- 1Section of Endocrinology and Internal Medicine, Department of Medical Sciences, University of Ferrara, Ferrara, Italy
- 2Laboratorio in rete del Tecnopolo “Tecnologie delle Terapie Avanzate” (LTTA), University of Ferrara, Ferrara, Italy
- 3Department of Surgical, Oncological and Gastroenterological Sciences, University of Padova, Padova, Italy
Context: Medullary thyroid carcinoma (MTC) is a rare tumor originating from thyroid parafollicular C cells. It has been previously demonstrated that insulin-like growth factor I (IGF-I) protects MTC from the effects of antiproliferative drugs. Everolimus, an mTOR inhibitor, has shown potent antiproliferative effects in a human MTC cell line, TT, and in two human MTC primary cultures.
Objective: To verify whether IGF-I may influence the effects of everolimus in a group of human MTC primary cultures.
Design: We collected 18 MTCs that were dispersed in primary cultures, treated without or with 10 nM–1 μM everolimus and/or 50 nM IGF-I. Cell viability was evaluated after 48 h, and calcitonin (CT) secretion was assessed after a 6 h incubation. IGF-I receptor downstream signaling protein expression profile was also investigated.
Results: Everolimus significantly reduced cell viability in eight MTC [by ~20%; P < 0.01 vs. control; everolimus-responders (E-R) MTCs], while cell viability did not change in 10 MTCs [everolimus-non-responders (E-NR) MTCs]. In E-R MTCs, IGF-I blocked the antiproliferative effects of everolimus that did not affect CT secretion, but blocked the stimulatory effects of IGF-I on this parameter. IGF-I receptor downstream signaling proteins were expressed at higher levels in E-NR MTC as compared to E-R MTCs.
Conclusion: IGF-I protects a subset of MTC primary cultures from the antiproliferative effects of everolimus and stimulates CT secretion by an mTOR mediated pathway that, in turn, may represent a therapeutic target in the treatment of aggressive MTCs.
Introduction
Insulin-like growth factor I (IGF-I) system has been described in both rat (1) and human medullary thyroid carcinoma (MTC) (2), where it stimulates DNA synthesis (3). In a human MTC cell line, the TT cells, IGF-I increases cell proliferation, DNA synthesis, and the percentage of cells in S phase, all effects counteracted by co-incubation with either an IGF-I antibody or an IGF-I receptor (IGF-I R) antibody (4). In addition, it has been previously demonstrated that IGF-I protects TT cells from drug-induced cytotoxicity (5). Therefore, IGF-I may hamper the effects of antiproliferative drugs in MTC.
New medical approaches became lately available for MTC, thanks to the development of several agents that target tyrosine kinases. Clinical trials have reported variable results, since patients may develop drug resistance with consequent tumor progression (6). An alternative medical approach for neuroendocrine tumors, including MTC, is represented by mTOR inhibitors, such as everolimus. The latter displays antiproliferative effects on a wide spectrum of tumors including neuroendocrine tumors, both in vitro and in vivo (7–11). Moreover, it has been recently demonstrated that everolimus inhibits cell viability in a dose- and time-dependent fashion and reduces mTOR phosphorylation in a human MTC cell line and in two human MTC primary cultures (12). In addition, everolimus treatment of two patients with progressive metastatic MTC was associated with disease stabilization in one and disease progression in the other patient (13), indicating that mTOR pathway may effectively control MTC cell proliferation in a subset of patients. The variable effects of mTOR inhibitors may be ascribed to independent signaling mechanisms, activated by several growth factors, including IGF-I.
The aim of our study is therefore to verify whether IGF-I may influence the effects of everolimus in a group of human MTC primary cultures.
Materials and Methods
Materials
All reagents, if not otherwise specified, were purchased from Sigma-Aldrich (Milano, Italy). Everolimus was provided by Novartis Pharma (Basel, Switzerland).
