- Department of Invertebrate Zoology and Geology, California Academy of Sciences, San Francisco, CA, United States
The Phanerozoic fossil record can be organized as a nested set of persistent paleoecological units, ranging from paleocommunities to Sepkoski’s Evolutionary Faunas. This paper argues that the basis for ecological persistence on geological timescales is rooted in the robustness of ecological communities, that is, the resistance and resilience of communities when perturbed by the environment. Here I present the Ecological Functional Networks Hypothesis (EFNH) that proposes that networks of species functions, or Ecological Functional Networks (EFNs), underlie ecological stasis and persistence, and that EFNs are both subject to selection and evolve. An EFN varies if the species composition and hence functional structures of its constituent communities vary, and EFNs may differ from each other based on the robustness of those constituent communities, numerical representation, and biogeographic distribution. That variation is subject to selection acting on EFN community composition, and determines both the persistence of an EFN and the differential persistence among multiple EFNs. Selection pressures on EFNs in turn exert top-down influence on species evolution and extinction. Evidence is presented to both establish the reality of EFNs in the fossil record, for example, community structures that persist even as species composition changes, and the selection of EFNs, which is apparent during and after episodes of severe biotic turnover such as mass extinctions. Finally, tests are suggested that make the EFNH falsifiable, including testing the correlation between EFNs or EFN emergent traits and geological persistence, and using models of paleocommunity dynamics to examine the relationship between community or EFN robustness and geological persistence. The tests should be applied broadly throughout the Phanerozoic and diverse environments. The EFNH is part of a growing body of hypotheses that address the selection, evolution and persistence of non-reproducing systems, including ecosystems and entire biospheres, and addresses those concepts on geological timescales.
1 Introduction
The Phanerozoic fossil record comprises hierarchically structured, multi-taxon, temporally bound, and compositionally persistent biotic units (Roopnarine and Banker, 2021). Its diversity of multicellular lineages has persisted for
The EFNH is presented as follows. First, evidence is presented to establish the reality of ecological functional networks, followed by discussion of the distinction from species communities. Second, mechanisms and supporting evidence are proposed to explain how EFNs arise via multi-level selection (MLS), and tests are discussed that could falsify the hypothesis. Finally, the relationship of EFNH to other proposals of ecological system selection, evolution and persistence are discussed, as well as additional hypotheses of multilevel system selection, evolution and persistence.
2 Evidence
Scientific hypotheses are proposed to address empirical observations that lie outside the domain of, or conflict with, current theory (Kuhn, 1970). The observations of concern here are paleoecological phenomena, including: (1) a nested hierarchy of geologically persistent paleoecological assemblages; (2) paleoecological communities characterized by morphologically or taxonomically static species; and (3) paleoecological functional frameworks that are geologically persistent even as species composition within a framework changes. The latter point implies that within the geological duration of a functional framework, the framework itself is a product of the functions that species perform, and not necessarily of species continuity. A major issue to be resolved then is the question of what maintains an EFN, and the nature of its relationship to species and communities.
2.1 Paleocommunity persistence
Despite a “haziness” inherent in the delineation of ecological communities (Yodzis, 1988), species biotic inter-dependencies, and environmental requirements do give rise to replicable species assemblages and networks of interactions. A classical ecology argument arises from contending views of communities as highly integrated and somewhat functionally inflexible species assemblages (Clements, 1916), versus more random assemblages of species based largely on autecological environmental requirements (Gleason, 1926). Yet replicability of species assemblages and networks of interactions, when extended to the fossil record and the temporal dimension, are the main bases for paleocommunity recognition, therefore lying somewhere between sp. Clementsian and Gleasonian concepts.
Brett and others (Morris et al., 1995; Brett et al., 1996) documented species-level compositional stability in mid-Paleozoic faunas from the U.S. Appalachian Basin, with units persistent for 3-7 my. The proposed Coordinated Stasis hypothesis postulates that the persistence of morphologically static species assemblages, and their synchronous turnovers, are driven by either rigid patterns of biotic dependencies (“ecological locking”), or environmental tracking. Ecological locking implies strong interspecific interactions, that is, individuals have significant per capita effects on the populations of other species. Theoretical ecology, however, predicts strong interactions to have destabilizing effects on community structure (May, 1972; McCann et al., 1998), and one would least expect ecological stasis under such conditions. The Appalachian Basin assemblages do exhibit very little change, and may be a limiting case of a more general phenomenon of ecological stasis, wherein taxon composition varies even as emergent community features remain stable. Ecological communities are rarely spatio-temporally homogeneous, but instead occupy variable environments that influence both species composition and therefore community functional structures. Compositional variation is therefore not surprising, but functional persistence is expected when that variation is phylogenetically constrained and within-lineage ecology is conserved. For example, Silurian brachiopod assemblages exhibited changes of species composition over
2.2 Ecological functional networks
At the heart of the EFNH are networks of biotically interacting ecological functions, EFNs, defined by species functional traits, such as body size, functional morphologies, and biogeochemical activities. It is now commonplace in paleoecology to characterize taxon assemblages and paleocommunities according to functional traits at both taxon and community levels–e.g. functional disparity– (Dineen et al., 2019; Cole and Hopkins, 2021) where the measures are often emergent properties. Paleocommunities are systems, with characteristics depending ultimately on the properties and relationships of constituent species, and functionally similar or redundant species, their interactions with the external environment, and the outcome of the feedback of system dynamics to constituent entities. Whereas paleocommunities are spatiotemporally bound, however, the functions performed by species in their interactions with each other, and with the abiotic environment as in biogeochemical cycles, need not be so bound because of either functional redundancy among species (Falkowski et al., 2008; Doolittle, 2024), or the recurrence of functions separated by temporal gaps (Banker et al., 2022).
EFNs are one level in an ecological hierarchy that ranges from individual organisms to local populations or avatars, to local communities and metacommunities (Damuth, 1985; Eldredge, 1989). The hierarchy occupies a dimension of spatial contemporaneity, encompassing population distributions and both species and community spatial connectivity, but there is also a temporal dimension that describes geological persistence. A significantly persistent paleoecological unit is one whose geological duration exceeds that of the transitional interval that separates it from preceding and succeeding intervals of the same type. Sepkoski’s Phanerozoic Evolutionary Faunas (Sepkoski, 1981) and Boucout and Sheehan’s Ecologic Evolutionary Units (EEUs) (Boucot, 1983; Sheehan, 1996), are canonical, inclusive, and rigorously defined units at the top of the hierarchy. The Evolutionary Faunas group Phanerozoic marine animal families and genera according to their ecological characteristics and origination-diversification-extinction histories (Alroy, 2004), and are separated by intervals of major turnover and extinction. Nested within the Sepkoskian Faunas are the EEUs, each comprising contemporaneous benthic marine communities of similar ecological structure, much of which is generated by persistent phylogenetic lineages. EEUs vary in duration from 30 to 140 my, separated by much shorter transitional intervals of 3-5 my, which are often associated with times of increased extinction. Species composition varies within EEUs, but compositional stability at higher taxonomic levels suggests significant ecological continuity, to the extent to which ecological functions are conserved within a particular lineage. This stratigraphically constrained variability of taxon composition is common in the Phanerozoic record (DiMichele et al., 2004), including Permian tetrapod terrestrial faunas (Olson, 1952), late Paleozoic terrestrial floras (DiMichele et al., 2002; Willard et al., 2007), and both late Cenozoic terrestrial mammalian assemblages (Barry et al., 2002) and tropical coral reefs (Pandolfi and Jackson, 2006). An EFN is more constrained because continuity of both functions and the network of interactions are required. Functions arise and disappear according to the persistence of the species performing them, but accordingly can recur discontinuously and be performed by phylogenetically distant species (Banker et al., 2022).
