- 1State Laboratory of Continental Dynamics, Shaanxi Key Laboratory of Early Life and Environment and Xi’an Key Laboratory of Paleo-bioinformatics, Northwest University, Xi’an, China
- 2Museum für Naturkunde, Leibniz Institute for Research on Evolution and Biodiversity, Berlin, Germany
- 3Department of Earth Science, Freie Universität Berlin, Berlin, Germany
- 4School of Earth Sciences and Resources, China University of Geosciences (Beijing), Beijing, China
Parvibellus atavus gen. et sp. nov. from the Early Cambrian Chengjiang fauna of China is a small fossil having a distinct cephalic region bearing a pair of lateral projections and a circular, ventral mouth. The trunk bears eleven pairs of probably flap-like appendages and a short pair of terminal projections. This character combination is unique for the Chengjiang biota. A circular ventral mouth is seen in Radiodonta and in some of the gilled lobopodians which are thought to be among the radiodont’s closest relatives. P. atavus, gilled lobopodians, opabiniids, and radiodonts also share the putative character of flap-like appendages along the trunk. However, the new fossil differs from radiodonts and gilled lobopodians by the absence of enlarged and/or raptorial frontal appendages. It also differs from gilled lobopodians by lacking in ventral lobopod limbs and from radiodonts by lacking in stalked eyes. It provisionally resolves as a sister-group to a clade containing the gilled lobopodians, opabiniids, and radiodonts, and could potentially be part of an early radiation of the nektonic lower stem—Euarthropoda.
Introduction
Cambrian fossils offer unique insights into the early stages of animal evolution. Two groups of particular significance, both of which first appear in the Cambrian, are lobopodians and radiodonts. Lobopodians have been characterized as “worms with legs” and are widely presumed to include the ancestors of water bears (Tardigrada), velvet worms (Onychophora), and arthropods; see, e.g., Liu and Dunlop (2014), Smith and Ortega-Hernández (2014), Daley et al. (2018), Edgecombe (2020) and Aria (2022), and the references therein. A remarkable lineage belonging to the euarthropod stem-group is Radiodonta. As the name implies, these animals are typically characterized by a ring-like mouth, or an oral cone. This structure is not unique to radiodonts, and may even be absent in some taxa (Cong et al., 2016; Cong et al., 2018), but when present, the radiodont oral cone is characterized by a series of plates with individual elements of different sizes (Daley and Bergström 2012; Daley and Edgecombe, 2014; Liu et al., 2018; Moysiuk and Caron 2021). Radiodonts also have a pair of large and sometimes raptorial frontal appendages, and a series of flaps along the body which are presumed to have been used for swimming (Usami, 2006). Some radiodonts achieved body lengths of more than 2 m (Van Roy et al., 2015), although others were considerably smaller (Lerosey-Aubril and Pates 2018; Pates et al., 2020). Based on the armature of the frontal appendages, evolutionary trends from active predation to suspension feeding have been proposed (Vinther et al., 2014; Cong et al., 2016; Liu et al., 2018; Moysiuk and Caron, 2019, 2021).
Several fossils have been proposed as possible transitional forms between the lobopodian grade of organization and the radiodonts. These are Opabinia Walcott (1912) from the Burgess Shale of Canada and probably the closely-related Utaurora Pates et al. (2022) from the Wheeler Formation of Utah, as well as Kerygmachela Budd (1993) and Pambdelurion Budd (1997) both from the Sirius Passet fauna of Greenland. These animals have been referred to as “gilled lobopodians,” although some recent studies would restrict this informal name to Kerygmachela and Pambdelurion only. In detail, Budd (1999) recognized an “AOPK” group comprising of Anomalocaris Whiteaves (1892) (a radiodont) plus Opabinia, Pambdelurion, and Kerygmachela. All of these taxa share the presence of lateral lobes (i.e. flaps) and enlarged frontal appendages. Several subsequent studies also recovered gilled lobopodians close to Radiodonta (e.g., Legg et al., 2013; Vinther et al., 2014; Van Roy et al., 2015: Supplementary Material). Ortega-Hernández (2016) cautioned that gilled lobopodians probably represent a grade, rather than an explicit clade. This is reflected in most of the recently published phylogenies (e.g., Daley et al., 2018; Aria 2022; Fu et al., 2022; Pates et al., 2022) in which the opabiniids (i.e., Opabinia and Utaurora) usually resolve as a sister-group to a wider clade including radiodonts and the remaining arthropods, with the gilled lobopodians (Pambdelurion and Kerygmachela) as their immediate outgroups.
