
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 22 January 2014
Sec. Translational and Clinical Endocrinology
Volume 4 - 2013 | https://doi.org/10.3389/fendo.2013.00195
This article is part of the Research Topic Circadian clocks and pregnancy View all 8 articles
Over the past two decades, it has become clear just how much of our physiology is under the control of the suprachiasmatic nucleus (SCN) and the cell-intrinsic molecular clock that ticks with a periodicity of approximately 24 h. The SCN prepares our digestive system for meals, our adrenal axis for the stress of waking up in the morning, and the genes expressed in our muscles when we prepare to exercise. Long before molecular studies of genes such as Clock, Bmal1, and the Per homologs were possible, it was obvious that female reproductive function was under strict circadian control at every level of the hypothalamic-pituitary-gonadal axis, and in the establishment and successful maintenance of pregnancy. This review highlights our current understanding of the role that the SCN plays in regulating female reproductive physiology, with a special emphasis on the advances made possible through the use of circadian mutant mice.
The light:dark cycle is the most predictable environmental cue available to animals, and determines basic environmental factors such as food and mate availability and predator/prey dynamics. Organisms have therefore developed a reliable biological mechanism to anticipate the changes in their environment, and to adjust their behavior and physiology appropriately. In mammals, the biological basis of this predictor is a cell-intrinsic molecular circadian pacemaker within the suprachiasmatic nuclei (SCN) of the hypothalamus. At its most basic level, the molecular circadian clock is composed of a transcription-translation loop: two proteins, CLOCK and BMAL1 (also known as MOP3), induce the transcription of four other genes, Per1, Per2, Cry1, and Cry2, which, after translation, dimerize, re-enter the nucleus, and inhibit their own transcription (1, 2). Following turnover of the inhibitory proteins, the transcription-translation cycle resumes. This molecular cycle occurs over a period of approximately 24 h and, in the absence of any external input, the neuronal network within the SCN maintains a stable period almost indefinitely. All tissues contain this basic molecular clock, but, in general the SCN act as the master pacemaker in a hierarchical system of multiple oscillators, receiving time-of-day input from photosensitive melanopsin-containing retinal ganglion cells, and phase-coordinating the activity of peripheral tissue-specific oscillators via neuronal and humoral output (3, 4).
This review will primarily focus on the female rodent, the most well-studied model of circadian reproductive neuroendocrinology. In the female, reproductive function is dependent on a tightly orchestrated cascade of events that originate from gonadotropin-releasing hormone (GnRH) neurons distributed throughout the preoptic area (POA) and septal areas of the basal forebrain. On the afternoon of proestrus, the stage prior to ovulation, a surge of GnRH released from neuronal terminals in the mediobasal hypothalamus induces the pituitary to release a timed bolus of luteinizing hormone (LH) and follicle stimulating hormone (FSH), which act on the ovary to induce ovulation and follicular recruitment. Regulation of the preovulatory GnRH surge itself is primarily controlled by two types of input to GnRH neurons and their surrounding afferent neurons: estradiol feedback from maturing ovarian follicles, and time-of-day output from the suprachiasmatic nucleus (SCN). The absence or dysregulation of either type of input disrupts GnRH surge release and results in an anovulatory cycle (5–8).
Although proper levels of estrogen and progesterone create a permissive state for GnRH release, a timing signal from the SCN is required to induce the level of GnRH release associated with the LH surge. The signal from the SCN to GnRH neurons occurs once daily: if rats are treated chronically with high levels of estradiol, an LH surge can be observed on multiple successive days, always restricted to the late afternoon (9). Even in the presence of high estradiol levels, ablation of the SCN or disruption of the neuronal projections from the SCN to the POA, including kisspeptin-secreting neurons, results in estrous acyclicity, indicating the crucial role the SCN play in reproductive function (10–13).
In addition to the estrous cycle, certain aspects of pregnancy are also under circadian control. The mating stimulus triggers a series of daily, biphasic surges in prolactin (PRL) occurring during the early morning and early evening hours (14). Knife cuts to the retrochiasmatic region prevent circadian prolactin release during pseudopregnancy (14) and prolactin surges during the estrous cycle, indicating that PRL release is SCN-dependent (15). Parturition is also under circadian regulation; although the major contributory factors to the onset of parturition vary among species, the timing of the onset of parturition generally occurring during the species-dependent inactive phase (e.g., day-time for nocturnal rodents and night-time for diurnal humans) (16).
Reproductive function can be disrupted by interfering with many of the core genetic components of the molecular pacemaker, including Clock (17), Bmal1 (18), and Per1 and Per2 (19). Recent studies of reproduction in circadian mutant mice have brought a new dimension of molecular specificity to our understanding of central circadian control over reproductive endocrinology. Here, we review the current literature regarding the role of the SCN in regulating female estrous cycles, the establishment and maintenance of pregnancy, and parturition. The importance of circadian rhythms in peripheral reproductive tissue is reviewed elsewhere in this issue.