Human MTCs
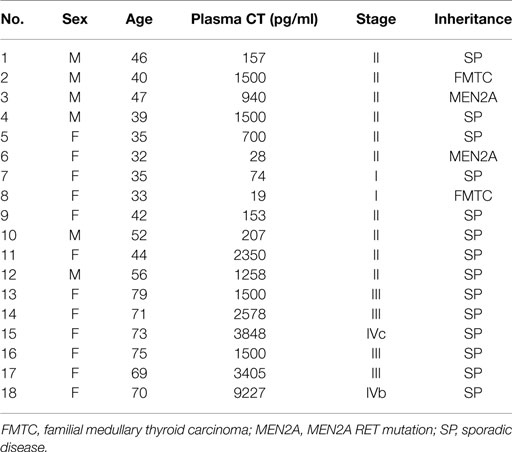
The samples derived from 18 patients diagnosed and operated on for MTC at the Section of Endocrinology and Internal Medicine of the University of Ferrara, and at the Department of Surgical, Oncological and Gastroenterological Sciences of the University of Padova. Table 1 shows patients’ characteristics and pre-operative hormonal values. All patients (six males and 12 females; age = 52.1 ± 3.9 years) underwent total thyroidectomy with central neck lymph node dissection and had histological and immunohistochemical diagnosis of MTC.
Tissue Collection and Primary Culture
Tissues were collected following the guidelines of the local committee on human research, and immediately minced in RPMI 1640 medium under sterile conditions. Primary cultures were then prepared are described previously (14, 15). Cells were then treated with test substances, with further evaluation of hormone secretion and cell viability. Informed consent of the patients was obtained for disclosing clinical investigation and performing the in vitro study.
Cell Viability
The effects of everolimus and IGF-I on MTC cell viability in vitro were assessed by ATPlite assay (Perkin-Elmer, Monza, Italy) on the Wallac Victor™ 1420 Multilabel Counter (Perkin-Elmer) as previously described (16). Cells were treated after 24 h with or without 10 nM–1 μM everolimus and/or 50 nM IGF-I. Treatments were renewed after the first 24 h of incubation. Cell viability was assessed after 48 h. Results were obtained by determining the mean value of six replicates.
Protein Expression Panel
Tissues were dissolved in cell lysis buffer (Bio-Rad, Milano, Italy) supplemented with cell lysis factor QG (Bio-Rad, Milano, Italy) and 2 mM phenylmethanesulfonylfluoride. Protein concentration was measured by BCA Protein Assay Reagent Kit (Pierce, Rockford, IL, USA), as previously described (17). Bio-plex®/Luminex® Technology (Bioclarma Research and Molecular Diagnostics, Torino, Italy) was employed to assess total protein levels of IGF-I receptor (IGF-I R), AKT, p70S6K, p38MAPK, ERK1/2, and CREB in MTC tissue samples. The following phosphorylated forms were also investigated: p(Tyr1131) IGF-I R, p(Ser473) AKT, p(Thr421/Ser424) p70S6K, p(Thr180/Tyr182) p38MAPK, p(Thr202/Thr204,Thr185/Thr187) ERK1/2, and p(Ser133) CREB levels.
Calcitonin Assay
Calcitonin (CT) was measured in conditioned medium from primary cultured cells by an ELISA kit (DRG, Springfield, NJ, USA), after a 6 h treatment without or with the test substances. The intra- and inter-assay variation coefficients were 2.8–5.7% and 6.1–7.4%, respectively. The detection limit was 1.0 pg/ml. Assays were performed in duplicate.
Statistical Analysis
Fisher exact test was used to evaluate the association between clinical characteristics of the patients and MTC primary culture responsivity to everolimus in terms of cell viability reduction.
Results are expressed as the mean ± standard error of the mean (SEM). A Student’s paired or unpaired t test was used to evaluate the individual differences between means.
P-values < 0.05 were considered significant.
Results
Effects of Everolimus on Cell Viability
To determine the effects of everolimus on cell viability of dispersed MTC cells, we assessed cell viability in MTC primary cultures after a 48 h treatment with 10 nM–1 μM everolimus. According to the extent of cell viability reduction recorded after treatment with everolimus, the primary cultures were divided in two groups: the cultures responding to any everolimus concentration with a significant (P < 0.05) cell viability reduction were considered as “everolimus-responders” (E-R), while those displaying a non-significant cell viability variation were considered as “everolimus-non-responders” (E-NR). According to this criterion, cultures from eight MTC were considered as E-R and 10 as E-NR.