An EFN is therefore the system of functions and their pattern of interaction, regardless of species composition. “Function” is used broadly here to refer to any action that members of a species undertake to ensure individual or reproductive success, and that has an impact on other individuals, including those belonging to other species. Examples include foraging, ecosystem engineering, and actions affecting biogeochemical cycles, such as microbial decomposition. Functions affect other functions, either facilitating or inhibiting them. For example, giant kelp that attain great height and form a canopy at the water’s surface to perform the vital role of photosynthesis and production, also dampen hydrodynamic forces thereby promoting local biodiversity, and shade the benthic understory thus inhibiting benthic primary productivity (Detmer et al., 2021). Multiple species may perform a function redundantly, but those species are unlikely to be completely redundant because they differ in other traits, functions and environmental requirements. This is a key mechanism of how biodiversity promotes community robustness. Therefore a function, as used in the current context, can be an abstraction of one or more species, and an EFN is not a system of species, but a system of abstracted functions. This definition requires clarification of the structure of an EFN, and the extent to which such structures could be both recognized in the fossil record, and identified with geologically persistent assemblages. An EFN is a network in which nodes are ecological functions, and the links between nodes indicate the directional, bidirectional, or looped impact between functions. A simple but clear example would be a network consisting of a “photosynthesis” node and an “herbivory” node. Each node obviously comprises the community’s primary producers and consumers at multiple levels of organization, including individuals, populations and species, and the link represents both the impact of herbivory on both nodes and the flow of energy through the EFN. The example would gain both feasibility and persistence with the addition of a decomposition node for the recycling of essential and often limiting raw nutrients. This closed-loop network is an accurate, although simplified, representation of a basic EFN, and the dynamics are multifaceted, including both ecological (demographic) and evolutionary dynamics.
Real EFNs are of course more diverse and complex in the sense that as nodes and links proliferate over time and environment, so do the number of feedback loops and indirect impacts. How does one translate an ecological community, comprising individual organisms, the populations to which they belong, and the ways in which they interact, into an EFN representation? The potential complexity of interactions in a species assemblage, and hence the combinatoric space of possible interaction networks, grows hyper-exponentially as richness increases. For example, in an assemblage of only 10 unique species, if one allows directional, bidirectional and self-looped (e.g., cannibalism) interactions, the number of networks possible exceeds
Have any EFNs been recognized in the fossils record? Not explicitly, but a point worth reiterating is that EFN nodes are functions, not taxa, although they are rooted in the functions performed by individual organisms. Organisms themselves are multifunctional, and will therefore be represented in multiple nodes. There are various viewpoints of communities and ecosystems that approach the EFN concept, such as energy flux and box models, recycling loops, and aggregated food webs, each of which represents functional interactions. Recent work on Miocene-Pleistocene terrestrial communities of the Iberian peninsula showed that their functional compositions, and networks of functional interactions, persisted in the face of species opportunistic and climate-influenced immigration and extinction (Blanco et al., 2021). Mammalian body size, diet and locomotion were used to assign species to “functional entities” (FEs), here interpreted as a type of node in an EFN. Network community detection analyses showed that communities of FEs, referred to as functional faunas, persisted differently from networks based on taxonomic composition. Blanco et al. termed this pattern of persistent functional units separated by abrupt transitions, “punctuated ecological equilibrium”. Network modules of species assemblages persisted an average of 0.9 my, modules defined on the basis of functions persisted
Such networks of functional modules typify EFNs, another example being late Permian networks of southern African terrestrial communities, which although spatio-temporally variable in taxon composition, were structurally cohesive for more than 10 my (Roopnarine et al., 2017), changing only during the Permian-Triassic mass extinction (PTME). Early Triassic successor communities represented new and structurally distinct EFNs. The functional networks here are metanetworks (Roopnarine, 2009), which aggregate paleocommunity species on the basis of overlapping morphological traits, environmental requirements and trophic interactions. Thus the nodes in a metanetwork, although derived from species traits, represent trophic functional groups present in a food web. Aggregated nodes thus share properties with Blanco et al.‘s FEs. The southern African networks, however, include a broader array of taxa, being representative of community food webs, including plants, insects and multiple vertebrate clades. They extend the functional fauna concept to a community scale. The metanetwork framework has been applied to multiple time periods and paleocommunities (e.g., Roopnarine et al., 2007; Mitchell et al., 2012; Kempf et al., 2020), and a similar pattern of persistence punctuated by the PTME, as in southern Africa, has been also found in both terrestrial and marine communities from northwestern and South China respectively (Huang et al., 2021; 2023).
Therefore, phylogenetic change and species turnover occur in EFNs, but community structure and processes are continuous. Taxon stability in an EFN is unsurprising if new species are descendants of earlier species in the EFN and retain ancestral functional traits, but taxonomic variability among communities can be great enough to generalize an EFN as a set of structurally similar, but compositionally varying communities. Modern tropical coral reefs are an example, with a diverse set of scleractinian taxa forming physiologically and ecologically similar systems, while varying in terms of species richness, composition and dominant species. This is a somewhat Clementsian view (Clements, 1916) of communities, but at a level of organization above that of individual species, with the focal units being species functions that interact with other species functions. Thus, it is the integrated systems of functions that persist, and not necessarily systems of particular species.
The types of organisms, environmental conditions, and ecosystems vary across this range of examples, but a common theme of geological persistence holds. In some cases, persistence is apparently limited to a subset of what must have been a series of communities, e.g., floras, and in other cases there is broader ecological continuity. Timescales also span a range of durations, from tens of millenia to millions of years. Yet what is noted is the apparent persistence of interacting ecological functions, and by extension community dynamics, because of the presumed conservation of functions and interactions among diverse sets of taxa.
3 Selection and evolution
EFNH proposes that: (1) EFNs belong to a class of entities above the species level, that includes clades, ecosystems and function interaction networks (Doolittle and Inkpen, 2018; Papale and Doolittle, 2024); (2) that geological persistence arises from selection acting on EFN properties which vary according to variance within and among communities; and (3) that EFNs represent evolved functional networks. The EFNH draws a distinction between network changes that are driven by changes of species composition or selection acting on species traits, versus system persistence based on properties of the system itself. Certainly there are alternative explanations for some of the evidence presented earlier that are consistent with a conventional framework of species-environment interactions and natural selection acting at the population level. For example, Hanimualoto island presents a fixed area, and areal and energetic constraints could explain a constancy of species richness. On the other hand, the persistence of a system as agents within the system come and go suggests a causal relationship between species properties, system properties, and system persistence. Given the large combinatoric space available to a set of interacting species, what constrains that set of species from exploring the space based on individual evolutionary histories? And why, if set composition changes, do those changes not, or not always, lead to exploration of the combinatoric space? Finally, why would an event such as the PTME seemingly release species from the constraint?
The EFNH proposes that EFN persistence may be the result of selection acting on EFNs themselves. Proposals of community or ecosystem evolution by natural selection invariably run up against the third of Lewontin’s three principles of evolution by natural selection (Lewontin, 1970), which requires that units of selection produce offspring. No argument will be presented here that EFNs reproduce or generate offspring in any sense similar to organismal reproduction or otherwise that meet Lewontin’s requirement. There is, however, a substantial body of work arguing that differential persistence is a more encompassing framework than differential reproduction (Dussault and Bouchard, 2017; Bouchard, 2008; Neto and Doolittle, 2023), including instances of persistent ecological functions (Doolittle and Booth, 2017; Doolittle, 2024). According to Hull’s replicator-interactor framework of evolution by natural selection (Hull, 1980), interaction with the environment may take place at a level of organization above that of replicators, with replicators causing the interaction which in turn affects replication. EFNs are interactors, and species are both interactors and replicators. It is unnecessary, however, for EFNs to be replicating units with heritable traits to evolve, for selection here is not causing differential reproductive success, but instead differential persistence (Dussault and Bouchard, 2017). It is also unnecessary for EFN-environment interactions to be reducible to the species level, for there are emergent EFN properties that are subject to selection by the environment, the outcome of which will feedback to species replication and evolution. EFNH further proposes that feedback constrains both functional innovation and divergence of species within an EFN, as well as the success of species introduced into an EFN, thus extending EFN persistence even if species composition varies. There are subsequently several challenges that EFNH must address, including: (1) identifying EFN traits acted upon by selection; (2) explaining how the concept differs from lineage-based evolution by natural selection; and (3) outlining how EFNH explains geological persistence.
