exp Biol (2015) 218(Pt 19):3002–9. doi:10.1242/jeb.118729
128. Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J (2002) 16(10):1280–2. doi:10.1096/fj.01-0913fje
129. Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res (2004) 19(3):343–51. doi:10.1359/JBMR.0301251
130. Wallace IJ, Rubin CT, Lieberman DE. Osteoporosis. Evol Med Public Health (2015) 2015(1):343. doi:10.1093/emph/eov032
131. Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci (1999) 54(8):B352–7. doi:10.1093/gerona/54.8.B352
132. Qin YX, Lam H, Ferreri S, Rubin C. Dynamic skeletal muscle stimulation and its potential in bone adaptation. J Musculoskelet Neuronal Interact (2010) 10(1):12–24.
133. Muir J, Judex S, Qin YX, Rubin C. Postural instability caused by extended bed rest is alleviated by brief daily exposure to low magnitude mechanical signals. Gait Posture (2011) 33(3):429–35. doi:10.1016/j.gaitpost.2010.12.019
134. Rubin C, Judex S, Hadjiargyrou M. Skeletal adaptation to mechanical stimuli in the absence of formation or resorption of bone. J Musculoskelet Neuronal Interact (2002) 2(3):264–7.
135. Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature (2001) 412(6847):603–4. doi:10.1038/35088119
136. Leonard MB, Shults J, Long J, Baldassano RN, Brown JK, Hommel K, et al. Effect of low-magnitude mechanical stimuli on bone density and structure in pediatric Crohn’s disease: a randomized placebo-controlled trial. J Bone Miner Res (2016) 31(6):1177–88. doi:10.1002/jbmr.2799
137. Mogil RJ, Kaste SC, Ferry RJ Jr, Hudson MM, Mulrooney DA, Howell CR, et al. Effect of low-magnitude, high-frequency mechanical stimulation on BMD among young childhood cancer survivors: a randomized clinical trial. JAMA Oncol (2016). doi:10.1001/jamaoncol.2015.6557
138. Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res (2004) 19(3):360–9. doi:10.1359/JBMR.040129
139. Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res (2006) 21(9):1464–74. doi:10.1359/jbmr.060612
140. Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev (2006) 86(1):205–43. doi:10.1152/physrev.00023.2004
141. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev (2004) 84(1):277–359. doi:10.1152/physrev.00015.2003
142. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab (2007) 293(2):E444–52. doi:10.1152/ajpendo.00691.2006
143. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell (2012) 150(2):366–76. doi:10.1016/j.cell.2012.05.016
144. Nicholls DG, Bernson VS, Heaton GM. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl (1978) 32:89–93. doi:10.1007/978-3-0348-5559-4_9
145. Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L, et al. Irisin – a myth rather than an exercise-inducible myokine. Sci Rep (2015) 5:8889. doi:10.1038/srep08889
146. Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A (2015) 112(39):12157–62. doi:10.1073/pnas.1516622112
147. Colaianni G, Grano M. Role of Irisin on the bone-muscle functional unit. Bonekey Rep (2015) 4:765. doi:10.1038/bonekey.2015.134
148. Yong Qiao X, Nie Y, Xian Ma Y, Chen Y, Cheng R, Yao Yinrg W, et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep (2016) 6:18732. doi:10.1038/srep18732
149. Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem (2009) 284(50):34607–17. doi:10.1074/jbc.M109.039453
150. Sen B, Guilluy C, Xie Z, Case N, Styner M, Thomas J, et al. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells (2011) 29(11):1829–36. doi:10.1002/stem.732
151. Styner M, Meyer MB, Galior K, Case N, Xie Z, Sen B, et al. Mechanical strain downregulates C/EBPbeta in MSC and decreases endoplasmic reticulum stress. PLoS One (2012) 7(12):e51613. doi:10.1371/journal.pone.0051613
Keywords: exercise, marrow adipose tissue, quantitative image analysis, bone microarchitecture, lipid, PPARγ, rosiglitazone, exercise
Citation: Pagnotti GM and Styner M (2016) Exercise Regulation of Marrow Adipose Tissue. Front. Endocrinol. 7:94. doi: 10.3389/fendo.2016.00094
Received: 21 May 2016; Accepted: 04 July 2016;
Published: 14 July 2016
Edited by:
William Peter Cawthorn, University of Edinburgh, UKReviewed by:
Petra Simic, Massachusetts Institute of Technology, USAJonathan Gooi, The University of Melbourne, Australia
Copyright: © 2016 Pagnotti and Styner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maya Styner, bXN0eW5lckBtZWQudW5jLmVkdQ==
 Gabriel M. Pagnotti
Gabriel M. Pagnotti Maya Styner
Maya Styner