- 1 Mount Sinai School of Medicine, New York, NY, USA
- 2 University of California San Diego, La Jolla, CA, USA
Hypoglycemia is well-recognized to limit the degree of glycemic control possible for many individuals for diabetes. Although the likelihood of hypoglycemia increases as A1c levels decrease in type 1 diabetes, insulin-treated type 2 diabetic persons with higher A1c appear paradoxically to have more hypoglycemia which may explain, in part, the adverse outcome reported in the ACCORD study. Approaches to glucose-lowering that cause lesser degrees of risk for hypoglycemia, technologies to better ascertain hypoglycemic events, and better understanding of patient characteristics associated with greater likelihood of hypoglycemia will all be required to reduce this limiting factor in optimizing glycemic treatment.
Attempts to determine whether intensive glycemic treatment would be associated with reduction in adverse cardiovascular outcomes led to three large recent randomized controlled trials: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial, and the Veteran’s Administration Diabetes Trial (VADT). A widely held, but inaccurate interpretation has been that none of these studies suggested benefit from intensive glycemic control. In ADVANCE, the combination of intensive glycemic treatment along with blood pressure-lowering with a diuretic and angiotensin-directed therapy reduced mortality (Zoungas et al., 2009), and an epidemiologic analysis of different levels of on-trial glycemia showed optimal outcome with normal to near-normal A1c levels (Zoungas et al., 2012). In ACCORD there was consistent evidence of reduction in microvascular endpoints, with the intensively treated subgroup showing 30% reduction in retinopathy, while the likelihood of non-fatal myocardial infarction was significantly reduced by 21% (Boussageon et al., 2011; Hemmingsen et al., 2011). There was, however, a significant 26% increase in mortality in this study, which has been a major source of concern.
Over the past few years, there has been increasing recognition of the importance of hypoglycemia as an adverse consequence of treatment of type 2 diabetes. Previously, severe hypoglycemia was thought to be relatively rare in type 2 diabetes and perhaps not as important as in type 1 diabetes. The ACCORD, VADT, and ADVANCE trials have, however, underscored the concern with hypoglycemia-related adverse outcomes. Furthermore, hypoglycemia in type 2 diabetes is associated with longer length of hospital stay, greater cost, and higher mortality during hospitalization (Curkendall et al., 2009; Turchin et al., 2009). Important observations have shown greater risk of specific types of hypoglycemia. Spontaneous hypoglycemia following myocardial infarction is associated with increased mortality, while insulin-induced hypoglycemia after myocardial infarction was not, leading to the conclusion that persons experiencing hypoglycemia might be particularly at risk of adverse outcome not directly related to glucose effects, but rather because of concomitant underlying characteristics such as greater degrees of renal insufficiency (Kosiborod et al., 2009). Based on these observations, it has become apparent that it is important to include the implications of hypoglycemia in realistic goal-setting for treatment approaches in type 2 diabetes.
It had been thought for some time that with improvement in glycemic control, i.e., overall lowering of average glucose, the likelihood of hypoglycemia would increase. The concept seemed logical, and was indeed confirmed among type 1 diabetics in the Diabetes Control and Complications Trial where annual severe hypoglycemia frequency increased from ∼30 to 90 incidents per 100 person-years as mean A1c decreased from 9 to 6% (The Diabetes Control and Complications Trial Research Group, 1993). It should be noted that this trial preceded the newer and more predictable analog insulin preparations, and the widespread use of self monitoring of blood glucose levels.
In type 2 diabetes, however, the situation is more complex. Among diet, sulfonylurea (SU), or metformin-treated type 2 diabetic patients in the United Kingdom Prospective Diabetes Study (UKPDS), total annual hypoglycemia rates increased from ∼2 to 6% as the most recent on-trial A1c level decreased from 9 to 6%. Paradoxically, however, for insulin-treated patients in the study, almost all of whom were receiving just one daily long-acting insulin dose, the respective hypoglycemia rates actually decreased from ∼25 to 18% (Wright et al., 2006). Those persons who, despite the use of insulin, did not achieve good glycemic control were, then, particularly at risk of hypoglycemia (Figure 1A). The implication appears to be that insulin treatment in type 2 diabetes alters the equation, perhaps because, as observed in other trials, patients with better control actually have less hypoglycemia. This may relate to lesser degrees of insulin secretory deficiency, or to factors such as adherence, which are crucial in the successful outcome of any efforts to improve glycemic control.
