- 1Geology Department, Faculty of Science, Assiut University, Assiut, Egypt
- 2Zoology Department, Faculty of Science, Assiut University, Assiut, Egypt
Trace elements such as titanium, zirconium, thorium, and uranium, are found in black sand (BS) after weathering and corrosion. Precious metals are not the only valuable elements in black sand, rare earth elements are also found. The aquatic life in lakes and reservoirs is negatively affected by lithophilic elements such as lithium, uranium, and tin. Accordingly, intensive experiments were conducted on Nile tilapia (Oreochromis niloticus) after exposure to isolated black sand. Blood biomarkers, antioxidant balance, morpho-nuclear erythrocyte’s alterations, and histopathological signs have been investigated after fish exposure for 15 days to a 6.4 g BS/kg diet, 9.6 g BS/kg diet, and 2.4 g BS/kg diet. The blood profile, including platelets and white blood cells, was pronouncedly decreased as a result. Functions of the liver and kidneys were impaired. An increase in serum-antioxidant enzymes such as catalase activities and superoxide dismutase was recorded. Also, exposure to black sand induced cellular and nuclear abnormalities in the erythrocytes. In conclusion, the black sand isolated from the Red sea beach influenced Oreochromis niloticus’s hematology, biochemistry, and antioxidant parameters. Poikilocytosis and RBC nuclear abnormalities were also associated with exposure to black sand. The resulting erosion of rocks and rocks’ access to water forces us to consider the seriousness of climatic change on the aquatic ecosystem.
1 Introduction
Black sands (BS) in Egypt are the resulting deposits of igneous and metamorphic material eroded and transported through the Nile River from southern regions (Aziz et al., 2020). Such deposits were initially placed along the Nile Delta with subsequent transportation to the east due to the Mediterranean Sea currents (Abdel-Karim and Barakat, 2017). While dissolution of some minerals prevents keeping them in solution, natural sorting of heavy minerals may occur through a mechanical action, separating lower from higher density minerals. The latter are less prone to transportation by currents and tend therefore to beach up, forming lag deposits (El-Arel et al., 2020).
Accumulations of black sand have been found in some places in Egypt, an important location of black sands along the Mediterranean and Red Sea beaches. Black sand is distributed in two forms: beach sediments and coastal dunes (Ibrahim et al., 2009).
Despite the high potential of the Red Sea coast for BS placer deposits, these are poorly explored (Ibrahim et al., 2018), and therefore a comprehensive understanding of BS physicochemical characteristics from the Red Sea remains very limited. Black sands are usually enriched in many minerals, namely, ilmenite, magnetite, zircon, monazite, garnet, and rutile. They also contain considerable amounts of metals such as Fe, Ti, Mg, Al, and trace quantities of gold and rare earth elements as well. In addition, BS from northern Egypt has higher levels of radioactive elements than the world average level, such as U and Th, but within a tolerable range (Hamdan and Hassan, 2020).
Most of the studies examined BS and its composition in terms of economic value, mainly in the Nile Delta and the Mediterranean region. Limited attention has been given to the BS assessment of potential BS ecological impacts, particularly its effects in biota, namely, in fish for human consumption.
A major source of food worldwide, the Nile tilapia is one of the most important freshwater fish in Africa (Hamed et al., 2020). Because the Nile tilapia can be easily handled and followed up in controlled conditions, as well as being less expensive than other species, it makes an ideal model for contamination assessment and toxicology analysis (Hamed et al., 2019a).
The physiological responses and stress caused by BS to fish were never reported. Accordingly, this study tested the hypothesis of large concentrations of BS causing changes in fish biological responses. It aimed therefore at 1) isolating and characterizing the physicochemical properties of BS from the Red Sea; 2) assessing and understanding the fish responses under controlled exposure to different BS concentrations; and 3) evaluating the potential negative impact of BS towards the biota, namely, to fish. To achieve these aims, fish were exposed to different doses of BS by ingestion and data were collected after analysis of hematological, biochemical, antioxidant, and histopathological lesions.
2 Materials and methods
2.1 Study area and sampling of the black sand
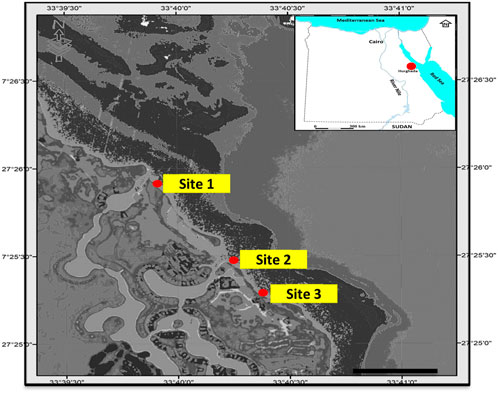
The black sand samples were collected along the Red Sea beach, Hurghada, Egypt (Figure 1). Three sites were chosen based on the presence and distribution of black sand in these sites; the samples were collected in August 2021.
About 5 kg of composite sand samples from every site were collected from the beach in plastic bags and black sand samples were isolated using magnets (Figure 2).
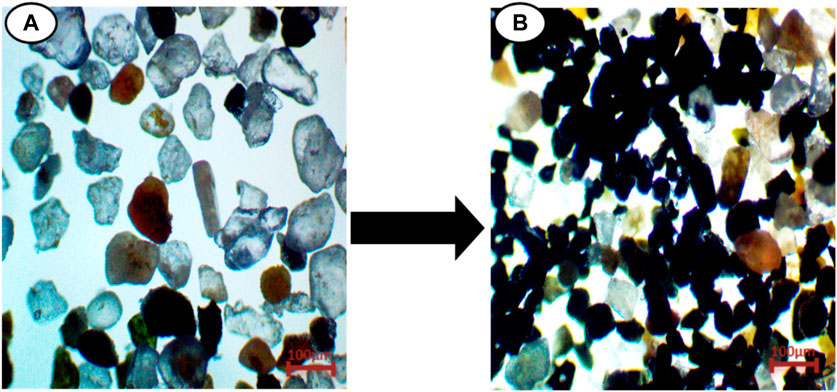
FIGURE 2. Showing (A), composite sand samples and (B), Black sand Isolation from composite sand samples.
The concentration of the black sand was 6.4 g/kg from Site 1, 9.6 g/kg from Site 2, and 12.4 g/kg from Site 3. Isolation, separation, and purification were done according to Ralph (2010).
2.2 Black sand characterization
2.2.1 Chemical composition
The chemical composition of the black sand sample was conducted using X-ray fluorescence (XRF) at the Assiut Cement Company (CEMEX).
2.2.2 FTIR and XRD patterns
The samples of black sand were examined by Fourier transform infrared (FTIR) spectrometer for identification of functional groups using a Nicollet spectrophotometer (Model 6,700) and KBr technology.
The black sand samples were measured by X-ray diffraction using a Philips PW 2103 diffractometer (Netherlands).
2.3 Fish collection
Oreochromis niloticus weighing 100–150 g and 10–15 cm in length were completely healthy and free of contaminants and parasites according to the American Fisheries Society, Fish Health Section (AFS-FHS, 2007). Four weeks’ acclimation period was made for the fish in a 250-liter water tank under laboratory conditions (conductivity 262.8 mM cm−1, pH 7.3, dissolved oxygen 6.7 mg L−1, temperature 20.6°C, photoperiod 12:12 h light: dark). During the acclimation period, fish were fed (5% body weight) twice/day on commercial food (30% protein) purchased from AlShurouq company, Cairo.
2.4 Experimental design
A total of 120 Nile Tilapia (Oreochromis niloticus) were randomly assigned to four groups. The samples were stored in glass containers (100L water) in each group (30 samples in triplicate). The control group (Group 1) was fed on a normal diet. Group 2 was fed on a diet containing 6.4 g black sand/kg, group 3 was fed on a diet containing 9.6 g black sand/kg, and group 4 was fed on a diet containing 12.4 g black sand/kg according to BS concentrations which were collected and isolated from the study area (Figure 1).
After the end of the experimental period (15 days), six samples from each treatment were randomly selected and placed on ice in order to reduce stress before dissection and sampling (Hamed et al., 2019b). Samples were taken for further analysis.
2.5 Hemato-biochemical parameters
Various hematological indicators (counts of red blood cells [RBCs] and white blood cells [WBCs]; differential WBCs; blood platelets; Erythrocyte indices (including mean corpuscular hemoglobin [MCH], mean corpuscular volume [MCV], and mean corpuscular hemoglobin concentration [MCHC]) were assessed following the method of (Blaxhall and Daisley, 1973; Bain et al., 2016) McKnight (1966).