The transition series from lobopodian to gilled lobopodian and eventually to radiodonts and arthropods is still being actively investigated. Drawing on the sequence of character acquisition in Fu et al. (2022), the lobopodian condition involves the appearance of walking limbs (lobopods) and the gilled lobopodian grade includes the addition of respiratory exites (the flaps). An important question (see also Aria 2022) is whether these original lobopods’ walking limbs were retained beyond the gilled lobopodian grade—and are thus homologous with the endopods of modern arthropods—or whether the lobopod limbs were lost during the transition to Radiodonta. In the remaining taxa above the radiodont level of organization, a group now usually referred to as Deuteropoda which includes all modern arthropods, an endopod is present (possibly as a novel acquisition) as part of the biramous limb, with the limbs and body undergoing arthropodization. Into this broad framework, we describe here an interesting new fossil from the early Cambrian Chengjiang fauna of China which reveals a combination of characters consistent with a lineage which may have been close to the origins of the grade containing both gilled lobopodians/opabiniids and radiodonts.
Material and methods
Fossil imaging and measurements
The holotype and the only known specimen consist of a part and a counterpart (Figure 1). The part shows the complete animal in a ventral view, the counterpart reveals fewer details and lacks some series of lateral projections as well as the posterior end of the body. It was collected from the Chengjiang Biota, originating specifically from Erjie, Haikou, in the Yunnan Province of south-western China. This locality dates to the Early Cambrian, Series 2, Stage 3, or about 518 Ma. It is deposited in the Early Life Institute (ELI) of Northwest University, Xi’an, China, under the repository number EJ 048A/B. The specimen was studied under a Leica stereomicroscope and drawn with a camera lucida attachment. Both low angle light and immersion in alcohol with a Leica M125 and VHX-1000 were used for taking detailed photographs. Energy-dispersive spectroscopy (EDS) analyses of the specimen without coating were conducted on a ZEISS-Supra40VP environmental scanning electron microscope with an Inca (EDS) system and an X-max 50 mm2 detector at FU Berlin. Micro-CT analyses of the specimen (Supplementary Video S3) were conducted on a ZEISS-Xradia 520 Versa at Northwest University, Xi’an.
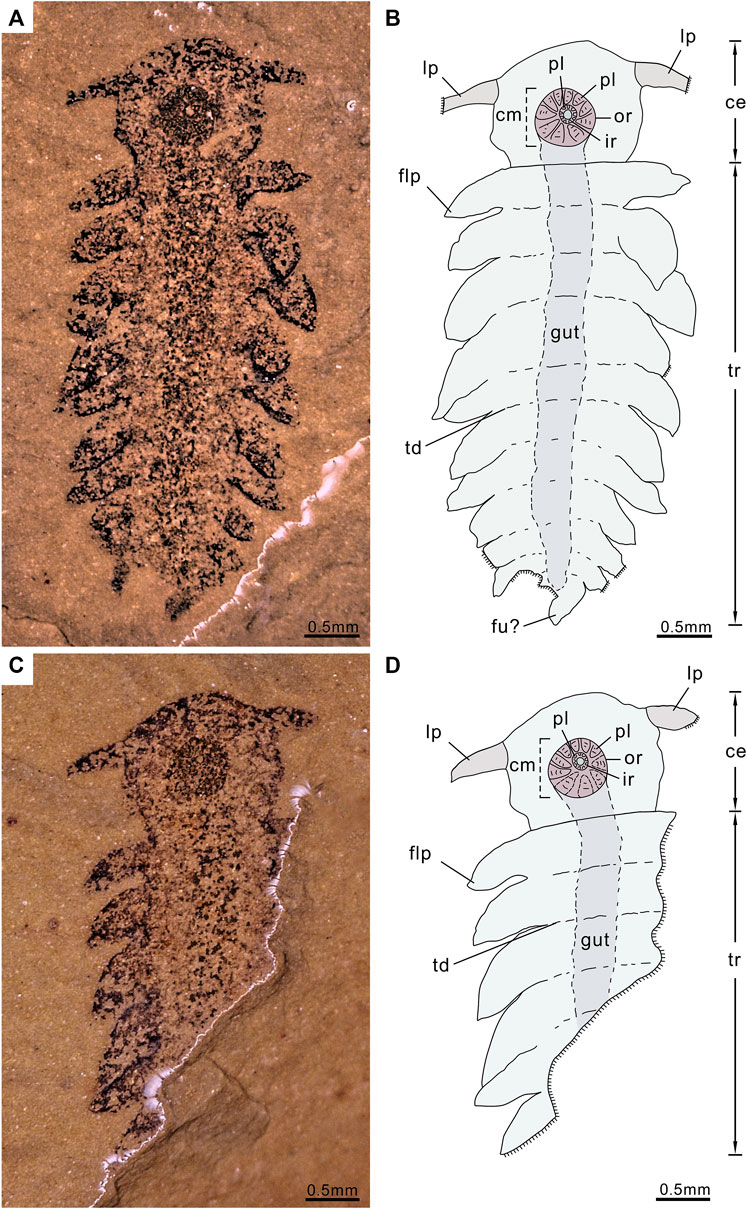