Ovulation in the female mammal is a complex and carefully timed process that is governed both centrally, by GnRH neurons in the hypothalamus, and locally, by hormones released by cells proximate to the maturing follicle; these same hormones modulate GnRH neuron activity (Figure 1). The first two stages of the cycle, metestrus and diestrus, are characterized by low levels of estradiol, as follicles recruited during the previous estrous cycle progress from the early antral to preovulatory stage. On late diestrus and early proestrus, serum levels of estradiol, produced primarily by the granulosa cells in the developing preovulatory (Graafian) follicles, peak. Elevated estradiol induces a number of molecular and morphological changes in GnRH neurons and the interneurons that surround and modulate GnRH neurons, including the upregulation of progesterone receptors and an increase in the amplitude and frequency of GnRH pulsatility (20–22). The end result of estradiol priming is a surge in GnRH release on late proestrus, the precise timing of which is gated by a neurally derived timing signal. The proestrus GnRH surge induces release of LH and FSH from gonadotropes in the pituitary; in the ovary, LH induces ovulation of Graafian follicles, while FSH stimulates the recruitment of a new cohort of antral follicles. In the mouse and rat, estrous cycles normally last 4–5 days.
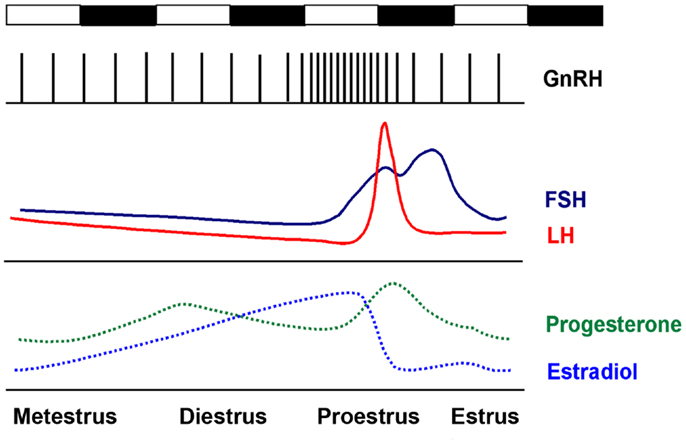
Figure 1. The rodent estrous cycle. In the mouse, ovulation occurs every 4–5 days. Metestrus and diestrus are characterized by low but slowly increasing levels of estradiol. On the late afternoon of proestrus, elevated estradiol levels induce a bolus of GnRH release from the hypothalamus, which induces the proestrus LH and FSH surge at approximately the start of the active (dark) period. Ovulation occurs 12–14 h later.
Elevated estradiol is mandatory for a GnRH surge to occur. It is not, however, sufficient. More than 50 years ago, it was shown that the preovulatory GnRH surge is controlled by two types of input to GnRH neurons and their surrounding interneurons: hormonal feedback from maturing ovarian follicles and a time-restricted neuronal signal (23). Everett and Sawyer demonstrated that female rats treated with pentobarbital at 1400 h, but not 1600 h, exhibit a 24-h delay in both the LH surge and ovulation. Bingel and Schwartz later demonstrated a similar effect of proestrus pentobarbital treatment on ovulation in the mouse (24). Both groups determined that the effect of the pentobarbital was to inhibit the then-unknown signal driving the LH surge and subsequent ovulation. It was later shown that the neural timing signal occurs once daily: if rats are treated chronically with high levels of estradiol, an LH surge can be observed on multiple successive days, always restricted to the late afternoon (9, 25).
The SCN was identified as the locus of the neural timing signal when it was shown that ablating the SCN, or severing neuronal connections between the SCN and the POA, resulted in estrous acyclicity (10–12). Tract-tracing and immunohistochemical studies have identified direct SCN-GnRH neuron connections, and indirect connections in which SCN neurons synapse on estradiol-concentrating interneurons adjacent to GnRH neurons in the anterior POA (26, 27). The SCN-derived signal is believed to be neural, rather than humoral, in nature: when a SCN-lesioned females hamster receives a transplanted SCN that is capable of sustaining molecular rhythms but does not form normal neural connections, certain rhythms, such as locomotor activity, are restored, but estrous cyclicity is not (28–30).
Gonadotropin-releasing hormone neurons represent the final point of convergence between the hormonal and timing signals, but also possess an intrinsic circadian clock that regulates GnRH transcription, translation, and release. Several groups have shown that core clock genes, including Bmal1 and the Period genes, are expressed and cycle rhythmically in both GnRH neurons and the immortalized GT1-7 GnRH neuronal cell line (31, 32). Dysregulation of certain circadian genes, such as Clock and Cry1, disrupts ultradian GnRH pulse amplitude and frequency (33), and the effect of the potent GnRH secretagogue kisspeptin is significantly reduced in preoptic explants from Bmal1 knockout mice (34).
Although the precise nature of the timing signal is still under debate, both vasopressin (AVP) and vasoactive intestinal polypeptide (VIP) appear to regulate the timing of GnRH release. Both polypeptides are rhythmically expressed and present in SCN neurons efferent to the mPOA, and, in rats, inhibition of either AVP or VIP signaling results in a reduction in the amplitude of an estradiol-induced LH surge (35–37), and VIP can regulate the timing of the LH surge within a specific timeframe (38). However, AVP alone is capable of inducing an LH surge in SCN-lesioned rats, and if the rhythms of AVP and VIP are phase-dissociated in SCN-POA co-cultures, AVP secretion occurs in phase with GnRH release, whereas VIP secretion does not (39, 40).