Blood samples for biochemical parameters were obtained from fish without the use of an anticoagulant agent in order to acquire serum. Colorimetric determinations of the following important biochemical indices, namely, alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), creatinine, uric acid, albumin, globulin, and glucose, were carried out according to (Hamed et al., 2019a) by using a spectrophotometer in a wavelength range of 340–546 nm (Biodiagonstic Company, Egypt).
2.6 Serum-antioxidant parameters
Catalase (CAT) was determined using the method found in Aebi. (1984). Superoxide dismutase (SOD) was measured according to (Nishikimi et al., 1972). Total antioxidant capacity (TAC) was measured according to the protocol given by (Koracevic et al., 2001). Total peroxide (TPX) was assessed following the procedure of (Harma et al., 2005).
2.7 Erythron profile abnormalities
Hematoxylin-Eosin-stained blood smears were selected, coded, randomized, and scored blindly for morphological and nuclear abnormalities of RBC’s according to the criteria of others (Schmid, 1975; al-Sabti and Metcalfe, 1995).
2.8 Histopathological analysis
Tissue samples of the liver, intestine, and brain were fixed in neutral buffered formalin before being processed using a standard automated process (dehydrated through graded ethanol concentration, cleared with methyl benzoate), embedded in paraffin wax, and sectioned (5 microns). De-waxed slides were rehydrated before being stained with hematoxylin and eosin (H & E) (Feldman and Wolfe, 2014).
2.9 Statistical analysis
One-way analysis of variance (ANOVA) was used to evaluate the pattern of variation for each variable, with a Tukey post-hoc test used in the multiple comparisons using SPSS package (SPSS, 1998). Data are presented as mean ± SD. Values in the same row with different superscript letters are significantly different (p < 0.05).
3 Results
3.1 Black sand characterization
3.1.1 Chemical composition
The elements of the black sand composition is given in Table 1. The oxides that presented higher concentrations were SiO2 (51.3 wt%), Fe3O4 (34.9 wt%), TiO2 (7.01 wt%), Al2O3 (3.01 wt%), and CaO (3.01 wt%). Lower fractions of MgO (0.27 wt%), SO3 (0.15 wt%), Na2O (0.13 wt%), Cr2O3, (0.1 wt%), K2O (0.07 wt%), and P2O5 (0.05 wt%) were also observed.
3.1.2 X-ray diffraction analysis (XRD) patterns
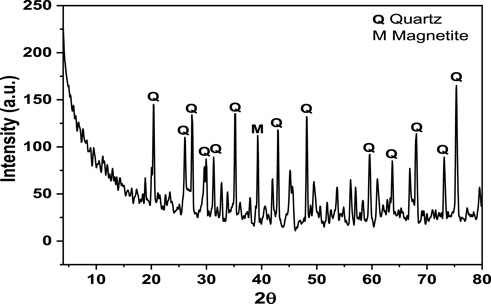
Figure 3 shows the XRD pattern of the black sand. The BS sample was composed principally by SiO2 and Fe3O4 phases. The diffraction peaks observed at 2θ values of 20.4°, 26.1°, 27.4°, 29.9°, 31.3°, 39.3°, 42.9o, 45.1o, 48.1o, 59.6o, 63.7o, 68o, 73.1o, and 75.3° agree with the COD database file (Entry number 96-900-5,023) for quartz. The peak observed at 35.2° corresponded to magnetite, in agreement with the COD database file (Entry number 96-900-7,645). Diffraction peaks for other oxides were not observed, which might be due to their low percentages in the sample.
3.1.3 Fourier transform infrared spectroscopy (FTIR) analysis
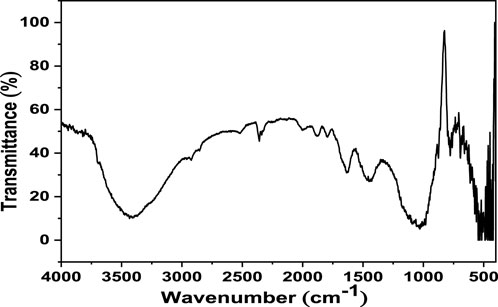
The FTIR spectrum of black sand is presented in Figure 4. The black bands showed various bands in the range of 400–600 cm-1, which may be due to the metal oxide contents in the sample. The two bands that are located at 1,030 cm-1 and 777 cm-1 are attributed to the asymmetric and symmetric stretching vibrations of Si-O, respectively. The O-H stretching and bending modes of adsorbed water molecules appeared at 3,434 cm-1 and 1,636 cm-1, respectively. The band that is observed at 1,450 cm-1 is characteristic of Ca-O stretching vibration.
3.2 Hematological parameters
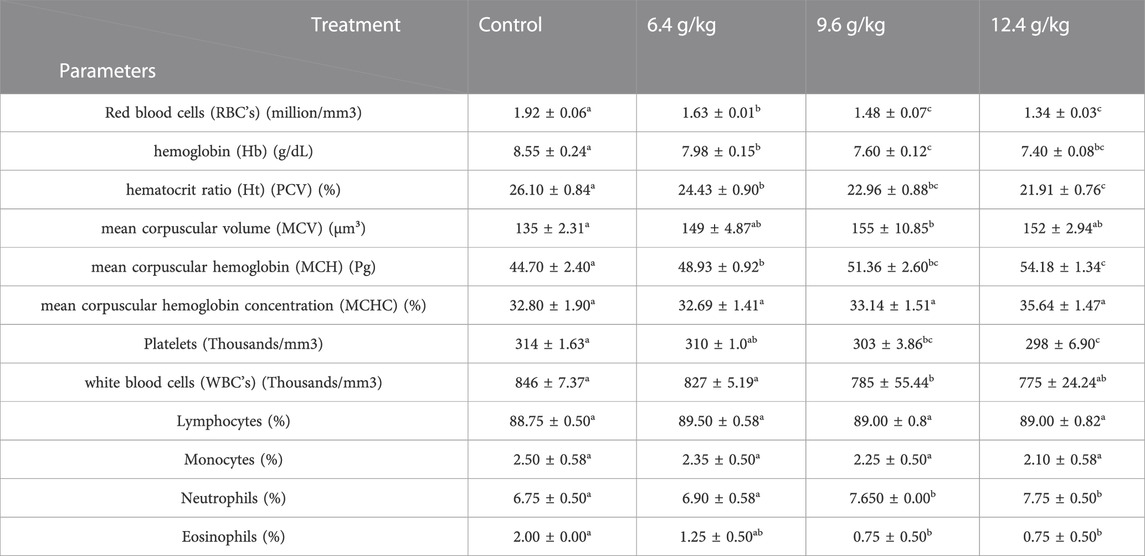
Our results on the hematological parameters are presented in Table 2 and demonstrate a significant decrease (p < 0.05) in the levels of the hematological parameters such as RBC’s, Hb, Hct, WBCs, and percent of monocytes after 15 days of exposure to a 12 g/kg diet of black sand, >9 g/kg diet, and >6 g/kg diet compared with the control group. Significant increases (p < 0.05) of MCV, MCH, MCHC, and percent of lymphocytes and neutrophils after 15 days of exposure to a 12.4 g/kg diet of black sand >, 9.6 g/kg diet >, and 6.4 g/kg diet compared to the control group were recorded.
3.3 Biochemical parameters
The impact of black sand on biochemical parameters of the Nile Tilapia (Oreochromis niloticus) is shown in Table 3. Albumin, globulin, total protein, glucose, AST, ALT, ALP, creatinine, and A/G ratio showed a significant increase (p < 0.05) after 15 days of exposure to a 12.4 g/kg diet of black sand, >9.6 g/kg diet, and >6.4 g/kg diet when compared to the control.
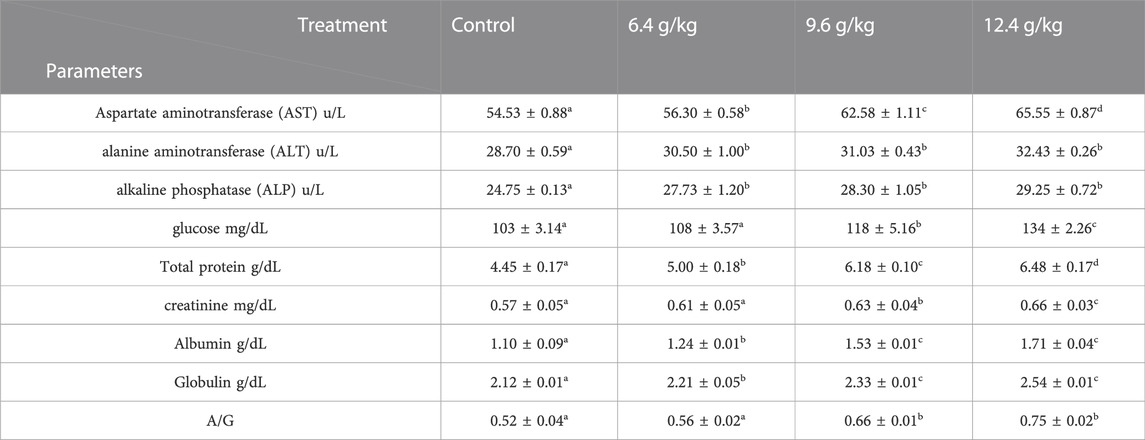
TABLE 3. Effects of black sand on the serum profile of Oreochromis niloticus after 15 days of exposure.
