
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 18 September 2013
Sec. Neuroendocrine Science
Volume 4 - 2013 | https://doi.org/10.3389/fendo.2013.00129
This article is part of the Research Topic The regulated secretory pathway in neuroendocrine cells View all 18 articles
Secretory epithelial cells of the proximal airways synthesize and secrete gel-forming polymeric mucins. The secreted mucins adsorb water to form mucus that is propelled by neighboring ciliated cells, providing a mobile barrier which removes inhaled particles and pathogens from the lungs. Several features of the intracellular trafficking of mucins make the airway secretory cell an interesting comparator for the cell biology of regulated exocytosis. Polymeric mucins are exceedingly large molecules (up to 3 × 106 Da per monomer) whose folding and initial polymerization in the ER requires the protein disulfide isomerase Agr2. In the Golgi, mucins further polymerize to form chains and possibly branched networks comprising more than 20 monomers. The large size of mucin polymers imposes constraints on their packaging into transport vesicles along the secretory pathway. Sugar side chains account for >70% of the mass of mucins, and their attachment to the protein core by O-glycosylation occurs in the Golgi. Mature polymeric mucins are stored in large secretory granules ∼1 μm in diameter. These are translocated to the apical membrane to be positioned for exocytosis by cooperative interactions among myristoylated alanine-rich C kinase substrate, cysteine string protein, heat shock protein 70, and the cytoskeleton. Mucin granules undergo exocytic fusion with the plasma membrane at a low basal rate and a high stimulated rate. Both rates are mediated by a regulated exocytic mechanism as indicated by phenotypes in both basal and stimulated secretion in mice lacking Munc13-2, a sensor of the second messengers calcium and diacylglycerol (DAG). Basal secretion is induced by low levels of activation of P2Y2 purinergic and A3 adenosine receptors by extracellular ATP released in paracrine fashion and its metabolite adenosine. Stimulated secretion is induced by high levels of the same ligands, and possibly by inflammatory mediators as well. Activated receptors are coupled to phospholipase C by Gq, resulting in the generation of DAG and of IP3 that releases calcium from apical ER. Stimulated secretion requires activation of the low affinity calcium sensor Synaptotagmin-2, while a corresponding high affinity calcium sensor in basal secretion is not known. The core exocytic machinery is comprised of the SNARE proteins VAMP8, SNAP23, and an unknown Syntaxin protein, together with the scaffolding protein Munc18b. Common and distinct features of this exocytic system in comparison to neuroendocrine cells and neurons are highlighted.
Mucus has physical characteristics on the border between a viscous fluid and a soft and elastic solid (1). These characteristics are conferred by a semi-dilute network of polymerized mucins in water. The secreted, polymeric mucins expressed in the airways are Muc5ac and Muc5b (2, 3). (Note, lower case letters are used to designate non-human mammalian mucins, while MUC5AC and MUC5B designate the human orthologs. In this review, we use lower case letters to designate all mammalian mucins, and only use upper case letters when referring specifically to human data.) In healthy airway mucus, water accounts for about 98% of the mass, mucins for about 0.7%, and salts and small amounts of other macromolecules for the rest. The mucus layer lies atop a denser periciliary layer containing membrane-tethered glycoconjugates, including glycosaminoglycans and membrane-spanning mucins (Muc 1, 4, and 16) (4–6). The mucus layer is continually swept from distal to proximal airways by beating cilia, and is eventually propelled out of the lungs into the pharynx and swallowed, removing entrapped particles, pathogens, and dissolved chemicals. The critical importance of the mucus layer in airway defense is shown by the spontaneous inflammatory lung and nasal disease that develops in mice in which the constitutively produced mucin, Muc5b, has been deleted (1).
In order to replenish the mucus layer, mucins are continuously synthesized and released by secretory cells that form a mosaic with ciliated cells, with similar numbers of both cell types (Figure 1, left). In allergic lung inflammation, which appears to be a parasitic defense gone awry (4, 7), the second polymeric airway mucin, Muc5ac, is produced in large quantities (Figure 1, center and right). Whereas increased mucin production alone does not appear to lead to pathology (8), the production of large amounts of mucin together with its rapid secretion (“mucus hypersecretion”) can overwhelm available liquid resulting in formation of excessively viscoelastic mucus that is poorly cleared by ciliary action or cough. When coupled with airway narrowing due to bronchoconstriction in asthma, this can lead to widespread airway closure with serious consequences. Mucus hypersecretion is also an important feature of chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and idiopathic bronchiectasis (1).
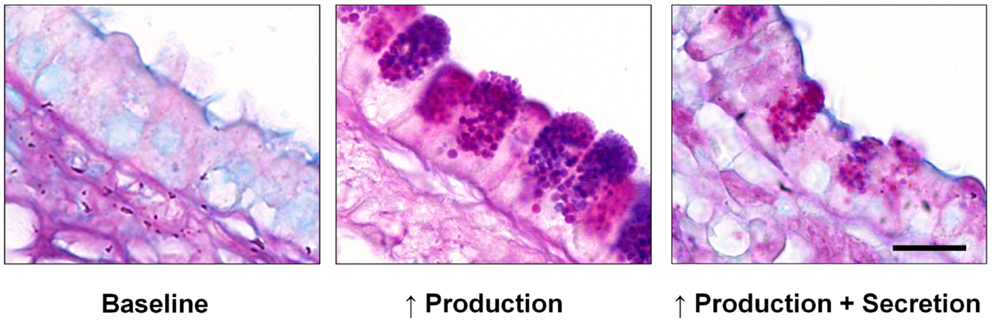
Figure 1. Mucin production and secretion in the mouse airway. Left – In the healthy baseline state, alternating ciliated and domed secretory cells are seen, with no mucin granules visible by Alcian blue and periodic acid Schiff (AB-PAS) staining. Center – Numerous large mucin granules are visible in secretory cells 3 days after mucin production is increased by IL-13-dependent allergic inflammation as described (51). Right – Exocytic secretion of the intraepithelial mucin stored in inflamed airway epithelium induced by brief exposure to an ATP aerosol as described (51). Scale bar is 10 μm.