Genetic mouse models have allowed for a more precise understanding of the timing signal. Expression of both AVP and its receptor, V1aR, are reduced in the SCN of both male and female mice that are homozygous for the CLOCK-Δ19 dominant negative mutation, whereas VIP expression is not affected by the Clock mutation (Figure 2). Moreover, AVP injection into the region of the medial POA on the afternoon of proestrus is sufficient to rescue the LH surge in Clock/Clock mutant female mice (41–43). The effect of AVP is time dependent: administration of AVP in the afternoon, but not morning, of proestrus induces LH release (44).

Figure 2. AVP and VIP expression in the SCN. AVP and VIP mRNA levels were measured by autoradiography in the SCN from wildtype and Clock/Clock mice. Both AVP and VIP showed circadian expression in wildtype SCN. In Clock mutants, VIP expression was similar to that observed in wildtype mice, but AVP expression and rhythmicity was significantly damped. Modified from data presented in Ref. (43).
Neurons expressing the GnRH secretagogue kisspeptin, which has been shown to play a critical role in the onset of puberty, also may control the timing of the proestrus GnRH surge. Mice lacking either Kiss1 or its receptor, GPR54, generally do not have an LH surge, and the circadian expression of Kiss1 and c-fos in KISS1-secreting neurons is dependent on the presence of estrogen (45–47). In vitro and in vivo experiments have shown that elevated estradiol levels produce circadian expression of the kisspeptin peptide receptor GPR54 (48). Additionally, Williams and colleagues showed that Kiss1R neurons are targeted by AVP neurons from the SCN, and that there is a strong circadian rhythm in the response of GnRH neurons to kisspeptin, suggesting that Kisspeptin neurons may represent the locus of integration of the estradiol and circadian signals (49). In this model, rising estradiol levels on proestrus upregulate factors that promote GnRH release, while daily rhythmic expression of AVP gates the timing of LH release via direct and indirect regulation of GnRH neurons (50, 51) (Figure 3).
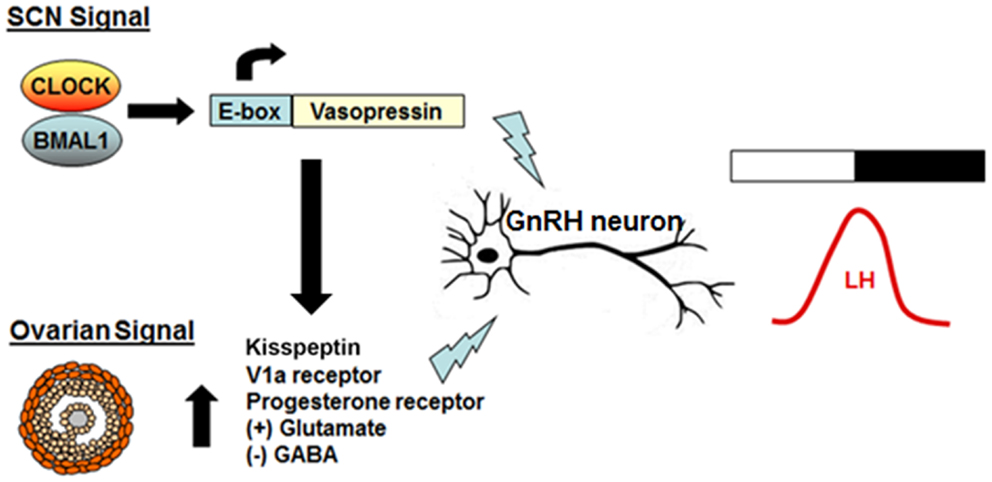
Figure 3. Model of the interaction between the daily timing signal and the ovarian signal on GnRH release. AVP is rhythmically transcribed in the SCN and forms the basis for the strong neural daily timing signal from the SCN. On the day of proestrus, estradiol produced by the developing follicles in the ovary upregulates expression of hypothalamic AVP and VIP receptors and primes kisspeptin neurons to become more sensitive to the SCN-derived signal. The end result is an increase in excitatory input to GnRH neurons, elevated GnRH release, and a subsequent surge in LH.
In addition to disruption or ablation of the LH surge in circadian mutant mice, the estrous cycle itself is often affected. Multiple groups have shown that Clock/Clock mutant mice have an extended and disrupted estrous cycle under both light:dark and continuous darkness, although this effect is somewhat dependent on strain background due to genetic variations in the recently identified CLOCK-Δ19 phenotype suppressor gene Usf1 (17, 52, 53) (Figure 4). Deletion of the CLOCK binding partner Bmal1 similarly results in prolonged estrous cycles (18), and although deletion of either Per1 or Per2 does not affect the estrous cycle in young mice, it accelerates age-related changes in the estrous cycle (19). Surprisingly, double knockout of either the Per or Cry paralogs has not been reported to affect estrous cyclicity, despite causing behavioral arrhythmicity in complete darkness (54, 55). These data suggest that CLOCK and BMAL1 have extra-circadian roles in regulating progression of the estrous cycle.