3.4 Measurement of antioxidants biomarkers
The activities of CAT, SOD, TPX, TAC, OSI, and MDA showed a significant increase (p < 0.05) after 15 days of exposure to 6.4, 9.6, and12.4 g black sand/Kg diet compared to the control group (Table 4).
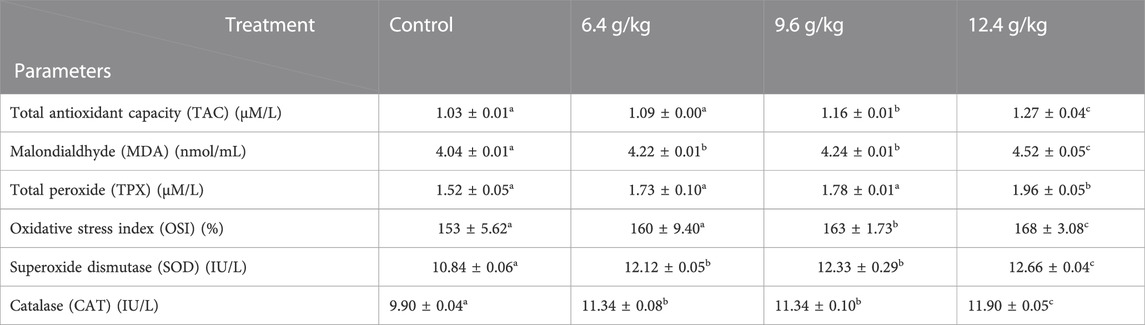
TABLE 4. Effects of black sand on the antioxidant’s parameters of Oreochromis niloticus after 15 days of exposure.
3.5 Erythron profile
Cell alteration and nuclear abnormalities of RBCs in Nile Tilapia after exposure to the black sand (6.4 g/kg, 9.6 g/kg, and 12.4 g/kg) was associated with a significant increase (p < 0.05) in the percentage of RBCs-alterations compared with the control group in a concentration (Figure 5).
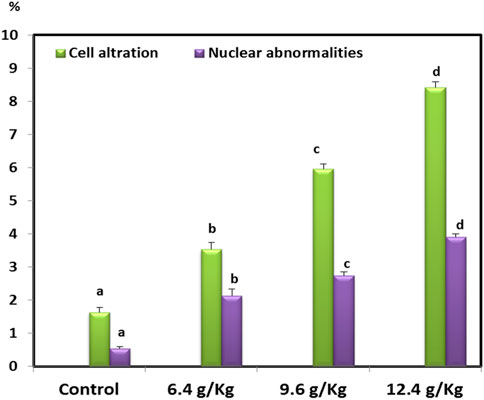
FIGURE 5. RBCs alteration and nuclear abnormalities of Oreochromis niloticus after 15 days of exposure to black sand.
The blood smears of the normal fish showed normal erythrocytes with a centrally located nucleus (Figure 6A). The blood smears of the fish exposed to black sand (6.4 g/kg, 9.6 g/kg and 12.4 g/kg) displayed poikilocytosis of the erythrocytes (Figures 6B–D) in the form of spinocyte, crenated cell, acanthocyte, sickle cell, tear-drop cell, notched nuclei, schistocyte, eccentric nucleus, and microcyte.
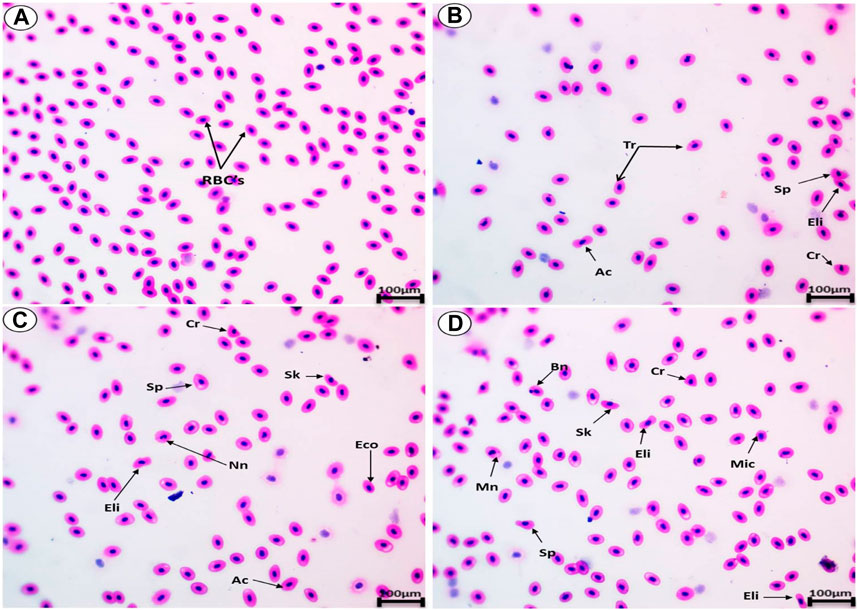
FIGURE 6. Blood smears from Oreochromis niloticus showing (A) the normal erythrocytes and the deformed erythrocytes after exposure to 6.4 g/kg (B), 9.6 g/kg (C), and 12.4 g/kg (D). Tr, tear-drop cell; Sp, spinocyte; Cr, crenated cell; Ac, acanthocyte; Eco, eccentric nucleus; Mn, micronucleus; e; Nn, Notched nucli; Mic, microcyte; Sh, schistocytic; and Sk, Sickle cell (H & E stain, scale bar: 100 µm).
3.6 Histological examination
3.6.1 The liver tissues
Hepatocytes with central nuclei are regularly arranged in wedge shapes in the liver of control Oreochromis niloticus (Figure 7B). Fish exposed to 6.4 g/kg, 9.2 g/kg, and 12.4 g/kg of black sand for 15 days showed damage in the liver architecture, the hepatocytes lost their normal shape, and ruptures occurred in the central vein and dilation. Accordingly, sinusoidal lumen collapsed. Also, infiltration of the inflammatory cells and increased numbers of Küpffer cells were observed. Vacuolation, hemorrhage, pyknotic nuclei, apoptotic cells, and fatty degeneration were observed. In addition to the previous changes, exposure to black sand led to many impacts on the liver including ruptured cell membranes in some areas and an increase in lipid droplets. Increased numbers of blood sinusoids and areas of hepatic necrosis started to appear in some regions (Figures 7B–D). The liver damage was dose-concentration dependent one.
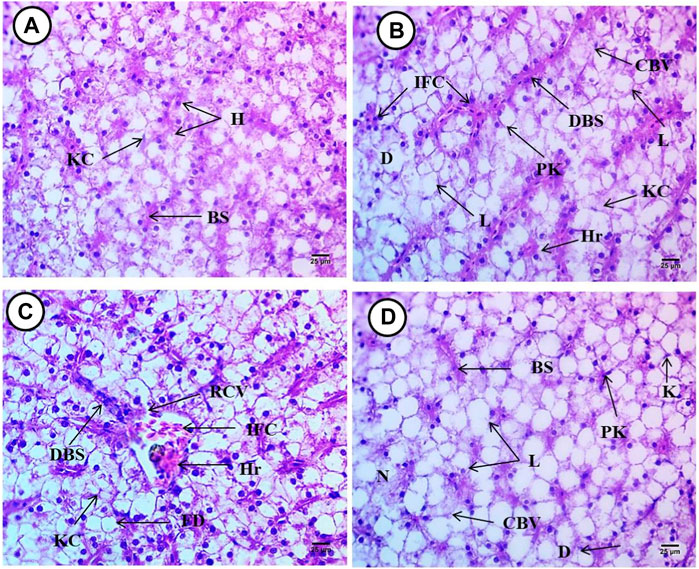
FIGURE 7. Liver from Oreochromis niloticus showing, (A) control fish, (B) fish exposed to 6.4 g/kg, (C) fish exposed to 9.2 g/kg, and (D) fish exposed to 12.4 g/kg of black sand. Blood sinusoids (Bs), hepatocytes (H), Küpffer cell (KC), infiltrations of inflammatory cell (IFC), pyknotic (PK), apoptotic (AP) nuclei, dilation of blood sinusoids (DBS), cytoplasmic vacuolation (CPV), hemorrhage (Hr), degeneration (D), rupture in the central vein (RCV), dilation in the blood sinusoid (DBS). Fatty degeneration (FD), and cytoplasmic vacuolation (CPV). (H&E X 400).