3.1 Emergent traits
An emergent system trait is one that is neither shared with, nor reducible to the traits of any single entity within the system, and thus exists at the level of the system. EFNH requires that EFNs be characterized by emergent traits and not the functional traits of individual species in any particular community of an EFN, for in that case any proposed stasis, persistence or selection of an EFN would be indistinguishable from those processes acting at the species level. EFN traits must therefore be emergent, above the species level. The traits are rooted ultimately in the emergent properties of an EFN’s constituent communities, in the same manner in which a community’s emergent traits are rooted in the traits of its constituent species, but similarly, an EFN will also exhibit traits that are not reducible to the level of any of its constituent communities, unless it consists of a single community. Thus there are emergent properties that are the result of aggregation or accumulation of properties at a lower hierarchical level, and those that are unique to the system itself. Their distinction is important to understanding the persistence of an EFN, and differential persistence between EFNs, although importantly, it will be explained below that selection and persistence at the species (Neto and Doolittle, 2023), community and EFN levels all play a role in the EFNH.
The question of whether a collection of overlapping and interacting species, a community, possesses emergent traits (Pianka, 1986), may similarly be posed of a community. I argue here that emergent community properties are one class of EFN traits, and include aggregate properties of species within individual communities such as total abundance, geographic distribution, and ecosystem functions, as well as the irreducible property of community robustness [resistance, resilience and anti-fragility (Taleb, 2014; Munoz et al., 2022)]. It is also important to distinguish those community emergent properties that are variable among communities that share an EFN, from properties that emerge collectively from all the communities sharing an EFN, and that therefore may distinguish among communities. Examples of the former are exhibited by modern coral reefs. Modern reefs are all descended from communities that arose from the earliest scleractinians in the Middle Triassic, and they share later important events, such as a late Neogene parrotfish (family Labridae) diversification in coral reefs (Choat et al., 2012), as well as both environmental requirements and physiographic impacts. In broad functional structure, modern reefs may all be assigned to a single EFN on the basis of shared history and functional structure, but are also distinguishable on the basis of other characteristics that are important to persistence. These include species richness, geographic distribution, and resilience founded on functional diversity. Among tropical western Atlantic reefs one can recognize differences of richness and composition between the Bahamas, the Gulf of Mexico, Greater Antilles, Lesser Antilles, and South America. There are also geologically persistent variants and variants of shorter duration (O’Dea et al., 2020). The second class of EFN traits are cumulative emergent properties that are not community -specific properties, but rather result from sets of communities, including the number of communities that share an EFN (e.g., the number of tropical western Atlantic coral reefs) and their total geographic distribution. Emergent EFN properties may also serve to distinguish between EFNs. For example, forests and grasslands, which often occupy the same geographic landscape as alternative ecological terrestrial ecosystem states, have at any given time and under specific environmental conditions different emergent properties. Those properties include the number of communities (forest versus grassland), their richnesses, robustness against perturbations such as drought and fire, and different agents, rates and mass transfers within biogeochemical cycles. Particular times and places may favour one EFN over another.
Thus there is a nested hierarchy of emergent properties that characterize the hierarchy of ecological units. Species have properties that are not reducible to individuals or populations, communities have properties that are not reducible to their member species but nonetheless can affect community persistence, and EFNs have properties that are not reducible to the community level and which are important to the persistence of the EFN. The interactions between these properties and environmental perturbations form the basis for selection leading to differential persistence, and selection at one level may affect emergent properties at a higher level. This is a central concept in Lenton et al. (2021) “survival of the systems”, and Dussault and Bouchard’s Persistence Enhancing Propensity (Dussault and Bouchard, 2017). Selection acting on a population affects the geographic distribution of the species, which in turn can affect community robustness depending on the presence or absence of the species or its abundance, and subsequently affects community persistence and hence the number and variability of communities within an EFN. The interactions of emergent properties at a higher level in turn feeds back to entities at lower levels because: (1) the number of communities within an EFN, an emergent property, can affect community persistence if individuals disperse among communities; and (2) community robustness can affect the survivability of member species. Ultimately the success of species and communities depends on selection acting on species traits and functions, but both community and EFN emergent properties are critical to species persistence and longevity.
3.2 EFN selection and evolution
There are several classes of evolutionary processes that occur at multiple levels within an EFN. First, species functions evolve according to the conventional framework of selection acting upon heritable traits and their variation, giving rise to genetic lineages (Lewontin, 1970). Second, species persistence and hence evolutionary success, may depend on the completion and persistence of emergent community processes (Dussault and Bouchard, 2017; Lenton et al., 2021; Doolittle, 2024), and the system’s capacity to withstand (resistance), recover from (resilience), or thrive (anti-fragility) when perturbed. Third, community persistence is dependent upon both the success of its constituent entities, and the responses of its emergent processes and robustness to environmental change. Fourth, the success and persistence of an EFN depends both upon its numerical representation, that is the number of communities sharing the EFN, which must exceed zero, and the persistences of those communities. But it is not necessary for the number of communities to exceed one for an EFN to be persistent.
3.2.1 Examples
The proposed process is best demonstrated with specific examples, for which I return first to the Karoo Basin and South China during the PTME. Imagine that either EFN consisted of a single community, in which case the taxon composition and functional structure of the EFN and community are identical. The extreme environmental disturbances at the end of the Permian would affect the robustness of the community because of direct impacts on some species (e.g., drought, lowered productivity, starvation, anoxia), and cascading secondary negative impacts on other species. Robustness, specifically the resistance of the community to a change of state, or resilience in recovery back to a pre-disturbance state, is an emergent community property and, because the EFN in this case consists of a single community, it is also an emergent EFN property. Thus there is a direct causal chain between population dynamics and persistence of the community/EFN.
In neither the Karoo Basin or South China systems, however, did significant initial species extinction (50.4% and 50.2%) lead to collapse and loss of the EFN (Roopnarine et al., 2019; Huang et al., 2023). Multiple population and community dynamics simulation models show that persistence of the terrestrial EFN was driven by its structure, that is, the network or pattern of interacting functions, and not taxon richness, functional richness or other unstructured measures of functionality such as disparity. Moreover, simulated extinctions of EFN species, combined with observed variation of taxonomic and functional composition, show that the EFNs were initially very robust, collapsing only after repeated waves of extinction. The estimated species richnesses of both the terrestrial and marine EFNs range in the hundreds of species (certainly underestimates), and thus their combinatoric spaces of possible EFNs are enormous. Manipulation of structure and simulated disturbance of the terrestrial EFN demonstrate that changes of richness and functional redundancy have no effect on robustness, but that the observed EFN is more robust than millions of counterfactual models in the combinatoric space Roopnarine and Angielczyk (2015), Roopnarine et al. (2019). This example, and by extension according to the EFNH, others described in earlier sections, strongly suggest that the observed EFN structures are not randomly assembled patterns.