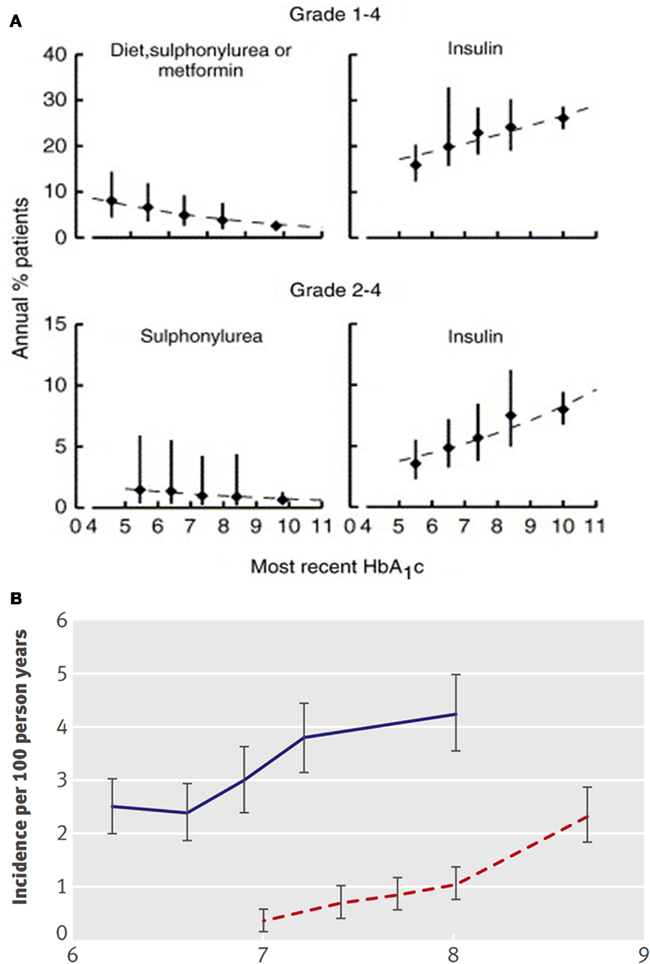
Figure 1. (A) total (grades 1–4) and severe (grades 2–4) hypoglycemia in UKPDS (Hemmingsen et al., 2011). (B) Hypoglycemia vs. on-trial A1c in the intensive (blue) and conventional treatment (dotted red) groups of ACCORD (Kendall et al., 2005).
These concepts are highly important in understanding the adverse outcome reported in the ACCORD study. The group with the worst outcome was the subset of the tight control group that could not, for whatever reason, achieve tight control (Figure 1B). An attractive hypothesis is that targeting near-normal levels of glucose may not be the problem, but rather that harm results from the treatments unsuccessfully employed to accomplish this in individuals who are nevertheless unable to improve glycemia. While we do not know what factors led to the failure of individuals to achieve best control, it seems reasonable to suspect that they had a higher rate of undocumented hypoglycemia which may have been responsible, at least in part, for their adverse outcome.