Evaluating the patients clinical charts, we found that mean CT plasma levels of patients which primary cultures belonged to the E-NR group were ~fourfold higher than the mean CT plasma levels of patients which primary cultures belonged to the E-R group (P = 0.054). In addition, disease stage and inheritance were differently distributed among patients which primary cultures belonged to the E-R and those which primary cultures belonged to the E-NR group. In particular, E-NR MTCs were significantly associated with a disease stage ≥III (P < 0.02) and with sporadic disease (P < 0.03).
In the E-R group (black bars), everolimus dose-dependently reduced cell viability, from –19% at 10 nM to –31% vs. control at 1 μM (P < 0.01). In the E-NR MTCs (white bars), everolimus did not significantly modify cell viability (Figure 1). Further experiments were carried out only in the E-R group.
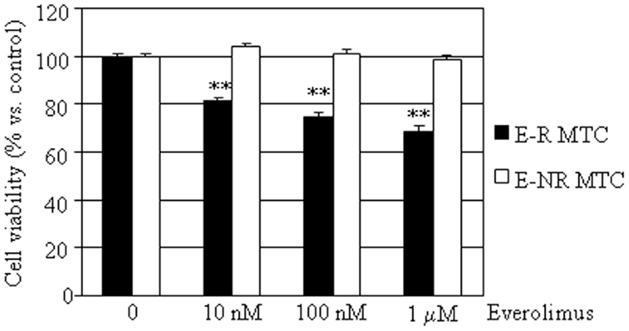
Figure 1. Effects of everolimus on MTC primary culture cell viability. MTC primary cultures were incubated in 96-well plates for 48 h in culture medium supplemented with everolimus at increasing concentrations from 10 nM to 1 μM, and control cells were treated with vehicle solution. Data from 18 MTC primary cultures were evaluated independently with six replicates each, and were expressed as the mean ± SEM percent cell viability vs. control cells. **P < 0.01 vs. control cells. As described in the Section “Results,” MTCs were divided according to cell viability inhibition after treatment with everolimus in E-R (eight samples, black bars) and E-NR (10 samples, white bars).
Protein Profiling
In a subset of MTC samples (including two E-R and four E-NR MTCs), we had a sufficient tissue amount that allowed us to investigate protein expression profile. We then assessed total and phosphorylated protein levels of IGF-I R, AKT, p70S6K, p38MAPK, ERK1/2, and CREB. We found that total IGF-I R, p70S6K, p38MAPK, ERK1/2, and CREB protein levels were higher in E-NR MTCs as compared to E-R MTCs (+153%, +259%, +68%, +53%, and +1735%, respectively). Statistical significance was observed for total CREB protein levels in E-NR MTCs vs. E-R MTCs (P < 0.02). On the contrary, total AKT protein levels were similar in the two groups (Figure 2A). In addition, phosphorylated IGF-I R, AKT, p70S6K, p38MAPK, and CREB protein levels were higher in E-NR MTCs as compared to E-R MTCs (+10%, +32%, +200%, +165%, and +426%, respectively). On the contrary, phosphorylated ERK1/2 protein levels were similar in the two groups (Figure 2B).