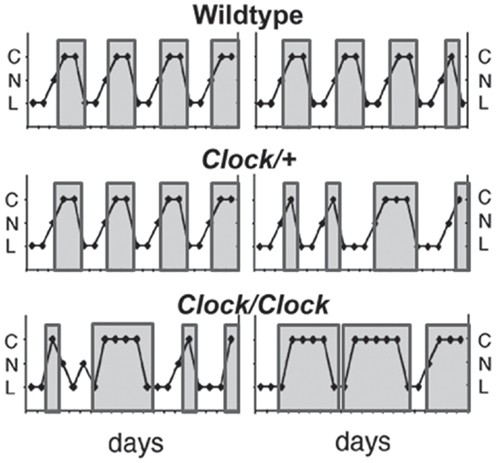
Figure 4. Clock/Clock females display lengthened and irregular estrous cycles. Representative estrous cycles as measured by vaginal cytology from wildtype (top), Clock/+ (middle), and Clock/Clock (bottom) females. Clock/Clock females have significantly fewer days of nucleated (proestrus) smears and significantly more days of cornified (estrus) smears compared to wildtype females. C, cornified; N, nucleated; L, leukocytic. Modified from a figure in Ref. (17).
Ovulation takes place on the early morning of estrus, approximately 12 h after the proestrus LH surge (24). If mating occurs, the copulatory stimulus induces the initiation of a pattern of biphasic circadian prolactin surges: a low-amplitude diurnal surge and a high-amplitude nocturnal surge (56). Regulation of PRL release is under SCN control, likely via inhibitory VIP output from the SCN to the dopaminergic neurons in the periventricular-arcuate nucleus (PVN) of the hypothalamus that generally suppress PRL release from the pituitary (57, 58). In non-pregnant females, VIP and PRL expression in the hypothalamus are phase-linked, dopamine neurons in the PVN express the VIP receptor VPAC2, and disruption of VIP can block circadian PRL release (59).
Pituitary prolactin release occurs for 10 days following a fertile mating, or for 12 days following a sterile mating (pseudopregnancy), the difference dependent on the presence of placental lactogen (PL), a prolactin-like hormone produced by the placenta that assumes control of the corpus luteum (CL) while inhibiting maternal prolactin secretion (14). The primary function of prolactin release appears to be to rescue and support the progesterone-producing CL for the first half of pregnancy (60). Placental production of PL is initiated on day 8–9 of pregnancy and continues through the end of pregnancy (61). Maintenance of the CL is an absolute requirement through late pregnancy: if CL maintenance is disrupted and progesterone levels drop, the developing fetuses are reabsorbed (60).
Placental PL production peaks at approximately day 14 of pregnancy and then declines, a pattern that parallels that of progesterone released from the CL (62). The precipitous decline in circulating progesterone levels relieves the inhibition of myometrial contractility, allowing parturition to progress. The drop in progesterone as fetuses near term is critical to successful labor: although estradiol levels increase in the final 24–48 h of pregnancy and estradiol plays an important role in the initiation of parturition, neither estradiol nor oxytocin treatment can overcome the inhibitory effects of elevated progesterone levels on uterine contractions (63).
Despite extensive observational data indicating a role for the central pacemaker in pregnancy, the molecular effects of circadian rhythms on pregnancy have received less attention than their effects on the estrous cycle and LH surge. However, Clock mutant mice and Bmal1, Per1, and Per2 knockout mice have all be shown to have deficiencies in embryonic implantation, maintenance of pregnancy, and/or parturition. In middle-aged female mice, deletion of either Per1 or Per2 results in an increase in the rate of fetal reabsorption (19), although this effect is not apparent in younger mice. Bmal1 knockout mice ovulate, but exhibit poor corpora luteum formation, reduced progesterone synthesis, and a complete lack of embryonic implantation, and it is not known whether this is due to a failure of embryonic development or of implantation (64, 65).
Here, comprehensive evaluation of the progression of pregnancy in Clock/Clock mice is instructive of the periods during pregnancy that are most sensitive to circadian disruption. Clock/Clock mutant females have been shown to have difficulties at multiple stages of pregnancy. Despite a reduced or absent proestrus LH surge, ovaries from non-pregnant homozygous mutant mice contain similar numbers of corpora lutea as wildtype mice (17), suggesting that ovulation, when it occurs, is generally normal. However, the duration of pseudopregnancy, an indirect measure of prolactin release, is significantly shortened in Clock/Clock mutants, as would be expected by recent data showing that inhibition of several core clock genes in the SCN can prevent the onset of cervical stimulation-induced biphasic PRL release (58). Indeed, the rate of homozygous mutants that become pregnant is significantly less than half that of wildtype mice, but the number of implanted fetuses at 11 days post-copulation (dpc) is the same in both genotypes. Thus, in Clock/Clock females that do become pregnant, the early processes, including embryonic development and implantation, appear normal.
However, Clock/Clock mutant serum progesterone levels are substantially lower at dpc 11, and significantly increased fetal reabsorption is observed by dpc 14, and more so at dpc 17 (Figure 5) (17). High midgestational (dpc 11–14) levels of progesterone are particularly important for maintaining blood flow to developing fetuses (66), and previous studies have shown a quantitative relationship between progesterone levels and maintenance of pregnancy (67). The low levels of progesterone in the Clock mutants at dpc 11 is the most likely explanation for increased pup reabsorption by mid-pregnancy; although PRL levels were not measured in these animals, the close association between the SCN and PRL release suggest a failure of maternal PRL release at the central level.