In UKPDS total hypoglycemia rates were more than 10-fold greater than rates of severe hypoglycemia, and insulin-treated patients had rates approximately three-fold greater than that of diet- and metformin-treated persons, while SU were associated with intermediate hypoglycemia rates [UK Prospective Diabetes Study (UKPDS) Group, 1998]. UKPDS was, it should be noted, conducted with older insulin preparations, which are less predictable, causing hypoglycemia with greater frequency than seen with newer analogs. The pattern of highest hypoglycemia rates with insulin and intermediate increase in hypoglycemia with SU has been reported in many studies of type 2 diabetes (Nissen et al., 2008), with further data suggesting that the SU are particularly likely to cause hypoglycemia during the first few months of their use (Bodmer et al., 2008), the period of their greatest glucose-lowering potency (Kahn et al., 2006). Increased hypoglycemia likelihood also is seen with SU in combination with metformin, with thiazolidinediones, and with incretin-based treatments (Buse et al., 2004; Kendall et al., 2005; Bolen et al., 2007; Nauck et al., 2007; Arechavaleta et al., 2011). In contrast, incretin-based treatments when given without a SU are noteworthy for the rarity of hypoglycemia, even with substantial glucose-lowering (Neumiller et al., 2010; MacConell et al., 2012).
Hypoglycemia is an important consideration in the choice of approaches to insulin treatment. Studies comparing insulin glargine with neutral protamine Hagedorn insulin show a reduction in total and severe hypoglycemia by ∼25% with the former (Riddle et al., 2003). The use of basal insulin detemir as primary treatment leads to two and threefold lower overall likelihood of hypoglycemia than multiple dose biphasic or prandial insulin (Holman et al., 2007, 2009). An even greater reduction of hypoglycemia was reported in a comparison of insulin glargine with insulin lispro three times daily (Bretzel et al., 2008).
Treatment associated with hypoglycemia may have substantial adverse clinical consequences. In the BARI 2D trial of therapies for type 2 diabetes in patients with coronary artery disease, treatment approaches based on use of SU and/or insulin were ∼50% more likely both to cause total and severe hypoglycemia than were insulin sensitization with metformin and rosiglitazone, with a suggestion of worse CV outcome (BARI 2D Study Group et al., 2009). One must wonder whether hypoglycemia, not only in the severe symptomatic form, but also in the much more common forms with minor symptoms or even altogether lacking symptoms, may have consequences in persons with coronary disease, cardiac arrhythmia, or diabetic autonomic neuropathy, because of increasing vasoactive cytokine release with consequent increase in myocardial ischemia. Certainly a number of arguments can be put forward to suggest that hypoglycemia may be pro-arrhythmic (Nordin, 2010) and/or that it may contribute to unstable atherosclerotic plaque and events weeks to months after the actual hypoglycemia.
In ACCORD, annual hypoglycemia rates were 3.3 vs. 1.1% in the intensive vs. standard control group (Miller et al., 2010). About 9,546 participants did not have documented severe hypoglycemia, and those randomized to intensive treatment had a 1.24-fold greater annual mortality than those in the standard treatment group. However, 705 participants had one or more episode of severe hypoglycemia, with annual mortality approximately 3-fold that in those not experiencing hypoglycemia. In the group with hypoglycemia, paradoxically, the mortality risk was ∼60% lower among those assigned to intensive glycemic treatment (Figure 2A). Severe hypoglycemia was more common with longer duration of diabetes, with lower body weight, with greater degrees of renal disease (both in terms of albuminuria and serum creatinine), with peripheral neuropathy, in African-Americans, and in females. Similarly, in ACCORD, in both the intensive and standard treatment groups the severe hypoglycemia rates were more frequent in those with higher, rather than lower, levels of A1c (Figure 1B). Furthermore, those in the intensive treatment group who failed to show at least a 0.5% reduction in A1c during the first year of the study had higher mortality, with higher mean on-trial A1c in this group associated with greater likelihood of mortality (Riddle et al., 2010). Two thirds of deaths were in the “unexpected/sudden” category (Action to Control Cardiovascular Risk in Diabetes Study Group et al., 2008), consistent with the association with hypoglycemia. In keeping with the supposition that those having greater difficulty attaining glycemic control might be at a particular risk due to intensive treatment, analysis showed no increase in mortality among those in the intensive treatment group with baseline A1c <7.5%, or 7.5–8.5%, while mortality was 1.7-fold increased in those in the intensive treatment group with baseline A1c >8.5% (Calles-Escandón et al., 2010). In other words, it bears repeated emphasis that it was the failure to achieve tight control despite best efforts that was the reason for excess mortality in the most intensively treated group in ACCORD. Those with lower A1c levels did better than those with higher A1c.