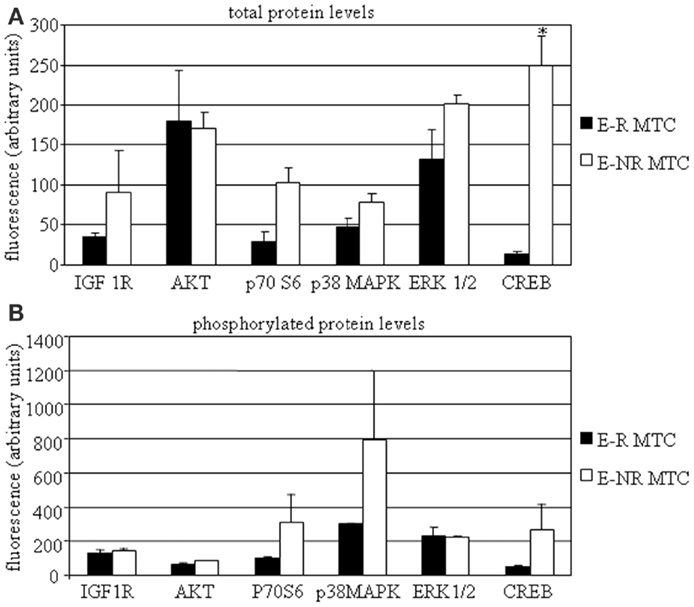
Figure 2. MTC protein profiling. MTC tissues were dissolved as described in the Section “Material and Methods” and assessed by Bio-plex®/Luminex® Technology. Total (A) and phosphorylated (B) protein levels of IGF-I R, AKT, p70S6K, p38MAPK, ERK1/2, and CREB were assayed in E-R (black bars) and E-NR (white bars) samples. *P < 0.05 vs. E-R MTCs.
Effects of IGF-I on Cell Viability in Everolimus-Responder MTCs
To investigate whether everolimus inhibitory effects might involve growth factor activated pathways, cell viability was assessed in E-R MTC cultures treated with or without 50 nM IGF-I, in the presence or in the absence of 10 nM–1 μM everolimus. IGF-I did not significantly affect cell viability, but counteracted the inhibitory effects of everolimus at all concentrations tested (Figure 3).
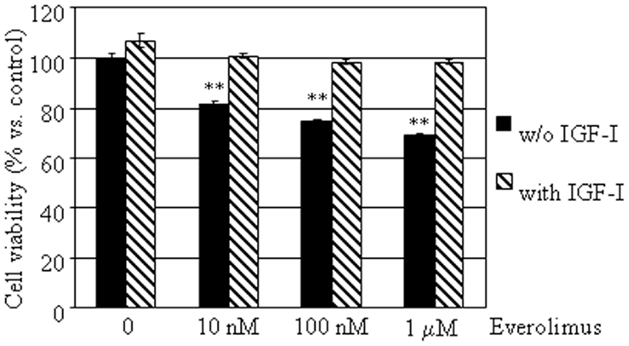
Figure 3. Effects of IGF-I on MTC primary culture cell viability. MTC primary cultures were incubated in 96-well plates for 48 h in culture medium supplemented with everolimus at increasing concentrations from 10 nM to 1 μM, and control cells were treated with vehicle solution. Data from 18 MTC primary cultures were evaluated independently with six replicates each, and were expressed as the mean ± SEM percent cell viability vs. control cells. **P < 0.01 vs. control cells. E-R MTC were incubated with (dashed bars) or without 50 nM IGF-I (black bars).
Effects of IGF-I on CT Secretion in Everolimus-Responder MTCs
To determine the effects of everolimus on CT secretion by dispersed MTC cells, we assessed CT concentrations in conditioned medium from the eight E-R MTC cultures. CT levels were not significantly influenced by treatment with everolimus at any concentration tested. On the other hand, IGF-I significantly induced CT secretion (+19%; P < 0.01 vs. control), an effect that was reduced by co-incubation with everolimus at all concentrations tested (Figure 4).