3.6.2 The intestine tissues
The intestine of Oreochromis niloticus in the control group had a normal morphology of layers; the mucosal had normal villi and goblet cells nuclei; the sub-mucosa constituting the lamina propria was composed of loose connective tissue with numerous collagen fibers; and muscular tissue consisted of longitudinal muscular layers and serosa (Figure 8A).
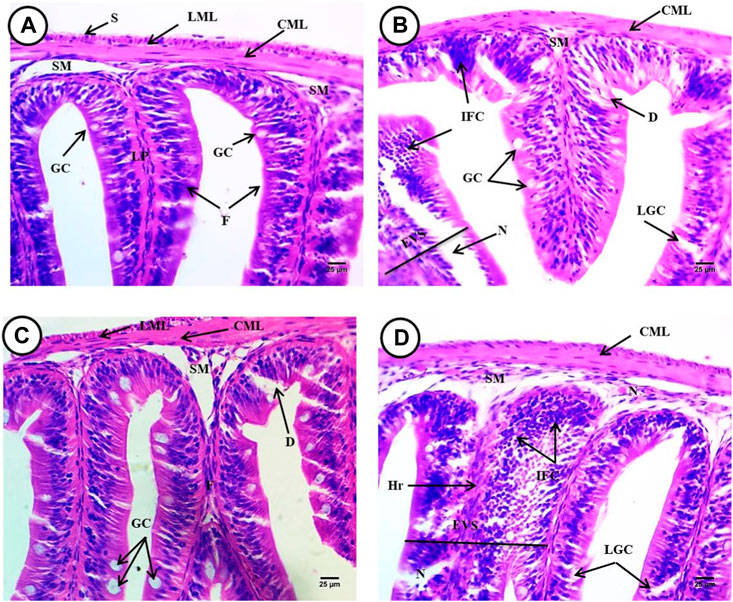
FIGURE 8. Intestine from Oreochromis niloticus showing, (A) control fish, (B) fish exposed to 6.4 g/kg, (C) fish exposed to 9.2 g/kg, and (D) fish exposed to 12.4 g/kg of black sand. Mucosal layer consists of mucosal folds (F), the lamina propria (LP), sub-mucosa (SM), circular (CML), longitudinal muscular layers (LML), serosa (S), and goblet cells (GC) degeneration (D) an increase in the number and size of goblet cells (LGC), infiltrations of inflammatory cells (IFC), expansion at villi structure (EVS) and necrosis (N), expansion at villi structure (EVS), hemorrhage (Hr). (H&E X 400).
However, photomicrographs of the Oreochromis niloticus intestine exposed to (6.4 g/kg, 9.2 g/kg and 12.4 g/kg) black sand had degeneration of the basement membrane, the serosa, and longitudinal muscular layers. Also, there was an increase in the number and size of goblet cells, infiltration of inflammatory cells, expansion of the villi structure, hemorrhage and necrosis that appeared in many regions and fusions in the mucosal folds, and degeneration of the columnar cells (Figures 8B–D).
3.6.3 The brain tissues
The normal structure of the brain tissues were considered as stratum and periventricular in the inner region, the second layer stratum album central, the third layer stratum griseum center tissues, fourth layer stratum fibro-sum et grisium superficiale, the fifth layer stratum optimum, and the last outer layer Stratum consists of many layers internal one (1) contains acidophilic neuropile with large region of spongiosis with blood capillaries and monnuclear inflammatroy cells nuclei, then another unstained region (2), and external layer (3) consists of connective tissues , epithelial cells and blood capillarie (Figure 9A, B).
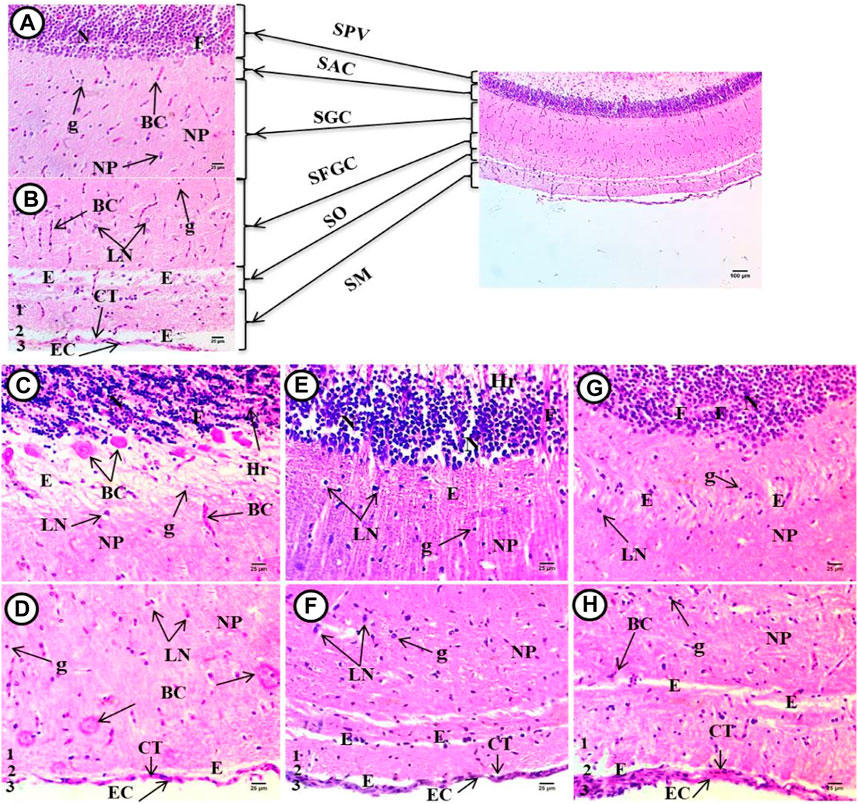
FIGURE 9. Optic tectum from Oreochromis niloticus showing, (A) control fish, (B) fish exposed to 6.4 g/kg, (C) fish exposed to 9.2 g/kg, and (D) fish exposed to 12.4 g/kg of black sand. Stratum periventricular (SPV), stratum album centrale (SAC), stratum griseum centrale (SGC), stratum fibrosum et grisium superficiale (SFGS), stratum opticum (SO), stratum marginale (SM), dense populated neurons (N), dema (E), contains fibers (F) separation of their apical regions, hemorrhage (Hr) with congested blood capillaries (BC), highly necrosed (NP), space edema/spongosis (E), small blood capillaries (BC), many mononuclear nuclei glial cells (G), and few large neurons with vesicular nuclei (LN), homogenous acidophilic neuropile (NP) with tiny unstained space edema (E), degenerated connective tissues (CT), and degenerated outer epithelial cell (EC). (H&E X 400).
Figure 9C-H showed the brain tissue in the treated groups, where densely populated differentiated neurons had vesicular stained nuclei, separated by space edema in the periventricular region, and fibers separating their apical regions, which contained degenerated cells, hemorrhage, and congested blood capillaries. The second layer was highly necrosed and contained unstained space edema/spongiosis, large deeply stained cells, and small deeply stained nuclei and small blood capillaries. The third layer consisted of unstained space surrounded by acidophilic neuropiles with blood capillaries and a large number of mononucleoid nuclei, glia nuclei, and large neurons with vesicular nuclei. The fourth layer contained heterogenous acidophilic neuropile with many tiny irregular unstained space edema/spongiosis and small blood capillaries. At the interface between the fourth and fifth strata, the fifth layer contained meshwork acidophilic neuropiles containing degenerated neurons and small blood vessels. The inner part of the last layer contained homogenous acidophilic neuropile with tiny unstained space edema surrounded blood capillaries. This was separated by small areas of unstained neuropile. The outer layer had degenerated connective tissues and edema and degenerated outer squamous cells (epithelial cell) were noticed.
4 Discussion
Although black sand on beaches has high levels of natural radioactivity, mainly due to its content of thorium and uranium radio nuclides (Ibrahim et al., 2009), to our knowledge little is known about their effects on the aquatic ecosystems. It has been reported that internal exposure of animals to black by either inhalation or ingestion could warrant a risk assessment (Ibrahim et al., 2009).