A key event in mucus secretion is its hydration immediately after exocytosis (4, 9, 10). Mucins are packaged dehydrated in secretory granules, and must adsorb more than 100-fold their mass of water soon after secretion in order to attain the appropriate viscoelasticity for ciliary clearance. Water in the airway lumen is controlled both by the release of chloride through CFTR, CaCC, and Slc26A9, and by the absorption of sodium by ENaC, with water passively following the flux of ions (11–13). Coupling of the secretion of chloride and mucins is accomplished by paracrine signaling through ATP, adenosine, and other extracellular signaling molecules. In CF, mutation of the principal chloride channel, CFTR, results in insufficient luminal water that causes the formation of underhydrated mucus which is excessively viscoelastic and difficult to clear. The underhydration is exacerbated because CFTR is also a channel for bicarbonate, which is needed to chelate calcium (9, 14). In acidic secretory granules of the intestinal epithelium, calcium binds to the N-terminus of MUC2, which is the secreted mucin most similar to MUC5AC in structure, and organizes the mucin in such a way that it can be secreted without entanglement (15). Too little bicarbonate at mucin release prevents normal mucin unfolding and leads to formation of abnormally dense mucus (16); similar mechanisms likely operate in the airway.
In summary, the mucus layer forms a mobile, essential barrier that protects the lungs when it is functioning properly, but dysfunction of the mucus layer plays a prominent role in all of the common diseases of the airways.
Muc5b/MUC5B is transcribed constitutively throughout the conducting airways from the trachea down to but not including terminal bronchioles (1, 17, 18). Muc5ac is produced in low amounts or not at all in healthy mice, although in humans MUC5AC is produced constitutively in proximal airways (trachea and bronchi) (1). In allergic inflammation the production of Muc5ac increases dramatically (40- to 200-fold) in the airways of mice and in cultured human airway epithelial cells (19–21). Both mucins are produced in the same secretory cells, but even in conditions of severe inflammation they are not produced in small airways, which makes teleologic sense in that their small luminal diameters (<200 μm) make them highly susceptible to occlusion.
After translation at the ER, Muc5ac, and Muc5b undergo initial polymerization as homodimers (3). These are among the largest macromolecules encoded in the mammalian genome, and their processing induces a stress response in the ER (22). Proper folding and polymerization require the protein disulfide isomerase Agr2, whose deletion in mice results in absent intestinal mucin (23) and in reduced airway mucin in the setting of allergic lung inflammation (24). The transport of polymeric mucins from the ER to the Golgi and through the Golgi has been little studied, but likely involves modulation of COP-II transport vesicle size to accommodate large cargoes as has been described for collagen (25). In the Golgi, Muc5b undergoes further polymerization in linear chains up to 20 monomers in length (3, 26). The structure of Muc5ac is less well studied, but appears to be more similar to Muc2 that forms branched polymers resulting in formation of a covalent net (9). Both mucins undergo O-glycosylation in the Golgi that result in mature glycoproteins that are more than 70% carbohydrate with a general negative charge due to sulfation or sialylation of many terminal sugars (27).
Export of polymeric mucins from the trans-Golgi and lateral fusion of post-Golgi vesicles to form secretory granules are additional transport steps that have been poorly studied. Similar to the case of COP-II vesicles, post-Golgi clathrin-coated vesicles have recently been shown to be capable of size variation to accommodate large cargo proteins (28). It is probable this mechanism is also utilized for mammalian mucins because, in fruit flies, assembly of large salivary mucin granules requires clathrin and the adaptor AP-1 (29). Mature mucin secretory granules are very large, with a mean diameter of 1 μm. Their exocytic fusion is highly regulated by extracellular secretagogues (Table 1), as described below. It should be noted that even though additional secretory pathways have been described in other cell types, such as a minor regulated pathway for secretion of immature granules and the compound exocytosis of mature granules (30), these are not well-described in airway secretory cells and will not be addressed in this review.
The movement of mature mucin granules to the plasma membrane for exocytosis has been the subject of work in numerous laboratories for many years. In the 1990s, work in the Adler laboratory turned to the Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) protein. MARCKS was a known actin-binding protein and protein kinase C (PKC) substrate (31), and it was known that PKC activation enhanced mucin secretion (32), so MARCKS was a logical candidate regulator of mucin granule movement.
MARCKS is a rod-shaped 87 kDa protein that is ubiquitously expressed. Three domains of MARCKS are conserved evolutionarily. First is the Phosphorylation Site Domain (PSD), also known as the “effector domain,” a highly basic 25 amino acid stretch containing a number of serine residues that are phosphorylated by PKC. This domain also binds calcium/calmodulin and crosslinks actin filaments. Second is the Multiple Homology (MH2) domain, whose function is unknown. Third is the N-terminal region containing 24 amino acids and a myristic acid moiety involved in binding to membranes. MARCKS knockout mice die at birth or soon afterward, so peptides that might compete with native MARCKS to inhibit its function were generated by the Adler laboratory in collaboration with the Blackshear laboratory. These were tested using normal human bronchial epithelial (NHBE) cells grown in air-liquid interface culture to maintain their well-differentiated state. Peptides identical to the PSD site tended to induce a toxic response, but a peptide identical to the N-terminus had a strong inhibitory effect on mucin secretion induced by a combination of phorbol ester, a PKC activator, and 8-bromo-cyclic GMP, a protein kinase G (PKG) activator (33), or by the more physiologically relevant stimulus UTP. This peptide was named Myristoylated N-terminal Sequence (MANS), and a control missense peptide was named Random N-terminal Sequence (RNS). In contrast to MANS, RNS was without effect on mucin secretion. Additional studies showed that MARCKS phosphorylation in response to protein kinase activation, followed by dephosphorylation catalyzed by a protein phosphatase type 2A (PP2A), were critical to MARCKS function. This was the first publication to show a specific biological function for MARCKS, and suggested a mechanism whereby MARCKS came off the inner face of the plasma membrane when phosphorylated by PKC, then bound to mucin granules at the N-terminus and the cytoskeleton at the PSD site, serving as a bridge for granule transport to the plasma membrane by the cytoskeleton (33).