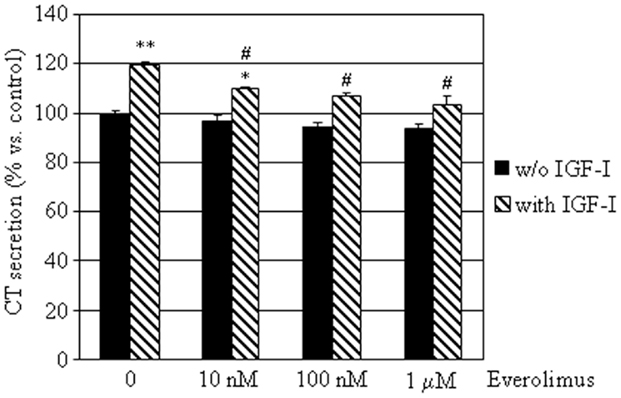
Figure 4. Effects of everolimus and IGF-I on CT secretion by E-R MTCs. E-R MTC primary cultures were incubated in 96-well plates for 6 h in culture medium supplemented with indicated substances. Control cells were treated with vehicle solution. CT secretion by each primary culture was then measured by ELISA. Data from eight E-R MTC primary cultures were evaluated independently with two replicates each and were expressed as the mean ± SEM percent CT secretion vs. control cells. *P < 0.05 and **P < 0.01 vs. control cells. #P < 0.05 vs. IGF-I treated cells.
Discussion
This study demonstrates that IGF-I influences the effects of everolimus in a sub-group of human MTC primary cultures, supporting the hypothesis that IGF-I may hamper the effects of antiproliferative drugs in MTC. In addition, our results indicate that mTOR inhibitors may be effective in restraining cell proliferation only in a subset of MTCs, in keeping with previously reported in vivo data (13, 18). Indeed, Lim et al. show that only one out of the nine MTC patients treated with everolimus displays a ~20% reduction in tumor bulk, while the other patients show either stable or progressive disease (18). These data support the hypothesis that other survival pathways, including those activated by RET mutations, are active in MTC and may hamper the effects of mTOR inhibitors. The latter may be cytostatic, since it has been demonstrated that everolimus treatment of pancreatic neuroendocrine tumor (pNET) and MTC cell lines inhibits cell growth by increasing the G0/G1 phase of the cell cycle (12, 19). Moreover, it is widely demonstrated that mTOR inhibition has a significant antiproliferative effect on pNET cell lines (20) as well as on other tumor cells (8, 9).
Insulin-like growth factor I is confirmed as a protective growth factor toward C-cell survival, in keeping with previous evidence (21). Our data confirm that IGF-I does not stimulate cell proliferation of MTC primary cultures, but protects them from the effects of everolimus, suggesting that escape from therapy may occur in the presence of IGF-I or similar growth factors. This hypothesis is further strengthened by the finding that E-NR MTC display higher levels of IGF-I R and therefore may possibly be more sensitive to the protective effects of IGF-I. Indeed, we observed that IGF-I R displays higher phosphorylation levels in E-NR MTC as compared to E-R MTCs. This finding is in keeping with a greater activation of the IGF-I pathway, as also supported by higher phosphorylation levels (i.e., activation) of IGF-I downstream signaling proteins. Moreover, MTCs belonging to the E-NR group are significantly associated with sporadic and higher disease stage, indicating that this group represents a more aggressive disease.
In addition, we found that mTOR pathway is activated in MTC, as previously reported (22), especially in E-NR as compared to E-R MTCs. This finding indicates that mTOR signaling pathway over-activation is not invariably a marker of sensitivity to mTOR inhibitors in all endocrine and endocrine-related tumors (7, 9, 23). However, further studies are needed to identify a prognostic protein panel that may accurately predict responsivity of MTCs to mTOR inhibitors on clinical grounds.
Our results confirm that IGF-I promotes CT secretion in a subset of MTC primary cultures, as previously demonstrated (21). In addition, we found that this effect is hampered by everolimus, indicating that IGF-I modulates CT secretion involving the mTOR pathway, which, in turn, may regulate CT secretion only in response to secretory stimuli, such as IGF-I. It has been previously shown that IGF-I stimulates hormone secretion in parathyroid cells by modulating calcium channels (24) that are present in MTC cells (25). Calcium-dependent signaling pathways are induced by IGF-I through CREB phosphorylation (26) that, in turn, regulates CT secretion (27). Higher levels of both total and phosphorylated CREB protein were found in E-NR MTCs, that also display higher IGF-I R levels and higher CT plasma levels, supporting the hypothesis that IGF-I system may be over-activated and may promote CT secretion in E-NR MTCs. These tumors, despite displaying aggressive characteristics, may anyway benefit from treatment with everolimus, since this drug may control CT secretion. Indeed, as shown in the available clinical studies, everolimus treatment is effective in significantly reducing circulating CT plasma levels also when no effect on tumor bulk was apparent (13, 18, 28).