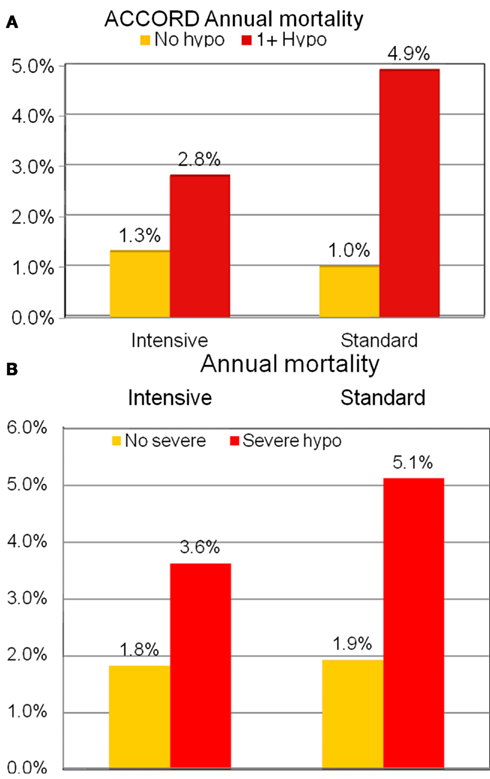
Figure 2. (A) Association of severe hypoglycemia with mortality in intensive and standard treatment groups in ACCORD replotted from Bonds et al. (2010). (B) Association of severe hypoglycemia with mortality in intensive and standard treatment groups in ADVANCE replotted from Holman et al. (2009).
The ADVANCE trial showed a remarkably similar relationship to that in ACCORD between severe hypoglycemia, treatment assigned, and mortality risk (Figure 2B). Severe hypoglycemia risk factors, as in ACCORD, were longer duration of diabetes, lower body weight, greater degrees of renal disease (both higher serum creatinine and albuminuria), and cigarette smoking. Interestingly, in ADVANCE there were associations of severe hypoglycemia with total and CV mortality, as well as with microvascular and macrovascular events, and respiratory, skin, and digestive tract illnesses. These associations have led some to hypothesize that hypoglycemia may be a marker of propensity to develop illness, rather than itself being causally related to the various complications (Zoungas et al., 2010). Macrovascular events and CV mortality not only occurred around the time of episodes of severe hypoglycemia, but continued to occur with increased frequency for 2 years after the index hypoglycemic event, supporting the hypothesis that hypoglycemia may be a marker of poor prognosis, rather than its cause. Interestingly, minor hypoglycemia had entirely different associations in the trial, being associated with lower rates of macrovascular disease and of total and cardiovascular mortality. In the VADT too, severe hypoglycemia rates were threefold greater with intensive treatment, and hypoglycemia increased mortality risk, with sudden death again appearing to account for the excess in mortality in the intensive treatment group (Bloomgarden, 2008).
The ACCORD investigators report, however, that severe hypoglycemia did not appear to explain the increase in mortality seen in the intensively treated group. It is difficult to fully support this viewpoint, as severe hypoglycemia certainly was associated with increased mortality in this and in the other trials, and as the glycemic intervention was itself associated with an increase in the likelihood of severe hypoglycemia. The lack of association of severe hypoglycemia with mortality may reflect incomplete ascertainment of events, as the investigators only tracked severe symptomatic hypoglycemia, while, in fact, episodes with minor symptoms or lacking symptoms occur more frequently than symptomatic ones (Swinnen et al., 2009). Clearly, full analysis of self-monitored blood glucose measurements performed by the participants in ACCORD would be likely to give much more information about hypoglycemia risk in this population (Kovatchev et al., 2000), which would clarify whether there is indeed a relationship between hypoglycemia and adverse outcome. The alternative hypothesis discussed above is that hypoglycemia may be seen as a marker of greater “illness,” in association with multiple adverse outcomes, some of which may not be directly related to the blood sugar, so that more frail patients may be at risk both for hypoglycemia and for mortality. Behavioral and psychological factors associated with being unable to adhere to a diabetes regimen have also been hypothesized.