To examine the function of MARCKS in vivo, mice with mucous metaplasia induced by allergic inflammation (see Mucin Exocytosis, below) were then exposed to aerosolized methacholine to induce mucin secretion. Intratracheal pretreatment with MANS dose-dependently inhibited mucin secretion (34), and it attenuated airflow obstruction about 40% (35). Gold-labeling of stimulated cells revealed MARCKS to be morphologically associated with mucin granules, and treatment with MANS but not RNS blocked the association (34). Additional studies performed in mice with human neutrophil elastase instilled in the airways to induce mucous metaplasia showed similar results, with MANS but not RNS attenuating both mucin secretion and airway hyperreactivity in response to serotonin (36).
Subsequent studies have revealed that PKC δ and ε isoforms are involved in stimulated mucin secretion (37, 38), and that PKCδ-provoked secretion depends on phosphorylation of MARCKS (38, 39). Another question was the mechanism of translocation of MARCKS from the plasma membrane to mucin granules. Co-immunoprecipitation studies revealed an association between MARCKS and two previously described chaperones – Heat Shock Protein 70 (HSP70) and Cysteine String Protein (CSP) (40). Of interest, there was previously known to be direct and specific interaction of HSP70 with CSP (41). Western blotting and proteomic analysis of mucin granule membranes, ultrastructural immunohistochemistry, and immunoprecipitation experiments showed that MARCKS, HSP70, and CSP form a trimeric complex associated with the granule membrane (42, 43). Functional studies in a bronchial epithelial cell line using siRNA to knock down expression of MARCKS, HSP70, or CSP resulted in the attenuation of stimulated mucin secretion (42).
Additional studies have examined interactions among MARCKS, the chaperones, and cytoskeletal proteins. Treatment of NHBE cells with the pyrimidinone MAL3-101, an HSP70 inhibitor, or siRNA against HSP70, attenuated phorbol ester-stimulated mucin secretion, and blocked trafficking of fluorescent-tagged MARCKS (42, 44). In preliminary studies, cell-permeant peptides that target different domains of CSP were utilized to show that the C-terminus of CSP, rather than the more frequently studied “J” domain, appears to be involved in attachment of MARCKS to mucin granule membranes and resultant secretion. MARCKS has been found to bind both actin and myosin (33), and recent experiments showed that the myosin family involved is Myosin V (45). A possible contributing mechanism of MARCKS action besides granule transport could be the remodeling of apical actin (30). Exocytic Rab GTPases of the 3 and 27 subfamilies interact with the cytoskeleton and catalyze loose tethering of secretory granules to the plasma membrane in other cell types; Rab3D and Rab27A are expressed in airway secretory cells (30, 46), though they have not yet been functionally implicated in mucin secretion or MARCKS interaction. Another possibly important interaction is with VAMP8 that has been identified as the principal t-SNARE in mucin secretion (47) (see Core Exocytic Machinery, below). Preliminary studies from the Adler laboratory show that MARCKS and CSP bind VAMP8 on mucin granules. In summary, MARCKS engages in multiple protein interactions that together help position mucin secretory granules for exocytotic release. For a listing of proteins known to localize to the mucin granule membrane, see Table 2.
Mucins are secreted into the airway lumen at a low basal rate and a high stimulated rate (1, 30). It is difficult to precisely define the difference in these rates because their measurement depends upon intracellular mucin content, the time interval of observation, and the post-exocytic release of mucins and their maturation to mucus for most assays. Despite these limitations, the rate of stimulated secretion has been generally found to exceed the rate of basal secretion by ∼5-fold over durations of 1 h or less by a variety of techniques (18, 37, 48, 49). The basal rate of secretion matches the basal rate of mucin synthesis in the distal airways of humans and all the airways of mice so that there is little intracellular mucin accumulation in the healthy state (Figure 1, left). Small amounts of intracellular mucin in this setting can be detected by sensitive immunohistochemical techniques that involve signal amplification (18, 50), but generally are not detectable by histochemical stains (51). The proximal airways of humans do contain histochemically apparent mucin associated with the constitutive expression of MUC5AC (1), but most functional studies of the exocytic machinery have been performed in mouse models so these will be the focus of further discussion. For comparison with in vitro systems studied by electrophysiologic (49) and videomicroscopic (52) techniques, the reader is referred to the referenced articles.
A regulated exocytic mechanism mediates both basal and stimulated mucin secretion as indicated by abnormal phenotypes in both basal and stimulated secretion when Munc13-2, a sensor of second messengers (see Extracellular Signaling and the Exocytic Regulatory Machinery), is deleted in mice (18). A defect in basal mucin secretion can be detected as the spontaneous accumulation of intracellular mucin in the absence of increased mucin synthesis (53). To measure stimulated secretion, it is useful to first induce increased mucin production and accumulation (mucous metaplasia) with allergic inflammation (Figure 1, center), such as by IL-13 instillation or ovalbumin immunization and challenge (51, 53). A defect in stimulated mucin secretion can then be detected as the failure to release intracellular mucin in response to a strong agonist such as ATP (Figure 1, right). Differential effects of the deletion of genes encoding various exocytic proteins on basal and stimulated mucin secretion indicate which proteins participate in which secretory state. In general, deletion of components of the core exocytic machinery give phenotypes in both basal and stimulated secretion, indicating that there is a single core exocytic machine, whereas deletion of components of the regulatory machinery give variable phenotypes, as described below.