Our results also indicate that everolimus treatment may not result in both tumor growth control and hormone secretion reduction in all MTCs, as also previously found for treatment with somatostatin analogs, that could exert antiproliferative effects despite the lack of antisecretory activity and vice versa (14). These finding suggest that proliferation and secretion are controlled by two different mechanisms in human MTC.
In conclusion, our data show that IGF-I protects MTC primary cultures from the effects of everolimus and stimulates CT secretion by an mTOR mediated pathway that, in turn, may represent a therapeutic target in the treatment of aggressive MTCs. A synergistic effect of IGF-IR inhibitors and everolimus has already been hypothesized (29, 30). Indeed, mTOR inhibition increases Akt activity via the IGF-IR pathway, leading to the reduction of mTOR inhibitor effects (30). Therefore, IGF-IR signaling inhibition may prevent this positive feedback mechanism. The results of ongoing clinical trial employing both everolimus and the somatostatin receptor pan-agonist Pasireotide (SOM230 alone or in combination with RAD001 in patients with medullary thyroid cancer; NCT01625520) may help in clarifying this issue, also on the basis of the potent inhibitory effects of Pasireotide on IGF-I secretion.
Author Contributions
EG: wrote the manuscript. CDP: performed protein expression profile. MR: performed primary cultures. FT: performed calcitonin secretion assay. TG: performed cell viability assay. RR: provided patients information. MP: provided patients information and surgical specimens. IMB: provided patients information and surgical specimens. EdU: supervised patients information and in vitro experiments. MCZ: supervised patients data and in vitro experiments, and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Novartis Pharma Italy for providing everolimus. Funding: This work was supported by grants from the Italian Ministry of Education, Research and University (FIRB RBAP11884M, RBAP1153LS, 2010TYCL9B_002), Fondazione Cassa di Risparmio di Ferrara, and Associazione Italiana per la Ricerca sul Cancro (AIRC) in collaboration with Laboratorio in rete del Tecnopolo “Tecnologie delle terapie avanzate” (LTTA) of the University of Ferrara, Ferrara.
References
1. Höppener JW, Steenbergh PH, Slebos RJ, de Pagter-Holthuizen P, Roos BA, Jansen M, et al. Expression of insulin-like growth factor-I and -II genes in rat medullary thyroid carcinoma. FEBS Lett (1987) 215:122–6. doi: 10.1016/0014-5793(87)80125-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. van der Laan BF, Freeman JL, Asa SL. Expression of growth factors and growth factor receptors in normal and tumorous human thyroid tissues. Thyroid (1995) 5:67–73. doi:10.1089/thy.1995.5.67
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Okimura Y, Kitajima N, Uchiyama T, Yagi H, Abe H, Shakutsui S, et al. Insulin-like growth factor I (IGF-I) production and the presence of IGF-I receptors in rat medullary thyroid carcinoma cell line 6-23 (clone 6). Biochem Biophys Res Commun (1989) 161:589–95. doi:10.1016/0006-291X(89)92640-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Yang KP, Samaan NA, Liang YF, Castillo SG. Role of insulin-like growth factor-I in the autocrine regulation of cell growth in TT human medullary thyroid carcinoma cells. Henry Ford Hosp Med J (1992) 40:293–5.