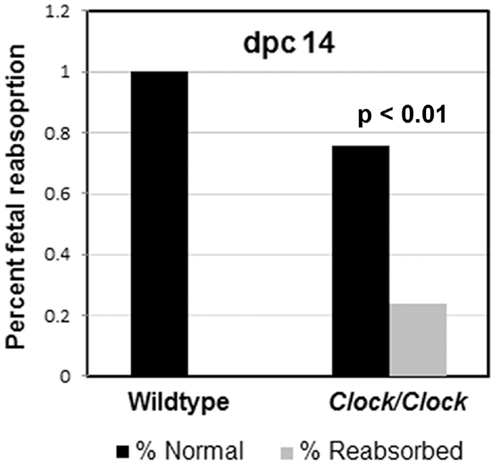
Figure 5. Clock/Clock pregnancies exhibit abnormal fetal reabsorption. In Clock mutants that do become pregnant, the early stages of pregnancy, including ovulation, implantation, and embryonic development appear normal. However, by mid-pregnancy, Clock/Clock females exhibit a significant increase in fetal reabsorption. Modified from data presented in Ref. (17).
Of the few Clock homozygotes who reached full term, half (as opposed to 0% of the wildtypes) displayed severe dystocia. Serum estradiol, progesterone, and glucocorticoid levels all regulate the timing and progression of parturition, but the specific cause of the parturition defects in Clock mutant mice has yet to be determined experimentally. Females who exhibited signs of unsuccessful labor for more than 24 h were sacrificed, and the uterine contents were examined. In all cases, the fetuses were clearly full term, but were dead and often exhibited gross morphological abnormalities (Figure 6). It is not known whether these abnormalities were the cause or the result of protracted labor, although it has been shown in rats that fetal death usually does not occur for at least 32 h after the onset of labor (68). This raises the possibility that fetal growth abnormalities may be more common in Clock mutants. In fact, the observed abnormalities are similar to those observed in wildtype mice nearing reproductive senescence (11–14 months of age), although the mice used in this study were <5 months old (69). Additionally, we have shown that mouse embryonic fibroblasts harvested from Clock/Clock mice exhibit a high rate of G1/S stage block, suggesting some difficulties with cell proliferation (70). Despite this evidence, when Clock/+ × Clock/+ matings are performed, there is no obvious imbalance in the expected percent of Clock homozygotes compared to either heterozygotes or wildtypes. Therefore, it is possible that defects in fetal growth and morphology are due to changes in the maternal hormonal milieu rather than a direct result of the Clock mutation on fetal development or maternal circadian rhythms.
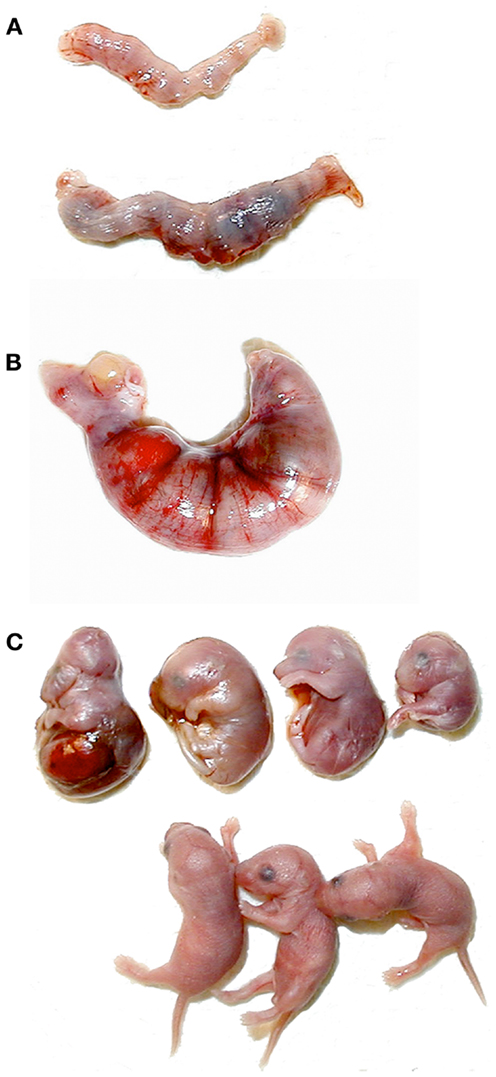
Figure 6. Abnormal pregnancy outcomes in Clock mutant females. (A) Uteri removed from postpartum wildtype (top) and Clock/Clock (bottom) females. The dark regions in the Clock mutant uterus are reabsorbing fetuses. (B) Uterus removed from a Clock mutant female after 24 h of dystocia. (C) Fetuses removed from the Clock/Clock uterus in (B), shown with age-matched live fetuses from a wildtype dam (bottom).
Although it has long been known that multiple aspects of female reproductive exhibit circadian regulation, our understanding of the role that the central molecular circadian pacemaker plays has been substantially advanced by the availability of multiple circadian mutant mice. Dysregulation of core circadian genes, including Clock, Bmal1, and Per1 and Per2, can disrupt the length and progression of the estrous cycle, the size and timing of the proestrus LH surge, the establishment and maintenance of pregnancy, and the success of parturition.
Human reproduction is similarly subject to circadian control. In women, the LH surge generally occurs immediately prior to the start of the active period, while the onset of parturition generally occurs during inactive period as a result of the circadian secretion of the pineal hormone melatonin (16, 71–74). Disruption of circadian rhythms due to rotating and night shift work or jet lag have been associated with an increase in the frequency of irregular, extended menstrual cycles, alterations in serum LH and FSH levels, an increased risk of pre-term birth, and overall reduced fecundity (75–77). Finally, polymorphisms in BMAL1 and the CLOCK paralog NPAS2 have been associated with the rate of pregnancy and risk of miscarriage (78). There is a long history of observational data among women, but a limited amount of molecular and genetic data to drawn upon.