Every step of vesicular transport on the exocytic and endocytic pathways involves the interactions of a four helix SNARE bundle with an SM protein (30, 54, 55). The SNARE proteins impart specificity to the pairing of transport vesicles with target membranes, mediate tight docking of vesicles to target membranes, and induce fusion of vesicle and target membranes when they fully coil. SM proteins provide an essential platform for sequential interactions of SNARE proteins, and also mediate interactions of the SNARE complex with tethering proteins (Figure 2). Three of the SNARE helices localize to the target membrane (called t-SNAREs for target SNAREs, or Q-SNAREs since the ionic amino acid of their SNARE domains is generally glutamine), and one SNARE helix is localized on the vesicle membrane (v-SNARE for vesicle SNARE, or R-SNARE since the ionic amino acid is generally arginine).
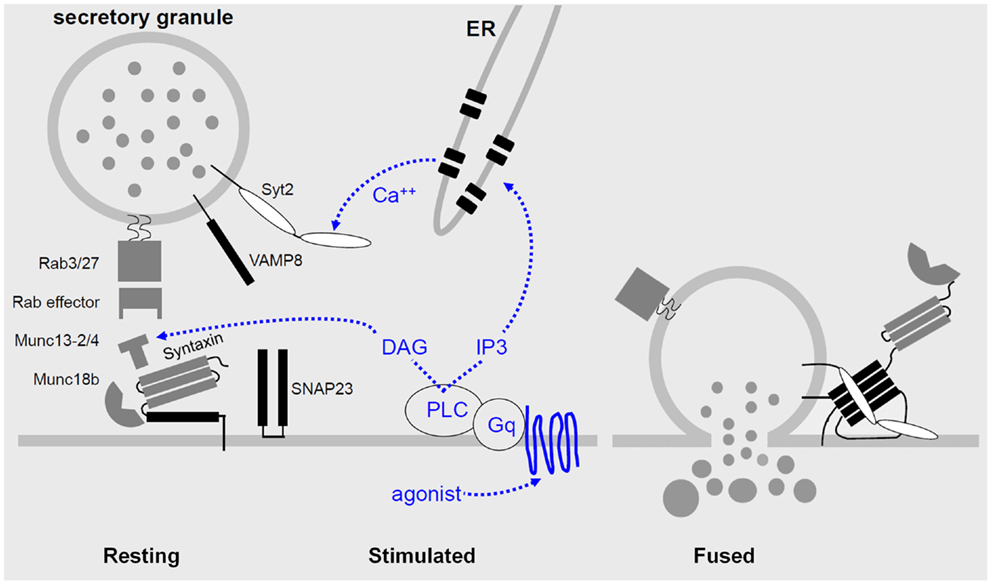
Figure 2. Regulated airway mucin secretion. Left – In the basal state, mucin granules are thought to become tethered to the plasma membrane by Rab proteins and effectors that have not yet been identified, in the vicinity of components of the exocytic machinery. Center – Activation of heptahelical receptors such as those for ATP (P2Y2) and adenosine (A3R) leads to activation of the trimeric G-protein, Gq, and phospholipase C (PLC), resulting in generation of the second messengers diacylglycerol (DAG) and inositol trisphosphate (IP3). Diacylglycerol activates the priming protein Munc13-2, and IP3 induces the release of calcium from apical ER to activate Synaptotagmin-2 (Syt2). Munc13-4 also participates in granule priming, and an unknown high affinity calcium sensor likely functions in basal secretion rather than Syt2. Right – Activation of the regulatory Munc13 and Syt proteins leads to full coiling of the SNARE proteins (SNAP23, VAMP8, and an unknown Syntaxin, all shown in black) to induce fusion of the granule and plasma membranes. The interactions of the SNARE proteins take place on a scaffold provided by Munc18b. In other secretory cells that form the basis for this model, exocytic Syntaxins contain four hydrophobic coiled-coil domains that must be opened to initiate secretion (left panel), and during fusion the associated Munc18 protein remains associated only by an interaction at the Syntaxin N-terminus (right panel).
Syntaxins are Qa SNAREs that can be considered the central component of the core machinery since they initiate formation of the SNARE complex and their structure is ordered even in the absence of interaction with the other SNARE components. The Syntaxin that mediates mucin granule exocytosis remains unknown. Candidates are Stx 2, 3, and 11, all of which have been shown to functionally pair with Munc18b in other cell types since Munc18b has been definitively implicated in airway mucin exocytosis (56) (see below). Efforts are underway in the Dickey laboratory using genetically modified mice to test the roles of these Syntaxins in mucin secretion.
In both yeast and neurons, the Qb and Qc SNAREs involved in exocytosis are contributed by a single protein with two SNARE domains connected by a linker region. In yeast this protein is Sec9, which is essential for cell viability. In neurons the cognate protein mediating axonal synaptic vesicle release is SNAP25 (57). While SNAP25 is essential for post-natal life, brain development to the time of birth is nearly normal. In unpublished work, the Dickey laboratory has obtained evidence that SNAP23 mediates both basal and stimulated airway mucin secretion. SNAP23 is expressed ubiquitously, and knockout mice experience early embryonic lethality (58, 59). However heterozygous knockout mice show spontaneous airway epithelial cell mucin accumulation, indicating a defect in basal mucin secretion, as well as epithelial mucin retention after stimulation with aerosolized ATP, indicating a defect in stimulated secretion. Thus, SNAP23 appears to mediate most or all Qbc function in both basal and stimulated mucin secretion.
Recently, the R-SNARE (v-SNARE) in airway mucin secretion was identified as VAMP8 by immunolocalization to mucin secretory granules, in vitro functional analysis by RNA interference, and in vivo analysis of knockout mice (47). Both basal and stimulated mucin secretion were reduced by loss of VAMP8, though the defects were not as severe as from the loss of some other exocytic proteins, consistent with the viability of knockout mice, and suggesting that other v-SNAREs also participate in mucin secretion.