The spectrometric measurements were done on the black sand samples. Mineralogical analysis has shown that almost the same minerals occur in black sand samples with varying percentages. The mineral components include SiO2, Fe3O4, TiO2, Al2O3, CaO, MgO, SO3, Na2O, Cr2O3, K2O, and P2O5. These results are consistent with previous studies that analyzed the mineral components of BS (Hammond, 1985; Dabbour, 1995)
X-ray spectrum of the black sand shows a high peak of O, indicating Fe may be present in the form of iron oxides, e.g., magnetite (Fe3O4) and hematite (Fe2O3). There are large quantities of C-bearing particles with a nm size in the sand, which gives it its black color. The black sand sample had the highest content of SiO2 (51.3 wt%), Fe3O4 (34.9 wt%), TiO2 (7.01 wt%), Al2O3 (3.01 wt%), and CaO (3.01 wt%). Combustion-generated particles can be traced using carbon, which is predominantly produced through combustion. Of the heavy metals, only Ti was present in levels approaching 7.01 wt%. Al2O3 and CaO were present at (3.01 wt%) and (3.01 wt%). Soot particles derived from fossil fuel combustion are evident in black sand due to the presence of S peaks, that are low intensity but discernible, as well as C and O peaks. In other studies of ambient particles using electron microscopy, similar results were observed (Chen et al., 2004; 2005).
To monitor and evaluate the health status of fish under the influence of various factors, hematological parameters must be measured (Thummabanca et al., 2016). Blood allows the body to mobilize defenses as soon as an injury or disease occurs (Adewumi et al., 2018). Research in the field and in the laboratory has clearly demonstrated that aquatic contaminants alter hematological parameters in animals involved in the study, including fish (Adewumi et al., 2018; Amaeze et al., 2020; Muthukumaravel et al., 2021a; Muthukumaravel et al., 2021b; Morshedi et al., 2022; Naguib et al., 2022). In the current study, a reduction in RBCs, hematocrit, hemoglobin, and thrombocytes may be caused by a disturbed hematopoiesis process as black sand concentrations rise. A mechanism causing erythrocyte alteration is denaturation of globin due to sulfhydryl oxidation (Harvey, 1997; Harvey et al., 2003; Kaneko et al., 2008). On the other hand, a drop in haemoglobin levels could result in different tissues not getting enough oxygen, which would slow down their metabolism and result in less energy production (Atamanalp and Yanik, 2003).
The decrease in RBC, Hb, Ht, and WBC with an increase in MCV and percent of lymphocytes observed in our results were consistent with the results of previous studies on Nile Tilapia exposed to MPs (Hamed et al., 2019a) and the common carp exposed to PE-MPs (Hamed et al., 2022b). Similar results were also seen in fish exposed to phosalone (Ali and RANI, 2009), as well as copper nanoparticles (Soliman et al., 2021), UVA in catfish (Osman et al., 2019), hydroxychloroquine in Catfish (Sayed et al., 2021), and 4-NP in catfish (Mekkawy et al., 2011). The exposure of Nile Tilapia fish to black sand resulted in anemia, which may attributed to RBC’s lysis or hematopoietic tissue damage (Arabi et al., 2016). The reduction in WBC’s may result from bioaccumulation of the pollutants in the tissues, which inhibited the ability of the organism to produce more new WBCs because of immunodepression. MCHC, RBC, and Hb values were decreased, with increases in MCV and MCH in our study (Bhattacharjee et al., 1993).
When monitoring and evaluating fish health impacts caused by environmental pollutants, biochemical parameters play a crucial role (Sayed and Soliman, 2018; Osman et al., 2019). Since AST and ALT are found in the cytoplasm of hepatocytes, they respond strongly to structural damage in the liver, resulting in their release in large amounts (Shah et al., 2002). Chronic liver diseases and excessive steroids following intoxication are the most common causes of elevated ALP in plasma (Okechukwu and Auta, 2007). Blood serum levels of ALT and AST are indicative of organ dysfunction, since they are distributed in tissues and cells of organs (Gabriel and George, 2005). A well-known biomarker of liver disease, obstructive jaundice, and intrahepatic cholestasis is alkaline phosphatase (ALP) (Matic et al., 2013). Contaminants present in water could cause fish to develop biliary blockages, bile duct dilatation, or hyperplasia of the bile ducts (Teh et al., 1997). In response to black sand exposure, increased cholesterol may be associated with defense mechanisms against apoptosis.
Most of the measured biochemical parameters (Table 3) were significantly increased after exposure to black sand compared to the control. The increase in CK, LDH, ALP, ALT, AST, TP, glucose, albumin, and globulin observed in our study were similarly reported by (Hamed et al., 2020) with the Nile tilapia exposed to MPs. Some parameters with hydroxychloroquine in Catfish (Sayed and Hamed, 2017; Sayed and Soliman, 2018; Sayed et al., 2021) with African catfish exposed to 4-NP, and common carp (Cyprinus carpio) exposed to PE-MPs (Hamed et al., 2022b) were also similar. Banaee et al. (2016) state that the higher levels of the enzymes (CK, AST, ALT, LDH, and ALP) are an indicator for cell membrane damage. Additionally, ALT, AST, and ALP activity were increased by paraquat and plastic particles (Oliveira et al., 2013). MPs and/or pyrene and nickel exposure also altered the biochemistry of the common goby (dos Santos Norberto, 2014). Among the most important functions of proteins is to maintain homeostasis and prevent fluid leaks from the body (Espinosa et al., 2017).
The observed increase in antioxidant enzyme activity of SOD, CAT, TPX, MDA, TAC, and OSI is in agreement with the results of other studies (Pedrajas et al., 1995; Paris-Palacios et al., 2000; Harma et al., 2005; Wang et al., 2009; Hamed et al., 2020; Iheanacho and Odo, 2020; Hamed et al., 2022b). After exposure to black sand, activities of these enzymes may increase as a result of increased production of H2O2 and oxidative stress mitigation (Gül et al., 2004; Iheanacho and Odo, 2020; Mohamed et al., 2022).
Poikilocytosis was caused by black sand on RBCs morphology in the current study. Micronucleated erythrocytes and nuclear lesions may be caused by the deleterious effects of black sand related to chromosomal fragmentation. Consistent with the present results, the data obtained by (Labieniec et al., 2007) reveal breaks in the nuclear DNA of the fresh-water mussel Unio tumidus exposed to gallic. In addition, studies show that hydrolyzable tannins and their derivatives can damage DNA (Khan et al., 2000). When pyrogallol and other toxicants cause increased oxidative stress, they cause a variety of cellular injuries, including necrosis and apoptosis (Xia et al., 2006), which can negatively impact lipids, proteins, nucleic acids, and carbohydrates (Cho et al., 2013) (Park, 2016).
The percentages of erythrocytes’ morphological alterations and nuclear abnormalities of BS-exposed fish were significantly increased compared to the control. In addition to membrane fluidity, the reduction in ATP levels and inhibition of the enzymes-membrane bound can all influence RBC poikilocytosis (Singh et al., 2009). Our results were similarly reported with different types of fish in the presence of heavy metals, MPs, nanoparticles, and herbicides (Ayllon and Garcia-Vazquez, 2000; Ergene et al., 2007; Sayed et al., 2018; Hamed et al., 2019b; Sayed et al., 2021; Soliman et al., 2021; Hamed et al., 2022b). Eukaryotic erythrocyte abnormalities are a powerful tool for studying genotoxic and cytotoxic damage in eukaryotes, since they are closely related to DNA damage and are simple, reliable, and sensitive (al-Sabti and Metcalfe, 1995; Mekkawy et al., 2011; Sayed and Hamed, 2017; Sayed et al., 2018).
During our study, Tilapia were exposed to BS continuously for 2 weeks, causing alterations in the liver, intestines, and brain. In exposed fish, hepatocytes were deformed and necrotic, many pyknotic nuclei were detected, blood sinusoids were dilated and congested, the central veins were hemorrhaging, the liver cells were shrunken and fatty, and the polysaccharide content was severely depleted. Similar alterations were reported in fish exposed to lead acetate (Mustafa et al., 2017), PE-MPs (Hamed et al., 2022b), 4-NP (Sayed and Soliman, 2018), MPs and chemical contaminants (Rainieri et al., 2018), and other types of toxins (Figueiredo-Fernandes et al., 2007; Espinosa et al., 2019b; Stalin et al., 2019; da Costa Araújo et al., 2020a; da Costa Araújo et al., 2020b). The morphological changes and vacuolation are associated with metabolic damage and hepatic lesions resulting from the excretion of toxic agents and degenerative processes caused by contaminated water (Valente et al., 1999; Nkwuda et al., 2020).