FIGURE 1. P. atavus gen. et sp. nov. Holotype (ELI-EJ 048A/B) and the only known specimen of a new lower stem-euarthropod from the Chengjiang fauna of China preserved in a ventral view, revealing a distinct cephalic region with lateral projections and a circular ventral mouth and a trunk with eleven pairs of flap-like appendages, the photograph was taken using a VHX-1000, immersing the fossil in alcohol: (A,B) Part of a near-complete specimen (ELI-EJ 048) and interpretative drawing; (C,D) Counterpart (mirrored) and interpretative drawing. ce, cephalic region; cm, circular mouth; flp, flap-like projections; fu, furcae; ir, inner ring; lp, lateral projections; or, outer ring; pl, plates; tr, trunk; td, trunk demarcations (trunk segmentation).
Repository and institutional abbreviation
The described and illustrated specimen P. atavus is deposited in the Early Life Institute of the Geology Department of Northwest University, Xi’an, China, as ELI-EJ 048A/B. The illustrated specimens Aysheaia pedunculata (365608) and Opabinia regalis (15559b) are deposited in the Smithsonian Institution, Washington (United States).
Morphological matrix
The morphological character matrix is the combination of Van Roy et al. (2015), Liu et al. (2018) and Pates et al. (2022). The updated matrix now includes 68 characters as described in the appendix and coded for 34 terminal taxa (Supplementary Data S2), the coding sources for which are also listed there.
Phylogenetic analyses
Phylogenetic analyses were performed using the Maximum parsimony (MP) method in PAUP* version 4.0b10 (Swofford, 2002) and the Baysian inference (BI) analysis in MrBayes version 3.2.6 (Ronquist et al., 2012). For the MP analysis, heuristic searches were performed with 1,000 replicates of random additions, one tree held at each step during the stepwise addition, tree-bisection-reconnection (TBR) branch swapping, MulTrees in effect, and steepest descent off. All character states were treated as unordered and equally weighted, with gaps as missing data. Nodal support was measured in PAUP by the bootstrap analysis (nReps = 1,000; ConLevel = 50) retaining those clades with a frequency greater than 50%.
In the Bayesian analysis, the Markov chain Monte Carlo (MCMC) algorithm was run with one cold and three heated chains running for 5,000,000 generations with a sampling frequency of 1,000 (less than 0.01 conducted in the average standard deviation of split frequencies). To estimate the appropriate burn-ins, posterior parameter distributions were viewed using Tracer v.1.7 (Rambaut et al., 2018).
Systematic palaeontology
Superphylum Panarthropoda (Nielsen, 1995)
(Stem-group) Arthropoda (Von Siebold, 1848)
Class, Order, Family uncertain.
Parvibellus gen. nov.
Etymology
From the Latin parvus (small) and bellus (cute) in recognition of its size and engaging appearance.