The risk of hypoglycemia per se should perhaps not be used as a rationale to not attempt best possible, safe glycemic control. Better A1c still provides better outcomes, recognizing that the degree of glucose-lowering must be individualized based on multiple different characteristics of individual patients (Blonde, 2012). Agents that can reduce A1c without hypoglycemia risk, such as the incretins and the TZDs, may deserve prominence in the treatment algorithm, and we look forward to studies designed to test the hypothesis of whether this is in fact the case. Glycemic variability may be another factor explaining adverse outcome (Hirsch and Brownlee, 2005), although the argument for this as a factor independent of hypoglycemia is by no means definite (Kilpatrick, 2009). We may, then, paraphrase Joslin’s remark, made shortly after the introduction of insulin, to state, “intensive diabetes management is a remedy primarily for the wise and not for the foolish, whether they be patients or doctors.” (Joslin, 1928).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Action to Control Cardiovascular Risk in Diabetes Study GroupGerstein, H. C., Miller, M. E., Byington, R. P., Goff, D. C. Jr., Bigger, J. T., Buse, J. B., Cushman, W. C., Genuth, S., Ismail-Beigi, F., Grimm, R. H. Jr., Probstfield, J. L., Simons-Morton, D. G., and Friedewald, W. T. (2008). Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559.
Arechavaleta, R., Seck, T., Chen, Y., Krobot, K. J., O’Neill, E. A., Duran, L., Kaufman, K. D., Williams-Herman, D., and Goldstein, B. J. (2011). Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double-blind, non-inferiority trial. Diabetes Obes. Metab. 13, 160–168.
BARI 2D Study GroupFrye, R. L., August, P., Brooks, M. M., Hardison, R. M., Kelsey, S. F., MacGregor, J. M., Orchard, T. J., Chaitman, B. R., Genuth, S. M., Goldberg, S. H., Hlatky, M. A., Jones, T. L., Molitch, M. E., Nesto, R. W., Sako, E. Y., and Sobel, B. E. (2009). A randomized trial of therapies for type 2 diabetes and coronary artery disease. N. Engl. J. Med. 360, 2503–2515.
Blonde, L. (2012). Benefits and risks for intensive glycemic control in patients with diabetes mellitus. Am. J. Med. Sci. 343, 17–20.
Bloomgarden, Z. T. (2008). Glycemic control in diabetes: a tale of three studies. Diabetes Care 31, 1913–1919.
Bodmer, M., Meier, C., Krähenbühl, S., Jick, S. S., and Meier, C. R. (2008). Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care 31, 2086–2091.
Bolen, S., Feldman, L., Vassy, J., Wilson, L., Yeh, H. C., Marinopoulos, S., Wiley, C., Selvin, E., Wilson, R., Bass, E. B., and Brancati, F. L. (2007). Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann. Intern. Med. 147, 386–399.
Bonds, D. E., Miller, M. E., Bergenstal, R. M., Buse, J. B., Byington, R. P., Cutler, J. A., Dudl, R. J., Ismail-Beigi, F., Kimel, A. R., Hoogwerf, B., Horowitz, K. R., Savage, P. J., Seaquist, E. R., Simmons, D. L., Sivitz, W. I., Speril-Hillen, J. M., and Sweeney, M. E. (2010). The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 340, b4909.
Boussageon, R., Bejan-Angoulvant, T., Saadatian-Elahi, M., Lafont, S., Bergeonneau, C., Kassaï, B., Erpeldinger, S., Wright, J. M., Gueyffier, F., and Cornu, C. (2011). Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 343, d4169.
Bretzel, R. G., Nuber, U., Landgraf, W., Owens, D. R., Bradley, C., and Linn, T. (2008). Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 371, 1073–1084.