Are EFNs then merely the results of the evolved properties (adaptations) of community species, or alternatively, is EFN structure itself an evolved one? An important piece of evidence in support of EFNs as evolved structures comes from the transitional intervals between long persistent paleoecological units. As mentioned earlier, those intervals tend to be relatively brief, occupy the aftermaths of ecological crises (Sheehan, 1996), and are often characterized by anomalous patterns of species dynamics, such as extreme uneveness and dominance, broad biogeographic distributions, and taxonomic compositions distinct from the preceding interval. Ecological recovery, meaning various things (Dineen et al., 2014) such as richness, functional disparity or diversity, or community robustness, is furthermore frequently delayed. A series of model experiments on recovery during the E. Triassic in the Karoo Basin, however, showed that within the combinatoric space of that community, the observed EFN was significantly less robust than millions of counterfactual alternatives (Roopnarine and Angielczyk, 2015; Roopnarine et al., 2019). In other words, increases of taxonomic richness and functional diversity relative to the extinction-depleted end Permian communities were not accompanied by the development of a robust EFN, and EFN persistence was predictably brief. The E. Triassic species survived for at least thousands of generations, but failed to assemble an EFN likely to be as geologically persistent as the preceding late Permian EFN. Failure of the species to co-evolve a robust EFN decoupled species adaptation from EFN robustness, with both EFN and species persistence compromised by the system-wide impacts of a fragile EFN. There is no initial cause or driver of the species-community-EFN interaction, but rather a dynamic interplay of causality.
3.3 EFN variance and selection
A persistent EFN may be represented by multiple communities distributed geographically and temporally. For example, a single type of community can vary spatially based on variable species environmental requirements, with some species being absent or varying demographically, or in per capita interaction strengths. Such are the cases as suggested earlier for regional modern coral reefs, and as observed in the Neogene Iberian mammalian and late Permian South China marine assemblages. There are no EFN-imposed constraints on species evolution or community compositional/functional variation if such evolution or variation have negligible effects on EFN robustness under prevailing environmental conditions. The EFN could then be broadly distributed, possibly represented by a metacommunity or a set of more isolated communities. The persistence of such EFNs despite species turnover (e.g., Blanco et al., 2021) supports this mechanism. It is distinct from constraints on ecological invasibility wherein species properties are the determining factors. In EFNH, immigration or invasion will fail if accompanied by a system-collapsing reduction of EFN robustness. An EFN undergoing this process, and represented by multiple communities, will appear as a uniform and persistent EFN in the fossil record if invaded or otherwise compromised communities fail.
Variation within an EFN may therefore arise if multiple member communities vary in species properties and emergent community properties. This variation may be subject to selection resulting in differential persistence among the communities and change to EFN emergent properties (number of communities and geographic distribution). The mechanism is a potential explanation for how extinction unfolded in the Karoo Basin EFN at the end of the Permian, where large tetrapods were more resistant to extinction, and persisted longer than smaller tetrapods (Roopnarine et al., 2017). Simulations of extinction targeting both size classes showed that communities lacking small species were more resistant to collapse than those lacking large species, attributed to the shorter food chains of larger tetrapods. Given that all these species populations were being subjected to physiological and ecological stresses during the PTME, and that the timing of population crashes across multiple communities distributed across the landscape was probably stochastic on an ecological timescale, the result would have been an EFN increasingly dominated by large species, as observed, and therefore a sorting among differentially impacted communities. This is not sorting of the species type (Vrba and Gould, 1986; Jablonski, 2008), whereby lineages become more or less numerically represented because of different rates of speciation or extinction; in the EFNH, it is communities within a metacommunity or dispersed framework that are selected, on the basis of varying emergent properties stemming from varying composition and/or environmental conditions.
Selection acting on an EFN subsequently feeds back to species, affecting their own persistences. In the Karoo communities described above, larger tetrapod species persisted longer than smaller species because of the greater robustness of local communities in which stochastic extirpation of larger species proceeded more slowly. Similarly, although surviving and new species in the very Early Triassic were obviously successful on that timescale and within that EFN, the lack of EFN robustness would have favored transformation of the EFN, again via differential persistence based on varying community composition.
3.3.1 A heuristic model
The discussion of EFN variability, selection and persistence can be summarized by a general model description. Here I use the familiar heuristic of a system state landscape to explain EFN selection (Figure 1). The landscape’s plane depicts a multidimensional space of EFN emergent properties occupied by communities. EFN structure, functional groups, network topology and interaction parameters, determine community position on the landscape. Elevation represents the likelihood of EFN and therefore community persistence,
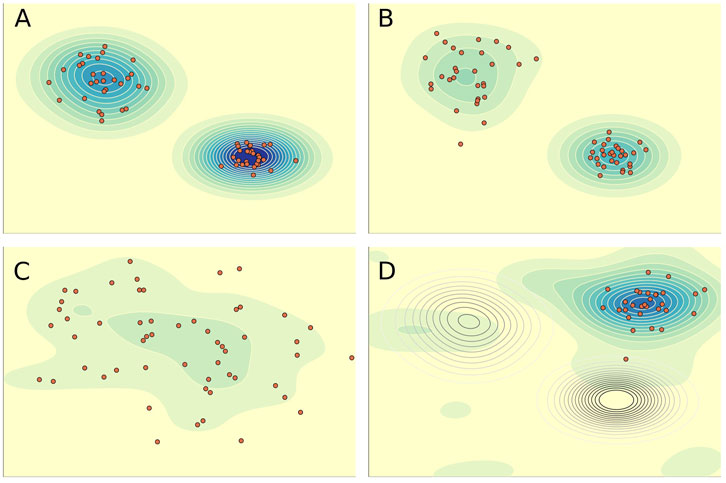
Figure 1. EFN heuristic state landscape. Contours measure the likelihood of persistence,
Landscape topology is dynamic, changing and varying over time, driven by the frequency and magnitude of perturbation regimes. The landscape is not a zero sum space, as there is no empirical evidence supporting zero sum constraints directing the history of life. Similarly, EFN persistence and the co-existence of multiple EFNs do not reflect steady state conditions, for these are thermodynamically open systems operating far from equilibrium, and constantly responding biogeographically, ecologically and evolutionarily to a dynamic environment. Reduced perturbation or frequency increases the
3.3.2 Evidence of EFN selection
Less powerful or extended perturbations, which can nonetheless have severe cumulative effects, e.g., the Permian-Triassic mass extinction (PTME), offer opportunities to examine landscape dynamics and EFN selection. For example, taxonomic extinction and EFN collapse were decoupled during the PTME (Roopnarine et al., 2019; Huang et al., 2023) as described above. The transition was marked by species extinctions that either occurred in multiple phases, or extended through the PTME, whereas EFN collapse, marked by the collapse of guild-level trophic networks, occurred toward the end of the PTME. Functional redundancy and the semi-independence of EFN robustness and species composition resulted in EFN persistence beyond the extinction of multiple constituent species. The Late Ordovician mass extinction (LOME) displays a similar pattern (Droser et al., 2000). EFN selection occurs under these circumstances. Consider a single EFN. Community variants have different
The short-lived paleoecological units observed to separate those of longer duration, an observation consistent with EFNH, which predicts that boundaries betwwen EEUs, marked by significant taxonomic and paleocological changes, also mark changing EFN landscapes. EFN destruction during environmentally challenging times releases species from top-down constraints on evolution and functional structure. What follows is an interval of landscape exploration by surviving and perhaps re-diversifying lineages, and communities that have no antecedents in the previous EFN (Figure 1C), a process that has been referred to as “random rewiring” (Lenton et al., 2021). These aftermath communities are predicted to be short-lived, not because of ecological instability, but because there are numerous alternative ways in which the evolving lineages can assemble into more efficient and robust EFNs. They are likely to do so incrementally via species coevolution (Roopnarine and Angielczyk, 2016), facilitating some continuity of system information (Lenton et al., 2021). Probable examples include LOME and PTME aftermaths. The counterfactual models of robustness of Early Triassic EFNs from the Karoo Basin of South Africa showed precisely this, with counterfactual EFNs being significantly more robust than the actual EFN (Roopnarine and Angielczyk, 2015; Roopnarine et al., 2019). The subsequent return of long persistent EFNs indicates selection for robust emergent properties, leading to increased