Type and only species
P. atavus gen. et sp. nov., by monotypy.
Diagnosis
Cephalic region subcircular with paired lateral projections and a large circular ventral mouth. The cephalic region is clearly differentiated from trunk, which bears eleven pairs of suboval lateral flaps generally decreasing in size from anterior to posterior; the trunk terminates in a short pair of projections.
P. atavus gen. et sp. nov.
Etymology
From the Latin atavus (ancestor), referring to its great age and the possibility that other nektonic stem, euarthropods could have evolved from this body plan.
Holotype and its only known specimen
ELI-EJ048A/B, part and counterpart. From the Chengjiang Biota, Erjie, Haikou, Yunnan, south-western China. Early Cambrian (Series 2, Stage 3), from the Yu’anshan (Heilinpu) Formation (Wutingaspis-Eoredlichia Zone).
Diagnosis
As done for the genus.
Description
The complete fossil is in ventral view. Total body length is 5.25 mm. The body is clearly divided into a subcircular cephalic region (length 1.18 mm, maximum width 1.38 mm) and a longer trunk (length 4.07 mm). The cephalic region with paired projections is emerging from the anterior third and directed laterally and is slightly posterior. Lateral projections 0.51 mm long, basal width 0.26 mm, tapering slightly from proximal to distal. The ventral surface of the cephalic region dominated by a large, circular mouth region. The mouth area consists of two circles, both composed of rings of plates; diameter of the outer ring 0.6 mm, of the inner ring 0.18 mm (Figures 1, 2, 3). Approximately ten plates in the outer ring and several tiny plates in the inner ring, but individual plates are not well preserved (Figures 1, 2).