5. Mitsiades CS, McMillin D, Kotoula V, Poulaki V, McMullan C, Negri J, et al. Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in vitro. J Clin Endocrinol Metab (2006) 91:4013–21. doi:10.1210/jc.2005-2472
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Wells SA Jr, Pacini F, Robinson BG, Santoro M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab (2013) 98:3149–64. doi:10.1210/jc.2013-1204
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Gagliano T, Bellio M, Gentilin E, Molè D, Tagliati F, Schiavon M, et al. mTOR, p70S6K, AKT, and ERK1/2 levels predict sensitivity to mTOR and PI3K/mTOR inhibitors in human bronchial carcinoids. Endocr Relat Cancer (2013) 20:463–75. doi:10.1530/ERC-13-0042
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Zatelli MC, Minoia M, Filieri C, Tagliati F, Buratto M, Ambrosio MR, et al. Effect of everolimus on cell viability in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab (2010) 95:968–76. doi:10.1210/jc.2009-1641
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Zatelli MC, Minoia M, Martini C, Tagliati F, Ambrosio MR, Schiavon M, et al. Everolimus as a new potential antiproliferative agent in aggressive human bronchial carcinoids. Endocr Relat Cancer (2010) 17:719–29. doi:10.1677/ERC-10-0097
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med (2011) 364:514–23. doi:10.1056/NEJMoa1009290
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Halperin DM, Kulke MH, Yao JC. A tale of two tumors: treating pancreatic and extrapancreatic neuroendocrine tumors. Annu Rev Med (2015) 66:1–16. doi:10.1146/annurev-med-061813-012908
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Grozinsky-Glasberg S, Rubinfeld H, Nordenberg Y, Gorshtein A, Praiss M, Kendler E, et al. The rapamycin-derivative RAD001 (everolimus) inhibits cell viability and interacts with the Akt-mTOR-p70S6K pathway in human medullary thyroid carcinoma cells. Mol Cell Endocrinol (2010) 315:87–94. doi:10.1016/j.mce.2009.09.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Faggiano A, Ramundo V, Dicitore A, Castiglioni S, Borghi MO, Severino R, et al. Everolimus is an active agent in medullary thyroid cancer: a clinical and in vitro study. J Cell Mol Med (2012) 16:1563–72. doi:10.1111/j.1582-4934.2011.01438.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Zatelli MC, Piccin D, Tagliati F, Bottoni A, Luchin A, Vignali C, et al. Selective activation of somatostatin receptor subtypes differentially modulates secretion and viability in human medullary thyroid carcinoma primary cultures: potential clinical perspectives. J Clin Endocrinol Metab (2006) 91:2218–24. doi:10.1210/jc.2006-0334
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Zatelli MC, Luchin A, Piccin D, Tagliati F, Bottoni A, Vignali C, et al. Cyclooxygenase-2 inhibitors reverse chemoresistance phenotype in medullary thyroid carcinoma by a permeability glycoprotein-mediated echanism. J Clin Endocrinol Metab (2005) 90:5754–60. doi:10.1210/jc.2005-1362
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Gentilin E, Tagliati F, Terzolo M, Zoli M, Lapparelli M, Minoia M, et al. Mitotane reduces human and mouse ACTH-secreting pituitary cell viability and function. J Endocrinol (2013) 218:275–85. doi:10.1530/JOE-13-0210
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Gagliano T, Gentilin E, Benfini K, Di Pasquale C, Tassinari M, Falletta S, et al. Mitotane enhances doxorubicin cytotoxic activity by inhibiting P-gp in human adrenocortical carcinoma cells. Endocrine (2014) 47:943–51. doi:10.1007/s12020-014-0374-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Lim SM, Chang H, Yoon MJ, Hong YK, Kim H, Chung WY, et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol (2013) 24:3089–94. doi:10.1093/annonc/mdt379
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Mulet-Margalef N, Capdevila J. Critical appraisal of the role of everolimus in advanced neuroendocrine tumors of pancreatic origin. Gastrointest Cancer: Targets and Therapy (2012) 2:29–37. doi:10.2147/GICTT.S24826