Notably, a number of the defects exhibited by circadian mutant mice resemble phenotypes observed during the transition to reproductive senescence in middle-age wildtype mice. In a series of basic studies, Finch and colleagues found that wildtype mice exhibit the most regular estrous cycles from 3 to 10 months of age, after which cycle length increases and frequency decreases; the onset of persistent estrus, as evidenced by continuously cornified cells, occurs at 13–16 months (79). In our hands, young (2–5-month-old) Clock/Clock and Bmal1 knockout mice show estrous cycle characteristics very similar to those described in middle-age wildtypes, and 7–8-month-old Clock/Clock females exhibit signs of persistent estrous similar to Finch’s 13–16-month-old wildtype mice. Pilorz and Steinlechner also noted that, while young Per1 and Per2 knockouts appeared normal, the knockouts exhibited an early onset of disrupted estrous cycles and pregnancy mice (19). Finally, although the Clock mutant dams described above were no more than 5 months old, the dystocia and fetal defects observed are similar to abnormalities described in 11–14-month-old wildtype mice (69). Thus, it is reasonable to consider alterations in central or peripheral pacemakers as a model for, or potential cause of, reproductive senescence.
Ultimately, molecular and genetic biology has confirmed long-standing observations regarding the role of circadian rhythms in female reproductive function. Almost all circadian mutant mice display reproductive defects to some extent, underlining the absolute importance that daily timing signals play in reproduction. These defects have multiple causes, not all of which are discussed here: in some cases, the daily timing signal needed to drive the LH surge is lacking, in other cases the clocks in peripheral reproductive organs are too disrupted to function properly, and in still other cases, the core circadian genes and transcription factors CLOCK and BMAL may alter the genetic landscape enough that reproduction fails.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by U01 MH61915 (Joseph S. Takahashi), Silvio O. Conte Center NIH Grant P50 MH074924 (Joseph S. Takahashi), F31NS047799 (Brooke H. Miller), F32MH084528 (Brooke H. Miller), and K99MH092321 (Brooke H. Miller). Joseph S. Takahashi is an Investigator of the Howard Hughes Medical Institute.
1. King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci (2000) 23:713–42. doi:10.1146/annurev.neuro.23.1.713
2. Lowrey PL, Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv Genet (2011) 74:175–230. doi:10.1016/B978-0-12-387690-4.00006-4
3. Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci U S A (1998) 95:6097–102. doi:10.1073/pnas.95.11.6097
4. Lowrey PL, Takahashi JS. Genetics of the mammalian circadian system: photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet (2000) 34:533–62. doi:10.1146/annurev.genet.34.1.533
5. van der Beek EM. Circadian control of reproduction in the female rat. Prog Brain Res (1996) 111:295–320. doi:10.1016/S0079-6123(08)60415-X
6. Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod (1997) 56:239–302. doi:10.1095/biolreprod56.2.293
7. Chappell PE. Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol (2005) 17:119–30. doi:10.1111/j.1365-2826.2005.01270.x
8. Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev (2010) 31:544–77. doi:10.1210/er.2009-0023
9. Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology (1975) 96:57–62. doi:10.1210/endo-96-1-57
10. Brown-Grant K, Raisman G. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci (1977) 198:279–96. doi:10.1098/rspb.1977.0098
11. Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology (1980) 31:147–57. doi:10.1159/000123066
12. Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures. Neuroendocrinology (1982) 34:395–404. doi:10.1159/000123335
13. Smarr BL, Morris E, de la Iglesia HO. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology (2012) 153:2839–50. doi:10.1210/en.2011-1857
14. Freeman ME, Smith MS, Nazian SJ, Neill JD. Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology (1973) 94:875–82. doi:10.1210/endo-94-3-875
15. Jakubowski M, Dow RC, Fink G. Preoptic-hypothalamic pathways controlling nocturnal prolactin surges, pseudopregnancy, and estrous cyclicity in the rat. Neuroendocrinology (1988) 47:13–9. doi:10.1159/000124884
16. Olcese J. Circadian aspects of mammalian parturition: a review. Mol Cell Endocrinol (2012) 349:62–7. doi:10.1016/j.mce.2011.06.041
17. Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol (2004) 14:1367–73. doi:10.1016/j.cub.2004.07.055
18. Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology (2009) 150:1879–85. doi:10.1210/en.2008-1021
19. Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction (2008) 135:559–68. doi:10.1530/REP-07-0434
20. Levine JE, Bauer-Dantoin AC, Besecke LM, Conaghan LA, Legan SJ, Meredith JM, et al. Neuroendocrine regulation of the luteinizing hormone-releasing hormone pulse generator in the rat. Recent Prog Horm Res (1991) 47:97–153.
21. Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol (1999) 411:346–58. doi:10.1002/(SICI)1096-9861(19990823)411:2<346::AID-CNE13>3.0.CO;2-S
22. Miller BH, Gore AC. Alterations in hypothalamic insulin-like growth factor-I and its associations with gonadotropin releasing hormone neurones during reproductive development and ageing. J Neuroendocrinol (2001) 13:728–36. doi:10.1046/j.1365-2826.2001.00686.x
23. Everett JW, Sawyer CH. A 24h periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology (1950) 46:196–216.
24. Bingel AS, Schwartz NB. Timing of LH release and ovulation in the cyclic mouse. J Reprod Fertil (1969) 19:223–9. doi:10.1530/jrf.0.0190223
25. Norman RL, Blake CA, Sawyer CH. Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology (1973) 93:965–70. doi:10.1210/endo-93-4-965
26. Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol (1997) 384:569–79. doi:10.1002/(SICI)1096-9861(19970811)384:4<569::AID-CNE6>3.0.CO;2-0
27. de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than humoral pathway. J Neurosci (2003) 23:7412–4.
28. Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci (1987) 7:1626–38.
29. Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature (1996) 382:810–3. doi:10.1038/382810a0
30. Meyer-Bernstein EL, Jetton AE, Matsumoto S-I, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology (1999) 140:207–18. doi:10.1210/en.140.1.207
31. Gillespie JMA, Chan BPK, Roy D, Cai F, Belsham DD. Expression of circadian rhythm genes in gonadotropin-releasing hormone-secreting GT1-7 neurons. Endocrinology (2003) 144:5285–92. doi:10.1210/en.2003-0802
32. Hickok JR, Tischkau SA. In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology (2010) 91:110–20. doi:10.1159/000243163
33. Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci (2003) 23:11202–13.
34. Choe HK, Kim HD, Park SH, Lee HW, Park JY, Seong JY, et al. Synchronous activation of gonadotropin-releasing hormone gene transcription and secretion by pulsatile kisspeptin stimulation. Proc Natl Acad Sci U S A (2013) 110:5677–82. doi:10.1073/pnas.1213594110
35. Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology (1996) 137:3696–701. doi:10.1210/en.137.9.3696
36. Funabashi T, Sachiko A, Sano A, Shinohara K, Kimura F. Intracerebroventricular injection of arginine-vasopressin V1 receptor antagonist attenuates the surge of luteinizing hormone and prolactin secretion in proestrus rats. Neurosci Lett (1999) 260:37–40. doi:10.1016/S0304-3940(98)00940-9
37. van der Beek EM, Swarts HJM, Wiegant VM. Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology (1999) 69:227–37. doi:10.1159/000054423
38. Sun Y, Shu J, Kyei K, Neal-Perry GS. Intracerebroventricular infusion of vasoactive intestinal peptide rescues the luteinizing hormone surge in middle-aged female rats. Front Endocrinol (2012) 3:24. doi:10.3389/fendo.2012.00024
39. Palm IF, Van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience (1999) 93:659–66. doi:10.1016/S0306-4522(99)00106-2
40. Funabashi T, Shinohara K, Mitsushima D, Kimura F. Gonadotropin-releasing hormone exhibits circadian rhythm in phase with arginine-vasopressin in co-cultures of the female rat preoptic area and suprachiasmatic nucleus. J Neuroendocrinol (2000) 12:521–8. doi:10.1046/j.1365-2826.2000.00481.x
41. Jin X, Shearman LP, Weaver DR, Zylka MJ, De Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell (1999) 96:57–68. doi:10.1016/S0092-8674(00)80959-9
42. Silver R, Sookhoo AI, Lesauter J, Stevens P, Jansen HT, Lehman MN. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in the mouse SCN. Neuroreport (1999) 10:3165–74. doi:10.1097/00001756-199910190-00008
43. Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod (2006) 75:778–84. doi:10.1095/biolreprod.106.052845
44. Palm IF, Van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res (2001) 901:109–16. doi:10.1016/S0006-8993(01)02309-5
45. Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, et al. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci (2007) 27:12088–95. doi:10.1523/JNEUROSCI.2748-07.2007
46. Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci (2008) 28:8691–7. doi:10.1523/JNEUROSCI.1775-08.2008
47. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology (2009) 150:3664–71. doi:10.1210/en.2009-0247
48. Tonsfeldt KJ, Goodall CP, Latham KL, Chappell PE. Oestrogen induces rhythmic expression of the Kisspeptin-1 receptor GPR54 in hypothalamic gonadotrophin-releasing hormone-secreting GT1-7 cells. J Neuroendocrinol (2011) 23:823–30. doi:10.1111/j.1365-2826.2011.02188.x
49. Williams WP III, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology (2011) 152:595–606. doi:10.1210/en.2010-0943
50. Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, et al. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol (2010) 22:1032–9. doi:10.1111/j.1365-2826.2010.02045.x
51. Smarr BL, Gile JJ, de la Iglesia HO. Oestrogen-independent circadian clock gene expression in the anteroventral periventricular nucleus in female rats: possible role as an integrator for circadian and ovarian signals timing the LH surge. J Neuroendocrinol (2013) 25:1273–9. doi:10.1111/jne.12104
52. Dolatshad H, Campbell EA, O’Hara L, Maywood ES, Hastings MH, Johnson MH. Developmental and reproductive performance in circadian mutant mice. Hum Reprod (2006) 21:68–79. doi:10.1093/humrep/dei313
53. Shimomura K, Kumar V, Koike N, Kim TK, Chong J, Buhr ED, et al. Usf1, a suppressor of the circadian Clock mutant, reveals the nature of the DNA-binding of the CLOCK:BMAL1 complex in mice. Elife (2013) 2:e00426. doi:10.7554/eLife.00426
54. van der Horst GTJ, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature (1999) 398:627–30. doi:10.1038/19323
55. Bae K, Xiaowei J, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron (2001) 30:525–36. doi:10.1016/S0896-6273(01)00302-6
56. Freeman ME, Neill J. The pattern of prolactin secretion during pseudopregnancy in the rat: a daily nocturnal surge. Endocrinology (1972) 90:1292–4. doi:10.1210/endo-90-5-1292
57. Mai LM, Shieh KR, Pan J-T. Circadian changes of serum prolactin levels and tuberoinfundibular dopaminergic neuron activities in ovariectomized rats treated with or without estrogen: the role of the suprachiasmatic nuclei. Neuroendocrinology (1994) 60:520–6. doi:10.1159/000126789
58. Poletini MO, Kennett JE, McKee DT, Freeman ME. Central clock regulates the cervically stimulated prolactin surges by modulation of dopamine and vasoactive intestinal polypeptide release in ovariectomized rats. Neuroendocrinology (2010) 91:179–88. doi:10.1159/000254379
59. Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology (2004) 145:3386–94. doi:10.1210/en.2003-1710
60. Smith MS. Role of prolactin in mammalian reproduction. In: Greep RO, editor. Reproductive Physiology III. Baltimore: University Park Press (1980). p. 249–76.