The scaffolding function of SM proteins in exocytosis in different cell types is mediated by three Munc18 proteins (54, 56). Munc18a (Stxbp1) and Munc18b (Stxbp2) appear to be paralogs functioning in axonal/apical secretion, whereas Munc18c (Stxbp3) is a ubiquitous isoform functioning in dendritic/basolateral secretion. Munc18a is expressed in neurons and neuroendocrine cells, whereas Munc18b is expressed in polarized epithelia. Together, these data suggested that Munc18b mediates airway mucin secretion, and localization and functional data support this. Munc18b is highly expressed in airway secretory cells where it localizes to the apical plasma membrane (56). Munc18b knockout mice are not viable postnatally, but heterozygous knockout mice show an ∼50% reduction in stimulated mucin secretion, indicating that Munc18b is a limiting component of the exocytic machinery (56). These heterozygous mutant mice do not show spontaneous mucin accumulation, unlike heterozygous SNAP23 mutant mice, suggesting that another SM protein besides Munc18b also plays a scaffolding role in basal mucin secretion whereas no other protein besides SNAP23 appears to function as a Qbc SNARE in mucin exocytosis. Ruling out the possibility that Munc18b functions only in stimulated and not basal mucin secretion, conditional mutant mice with Munc18b deleted only in airway secretory cells are viable and show spontaneous mucin accumulation, although preliminary results suggest that the accumulation is less than in Munc13-2 mice.
The extracellular ligands and signal transduction pathways controlling mucin secretion have been studied for longer and in more depth than the exocytic machinery itself (30). The best-studied ligand is ATP that acts on the P2Y2 receptor to activate Gq and PLC-β1, resulting in generation of the second messengers IP3 and diacylglycerol (DAG). ATP is released in a paracrine fashion from ciliated cells in response to mechanical shear stress and in an autocrine fashion along with uridine nucleotides from secretory granules (60, 61). The ATP metabolite adenosine acting on the A3 adenosine receptor appears to activate the same Gq-PLC pathway (62). It is possible that other G-protein coupled receptors, such as those sensing serotonin or acetylcholine, also function on airway secretory cells since those ligands induce mucin secretion in vivo (34, 36), however they may be acting in a paracrine fashion by inducing contraction of smooth muscle cells leading to the release of ATP that in turn induces mucin release (Table 1).
In airway secretory cells, the second messenger IP3 activates receptors on apical ER to induce the release of calcium. In contrast to excitable cells in which calcium enters the cytoplasm from outside through voltage-gated channels, or secretory hematopoietic cells such as mast cells in which an initial release of calcium from intracellular stores triggers further calcium entry from outside through ICRAC, all of the cytoplasmic calcium involved in exocytic signaling in airway secretory cells appears to come from intracellular stores (30). This may be an adaptation to the fact that the calcium concentration in the thin layer of airway surface liquid is not stable due to the variable release of mucins that carry calcium as a counterion and the variable secretion via CFTR of bicarbonate that chelates calcium. Calcium does enter airway secretory cells from the basolateral surface to maintain intracellular stores, presumably by communication between the basolateral and apical ER since mitochondrial barriers segregate cytoplasmic calcium signals (63). Nonetheless, the chelation of extracellular calcium in vitro does not acutely affect mucin secretion. Rough ER at the apical pole of airway secretory cells lies in close apposition to mucin granules (46, 64), which should allow localized calcium signaling to the exocytic machinery through proteins such as Synaptotagmins and Munc13s.
Synaptotagmins are a family of proteins containing two C2 domains capable of calcium-dependent phospholipid binding, of which several members mediate calcium-dependent exocytosis. Using Syt2 knockout mice, we have found that Syt2 serves as a critical sensor of stimulated but not of basal mucin secretion (46). There was no spontaneous mucin accumulation in these mice, consistent with the fact that Syt2 and its close homolog Syt1 inhibit rather than promote synaptic vesicle release at baseline levels of cytoplasmic calcium (65). In contrast, there was a complete failure of ATP-stimulated mucin release in homozygous knockout mice and a dose-dependent failure in heterozygous knockout mice (46). This was a surprising result for several reasons. First, there is no impairment of synaptic vesicle release in heterozygous mutant Syt1 or Syt2 mice, indicating that some structural or functional feature of stimulated exocytosis in airway secretory cells differs from that in neurons to make Syt2 levels limiting, such as the difference in size of the secretory vesicles (50 nm in neurons versus 1000 nm in airway secretory cells) or the concentration of exocytic proteins at the active zone. Second, Syt2 is the fastest among the low affinity, fast calcium exocytic sensors Syt 1, 2, and 9, yet mucin secretion is a slow exocytic process (measured in hundreds of milliseconds) compared to synaptic vesicle release (measured in milliseconds). This suggests that some other feature of Syt2 besides its kinetics makes it a suitable regulator of mucin release. The calcium-sensing protein in basal mucin secretion that performs a role comparable to that of Syt2 in stimulated secretion is not yet known. Nonetheless such a protein likely exists since a second, high affinity calcium sensor functions in neurons and neuroendocrine cells, and basal mucin secretion has been shown to be calcium dependent (66).
Munc13 comprises a family of four calcium and lipid sensing proteins with variable numbers of C1 and C2 domains that function in the priming of secretory vesicles. As mentioned above, Munc13-2 knockout mice show defects in both basal and stimulated mucin secretion, with the basal defect being more dramatic than the stimulated defect (18). Munc13-2 contains a C1 domain that binds the second messenger DAG. Another member of this family, Munc13-4, is also expressed in airway secretory cells (67). Munc13-4 does not contain a C1 domain, though it does contain two C2 domains that may bind phospholipids in a calcium-dependent manner. In unpublished results, the Dickey laboratory has found that deletion of Munc13-4 causes a mild defect in stimulated secretion, and that deletion of both Munc13-2 and Munc13-4 together causes a severe (though incomplete) defect in stimulated secretion. Whether a third protein also functions in mucin granule priming to account for the residual secretion or whether mucin granule exocytosis depends only partially on priming function is not yet known.