20. Moreno A, Akcakanat A, Munsell MF, Soni A, Yao JC, Meric-Bernstam F. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer (2008) 15:257–66. doi:10.1677/ERC-07-0202
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Molè D, Gentilin E, Gagliano T, Tagliati F, Bondanelli M, Pelizzo MR, et al. Protein kinase C: a putative new target for the control of human medullary thyroid carcinoma cell proliferation in vitro. Endocrinology (2012) 153:2088–98. doi:10.1210/en.2011-1988
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Tamburrino A, Molinolo AA, Salerno P, Chernock RD, Raffeld M, Xi L, et al. Activation of the mTOR pathway in primary medullary thyroid carcinoma and lymph node metastases. Clin Cancer Res (2012) 18:3532–40. doi:10.1158/1078-0432.CCR-11-2700
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res (2004) 10:1013–23. doi:10.1158/1078-0432.CCR-03-0043
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Wong CK, Lai T, Holly JM, Wheeler MH, Stewart CE, Farndon JR. Insulin-like growth factors (IGF) I and II utilize different calcium signaling pathways in a primary human parathyroid cell culture model. World J Surg (2006) 30:333–45. doi:10.1007/s00268-005-0339-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Freichel M, Zink-Lorenz A, Holloschi A, Hafner M, Flockerzi V, Raue F. Expression of a calcium-sensing receptor in a human medullary thyroid carcinoma cell line and its contribution to calcitonin secretion. Endocrinology (1996) 137:3842–8. doi:10.1210/en.137.9.3842
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Zuloaga R, Fuentes EN, Molina A, Valdés JA. The cAMP response element binding protein (CREB) is activated by insulin-like growth factor-1 (IGF-1) and regulates myostatin gene expression in skeletal myoblast. Biochem Biophys Res Commun (2013) 440:258–64. doi:10.1016/j.bbrc.2013.09.067
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Zatelli MC, Tagliati F, Piccin D, Taylor JE, Culler MD, Bondanelli M, et al. Somatostatin receptor subtype 1-selective activation reduces cell growth and calcitonin secretion in a human medullary thyroid carcinoma cell line. Biochem Biophys Res Commun (2002) 297:828–34. doi:10.1016/S0006-291X(02)02307-0
28. Druce M, Chung TT, Grozinsky-Glasberg S, Gross DJ, Grossman AB. Preliminary report of the use of everolimus in a patient with progressive medullary thyroid carcinoma. Clin Endocrinol (Oxf) (2012) 77:154–5. doi:10.1111/j.1365-2265.2011.04296.x
29. Quek R, Wang Q, Morgan JA, Shapiro GI, Butrynski JE, Ramaiya N, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res (2011) 17:871–9. doi:10.1158/1078-0432.CCR-10-2621
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Yim KL. Everolimus and mTOR inhibition in pancreatic neuroendocrine tumors. Cancer Manag Res (2012) 4:207–14. doi:10.2147/CMAR.S25979
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: IGF-I, medullary thyroid carcinoma, everolimus, mTOR, calcitonin
Citation: Gentilin E, Di Pasquale C, Rossi M, Tagliati F, Gagliano T, Rossi R, Pelizzo M, Merante Boschin I, degli Uberti EC and Zatelli MC (2015) IGF-I influences everolimus activity in medullary thyroid carcinoma. Front. Endocrinol. 6:63. doi: 10.3389/fendo.2015.00063
Received: 12 February 2015; Accepted: 10 April 2015;
Published: 05 May 2015
Edited by:
Antonino Belfiore, University Magna Graecia of Catanzaro, ItalyReviewed by:
Piero Ferolla, Multidisciplinary Group for Diagnosis and Treatment of Neuroendocrine Tumors, ItalyDaniela Pasquali, Seconda Università degli Studi di Napoli, Italy
Copyright: © 2015 Gentilin, Di Pasquale, Rossi, Tagliati, Gagliano, Rossi, Pelizzo, Merante Boschin, degli Uberti and Zatelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Chiara Zatelli, Section of Endocrinology and Internal Medicine, Department of Medical Sciences, University of Ferrara, Via Savonarola 9, Ferrara 44100, Italy,enRsbWNoQHVuaWZlLml0
 Erica Gentilin
Erica Gentilin Carmelina Di Pasquale1
Carmelina Di Pasquale1 Mariarosa Pelizzo
Mariarosa Pelizzo Ettore C. degli Uberti
Ettore C. degli Uberti Maria Chiara Zatelli
Maria Chiara Zatelli