Buse, J. B., Henry, R. R., Han, J., Kim, D. D., Fineman, M. S., Baron, A. D., Exenatide-113 Clinical Study Group. (2004). Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27, 2628–2635.
Calles-Escandón, J., Lovato, L. C., Simons-Morton, D. G., Kendall, D. M., Pop-Busui, R., Cohen, R. M., Bonds, D. E., Fonseca, V. A., Ismail-Beigi, F., Banerji, M. A., Failor, A., and Hamilton, B. (2010). Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 33, 721–727.
Curkendall, S. M., Natoli, J. L., Alexander, C. M., Nathanson, B. H., Haidar, T., and Dubois, R. W. (2009). Economic and clinical impact of inpatient diabetic hypoglycemia. Endocr. Pract. 15, 302–312.
Hemmingsen, B., Lund, S. S., Gluud, C., Vaag, A., Almdal, T., Hemmingsen, C., and Wetterslev, J. (2011). Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ 343, d6898.
Hirsch, I. B., and Brownlee, M. (2005). Should minimal blood glucose variability become the gold standard of glycemic control? J. Diabetes Complicat. 19, 178–181.
Holman, R. R., Farmer, A. J., Davies, M. J., Levy, J. C., Darbyshire, J. L., Keenan, J. F., Paul, S. K., 4-T Study Group. (2009). Three-year efficacy of complex insulin regimens in type 2 diabetes. N. Engl. J. Med. 361, 1736–1747.
Holman, R. R., Thorne, K. I., Farmer, A. J., Davies, M. J., Keenan, J. F., Paul, S., Levy, J. C., 4-T Study Group. (2007). Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N. Engl. J. Med. 357, 1716–1730.
Joslin, E. P. (1928). Insulin, Section II: The Treatment of Diabetes Mellitus. Philadelphia: Lea & Febiger, 69.
Kahn, S. E., Haffner, S. M., Heise, M. A., Herman, W. H., Holman, R. R., Jones, N. P., Kravitz, B. G., Lachin, J. M., O’Neill, M. C., Zinman, B., Viberti, G., ADOPT Study Group. (2006). Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443.
Kendall, D. M., Riddle, M. C., Rosenstock, J., Zhuang, D., Kim, D. D., Fineman, M. S., and Baron, A. D. (2005). Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 28, 1083–1091.
Kilpatrick, E. S. (2009). Arguments for and against the role of glucose variability in the development of diabetes complications. J. Diabetes Sci. Technol. 3, 649–655.
Kosiborod, M., Inzucchi, S. E., Goyal, A., Krumholz, H. M., Masoudi, F. A., Xiao, L., and Spertus, J. A. (2009). Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 301, 1556–1564.
Kovatchev, B. P., Cox, D. J., Farhy, L. S., Straume, M., Gonder-Frederick, L., and Clarke, W. L. (2000). Episodes of severe hypoglycemia in type 1 diabetes are preceded and followed within 48 hours by measurable disturbances in blood glucose. J. Clin. Endocrinol. Metab. 85, 4287–4292.
MacConell, L., Brown, C., Gurney, K., and Han, J. (2012). Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab. Syndr. Obes. 5, 29–41.
Miller, M. E., Bonds, D. E., Gerstein, H. C., Seaquist, E. R., Bergenstal, R. M., Calles-Escandon, J., Childress, R. D., Craven, T. E., Cuddihy, R. M., Dailey, G., Feinglos, M. N., Ismail-Beigi, F., Largay, J. F., O’Connor, P. J., Paul, T., Savage, P. J., Schubart, U. K., Sood, A., Genuth, S., ACCORD Investigators. (2010). The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 340, b5444.
Nauck, M. A., Meininger, G., Sheng, D., Terranella, L., Stein, P. P., Sitagliptin Study 024 Group. (2007). Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes. Metab. 9, 194–205.
Neumiller, J. J., Wood, L., and Campbell, R. K. (2010). Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy 30, 463–484.