There are additional targets of the regulation of mucin secretion besides Synaptotagmin and Munc13 proteins, such as PKC that also binds DAG and calcium (37, 38). Here we have focused on components of the exocytic machinery. A full accounting of the regulation of mucin secretion will require further knowledge of second messengers and their targets, together with analysis of their integrated function.
Airway secretory cells continuously synthesize and secrete polymeric mucins that form a protective mucus layer. Both the synthesis and secretion of mucins are highly regulated, with low basal rates and high stimulated rates for each. Mature mucin granules are positioned for secretion by interactions of MARCKS, CSP, HSP70, Rab proteins, and the cytoskeleton. A core exocytic machine consisting of the SNARE proteins VAMP8, SNAP23, and an unknown Syntaxin, along with the scaffolding protein Munc18b, mediates both basal and stimulated mucin secretion. Regulatory proteins including Munc13-2, Munc13-4, and Syt2 respond to second messengers to control the rate of mucin secretion in response to extracellular signals. These regulatory proteins show differential activities in basal and stimulated secretion, suggesting that they variably associate with the core machinery depending on the levels of second messengers. Close coordination of mucin production and secretion with physiologic need are essential to lung health since either a deficiency or excess of airway mucus causes disease. The medical importance of airway mucin secretion and its scientific value as a model of large-granule exocytosis in polarized epithelia insure its continued study.
Kenneth B. Adler has an ownership interest in BioMarck Pharmaceuticals that is developing the MANS peptide for commercial use. Michael J. Tuvim and Burton F. Dickey have no potential conflicts of interest.
This work was supported by grants from the U.S. National Institutes of Health HL36982 (Kenneth B. Adler) and HL097000 and HL094848 (Michael J. Tuvim and Burton F. Dickey).
1. Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med (2010) 363:2233–47. doi:10.1056/NEJMra0910061
2. Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev (2006) 86:245–78. doi:10.1152/physrev.00010.2005
3. Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol (2008) 70:459–86. doi:10.1146/annurev.physiol.70.113006.100702
4. Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science (2012) 337:937–41. doi:10.1126/science.1223012
5. Dickey BF. Walking on solid ground: a gel-on-brush model of airway mucosal surfaces. Science (2012) 337:924–5. doi:10.1126/science.1227091
6. Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol (2008) 70:431–57. doi:10.1146/annurev.physiol.70.113006.100659
8. Ehre C, Worthington EN, Liesman RM, Grubb BR, Barbier D, O’Neal WK, et al. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci U S A (2012) 109:16528–33. doi:10.1073/pnas.1206552109
9. Ambort D, Johansson ME, Gustafsson JK, Ermund A, Hansson GC. Perspectives on mucus properties and formation – lessons from the biochemical world. Cold Spring Harb Perspect Med (2012) 2(11):a014159. doi:10.1101/cshperspect.a014159
10. Verdugo P. Supramolecular dynamics of mucus. Cold Spring Harb Perspect Med (2012) 2(11):a009597. doi:10.1101/cshperspect.a009597
11. Anagnostopoulou P, Riederer B, Duerr J, Michel S, Binia A, Agrawal R, et al. SLC26A9-mediated chloride secretion prevents mucus obstruction in airway inflammation. J Clin Invest (2012) 122:3629–34. doi:10.1172/JCI60429
12. Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med (2007) 13:231–40. doi:10.1016/j.molmed.2007.05.001
13. Garcia GJ, Boucher RC, Elston TC. Biophysical model of ion transport across human respiratory epithelia allows quantification of ion permeabilities. Biophys J (2013) 104:716–26. doi:10.1016/j.bpj.2012.12.040
14. Quinton PM. Role of epithelial HCO3 transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol (2010) 299:C1222–33. doi:10.1152/ajpcell.00362.2010
15. Ambort D, Johansson ME, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci U S A (2012) 109:5645–50. doi:10.1073/pnas.1120269109
16. Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med (2012) 209:1263–72. doi:10.1084/jem.20120562
17. Wickstrom C, Davies JR, Eriksen GV, Veerman EC, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J (1998) 334(Pt 3):685–93.
18. Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, et al. Munc13-2-/- baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol (2008) 586:1977–92. doi:10.1113/jphysiol.2007.149310
19. Alevy YG, Patel AC, Romero AG, Patel DA, Tucker J, Roswit WT, et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest (2012) 122:4555–68. doi:10.1172/JCI64896
20. Young HW, Williams OW, Chandra D, Bellinghausen LK, Perez G, Suarez A, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5’ elements. Am J Respir Cell Mol Biol (2007) 37:273–90. doi:10.1165/rcmb.2005-0460OC
21. Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol (2007) 36:244–53. doi:10.1165/rcmb.2006-0180OC
22. Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, et al. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol (2013) 6:639–54. doi:10.1038/mi.2012.105
23. Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A (2009) 106:6950–5. doi:10.1073/pnas.0808722106
24. Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, et al. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol (2012) 47:178–85. doi:10.1165/rcmb.2011-0421OC
25. Stephens DJ. Cell biology: collagen secretion explained. Nature (2012) 482:474–5. doi:10.1038/482474a
26. Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol (2010) 298:L15–22. doi:10.1152/ajplung.00194.2009
27. Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology (2012) 22:736–56. doi:10.1093/glycob/cwr182
28. Philippe M, Leger T, Desvaux R, Walch L. Discs large 1 (dlg1) scaffolding protein participates with clathrin and adaptor protein complex 1 (AP-1) in forming Weibel-Palade bodies of endothelial cells. J Biol Chem (2013) 288:13046–56. doi:10.1074/jbc.M112.441261
29. Burgess J, Jauregui M, Tan J, Rollins J, Lallet S, Leventis PA, et al. AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila. Mol Biol Cell (2011) 22:2094–105. doi:10.1091/mbc.E11-01-0054
30. Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol (2008) 70:487–512. doi:10.1146/annurev.physiol.70.113006.100638
31. Stumpo DJ, Graff JM, Albert KA, Greengard P, Blackshear PJ. Molecular cloning, characterization, and expression of a cDNA encoding the “80- to 87-kDa” myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A (1989) 86:4012–6. doi:10.1073/pnas.86.11.4012
32. Abdullah LH, Conway JD, Cohn JA, Davis CW. Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells. Am J Physiol (1997) 273:L201–10.
33. Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem (2001) 276:40982–90. doi:10.1074/jbc.M105614200
34. Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med (2004) 10:193–6. doi:10.1038/nm983
35. Agrawal A, Rengarajan S, Adler KB, Ram A, Ghosh B, Fahim M, et al. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J Appl Physiol (2007) 102:399–405. doi:10.1152/japplphysiol.00630.2006
36. Foster WM, Adler KB, Crews AL, Potts EN, Fischer BM, Voynow JA. MARCKS-related peptide modulates in vivo the secretion of airway Muc5ac. Am J Physiol Lung Cell Mol Physiol (2010) 299:L345–52. doi:10.1152/ajplung.00067.2010
37. Ehre C, Zhu Y, Abdullah LH, Olsen J, Nakayama KI, Nakayama K, et al. nPKCepsilon, a P2Y2-R downstream effector in regulated mucin secretion from airway goblet cells. Am J Physiol Cell Physiol (2007) 293:C1445–54. doi:10.1152/ajpcell.00051.2007
38. Park JA, Crews AL, Lampe WR, Fang S, Park J, Adler KB. Protein kinase C delta regulates airway mucin secretion via phosphorylation of MARCKS protein. Am J Pathol (2007) 171:1822–30. doi:10.2353/ajpath.2007.070318
39. Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase C{delta}-mediated mechanism. Am J Pathol (2005) 167:651–61. doi:10.1016/S0002-9440(10)62040-8
40. Park J, Fang S, Crews AL, Lin KW, Adler KB. MARCKS regulation of mucin secretion by airway epithelium in vitro: interaction with chaperones. Am J Respir Cell Mol Biol (2008) 39:68–76. doi:10.1165/rcmb.2007-0139OC
41. Stahl B, Tobaben S, Sudhof TC. Two distinct domains in hsc70 are essential for the interaction with the synaptic vesicle cysteine string protein. Eur J Cell Biol (1999) 78:375–81. doi:10.1016/S0171-9335(99)80079-X
42. Park J, Fang S, Adler KB. Regulation of airway mucin secretion by MARCKS protein involves the chaperones heat shock protein 70 and cysteine string protein. Proc Am Thorac Soc (2006) 3:493. doi:10.1513/pats.200603-067MS
43. Raiford KL, Park J, Lin KW, Fang S, Crews AL, Adler KB. Mucin granule-associated proteins in human bronchial epithelial cells: the airway goblet cell “granulome”. Respir Res (2011) 12:118. doi:10.1186/1465-9921-12-118
44. Fang S, Crews AL, Chen W, Park J, Yin Q, Ren XR, et al. MARCKS and HSP70 interactions regulate mucin secretion by human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol (2013) 304:L511–8. doi:10.1152/ajplung.00337.2012
45. Lin KW, Fang S, Park J, Crews AL, Adler KB. MARCKS and related chaperones bind to unconventional myosin V isoforms in airway epithelial cells. Am J Respir Cell Mol Biol (2010) 43:131–6. doi:10.1165/rcmb.2010-0016RC
46. Tuvim MJ, Mospan AR, Burns KA, Chua M, Mohler PJ, Melicoff E, et al. Synaptotagmin 2 couples mucin granule exocytosis to Ca2+ signaling from endoplasmic reticulum. J Biol Chem (2009) 284:9781–7. doi:10.1074/jbc.M807849200
47. Jones LC, Moussa L, Fulcher ML, Zhu Y, Hudson EJ, O’Neal WK, et al. VAMP8 is a vesicle SNARE that regulates mucin secretion in airway goblet cells. J Physiol (2012) 590:545–62.
48. Abdullah LH, Davis SW, Burch L, Yamauchi M, Randell SH, Nettesheim P, et al. P2u purinoceptor regulation of mucin secretion in SPOC1 cells, a goblet cell line from the airways. Biochem J (1996) 316(Pt 3):943–51.
49. Danahay H, Atherton HC, Jackson AD, Kreindler JL, Poll CT, Bridges RJ. Membrane capacitance and conductance changes parallel mucin secretion in the human airway epithelium. Am J Physiol Lung Cell Mol Physiol (2006) 290:L558–69. doi:10.1152/ajplung.00351.2005
50. Nguyen LP, Omoluabi O, Parra S, Frieske JM, Clement C, Ammar-Aouchiche Z, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol (2008) 38:256–62. doi:10.1165/rcmb.2007-0279RC
51. Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol (2004) 31:382–94. doi:10.1165/rcmb.2004-0060OC
52. Davis CW, Dowell ML, Lethem M, Van Scott M. Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am J Physiol (1992) 262:C1313–23.