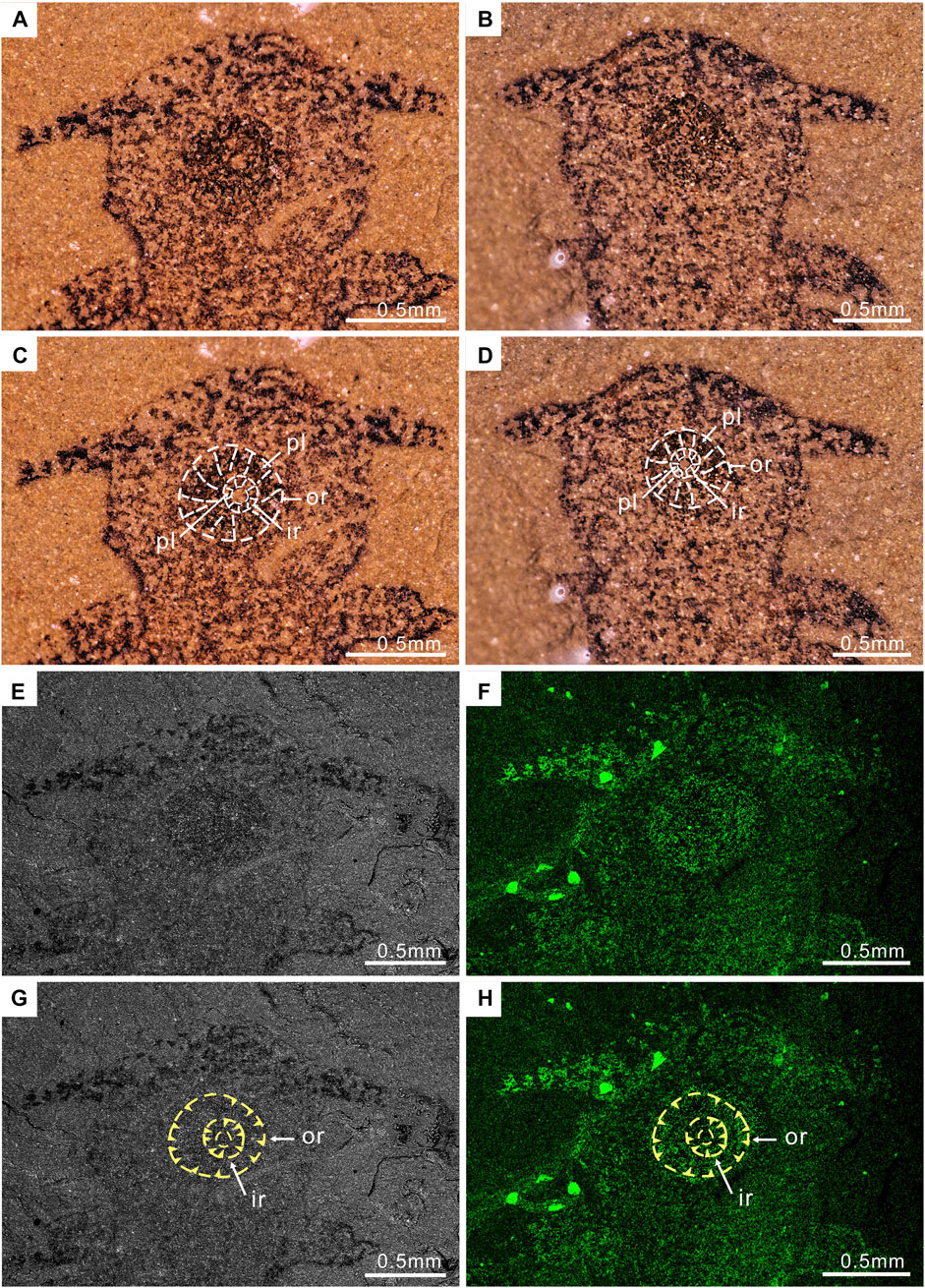
FIGURE 2. Enlargement of the head region of P. atavus: (A,B) Photographs of the part and counterpart (taken by VHX-1000, immersed the fossil in alcohol); (C,D) Outlines of the circular mouth with plates; (E,F) Backscattered electron micrographs and the iron element map of part ELI-EJ 048A; (G,H) showing the circular mouth with plates, outer ring (or), inner ring (ir), and plates (yellow arrowheads). Abbreviations as for Figure 1.
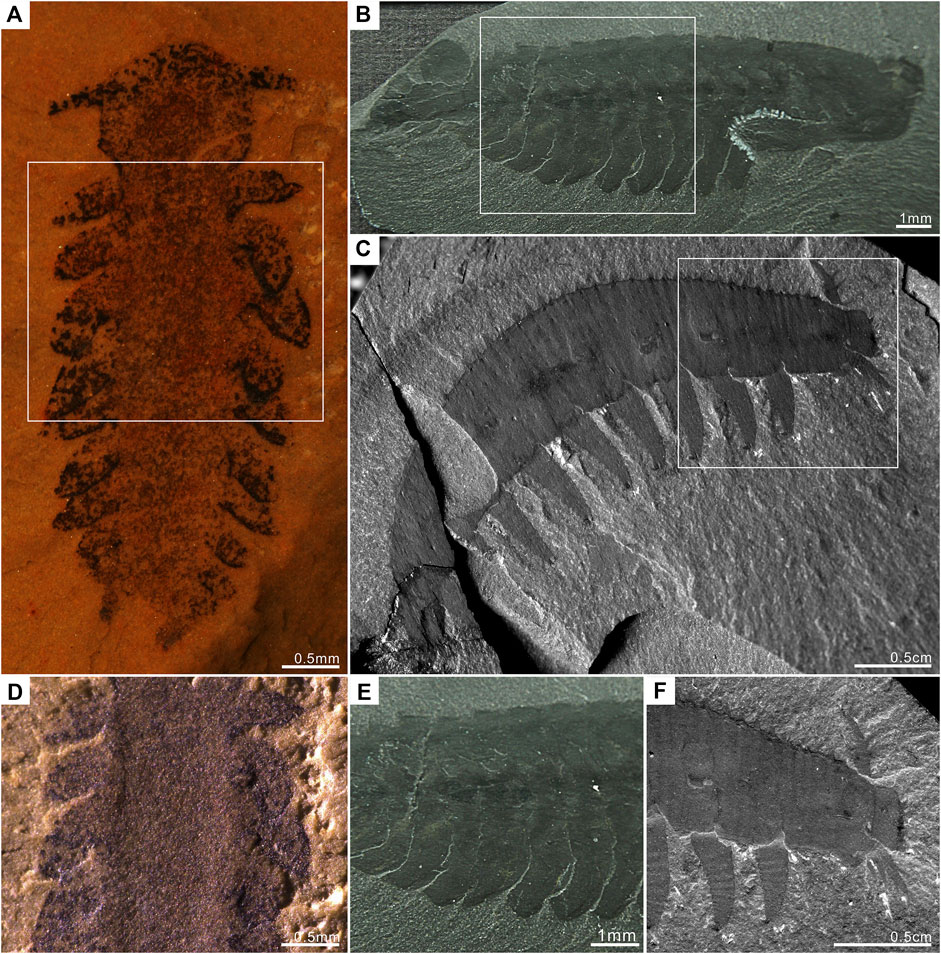
FIGURE 3. Comparison of P. atavus with A. pedunculata, and O. regalis. P. atavus is deposited in the Early Life Institute, Xi’an (China). A. pedunculata and O. regalis are deposited in the Smithsonian Institution, Washington (United States). (A) P. atavus ELI-EJ 048A (fossil immersed in alcohol using a Leica M125 for photography). (B) The complete specimen of O. regalis 15559b from the Burgess Shale. (C) The complete specimen of A. pedunculata 365608 from the Burgess Shale. (D–F) Detailed view of boxes in (A–C) showing details of appendages. Figure 3D shows that the appendages of P. atavus are clearly protruding ventro-laterally.