Another potential line of evidence crops up in community food web networks. One property of those networks, and all other networks with directional links between network nodes, is the node in-degree distribution,
An alternative explanation that is consistent with EFN selection and evolution comes from modern network theory. A key finding there is that the link distributions of real networks, e.g., the Internet, transportation networks and food webs, deviate strongly from random networks (e.g., Poisson or normal distributions) and instead tend to be decay distributions. Networks with such distributions are topologically robust against the random removal (extinction) of nodes or links. For example, because most Internet servers are linked to only a few other computers (specialists), the loss of a single server will on average have no noticeable impact on the functioning of the Internet. The same would hold true for the random removal of a species from a sparsely connected food web in which species are predominantly specialized (Roopnarine, 2006; Dunne et al., 2002). In contrast, the loss of a highly connected server or species could have system-wide effects. The connection to EFNH arises when we consider how different types of networks come to have decay degree distributions. Whereas the robustness of artificial networks often arises from intentional design [e.g., Mozafari and Khansari, 2019), preferential attachment (“the rich get richer” (D’souza et al., 2007)], or iterative correction of link distributions in response to negative perturbations (e.g., Yang et al., 2015), ecological community robustness is less easily explained. The distributions in modern communities could have arisen either fortuitously as a result of species-level eco-evolutionary constraints and opportunities as described above, in which case community robustness would not be a factor as specialist species would be more prone to extinction; or by the iterative evolution of the communities and distributions themselves. The latter would imply: (1) a differentiation of
3.4 Testing the EFNH
EFNH is consistent with a range of observed phenomena, but must be falsifiable. Two tests are suggested: (1) constructing community functional networks throughout the Phanerozoic; and (2) testing predictions with models of paleocommunity dynamics. The tests require the construction of networks of taxa aggregated according to function, with links corresponding to classes of biotic interactions, and interactions between groups and the environment. The first step is the derivation of an EFN from a community or set of communities. Determining whether multiple communities are assignable to the same EFN requires comparison of their functional structures, not taxon overlap or similarity. An accessible means of doing this is multidimensional scaling (MDS) of community data where taxa are aggregated according to function (e.g., Roopnarine et al., 2017) and clusters of communities are then inferred qualitatively to constitute EFNs. More rigorous EFN identification, which has not yet been applied to paleo- or Recent community functional networks, would involve measuring the similarities of the network structures themselves, for example, using approximate network or graph isomorphisms (Roopnarine, 2009; Arvind et al., 2012) or inexact network matching (e.g., Bengoetxea et al., 2002) when the number of functions (nodes) in the networks are unequal. These latter methods are computationally hard, but increasingly available. EFNs also may be suitably represented by hypergraphs, where species are nodes or vertices as in conventional network representations, but where (hyper)edges represent multidimensional relationships and interactions among species. Given the divisibility of Earth’s history into intervals characterizable by particular geophysical and geochemical conditions, secular trends or transitions such as continental configurations and climatic conditions, and biological increases of organizational complexity, body size, metabolic rates and power, colonization of ecospaces, and both ecosystem and Earth system engineering, EFNH predicts that at any given time multiple communities will share hypergraph properties, such as motifs (e.g., Contisciani et al., 2022; Lotito et al., 2022), and potentially be isomorphic when species are abstracted as functions (Feng et al., 2024), although the interpretation of hypergraph motifs in this context, and establishing community functional hypergraph isomorphism, remain unexplored problems. Tests of this nature will rely heavily on the continuing assembly of paleocommmunity data and functional interpretation. EFNH further predicts that paleocommunities that share the characteristics of an EFN will do so during the same interval of time regardless of taxon variation. Such uniformity will disappear during transitional intervals, perhaps being replaced by communities of more singular character. Furthermore, if transitory intervals have sufficient stratigraphic resolution, taxon turnover and extinction will precede final EFN breakdown, as already described in earlier examples. Finally, the EFNH predicts a positive correlation between system persistence and the presence of decay in-degree food web distributions, with the dataset of the latter increasing as the number of measured networks increases.
The second test examines EFN robustness using models of community dynamics and perturbation. Current network models of cascading secondary extinctions chronically underestimate dynamic impacts because they lack feedback processes (Dunne and Williams, 2009; Bodini et al., 2009), whereas available feedback models can be improved with incorporation of expanded species functional traits (Roopnarine et al., 2017; Huang et al., 2023). Suitable models that simulate community dynamics require parameters that are difficult to gather or infer for fossil taxa, such as population sizes (but see Marshall et al., 2021), intrinsic rates of population increase, mortality rates, and interaction strengths, but recent advances that rely largely on scaling relationships of modern taxa (Brown et al., 2004; Savage et al., 2004) and uniformitarian assumptions, plus increasingly sophisticated inferences from fossils themselves, place enhanced models well within reach.
Ultimately, EFN structure, whether represented as ordinations of functional traits, conventional networks, or hypergraphs, may be combined with dynamic models to understand both the changing
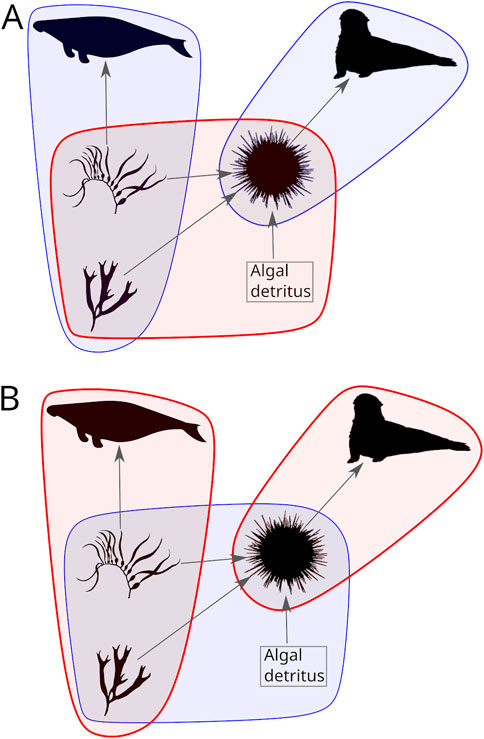
Figure 2. EFN hypergraphs of species functions and interactions in a model of historical North Pacific giant kelp forest ecosystems including the extinct megaherbivore Hydrodamalis gigas. Hypergraph edges connect two or more taxa (including algal detritus), with thick-outlined and red edges representing strong functional interactions in a particular community state, and thin-outlined blue edges representing weaker interactions. (A) Model EFN under conditions of sea star wasting disease, which has removed the urchin predator sunflower sea star Pycnopodia gigas. (B) EFN under conditions of both disease and a persistent marine heat wave. Note the reversal of edge strengths between the two EFN states. Gray arrows in both graphs, showing food web interactions, do not capture the full functionality of the system, ignoring competitive interactions and indirect relationships, e.g., via engineering impacts.
Finally, positive results of the above types of tests could be applied to conjectures that rates of background extinction have been steadily declining during the Phanerozoic. It has been suggested that the decline could result from changes to the ways in which perturbations propagate through communities because of Phanerozoic changes of community structures (Foote, 2000; Roopnarine, 2006). Again, this would be a system-level phenomenon, offering a complimentary although not exclusive explanation from species-based evolution. The EFNH provides a legitimate mechanism.