53. Piccotti L, Dickey BF, Evans CM. Assessment of intracellular mucin content in vivo. Methods Mol Biol (2012) 842:279–95. doi:10.1007/978-1-61779-513-8_17
54. Carr CM, Rizo J. At the junction of SNARE and SM protein function. Curr Opin Cell Biol (2010) 22:488–95. doi:10.1016/j.ceb.2010.04.006
55. Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science (2009) 323:474–7. doi:10.1126/science.1161748
56. Kim K, Petrova YM, Scott BL, Nigam R, Agrawal A, Evans CM, et al. Munc18b is an essential gene in mice whose expression is limiting for secretion by airway epithelial and mast cells. Biochem J (2012) 446:383–94. doi:10.1042/BJ20120057
57. Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci (2002) 5:19–26.
58. Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, et al. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat Neurosci (2010) 13:338–43. doi:10.1038/nn.2488
59. Suh YH, Yoshimoto-Furusawa A, Weih KA, Tessarollo L, Roche KW, Mackem S, et al. Deletion of SNAP-23 results in pre-implantation embryonic lethality in mice. PLoS One (2011) 6:e18444. doi:10.1371/journal.pone.0018444
60. Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol (2009) 9:262–7. doi:10.1016/j.coph.2009.02.004
61. Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol (2006) 68:543–61. doi:10.1146/annurev.physiol.68.072304.112754
62. Young HW, Sun CX, Evans CM, Dickey BF, Blackburn MR. A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol (2006) 35:549–58. doi:10.1165/rcmb.2006-0060OC
63. Ribeiro CM, Paradiso AM, Livraghi A, Boucher RC. The mitochondrial barriers segregate agonist-induced calcium-dependent functions in human airway epithelia. J Gen Physiol (2003) 122:377–87. doi:10.1085/jgp.200308893
64. Perez-Vilar J, Ribeiro CM, Salmon WC, Mabolo R, Boucher RC. Mucin granules are in close contact with tubular elements of the endoplasmic reticulum. J Histochem Cytochem (2005) 53:1305–9. doi:10.1369/jhc.5B6713.2005
65. Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, et al. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci (2006) 26:13493–504. doi:10.1523/JNEUROSCI.3519-06.2006
66. Rossi AH, Sears PR, Davis CW. Ca2+ dependency of ‘Ca2+-independent’ exocytosis in SPOC1 airway goblet cells. J Physiol (2004) 559:555–65. doi:10.1113/jphysiol.2004.070433
67. Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem J (2000) 349:247–53. doi:10.1042/0264-6021:3490247
68. Chen Y, Zhao YH, Wu R. Differential regulation of airway mucin gene expression and mucin secretion by extracellular nucleotide triphosphates. Am J Respir Cell Mol Biol (2001) 25:409–17. doi:10.1165/ajrcmb.25.4.4413
69. Kemp PA, Sugar RA, Jackson AD. Nucleotide-mediated mucin secretion from differentiated human bronchial epithelial cells. Am J Respir Cell Mol Biol (2004) 31:446–55. doi:10.1165/rcmb.2003-0211OC
70. Kim KC, Lee BC. P2 purinoceptor regulation of mucin release by airway goblet cells in primary culture. Br J Pharmacol (1991) 103:1053–6. doi:10.1111/j.1476-5381.1991.tb12299.x
71. Breuer R, Christensen TG, Lucey EC, Stone PJ, Snider GL. An ultrastructural morphometric analysis of elastase-treated hamster bronchi shows discharge followed by progressive accumulation of secretory granules. Am Rev Respir Dis (1987) 136:698–703. doi:10.1164/ajrccm/136.3.698
72. Liu C, Li Q, Zhou X, Kolosov VP, Perelman JM. Human airway trypsin-like protease induces mucin5AC hypersecretion via a protease-activated receptor 2-mediated pathway in human airway epithelial cells. Arch Biochem Biophys (2013) 535:234–40. doi:10.1016/j.abb.2013.02.013
73. Huang HT, Guo JJ, Huang YH, Fu YS. Histamine-induced changes in rat tracheal goblet cell mucin store and mucosal edema. Histochem Cell Biol (2013) 139:717–26. doi:10.1007/s00418-012-1060-y
74. Guo JJ, Wang DS, Huang HT. Spontaneous remission of edema and regranulation of goblet cells in rat tracheae after capsaicin-induced acute inflammation. Anat Embryol (Berl) (2003) 206:301–9.
75. Kuo HP, Rohde JA, Tokuyama K, Barnes PJ, Rogers DF. Capsaicin and sensory neuropeptide stimulation of goblet cell secretion in guinea-pig trachea. J Physiol (1990) 431:629–41.
76. Leverkoehne I, Gruber AD. The murine mCLCA3 (alias gob-5) protein is located in the mucin granule membranes of intestinal, respiratory, and uterine goblet cells. J Histochem Cytochem (2002) 50:829–38. doi:10.1177/002215540205000609
77. Lesimple P, Goepp J, Palmer ML, Fahrenkrug SC, O’Grady SM, Ferraro P, et al. CFTR is expressed in mucin granules from Calu-3 and primary human airway epithelial cells. Am J Respir Cell Mol Biol (2013) 49. doi:10.1165/rcmb.2012-0419RC
Keywords: secretion, exocytosis, mucin, mucus, MARCKS, Munc18, Munc13, synaptotagmin
Citation: Adler KB, Tuvim MJ and Dickey BF (2013) Regulated mucin secretion from airway epithelial cells. Front. Endocrinol. 4:129. doi: 10.3389/fendo.2013.00129
Received: 04 June 2013; Accepted: 03 September 2013;
Published online: 18 September 2013.
Edited by:
Rafael Vazquez-Martinez, University of Cordoba, SpainReviewed by:
Ricardo Borges, University of La Laguna, SpainCopyright: © 2013 Adler, Tuvim and Dickey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Burton F. Dickey, Department of Pulmonary Medicine, University of Texas MD Anderson Cancer Center, Unit 1462, 1515 Holcombe Boulevard, Houston, TX 77030-4009, USA e-mail:YmRpY2tleUBtZGFuZGVyc29uLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.