A number of histopathological alterations were observed in the intestine in the present study, similar to the histopathological changes observed in previous studies on fish exposed to MPs, gibberellic acid, hydroxychloroquine, copper sulfate and copper oxide nanoparticles, UVA, and other pollutants (Jabeen et al., 2018; Lei et al., 2018; Espinosa et al., 2019a; Qiao et al., 2019; Song et al., 2019; Zhang et al., 2019; Iheanacho and Odo, 2020). Moreover, when stressed, numerous leukocytes infiltrate the body (Ped`a et al., 2016). Moreover, (Karami et al., 2016), which suggests that such damage may have been caused by sharp black sand particles. In addition, epithelial breakdown could lead to the entry of cytotoxic substances from the gut lumen into the bloodstream, possibly altering organ functions (Mobin et al., 2000).
Deformations of the brain were evident, such as edema/spongiosis, hemorrhage, enlargement of the SPV, and hydrops development. Similar alterations were observed in common carp exposed to different sizes of plastics (Hamed et al., 2022a), Channa punctatus exposed to the pesticide chlorpyrifos (Devi and Mishra, 2013), Gambusia affinis exposed to lead chloride (Alkshab and Taha, 2021), carp exposed to quinalphos (Chamarthi et al., 2014), carp exposed to organophosphate insecticide (Lakshmaiah, 2017), Catla catla exposed to heavy metals (Bose et al., 2015), and Carassius gibelio exposed to toxic cyanobacteria (Panagiotis et al., 2014).
In conclusion, the black sand isolated from the Red sea beach influenced Oreochromis niloticus’s hematology, biochemistry, and antioxidant parameters. Poikilocytosis and RBC nuclear abnormalities were also associated with exposure to black sand. The resulting erosion of rocks and rocks’ access to water forces us to consider the seriousness of climatic change on the aquatic ecosystem.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The animal study was approved by the ethics committee of the Faculty of Science, Assuit University, Assuit, Egypt. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ES: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. AHS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Karim, A.-A. M., and Barakat, M. G. (2017). Separation, upgrading, and mineralogy of placer magnetite in the black sands, northern coast of Egypt. Arabian J. Geosciences 10, 298–317. doi:10.1007/s12517-017-3075-0
Adewumi, B., Ogunwole, G. A., Akingunsola, E., Falope, O. C., and Eniade, A. (2018). Effects of sub-lethal toxicity of chlorpyrifos and DDforce pesticides on haematological parameters of Clarias gariepinus. Int. J. Environ. Res. Publ. Health 5, 62–71. doi:10.15739/irjpeh.18.010
Aebi, H. (1984). “[13] catalase in vitro,” in Methods in enzymology (Amsterdam, Netherlands: Elsevier), 121–126.
Ali, H., and Rani, V. (2009). Effect of phosalone on haematological indices in the tilapia, Oreochromis mossambicus. Turkish J. Veterinary & Animal Sci. 33, 407–411. doi:10.3906/vet-0804-43
Alkshab, A. A., and Taha, A. M. (2021). “Histological effect of lead chloride on the brain of Gambusia affinis,” in Journal of Physics: Conference Series, Coimbatore, India, 25-26 March 2021 (IOP Publishing).012024
Al-Sabti, K., and Metcalfe, C. D. (1995). Fish micronuclei for assessing genotoxicity in water. Mutat. Res. 343, 121–135. doi:10.1016/0165-1218(95)90078-0
Amaeze, N. H., Komolafe, B. O., Salako, A. F., Akagha, K. K., Briggs, T. D., Olatinwo, O. O., et al. (2020). Comparative assessment of the acute toxicity, haematological and genotoxic effects of ten commonly used pesticides on the African Catfish, Clarias gariepinus Burchell 1822. Heliyon 6, e04768. doi:10.1016/j.heliyon.2020.e04768
Arabi, H., Gholipour Kanani, H., Shahsavani, D., and Harsij, M. (2016). Improving effect of Spirulina platensis on hematological parameters in Cyprinus carpio exposed to sublethal doses of cyanide. Comp. Clin. Pathol. 25, 335–342. doi:10.1007/s00580-015-2187-8
Atamanalp, M., and Yanik, T. (2003). Alterations in hematological parameters of rainbow trout (Oncorhynchus mykiss) exposed to mancozeb. Turkish J. Veterinary & Animal Sci. 27, 1213–1217.
Ayllon, F., and Garcia-Vazquez, E. (2000). Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna: an assessment of the fish micronucleus test. Mutat. Research/Genetic Toxicol. Environ. Mutagen. 467, 177–186. doi:10.1016/s1383-5718(00)00033-4
Aziz, A., Sief, R., Ghieth, B., and Kaiser, M. (2020). Black sand deposits; their spatial distribution and hazards along the northern coast of Sinai Peninsula, Egypt. J. Appl. Geophys. 183, 104219. doi:10.1016/j.jappgeo.2020.104219
Bain, B. J., Bates, I., and Laffan, M. A. (2016). Dacie and lewis practical haematology E-book: expert consult: online and print. Amsterdam, Netherlands: Elsevier Health Sciences.
Banaee, M., Nemadoost Haghi, B., Tahery, S., Shahafve, S., and Vaziriyan, M. (2016). Effects of sub-lethal toxicity of paraquat on blood biochemical parameters of common carp, Cyprinus carpio (Linnaeus, 1758). Iran. J. Toxicol. 10, 1–5. doi:10.29252/arakmu.10.6.1
Bhattacharjee, N., Mukherjee, K., Chakravarti, S., Mukherjee, M., De, P., Sengupta, M., et al. (1993). Dengue haemorrhagic fever (DHF) outbreak in Calcutta--1990. J. Commun. Dis. 25, 10–14.
Blaxhall, P., and Daisley, K. (1973). Routine haematological methods for use with fish blood. J. Fish Biol. 5, 771–781. doi:10.1111/j.1095-8649.1973.tb04510.x
Bose, M. J., Ilavazhahan, M., Tamilselvi, R., and Viswanathan, M. (2015). Effect of heavy metals on the histopathology of gills and brain of fresh water fish Catla catla. Biomed. Pharmacol. J. 6, 99–105. doi:10.13005/bpj/390
Chen, Y., Shah, N., Huggins, F. E., and Huffman, G. P. (2004). Investigation of the microcharacteristics of PM2. 5 in residual oil fly ash by analytical transmission electron microscopy. Environ. Sci. Technol. 38, 6553–6560. doi:10.1021/es049872h
Chen, Y., Shah, N., Huggins, F. E., and Huffman, G. P. (2005). Transmission electron microscopy investigation of ultrafine coal fly ash particles. Environ. Sci. Technol. 39, 1144–1151. doi:10.1021/es049871p
Dabbour, G. A. (1995). Estimation of the economic minerals reserves in Rosetta beach sands. Egypt. Mineral. 7, 153–166.
da Costa Araújo, A. P., de Andrade Vieira, J. E., and Malafaia, G. (2020a). Toxicity and trophic transfer of polyethylene microplastics from Poecilia reticulata to Danio rerio. Sci. Total Environ. 742, 140217. doi:10.1016/j.scitotenv.2020.140217
da Costa Araújo, A. P., de Melo, N. F. S., de Oliveira Junior, A. G., Rodrigues, F. P., Fernandes, T., de Andrade Vieira, J. E., et al. (2020b). How much are microplastics harmful to the health of amphibians? A study with pristine polyethylene microplastics and Physalaemus cuvieri. J. Hazard. Mater. 382, 121066. doi:10.1016/j.jhazmat.2019.121066
Devi, Y., and Mishra, A. (2013). Histopathological alterations in gill and liver anotomy of fresh water, air breathing fish Channa punctatus after Pesticide Hilban® (Chlorpyrifos) Treatment. Adv. Bioresearch 4.
Dos Santos Norberto, R. S. (2014). Toxic effects of nickel alone and in combination with microplastics on early juveniles of the common goby (Pomatoschistus microps).