4 Systems selection and evolution
In closing, the EFNH should be placed in a proper context alongside other hypotheses that extend selection and evolution to systems comprising multiple phylogenetic lineages. The primary distinction typically drawn between systems evolution and evolution within a phylogenetic lineage is essentially one of replication and a vertical (genealogical) transmission of information (Papale and Doolittle, 2024). Whether one focuses on very reductionist levels such as the gene, or more holistic ones such as individual organisms, the conventional view of evolution by natural selection hinges upon replication. Nevertheless, there is an increasing body of thought positing that persistent ecological systems are products of selection of systems on the basis of properties that differentiate them from less persistent systems, and that systems bearing those properties can come to dominate the types of systems that may be assembled from multiple, interacting, phylogenetically distinct entities. Examples of this work includes Persistence Enhancing Propensity (PEP) (Dussault and Bouchard, 2017), the “survival of the systems” (STS) (Lenton et al., 2021), and ITSNTS (it is the singer, not the song) (Doolittle and Booth, 2017; Doolittle and Inkpen, 2018). PEP, by assuming that ecosystems are complex adaptive systems (Levin, 1998), proposes that such systems may evolve, without replication or the formation of lineages, because more resilient systems are more likely to persist. Persistence increases the chances of gaining more persistence enhancing properties, and such systems will increase in numerical representation, propositions consistent with the EFNH, where resilience is considered one facet of robustness. The STS hypothesis proposes that self-perpetuating feedbacks are a mechanism for system persistence, and importantly shows that such feedback cycles, built from organismal processes which in turn are active expressions of organismal functional traits, resolve the potential conflict “‘between levels and types of selection” (Lenton et al., 2021). Perhaps the most developed examination of systems selection and evolution are those put forward by Doolittle and colleagues, The context has focused primarily on microbial communities, but there are no obvious barriers to a broader application to macroscopic organisms. The ITSNTS hypothesis shifts the emphasis of evolution by natural selection from differential reproduction to differential persistence by proposing processes and patterns of functional interactions as legitimate units of selection (Doolittle and Inkpen, 2018). It is supported by the observation that functions can persist in communities beyond the persistence of the taxa that perform them, an observation consistent with the EFNH. In some sense, ITSNTS theory allows the projection of EFNH back into the Proterozoic where there is (currently) no evidence to support it. The common thread tying all these ideas together is that systems robustness and persistence benefits species (Doolittle, 2024), and their evolutionary histories are frequently coupled.
The nested hierarchy of species to systems extends further upward, both materially and conceptually, because the organization, selection and evolution of systems are extendable to entire planetary systems, wherein the living Earth system is the result of selection for properties, rooted in evolved lineages, that promote the persistence of the system. This re-imagining of the Gaia Hypothesis (Lovelock, 1983) recasts the concept of Gaia as a superorganism, to one of a complex adaptive system of functions, processes and systems (Doolittle, 2014; Lenton et al., 2018; Doolittle, 2019; Doolittle, 2024). A necessary feature of the hierarchy is the acceptance that selection and evolution do act at levels above those of genealogical units, the highest of which are species, without the necessity of replication, but with the possibilities of persistence, “re-production” and recurrence. It is difficult to speculate how far the principle of selection extends, but there is at least the proposal that fine-tuned fundamental parameters of the Standard Model are the result of selection, as in the hypothesis of Cosmological Natural Selection (Smolin, 2004). Emergent variation, system selection, and evolution likely represent universal principles and historical mechanisms that not only explain temporal change and stability on multiple timescales but, through multi-level feedbacks, unite multiple levels of material organization and complexity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
PR: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing–original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The author acknowledges Kenneth Angielczyk, Ashley Dineen, Yuangeng Huang and Roxanne Banker for many useful discussions. W. Ford Doolittle and two reviewers provided many helpful and insightful comments.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2025.1528448/full#supplementary-material
References
Alroy, J. (2004). Are Sepkoski’s evolutionary faunas dynamically coherent? Evol. Ecol. Res. 6, 1–32.
Arvind, V., Köbler, J., Kuhnert, S., and Vasudev, Y. (2012). “Approximate graph isomorphism,” in Mathematical foundations of computer science 2012: 37th international symposium, MFCS 2012, bratislava, Slovakia, august 27-31, 2012. Proceedings 37 (Springer), 100–111.
Banker, R. M., Dineen, A. A., Sorman, M. G., Tyler, C. L., and Roopnarine, P. D. (2022). Beyond functional diversity: the importance of trophic position to understanding functional processes in community evolution. Front. Ecol. Evol. 10, 983374. doi:10.3389/fevo.2022.983374
Barry, J. C., Morgan, M. E., Flynn, L. J., Pilbeam, D., Behrensmeyer, A. K., Raza, S. M., et al. (2002). Faunal and environmental change in the late Miocene Siwaliks of northern Pakistan. Paleobiology 28, 1–71. doi:10.1666/0094-8373(2002)28[1:faecit]2.0.co;2
Bengoetxea, E., Larranaga, P., Bloch, I., Perchant, A., and Boeres, C. (2002). Inexact graph matching by means of estimation of distribution algorithms. Pattern Recognit. 35, 2867–2880. doi:10.1016/s0031-3203(01)00232-1
Blanco, F., Calatayud, J., Martín-Perea, D. M., Domingo, M. S., Menéndez, I., Müller, J., et al. (2021). Punctuated ecological equilibrium in mammal communities over evolutionary time scales. Science 372, 300–303. doi:10.1126/science.abd5110
Bodini, A., Bellingeri, M., Allesina, S., and Bondavalli, C. (2009). Using food web dominator trees to catch secondary extinctions in action. Philosophical Trans. R. Soc. B Biol. Sci. 364, 1725–1731. doi:10.1098/rstb.2008.0278
Bouchard, F. (2008). Causal processes, fitness, and the differential persistence of lineages. Philosophy Sci. 75, 560–570. doi:10.1086/594507
Boucot, A. J. (1983). Does evolution take place in an ecological vacuum II the time has come’the walrus said. J. Paleontology, 1–30.
Brett, C. E., Ivany, L. C., and Schopf, K. M. (1996). Coordinated stasis: an overview. Palaeogeogr. Palaeoclimatol. Palaeoecol. 127, 1–20. doi:10.1016/s0031-0182(96)00085-5
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., and West, G. B. (2004). Toward a metabolic theory of ecology. Ecology 85, 1771–1789. doi:10.1890/03-9000
Choat, J. H., Klanten, O. S., Van Herwerden, L., Robertson, D. R., and Clements, K. D. (2012). Patterns and processes in the evolutionary history of parrotfishes (Family Labridae). Biol. J. Linn. Soc. 107, 529–557. doi:10.1111/j.1095-8312.2012.01959.x
Clements, F. E. (1916). Plant succession: an analysis of the development of vegetation. 242. Washington, D.C., USA: Carnegie Institution of Washington.
Cole, S. R., and Hopkins, M. J. (2021). Selectivity and the effect of mass extinctions on disparity and functional ecology. Sci. Adv. 7, eabf4072. doi:10.1126/sciadv.abf4072
Contisciani, M., Battiston, F., and De Bacco, C. (2022). Inference of hyperedges and overlapping communities in hypergraphs. Nat. Commun. 13, 7229. doi:10.1038/s41467-022-34714-7
Damuth, J. (1985). Selection among “species”: a formulation in terms of natural functional units. Evolution 39, 1132–1146. doi:10.2307/2408740
Detmer, A. R., Miller, R. J., Reed, D. C., Bell, T. W., Stier, A. C., and Moeller, H. V. (2021). Variation in disturbance to a foundation species structures the dynamics of a benthic reef community. Ecology 102, e03304. doi:10.1002/ecy.3304
DiMichele, W. A., Behrensmeyer, A. K., Olszewski, T., Labandeira, C. C., Pandolfi, J. M., Wing, S. L., et al. (2004). Long-term stasis in ecological assemblages: evidence from the fossil record. Annu. Rev. Ecol. Evol. Syst. 35, 285–322. doi:10.1146/annurev.ecolsys.35.120202.110110
DiMichele, W. A., Phillips, T. L., and Nelson, W. J. (2002). Place vs. time and vegetational persistence: a comparison of four tropical mires from the Illinois Basin during the height of the Pennsylvanian Ice Age. Int. J. Coal Geol. 50, 43–72. doi:10.1016/s0166-5162(02)00113-1
Dineen, A. A., Fraiser, M. L., and Sheehan, P. M. (2014). Quantifying functional diversity in pre-and post-extinction paleocommunities: a test of ecological restructuring after the end-Permian mass extinction. Earth-Science Rev. 136, 339–349. doi:10.1016/j.earscirev.2014.06.002
Dineen, A. A., Roopnarine, P. D., and Fraiser, M. L. (2019). Ecological continuity and transformation after the Permo-Triassic mass extinction in northeastern Panthalassa. Biol. Lett. 15, 20180902. doi:10.1098/rsbl.2018.0902
Doolittle, W. F. (2014). Natural selection through survival alone, and the possibility of Gaia. Biol. and Philosophy 29, 415–423. doi:10.1007/s10539-013-9384-0
Doolittle, W. F. (2019). Making evolutionary sense of Gaia. Trends Ecol. and Evol. 34, 889–894. doi:10.1016/j.tree.2019.05.001
Doolittle, W. F. (2024). Darwinizing Gaia: natural selection and multispecies community evolution. Cambridge,Massachusetts: The MIT Press.