Trunk width ca. 1.03 mm, preserving hints of transverse demarcations and a possible median gut trace. The trunk is mostly straight, bending slightly to the left posteriorly (Figure 1). The trunk bears eleven pairs of flap-like projections along its entire length. Flap-like projections are interpreted as appendages since they 1) show a one-to-one correspondence with the trunk demarcations; 2) they are flap-like, similar to the lateral lobes of taxa like Opabinia and different from the walking legs with claws of fossils like Aysheaia (Figure 3); and 3) protrude ventro-laterally as if adapted for swimming. Individual flaps are suboval to subtriangular in outline; transition from the trunk to the flap is indistinct, with the flap length ranging from ca. 0.82 to 0.27 mm. Trunk flaps three to four are the largest, with subsequent flaps becoming successively smaller toward the posterior end (Figures 1, 3A,D, 4). The anterior pair of flaps separate from the rest and project more laterally. Other flaps orientated postero-laterally and overlapping slightly. Disposition of the flaps gives the entire trunk a broadly oval outline in overview; maximum preserved width of entire animal is ca. 2.37 mm. The trunk terminates into a pair of short projections (left one mostly missing), or furcae probably, with a length of 0.25 mm (Figures 1, 4A,B; Supplementary Figure S1).
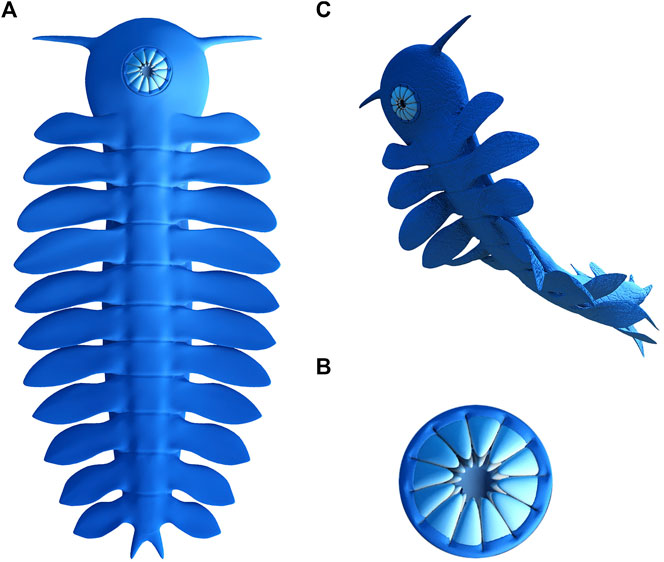
FIGURE 4. 3D-reconstruction of P. atavus: (A) Complete body in the ventral view; (B) Circular mouth with plates in a ventral view, showing the out ring and the inner ring; (C) Artistic representation of the animal.