61. Robertson MC, Friesen HG. Two forms of rat placental lactogen revealed by radioimmunoassay. Endocrinology (1981) 108:2388–90. doi:10.1210/endo-108-6-2388
62. Shiu RPC, Kelly PA, Friesen HG. Radioreceptor assay for prolactin and other lactogenic hormones. Science (1973) 80:968–71. doi:10.1126/science.180.4089.968
63. Csapo A. The four direct regulatory factors of myometrial function. In: Wolstenholme GEW, Knight J, editors. Progesterone: Its Regulatory Effect on the Myometrium. London: Churchill (1969). p. 13–55.
64. Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, et al. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms (2008) 23:26–36. doi:10.1177/0748730407311254
65. Boden MJ, Varcoe TJ, Voultsios A, Kennaway DJ. Reproductive biology of female Bmal1 null mice. Reproduction (2010) 139:1077–90. doi:10.1530/REP-09-0523
66. Milligan SR, Finn CA. Minimal progesterone support required for the maintenance of pregnancy in mice. Hum Reprod (1997) 12:602–7. doi:10.1093/humrep/12.3.602
67. Csapo I, Wiest WG. An examination of the quantitative relationship between progesterone and the maintenance of pregnancy. Endocrinology (1969) 85:735–46. doi:10.1210/endo-85-4-735
68. Arkaravichien W, Kendle KE. Fetal viability and fetal growth after prolonged uterine contractions induced by progesterone withdrawal in late pregnancy in rats. J Reprod Fertil (1992) 96:299–308. doi:10.1530/jrf.0.0960299
69. Gosden RG, Holinka CF, Finch CE. The distribution of fetal mortality in ageing C57BL/6J mice: a statistical analysis. Exp Gerontol (1981) 16:127–30. doi:10.1016/0531-5565(81)90035-8
70. Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A (2007) 104:3342–7. doi:10.1073/pnas.0611724104
71. Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol (1980) 87:737–56. doi:10.1111/j.1471-0528.1980.tb04610.x
73. Cahill DJ, Wardle PG, Harlow CR, Hull MG. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertil Steril (1998) 70:56–9. doi:10.1016/S0015-0282(98)00113-7
74. Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol (2010) 2010:813764. doi:10.1155/2010/813764
75. Nurminen T. Shift work and reproductive health. Scand J Work Environ Health (1998) 24(Suppl 3):28–34.
76. Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med (2007) 8:613–22. doi:10.1016/j.sleep.2006.09.011
77. Lawson CC, Whelan EA, Lividoti Hibert EN, Spiegelman D, Schernhammer ES, Rich-Edwards JW. Rotating shift work and menstrual cycle characteristics. Epidemiology (2011) 22:305–12. doi:10.1097/EDE.0b013e3182130016
78. Kovanen L, Saarikoski ST, Aromaa A, Lonnqvist J, Partonen T. ARNTL (BMAL1) and NPAS2 gene variants contribute to fertility and seasonality. PLoS One (2010) 5:e10007. doi:10.1371/journal.pone.0010007
Keywords: circadian rhythms, estrous cycle, clock gene, proestrus, parturition
Citation: Miller BH and Takahashi JS (2014) Central circadian control of female reproductive function. Front. Endocrinol. 4:195. doi: 10.3389/fendo.2013.00195
Received: 19 September 2013; Accepted: 06 December 2013;
Published online: 22 January 2014.
Edited by:
James Olcese, Florida State University College of Medicine, USAReviewed by:
Patrick Chappell, Oregon State University, USACopyright: © 2014 Miller and Takahashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brooke H. Miller, Departments of Psychiatry and Medicine, University of Florida College of Medicine, McKnight Brain Institute, 1149 S. Newell Drive, Suite L4-100, Gainesville, FL 32610, USA e-mail:YnJvb2tlbWlsbGVyQHVmbC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.