Doolittle, W. F., and Booth, A. (2017). It’s the song, not the singer: an exploration of holobiosis and evolutionary theory. Biol. and Philosophy 32, 5–24. doi:10.1007/s10539-016-9542-2
Doolittle, W. F., and Inkpen, S. A. (2018). Processes and patterns of interaction as units of selection: an introduction to ITSNTS thinking. Proc. Natl. Acad. Sci. 115, 4006–4014. doi:10.1073/pnas.1722232115
Droser, M. L., Bottjer, D. J., Sheehan, P. M., and McGhee Jr, G. R. (2000). Decoupling of taxonomic and ecologic severity of Phanerozoic marine mass extinctions. Geology 28, 675–678. doi:10.1130/0091-7613(2000)028<0675:dotaes>2.3.co;2
D’souza, R. M., Borgs, C., Chayes, J. T., Berger, N., and Kleinberg, R. D. (2007). Emergence of tempered preferential attachment from optimization, Proc. Natl. Acad. Sci. U. S. A. Emergence tempered Prefer. attachment Optim. 104, 6112–6117. doi:10.1073/pnas.0606779104
Dunne, J. A., and Williams, R. J. (2009). Cascading extinctions and community collapse in model food webs. Philosophical Trans. R. Soc. B Biol. Sci. 364, 1711–1723. doi:10.1098/rstb.2008.0219
Dunne, J. A., Williams, R. J., and Martinez, N. D. (2002). Food-web structure and network theory: the role of connectance and size. Proc. Natl. Acad. Sci. 99, 12917–12922. doi:10.1073/pnas.192407699
Dussault, A. C., and Bouchard, F. (2017). A persistence enhancing propensity account of ecological function to explain ecosystem evolution. Synthese 194, 1115–1145. doi:10.1007/s11229-016-1065-5
Eldredge, N. (1989). Macroevolutionary Dynamics. Species, niches and adaptive peaks. New York: McGraw-Hill Publishing Company.
Falkowski, P. G., Fenchel, T., and Delong, E. F. (2008). The microbial engines that drive Earth’s biogeochemical cycles. science 320, 1034–1039. doi:10.1126/science.1153213
Feng, Y., Han, J., Ying, S., and Gao, Y. (2024). Hypergraph isomorphism computation. IEEE Trans. Pattern Analysis Mach. Intell. 46, 3880–3896. doi:10.1109/tpami.2024.3353199
Foote, M. (2000). Origination and extinction components of taxonomic diversity: Paleozoic and post-Paleozoic dynamics. Paleobiology 26, 578–605. doi:10.1666/0094-8373(2000)026<0578:oaecot>2.0.co;2
Gleason, H. A. (1926). The individualistic concept of the plant association. Bull. Torrey botanical club 53, 7–26. doi:10.2307/2479933
Huang, Y., Chen, Z.-Q., Roopnarine, P. D., Benton, M. J., Yang, W., Liu, J., et al. (2021). Ecological dynamics of terrestrial and freshwater ecosystems across three mid-Phanerozoic mass extinctions from northwest China. Proc. R. Soc. B 288, 20210148. doi:10.1098/rspb.2021.0148
Huang, Y., Chen, Z.-Q., Roopnarine, P. D., Benton, M. J., Zhao, L., Feng, X., et al. (2023). The stability and collapse of marine ecosystems during the Permian-Triassic mass extinction. Curr. Biol. 33, 1059–1070.e4. doi:10.1016/j.cub.2023.02.007
Hull, D. L. (1980). Individuality and selection. Annu. Rev. Ecol. Syst. 11, 311–332. doi:10.1146/annurev.es.11.110180.001523
Jablonski, D. (2008). Species selection: theory and data. Annu. Rev. Ecol. Evol. Syst. 39, 501–524. doi:10.1146/annurev.ecolsys.39.110707.173510
Kempf, H. L., Castro, I. O., Dineen, A. A., Tyler, C. L., and Roopnarine, P. D. (2020). Comparisons of Late Ordovician ecosystem dynamics before and after the Richmondian invasion reveal consequences of invasive species in benthic marine paleocommunities. Paleobiology 46, 320–336. doi:10.1017/pab.2020.26
Kuhn, T. S. (1970). The structure of scientific revolutions. Chicago, USA: University of Chicago Press.
Lenton, T. M., Daines, S. J., Dyke, J. G., Nicholson, A. E., Wilkinson, D. M., and Williams, H. T. (2018). Selection for Gaia across multiple scales. Trends Ecol. and Evol. 33, 633–645. doi:10.1016/j.tree.2018.05.006
Lenton, T. M., Kohler, T. A., Marquet, P. A., Boyle, R. A., Crucifix, M., Wilkinson, D. M., et al. (2021). Survival of the systems. Trends Ecol. and Evol. 36, 333–344. doi:10.1016/j.tree.2020.12.003
Levin, S. A. (1998). Ecosystems and the biosphere as complex adaptive systems. Ecosystems 1, 431–436. doi:10.1007/s100219900037
Lewontin, R. C. (1970). The units of selection. Annu. Rev. Ecol. Syst. 1, 1–18. doi:10.1146/annurev.es.01.110170.000245
Lotito, Q. F., Musciotto, F., Montresor, A., and Battiston, F. (2022). Higher-order motif analysis in hypergraphs. Commun. Phys. 5, 79. doi:10.1038/s42005-022-00858-7
Lovelock, J. E. (1983). “Gaia as seen through the atmosphere,” in Biomineralization and biological metal accumulation: biological and geological perspectives papers presented at the fourth international symposium on biomineralization, renesse, The Netherlands, june 2–5, 1982 (Springer), 15–25.