Nissen, S. E., Nicholls, S. J., Wolski, K., Nesto, R., Kupfer, S., Perez, A., Jure, H., De Larochellière, R., Staniloae, C. S., Mavromatis, K., Saw, J., Hu, B., Lincoff, A. M., Tuzcu, E. M., PERISCOPE Investigators. (2008). Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299, 1561–1573.
Nordin, C. (2010). The case for hypoglycaemia as a proarrhythmic event: basic and clinical evidence. Diabetologia 53, 1552–1561.
Riddle, M. C., Ambrosius, W. T., Brillon, D. J., Buse, J. B., Byington, R. P., Cohen, R. M., Goff, D. C. Jr., Malozowski, S., Margolis, K. L., Probstfield, J. L., Schnall, A., Seaquist, E. R., Action to Control Cardiovascular Risk in Diabetes Investigators. (2010). Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 33, 983–990.
Riddle, M. C., Rosenstock, J., Gerich, J., Insulin Glargine 4002 Study Investigators. (2003). The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 26, 3080–3086.
Swinnen, S. G., Mullins, P., Miller, M., Hoekstra, J. B., and Holleman, F. (2009). Changing the glucose cut-off values that define hypoglycaemia has a major effect on reported frequencies of hypoglycaemia. Diabetologia 52, 38–41.
The Diabetes Control and Complications Trial Research Group. (1993). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986.
Turchin, A., Matheny, M. E., Shubina, M., Scanlon, J. V., Greenwood, B., and Pendergrass, M. L. (2009). Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 32, 1153–1157.
UK Prospective Diabetes Study (UKPDS) Group. (1998). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853.
Wright, A. D., Cull, C. A., Macleod, K. M., Holman, R. R., for the UKPDS Group. (2006). Hypoglycemia in Type 2 diabetic patients randomized to and maintained on monotherapy with diet, sulfonylurea, metformin, or insulin for 6 years from diagnosis: UKPDS73. J. Diabetes Complicat. 20, 395–401.
Zoungas, S., Chalmers, J., Ninomiya, T., Li, Q., Cooper, M. E., Colagiuri, S., Fulcher, G., de Galan, B. E., Harrap, S., Hamet, P., Heller, S., Macmahon, S., Marre, M., Poulter, N., Travert, F., Patel, A., Neal, B., Woodward, M., ADVANCE Collaborative Group. (2012). Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 55, 636–643.
Zoungas, S., de Galan, B. E., Ninomiya, T., Grobbee, D., Hamet, P., Heller, S., MacMahon, S., Marre, M., Neal, B., Patel, A., Woodward, M., Chalmers, J., ADVANCE Collaborative GroupCass, A., Glasziou, P., Harrap, S., Lisheng, L., Mancia, G., Pillai, A., Poulter, N., Perkovic, V., and Travert, F. (2009). Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diabetes Care 32, 2068–2074.
Zoungas, S., Patel, A., Chalmers, J., de Galan, B. E., Li, Q., Billot, L., Woodward, M., Ninomiya, T., Neal, B., MacMahon, S., Grobbee, D. E., Kengne, A. P., Marre, M., Heller, S., ADVANCE Collaborative Group. (2010). Severe hypoglycemia and risks of vascular events and death. N. Engl. J. Med. 363, 1410–1418.
Keywords: hypoglycemia, hemoglobin A1c, type 2 diabetes, glycemic control, insulin therapy
Citation: Bloomgarden ZT and Einhorn D (2012) Hypoglycemia in type 2 diabetes: current controversies and changing practices. Front. Endocrin. 3:66. doi: 10.3389/fendo.2012.00066
Received: 09 January 2012; Accepted: 29 April 2012;
Published online: 21 May 2012.
Edited by:
Charles M. Alexander, Merck, USAReviewed by:
Charles M. Alexander, Merck, USADinesh Selvarajah, Sheffield Teaching Hospitals NHS Foundation Trust, UK
Copyright: © 2012 Bloomgarden and Einhorn. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Zachary T. Bloomgarden, Mount Sinai School of Medicine, 35 East 85th Street, New York, NY, USA. e-mail:emJsb29tQGdtYWlsLmNvbQ==