Discussion
The preserved morphology of P. atavus gen. et sp. nov. appears to be unique among the animals found so far at Chengjiang, and from comparable Cambrian to Ordovician Lagerstätte. In the overview, the fossil is fairly small (length ca. 5 mm), with a distinct cephalic region bearing a single pair of tapering lateral projections. What we interpret as the mouth is circular and appears to be located ventrally in the middle of the cephalic region. It may have been formed from circlets of plates arranged as two rings: an outer ring with larger plates and the inner ring with smaller plates. There is no evidence that the lateral projections represent stalked eyes, i.e., they are not bulbous at the tips, and these same projections are neither articulated into limb podomeres nor enlarged or raptorial with either spines or setae. A sensory function as the antennae is conceivable, but their precise holomology to other arthropod structures remains equivocal. In early lobopodians/euarthropods, the mouth is often flanked by the protocerebral appendages; for detailed account of these structures, see Budd (2021). Alternatively, the projections in our new fossil could be novel developments, perhaps similar to the rostral spines (Park et al., 2018) of animals like Pambdelurion and Kerygmachela.
The trunk expresses, at best, only weak ventral demarcations into a series of what we interpret as limb-bearing regions, albeit not segmentation per se. The trunk bears eleven pairs of flap-like projections along its length with the flaps often overlapping slightly, especially towards the posterior end. In real life, these flaps probably showed reversed imbrication, in other words the posterior margin of each flap sloped below the anterior margin of the flap behind it. This condition is also seen in the opabiniids Opabinia (Figures 3B,E) and the recently described Utaurora (Pates et al., 2022) and in, for example, the radiodont Anomalocaris where it may have been an adaptation for swimming (Usami, 2006) by enabling the entire lateral body margin function as a single fin flap. In the new fossil there is no evidence for ventral leg-like appendages (i.e., lobopods) associated with these flaps. The trunk terminates into a pair of short projections.
Excluding a juvenile radiodont
A possible interpretation of P. atavus gen. et sp. nov. is an early instar of a radiodont in which the circular ventral mouth and flaps along the trunk are present, but the stalked eyes and raptorial frontal appendages have not yet developed. Arguing against this is the fact that unequivocal juvenile radiodonts such as the ca. 18 mm long specimen of Lyrarapax unguispinus Cong et al. (2014) described by Liu et al. (2018) resemble adults (cf. Cong et al., 2016) and have fully developed stalked eyes and spiny frontal appendages. If the new fossil were a juvenile radiodont, it would imply a fundamental shift in their morphology during early post-embryonic development.
Excluding lobopodians
Affinities with any of the fossils currently interpreted as lobopodians also appear unlikely as P. atavus gen. et sp. nov. lacks an elongated, worm-like body. The closest match would perhaps be A. pedunculata Walcott (1911) which also has laterally projecting (albeit here branching) frontal appendages and ten pairs of fairly broad lobopod limbs (Whittington, 1978; Liu and Dunlop, 2014). By contrast, the trunk appendages of our new fossil appear leaf- or flap-like, lacking both the annulations and terminal claws seen in the limbs of A. pedunculata (Figures 3C,F). While we cannot rule out a degree of flattening having contributed to the shape of the limbs in P. atavus gen. et sp. nov., we reiterate the fact that these appendages do appear to overlap one another and thus appear more consistent with a nektonic animal as opposed to one walking on the substrate. A ventral mouth is not usually present in Early Paleozoic lobopodians (Liu and Dunlop, 2014), although it was reported for the lobopodian Antennacanthopodia Ou et al. (2011) and the gilled lobopodian Pambdelurion (Vinther et al., 2016); see also below. We should also note that a mouth in this position is present in modern velvet worms (Onychophora).
The AOPK group
The circular ventral mouth consisting of at least two rings together with the flap-like appendages along the trunk could support affinities with both radiodonts and the gilled lobopodians/opabiniids; in other words, the AOPK group sensu Budd (1999). It has been suggested (Ortega-Hernández 2016) that within this assemblage, the position of the mouth may have shifted from an anterior position (Kerygmachela) to a ventral position (Pambdelurion, Radiodonta). We should note that Pates et al. (2022) consider the mouth of opabiniids to be orientated more posteriorly rather than ventrally (see also Whittington 1975; Budd and Daley 2012), although some studies (e.g., Budd, 2001) claimed that the mouth was ventral. Mouth position alone may thus not be decisive as a phylogenetic character.