Marshall, C. R., Latorre, D. V., Wilson, C. J., Frank, T. M., Magoulick, K. M., Zimmt, J. B., et al. (2021). Absolute abundance and preservation rate of Tyrannosaurus rex. Science 372, 284–287. doi:10.1126/science.abc8300
McCann, K., Hastings, A., and Huxel, G. R. (1998). Weak trophic interactions and the balance of nature. Nature 395, 794–798. doi:10.1038/27427
Mitchell, J. S., Roopnarine, P. D., and Angielczyk, K. D. (2012). Late Cretaceous restructuring of terrestrial communities facilitated the end-Cretaceous mass extinction in North America. Proc. Natl. Acad. Sci. 109, 18857–18861. doi:10.1073/pnas.1202196109
Morris, P. J., Ivany, L. C., Schopf, K. M., and Brett, C. E. (1995). The challenge of paleoecological stasis: reassessing sources of evolutionary stability, Proc. Natl. Acad. Sci. U. S. A. Chall. paleoecological stasis reassessing sources Evol. Stab. 92, 11269–11273. doi:10.1073/pnas.92.24.11269
Mozafari, M., and Khansari, M. (2019). Improving the robustness of scale-free networks by maintaining community structure. J. Complex Netw. 7, 838–864. doi:10.1093/comnet/cnz009
Munoz, A., Billsberry, J., and Ambrosini, V. (2022). Resilience, robustness, and antifragility: towards an appreciation of distinct organizational responses to adversity. Int. J. Manag. Rev. 24, 181–187. doi:10.1111/ijmr.12289
Neto, C., and Doolittle, W. F. (2023). A chemostat model for evolution by persistence: clade selection and its explanatory autonomy. Philosophy Sci. 90, 21–38. doi:10.1017/psa.2022.11
O’Dea, A., Lepore, M., Altieri, A. H., Chan, M., Morales-Saldaña, J. M., Muñoz, N.-H., et al. (2020). Defining variation in pre-human ecosystems can guide conservation: an example from a Caribbean coral reef. Sci. Rep. 10, 2922. doi:10.1038/s41598-020-59436-y
Olson, E. C. (1952). The evolution of a Permian vertebrate chronofauna. Evolution 6, 181–196. doi:10.2307/2405622
Pandolfi, J. M., and Jackson, J. B. (2006). Ecological persistence interrupted in Caribbean coral reefs. Ecol. Lett. 9, 818–826. doi:10.1111/j.1461-0248.2006.00933.x
Papale, F., and Doolittle, W. F. (2024). Towards a more general theory of evolution by natural selection: a manifesto. Philosophy, Theory, and Practice in Biology 16. doi:10.3998/ptpbio.5563
Pianka, E. R. (1986). Ecology and natural history of desert lizards: analyses of the ecological niche and community structure, 5153. Princeton, NJ: Princeton University Press.
Roopnarine, P. D. (2006). Extinction cascades and catastrophe in ancient food webs. Paleobiology 32, 1–19. doi:10.1666/05008.1
Roopnarine, P. D. (2009). Ecological modeling of paleocommunity food webs. Paleontol. Soc. Pap. 15, 195–220.
Roopnarine, P. D., Angielczyk, K., Weik, A., and Dineen, A. (2019). Ecological persistence, incumbency and reorganization in the Karoo Basin during the Permian-Triassic transition. Earth-Science Rev. 189, 244–263. doi:10.1016/j.earscirev.2018.10.014
Roopnarine, P. D., and Angielczyk, K. D. (2012). The evolutionary palaeoecology of species and the tragedy of the commons. Biol. Lett. 8, 147–150. doi:10.1098/rsbl.2011.0662
Roopnarine, P. D., and Angielczyk, K. D. (2015). Community stability and selective extinction during the Permian-Triassic mass extinction. Science 350, 90–93. doi:10.1126/science.aab1371
Roopnarine, P. D., and Angielczyk, K. D. (2016). “The stability of ecological communities as an agent of evolutionary selection: evidence from the Permian Triassic mass extinction,” in Evolutionary theory: a hierarchical perspective. Editors E. S. Niles Eldredge, T. Pievani, and I. Tëmkin (University of Chicago Press).
Roopnarine, P. D., Angielczyk, K. D., Olroyd, S. L., Nesbitt, S. J., Botha-Brink, J., Peecook, B. R., et al. (2017). Comparative ecological dynamics of permian-triassic communities from the Karoo, luangwa, and ruhuhu basins of southern Africa. J. Vertebrate Paleontology 37, 254–272. doi:10.1080/02724634.2018.1424714
Roopnarine, P. D., Angielczyk, K. D., Wang, S. C., and Hertog, R. (2007). Trophic network models explain instability of Early Triassic terrestrial communities. Proc. R. Soc. B Biol. Sci. 274, 2077–2086. doi:10.1098/rspb.2007.0515
Roopnarine, P. D., and Banker, R. M. (2021). Ecological stasis on geological time scales. Science 372, 237–238. doi:10.1126/science.abh2853
Roopnarine, P. D., Banker, R. M., and Sampson, S. D. (2022). Impact of the extinct megaherbivore Steller’s sea cow (Hydrodamalis gigas) on kelp forest resilience. Front. Ecol. Evol. 10, 983558. doi:10.3389/fevo.2022.983558
Roopnarine, P. D., and Hertog, R. (2013). Detailed food web networks of three Greater Antillean coral reef systems: the Cayman Islands, Cuba, and Jamaica. Dataset Pap. Sci. 2013, 1–9. doi:10.7167/2013/857470
Savage, V. M., Gillooly, J. F., Brown, J. H., West, G. B., and Charnov, E. L. (2004). Effects of body size and temperature on population growth. Am. Nat. 163, 429–441. doi:10.1086/381872
Sepkoski, J. J. (1981). A factor analytic description of the Phanerozoic marine fossil record. Paleobiology 7, 36–53. doi:10.1017/s0094837300003778
Sheehan, P. M. (1996). A new look at ecologic evolutionary units (EEUs). Palaeogeogr. Palaeoclimatol. Palaeoecol. 127, 21–32. doi:10.1016/s0031-0182(96)00086-7
Smith, J. M., and Szathmary, E. (1997). The major transitions in evolution (Oxford, United Kingdom: OUP)
Smolin, L. (2004). Cosmological natural selection as the explanation for the complexity of the universe. Phys. A Stat. Mech. its Appl. 340, 705–713. doi:10.1016/j.physa.2004.05.021
Tang, C. M., and Bottjer, D. J. (1996). Long-term faunal stasis without evolutionary coordination: Jurassic benthic marine paleocommunities, Western Interior, United States. Geology 24, 815–818. doi:10.1130/0091-7613(1996)024<0815:ltfswe>2.3.co;2
Vermeij, G. (2023). The evolution of power: a new understanding of the history of life. Princeton University Press.
Vrba, E. S., and Gould, S. J. (1986). The hierarchical expansion of sorting and selection: sorting and selection cannot be equated. Paleobiology 12, 217–228. doi:10.1017/s0094837300013671
Watkins, R., Coorough, P. J., and Mayer, P. S. (2000). The Silurian Dicoelosia communities: temporal stability within an ecologic evolutionary unit. Palaeogeogr. Palaeoclimatol. Palaeoecol. 162, 225–237. doi:10.1016/s0031-0182(00)00129-2
Willard, D. A., Phillips, T. L., Lesnikowska, A. D., and DiMichele, W. A. (2007). Paleoecology of the Late Pennsylvanian-age Calhoun coal bed and implications for long-term dynamics of wetland ecosystems. Int. J. Coal Geol. 69, 21–54. doi:10.1016/j.coal.2006.03.011
Yang, Y., Li, Z., Chen, Y., Zhang, X., and Wang, S. (2015). Improving the robustness of complex networks with preserving community structure. PloS one 10, e0116551. doi:10.1371/journal.pone.0116551
Keywords: paleoecological persistence, ecological stasis, systems evolution, functional diversity, systems selection, systems persistence
Citation: Roopnarine PD (2025) Selection, evolution and persistence of paleoecological systems. Front. Earth Sci. 13:1528448. doi: 10.3389/feart.2025.1528448
Received: 14 November 2024; Accepted: 27 January 2025;
Published: 13 February 2025.
Edited by:
Shuhai Xiao, Virginia Tech, United StatesReviewed by:
Anshuman Swain, Harvard University, United StatesGregory P. Dietl, Paleontological Research Institution (PRI), United States
Copyright © 2025 Roopnarine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter D. Roopnarine, cHJvb3BuYXJpbmVAY2FsYWNhZGVteS5vcmc=
 Peter D. Roopnarine
Peter D. Roopnarine