Ergene, S., Çavaş, T., Celik, A., Köleli, N., Kaya, F., and Karahan, A. (2007). Monitoring of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): genotoxic damage in relation to water pollution. Ecotoxicology 16, 385–391. doi:10.1007/s10646-007-0142-4
Espinosa, C., Cuesta, A., and Esteban, M. Á. (2017). Effects of dietary polyvinylchloride microparticles on general health, immune status and expression of several genes related to stress in gilthead seabream (Sparus aurata L). Fish shellfish Immunol. 68, 251–259. doi:10.1016/j.fsi.2017.07.006
Espinosa, C., Esteban, M., and Cuesta, A. (2019a). Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 95, 574–583. doi:10.1016/j.fsi.2019.10.072
Espinosa, C., Esteban, M. Á., and Cuesta, A. (2019b). Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L). Fish shellfish Immunol. 95, 574–583. doi:10.1016/j.fsi.2019.10.072
Feldman, A. T., and Wolfe, D. (2014). Tissue processing and hematoxylin and eosin staining. Histopathol. Methods Protoc. 1180, 31–43. doi:10.1007/978-1-4939-1050-2_3
Figueiredo-Fernandes, A. M., Fontaínhas-Fernandes, A. A., Monteiro, R. A., Reis-Henriques, M. A., and Rocha, E. (2007). Spatial relationships of the intrahepatic vascular–biliary tracts and associated pancreatic acini of Nile tilapia, Oreochromis niloticus (Teleostei, Cichlidae): a serial section study by light microscopy. Ann. Anatomy-Anatomischer Anzeiger 189, 17–30. doi:10.1016/j.aanat.2006.06.009
Gabriel, U., and George, A. (2005). Plasma enzymes in Clarias gariepinus exposed to chronic levels of round up (glyphosate). Environ. Ecol. 23, 271–276. doi:10.15192/PSCP.ASR.2017.19.3.9598
Gül, Ş., Belge-Kurutaş, E., Yıldız, E., Şahan, A., and Doran, F. (2004). Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in Seyhan Dam Lake, Turkey. Environ. Int. 30, 605–609. doi:10.1016/s0160-4120(03)00059-x
Hamdan, M. A., and Hassan, F. A. (2020). Quaternary of Egypt. The geology of Egypt. Cham: Springer, 445–493.
Hamed, M., Martyniuk, C. J., Naguib, M., Lee, J. S., and Sayed, A. E. H. (2022a). Neurotoxic effects of different sizes of plastics (nano, micro, and macro) on juvenile common carp (Cyprinus carpio). Front. Mol. Neurosci. 15, 1028364. doi:10.3389/fnmol.2022.1028364
Hamed, M., Monteiro, C. E., and Sayed, A.E.-D. H. (2022b). Investigation of the impact caused by different sizes of polyethylene plastics (nano, micro, and macro) in common carp juveniles, Cyprinus carpio L., using multi-biomarkers. Sci. Total Environ. 803, 149921. doi:10.1016/j.scitotenv.2021.149921
Hamed, M., Soliman, H. A., Osman, A. G., and Sayed, A.E.-D. H. (2019a). Assessment the effect of exposure to microplastics in Nile Tilapia (Oreochromis niloticus) early juvenile: I. blood biomarkers. Chemosphere 228, 345–350. doi:10.1016/j.chemosphere.2019.04.153
Hamed, M., Soliman, H. A., Osman, A. G., and Sayed, A.E.-D. H. (2020). Antioxidants and molecular damage in Nile Tilapia (Oreochromis niloticus) after exposure to microplastics. Environ. Sci. Pollut. Res. 27, 14581–14588. doi:10.1007/s11356-020-07898-y
Hamed, M., Soliman, H. A. M., and Sayed, A.E.-D. H. (2019b). Ameliorative effect of Spirulina platensis against lead nitrate-induced cytotoxicity and genotoxicity in catfish Clarias gariepinus. Environ. Sci. Pollut. Res. Int. 26, 20610–20618. doi:10.1007/s11356-019-05319-3
Hammond, N. S. (1985). Contribution to the evaluation problems of Egyptian beach economic minerals. Ann. Geol. Sur. Egypt 15, 45–59.
Harma, M., Harma, M., and Erel, O. (2005). Measurement of the total antioxidant response in preeclampsia with a novel automated method. Eur. J. Obstetrics Gynecol. Reproductive Biol. 118, 47–51. doi:10.1016/j.ejogrb.2004.04.012
Harvey, J. W. (1997). “The erythrocyte: physiology, metabolism, and biochemical disorders,” in Clinical biochemistry of domestic animals (Amsterdam, Netherlands: Elsevier), 157–203.
Harvey, J. W., Stockham, S. L., Scott, M. A., Johnson, P. J., Donald, J. J., and Chandler, C. J. (2003). Methemoglobinemia and eccentrocytosis in equine erythrocyte flavin adenine dinucleotide deficiency. Vet. Pathol. 40, 632–642. doi:10.1354/vp.40-6-632
Ibrahim, T., Dabour, G., Ren, M., El-Qady, G., Goodell, P., Gaafar, I., et al. (2018). “Potential heavy mineral-enriched black sand deposits south ras banas Red Sea coast (Egypt),” in Conference of the arabian journal of geosciences (Cham: Springer), 129–132.
Ibrahim, T. M., Ali, K., and Gaafar, I. (2009). Occurrence of black sand deposits on the Red Sea coastal plain of wadi diit, south eastern desert, Egypt: a preliminary study. Egypt 17, 107–116.
Iheanacho, S. C., and Odo, G. E. (2020). Neurotoxicity, oxidative stress biomarkers and haematological responses in African catfish (Clarias gariepinus) exposed to polyvinyl chloride microparticles. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 232, 108741. doi:10.1016/j.cbpc.2020.108741
Jabeen, K., Li, B., Chen, Q., Su, L., Wu, C., Hollert, H., et al. (2018). Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 213, 323–332. doi:10.1016/j.chemosphere.2018.09.031
Kaneko, J. J., Harvey, J. W., and Bruss, M. L. (2008). Clinical biochemistry of domestic animals. 6. San Diego: Academic Press.
Karami, A., Romano, N., Galloway, T., and Hamzah, H. (2016). Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res. 151, 58–70. doi:10.1016/j.envres.2016.07.024
Khan, N. S., Ahmad, A., and Hadi, S. M. (2000). Anti-oxidant, pro-oxidant properties of tannic acid and its binding to DNA. Chem. Biol. Interact. 125, 177–189. doi:10.1016/s0009-2797(00)00143-5
Koracevic, D., Koracevic, G., Djordjevic, V., Andrejevic, S., and Cosic, V. (2001). Method for the measurement of antioxidant activity in human fluids. J. Clin. pathology 54, 356–361. doi:10.1136/jcp.54.5.356
Labieniec, M., Biernat, M., and Gabryelak, T. (2007). Response of digestive gland cells of freshwater mussel Unio tumidus to phenolic compound exposure in vivo. Cell Biol. Int. 31, 683–690. doi:10.1016/j.cellbi.2006.12.005
Lakshmaiah, G. (2017). Brain histopathology of the fish Cyprinus carpio exposed to lethal concentrations of an organophosphate insecticide phorate. Int. J. Adv. Res. Dev. 2, 668–667.
Lei, L., Wu, S., Lu, S., Liu, M., Song, Y., Fu, Z., et al. (2018). Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 620, 1–8. doi:10.1016/j.scitotenv.2017.11.103
Matic, S., Stanic, S., Bogojevic, D., Vidakovic, M., Grdovic, N., Dinic, S., et al. (2013). Methanol extract from the stem of Cotinus coggygria Scop., and its major bioactive phytochemical constituent myricetin modulate pyrogallol-induced DNA damage and liver injury. Mutat. Res. 755, 81–89. doi:10.1016/j.mrgentox.2013.03.011
Mekkawy, I. A., Mahmoud, U. M., and Sayed, A.E.-D. H. (2011). Effects of 4-nonylphenol on blood cells of the African catfish Clarias gariepinus (Burchell, 1822). Tissue Cell 43, 223–229. doi:10.1016/j.tice.2011.03.006
Mobin, S., Kanai, K., and Yoshikoshi, K. (2000). Histopathological alterations in the digestive system of larval and juvenile Japanese flounder Paralichthys olivaceus reared on four feeding levels. J. Aquatic Animal Health 12, 196–208. doi:10.1577/1548-8667(2000)012<0196:haitds>2.0.co;2
Mohamed, I. A., Hamed, M., Abdel-Tawab, H. S., Mansour, S., Soliman, H. A. M., Lee, J.-S., et al. (2022). Multi-biomarkers approach to assess the toxicity of novel insecticide (Voliam flexi®) on Clarias gariepinus: from behavior to immunotoxicity. Fish Shellfish Immunol. 125, 54–64. doi:10.1016/j.fsi.2022.04.051
Morshedi, V., Pradhoshini, K. P., Tangestani, N., Ghasemi, A., Sotoudeh, E., Gamoori, R., et al. (2022). Effects of rearing tank colour on growth indices, blood chemistry, digestive enzymes, expression of stress and growth-related genes of Asian sea bass juvenile (Lates calcarifer). Agricutural Res. 53, 3780–3787. doi:10.1111/are.15884
Mustafa, S., Al-Faragi, J., Salman, N., and Al-Rudainy, A. (2017). Histopathological alterations in gills, liver and kidney of common carp, Cyprinus carpio exposed to lead Acetate. Adv. Anim. Vet. Sci. 5, 371–376. doi:10.17582/journal.aavs/2017/5.9.371.376
Muthukumaravel, K., Pradhoshini, K. P., Vasanthi, N., Kanagavalli, V., Ahadu Shareef, M., Musthafa, M. S., et al. (2021a). Sublethal effects of arsenic on oxygen consumption, hematological and gill histopathological indices in chanos chanos. Int. J. Environ. Res. public health 18 (24), 12967. doi:10.3390/ijerph182412967
Muthukumaravel, K., Vasanthi, N., Stalin, A., Alam, L., Santhanabharathi, B., and Musthafa, M. S. (2021b). Sublethal effects of phenol on histology of selected organs of freshwater fish Mystus vittatus. Environ. Sci. Pollut. Res. 28, 13752–13760. doi:10.1007/s11356-020-11434-3
Naguib, M., Mekkawy, I. A., Mahmoud, U. M., and Sayed, A.E.-D. H. (2022). Genotoxic evaluation of silver nanoparticles in catfish Clarias gariepinus erythrocytes; DNA strand breakage using comet assay. Sci. Afr. 16, e01260. doi:10.1016/j.sciaf.2022.e01260
Nishikimi, M., Rao, N. A., and Yagi, K. (1972). The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. biophysical Res. Commun. 46, 849–854. doi:10.1016/s0006-291x(72)80218-3
Nkwuda, P. J., Awoke, J. S., and Nwakpa, J. N. (2020). Histological changes in liver and kidney of Clarias gariepinus (burchell, 1822) juvenile exposed to sub-lethal doses of chloramphenicol (CAP). Aquac. Stud. 20, 19–28. doi:10.4194/2618-6381-v20_1_03
Okechukwu, E. O., and Auta, J. (2007). The effects of sub-lethal doses of lambda-cyhalothrin on some biochemical characteristics of the African catfish Clarias gariepinus. J. Biol. Sci. 7, 1473–1477. doi:10.3923/jbs.2007.1473.1477
Osman, A., Hamed, M., and Sayed, A. (2019). Protective role of Spirulina platensis against UVA-induced haemato-biochemical and cellular alterations in Clarias gariepinus. J. Photochem. Photobiol. B Biol. 191, 59–64. doi:10.1016/j.jphotobiol.2018.12.013
Panagiotis, B., Theodoti, P., Petridou, E., Konstantinos, K., and Ifigenia, K. (2014). Brain and liver histopathological examination of Carassius gibelio from a newly reconstructed lake with toxic cyanobacteria. Turkish J. Fish. Aquatic Sci. 14, 213–219. doi:10.4194/1303-2712-v14_1_23
Paris-Palacios, S., Biagianti-Risbourg, S., and Vernet, G. (2000). Biochemical and (ultra) structural hepatic perturbations of Brachydanio rerio (Teleostei, Cyprinidae) exposed to two sublethal concentrations of copper sulfate. Aquat. Toxicol. 50, 109–124. doi:10.1016/s0166-445x(99)00090-9
Park, W. H. (2016). Pyrogallol induces the death of human pulmonary fibroblast cells through ROS increase and GSH depletion. Int. J. Oncol. 49, 785–792. doi:10.3892/ijo.2016.3543
Pedrajas, J., Peinado, J., and Lopez-Barea, J. (1995). Oxidative stress in fish exposed to model xenobiotics. Oxidatively modified forms of Cu, Zn-superoxide dismutase as potential biomarkers. Chemico-Biological Interact. 98, 267–282. doi:10.1016/0009-2797(95)03651-2
Qiao, R., Sheng, C., Lu, Y., Zhang, Y., Ren, H., and Lemos, B. (2019). Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. total Environ. 662, 246–253. doi:10.1016/j.scitotenv.2019.01.245
Rainieri, S., Conlledo, N., Larsen, B. K., Granby, K., and Barranco, A. (2018). Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 162, 135–143. doi:10.1016/j.envres.2017.12.019
Sayed, A. E., and Hamed, H. S. (2017). Induction of apoptosis and DNA damage by 4-nonylphenol in African catfish (Clarias gariepinus) and the antioxidant role of Cydonia oblonga. Ecotoxicol. Environ. Saf. 139, 97–101. doi:10.1016/j.ecoenv.2017.01.024
Sayed, A.E.-D. H., Hamed, M., and Soliman, H. A. (2021). Spirulina platensis alleviated the hemotoxicity, oxidative damage and histopathological alterations of hydroxychloroquine in catfish (Clarias gariepinus). Front. Physiology 12, 683669. doi:10.3389/fphys.2021.683669
Sayed, A.E.-D. H., Kataoka, C., Oda, S., Kashiwada, S., and Mitani, H. (2018). Sensitivity of medaka (Oryzias latipes) to 4-nonylphenol subacute exposure; erythrocyte alterations and apoptosis. Environ. Toxicol. Pharmacol. 58, 98–104. doi:10.1016/j.etap.2017.12.023
Sayed, A.E.-D. H., and Soliman, H. A. (2018). Modulatory effects of green tea extract against the hepatotoxic effects of 4-nonylphenol in catfish (Clarias gariepinus). Ecotoxicol. Environ. Saf. 149, 159–165. doi:10.1016/j.ecoenv.2017.11.007
Shah, M., Patel, P., Phadke, M., Menon, S., Mary, F., and Sane, R. (2002). Evaluation ofthe effect of aqueous extract from powders of root, stem, leaves and whole plant of phyllanthus debilis against CCL4 induced rat liver dysfunction. Indian Drugs 39, 333–337.
Singh, R., Lemire, J., Mailloux, R. J., Chénier, D., Hamel, R., and Appanna, V. D. (2009). An ATP and oxalate generating variant tricarboxylic acid cycle counters aluminum toxicity in Pseudomonas fluorescens. PLoS one 4, e7344. doi:10.1371/journal.pone.0007344
Soliman, H. A., Hamed, M., and Sayed, A.E.-D. H. (2021). Investigating the effects of copper sulfate and copper oxide nanoparticles in Nile tilapia (Oreochromis niloticus) using multiple biomarkers: the prophylactic role of Spirulina. Environ. Sci. Pollut. Res. 28, 30046–30057. doi:10.1007/s11356-021-12859-0
Song, Y., Cao, C., Qiu, R., Hu, J., Liu, M., Lu, S., et al. (2019). Uptake and adverse effects of polyethylene terephthalate microplastics fibers on terrestrial snails (Achatina fulica) after soil exposure. Environ. Pollut. 250, 447–455. doi:10.1016/j.envpol.2019.04.066
Stalin, A., Suganthi, P., Mathivani, S., Paray, B. A., Al-Sadoon, M. K., Gokula, V., et al. (2019). Impact of chlorpyrifos on behavior and histopathological indices in different tissues of freshwater fish Channa punctatus (Bloch). Environ. Sci. Pollut. Res. Int. 26, 17623–17631. doi:10.1007/s11356-019-05165-3
Teh, S. J., Adams, S., and Hinton, D. E. (1997). Histopathologic biomarkers in feral freshwater fish populations exposed to different types of contaminant stress. Aquat. Toxicol. 37, 51–70. doi:10.1016/s0166-445x(96)00808-9
Valente, L., Rocha, E., Gomes, E., Silva, M., Oliveira, M., Monteiro, R., et al. (1999). Growth dynamics of white and red muscle fibres in fast-and slow-growing strains of rainbow trout. J. fish Biol. 55, 675–691. doi:10.1111/j.1095-8649.1999.tb00710.x
Wang, W.-N., Zhou, J., Wang, P., Tian, T.-T., Zheng, Y., Liu, Y., et al. (2009). Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp. Biochem. Physiology Part C Toxicol. Pharmacol. 150, 428–435. doi:10.1016/j.cbpc.2009.06.010
Zhang, S., Ding, J., Razanajatovo, R. M., Jiang, H., Zou, H., and Zhu, W. (2019). Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci. total Environ. 648, 1431–1439. doi:10.1016/j.scitotenv.2018.08.266
Keywords: black sand, SOD, poikilocytosis, histopathology, catalase, hematology
Citation: Saad E and Sayed AE-DH (2023) Effects of black sand on Oreochromis niloticus: insights into the biogeochemical impacts through an experimental study. Front. Earth Sci. 11:1289665. doi: 10.3389/feart.2023.1289665
Received: 06 September 2023; Accepted: 24 October 2023;
Published: 15 November 2023.
Edited by:
Ramanathan Alagappan, Jawaharlal Nehru University, IndiaReviewed by:
M. Saiyad Musthafa, The New College, IndiaMohamed Hamed, Al Azhar University, Egypt
Stanley Iheanacho, University of Kiel, Germany
Copyright © 2023 Saad and Sayed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alaa El-Din H. Sayed, alaasayed@aun.edu.eg; Eman Saad, eman.saad@aun.edu.eg
 Eman Saad
Eman Saad Alaa El-Din H. Sayed
Alaa El-Din H. Sayed