Perhaps more important are the trunk projections. P. atavus gen. et sp. nov. reveals eleven pairs of flaps, exactly matching the count in Pambdelurion and Kerygmachela (Budd, 1997, Budd, 1999) and at least some radiodonts where the complete body is known (Whittington and Briggs, 1985; Van Roy et al., 2015). Other radiodonts have been described with eight to ten pairs of trunk flaps (e.g., Daley et al., 2009; Cong et al., 2016; Moysiuk and Caron, 2019), while Opabinia and Utaurora both have fifteen. Additionally, Opabinia, Pambdelurion, and Kerygmachela have all been interpreted as having both lateral flaps and lobopod limbs (e.g., Budd, 1999; Budd and Daley, 2012). We should caution that in Opabinia, the lobopod limbs are less obvious, and have not been universally accepted (Zhang and Briggs, 2007). Their presence or absence in Utaurora as ambiguous according to Pates et al. (2022) and uncertainty about the lobopod limbs in opabiniids was also discussed by Aria (2022).
In this context, radiodonts were traditionally assumed to have lost the lobopod limbs and retained only the flaps from the gilled lobopodians. Van Roy et al. (2015), see also Edgecombe (2015), proposed that radiodonts actually possessed both dorsal and ventral flaps: the dorsal flaps are homologous with the flaps of the gilled lobopodians, the ventral flaps are homologous with their stubby lobopodian limbs. In this hypothesis, these dorsal/ventral structures may eventually have fused to form the typical arthropod biramous limb (Van Roy et al., 2015; Figure 4); but compare this with the alternative interpretations of biramous limb origins in, e.g., Fu et al. (2022) as outlined in the Introduction. Given that P. atavus gen. et sp. nov. apparently lacks lobopodian limbs, an interesting question is whether its lateral projections are homologous with the flaps of gilled lobopodians and opabiniids and with either the dorsal or the ventral flaps of radiodonts. This has ramifications for resolving the affinities of the new fossil given that ventral flaps are, so far, only known from radiodonts. However, we currently lack objective criteria for testing this homology since the flattened nature of the new fossil does not allow us to determine, for example, whether the flaps originated toward the dorsal side or the ventral side of the trunk. Our working hypothesis is that the flaps of P. atavus gen. et sp. nov. are probably equivalent to the flaps of the gilled lobopodians and the dorsal flaps of radiodonts.
Affinities of Parvibellus
As noted previously, a circular ventral mouth and eleven pairs of lateral flaps are strongly reminiscent of radiodonts, and similar flaps are also seen in opabiniids and the gilled lobopodians. A ventral mouth is also present in Pambdelurion (Vinther et al., 2016). The cephalic projections in the new fossil are primarily orientated laterally and are not demonstrably raptorial. In this sense, they differ from the more anteriorly directed and spiny frontal appendages of at least Pambdelurion, Kerygmachela, and the radiodonts. Opabiniids, by contrast, have a proboscis. There is no evidence in the new fossil for stalked eyes projecting laterally as in radiodonts. Pambdelurion and Kerygmachela have been reconstructed without eyes (Budd, 1997; Budd, 1999), while Opabinia has an unusual pattern of five stalked eyes on the dorsal surface. The dorsal surface of the new fossil, and any eyes it may have borne, remains equivocal.
One solution would be to place the new fossil in a sister-group to the clade encompassing gilled lobopodians, opabiniids, and radiodonts: the “AOPK” group sensu Budd (1999) (Figure 5A). The entire lineage could be defined by the acquisition of lateral flaps and, presumably, a shift from walking to swimming as the primary mode of locomotion. The gilled lobopodians and radiodonts could be further characterized by a probably predatory lifestyle facilitated by the development of large, raptorial forelimbs. The implication would be that the lateral cephalic projections in the new fossil could be homologous with the radiodontan frontal appendages. The problem with this hypothesis is the apparent absence of lobopodian limbs in P. atavus gen. et sp. nov. Either lobopod limbs were present in the new fossil, but have not been preserved, or there were multiple independent losses and/or transformations (sensu Van Roy et al., 2015) of these appendages.
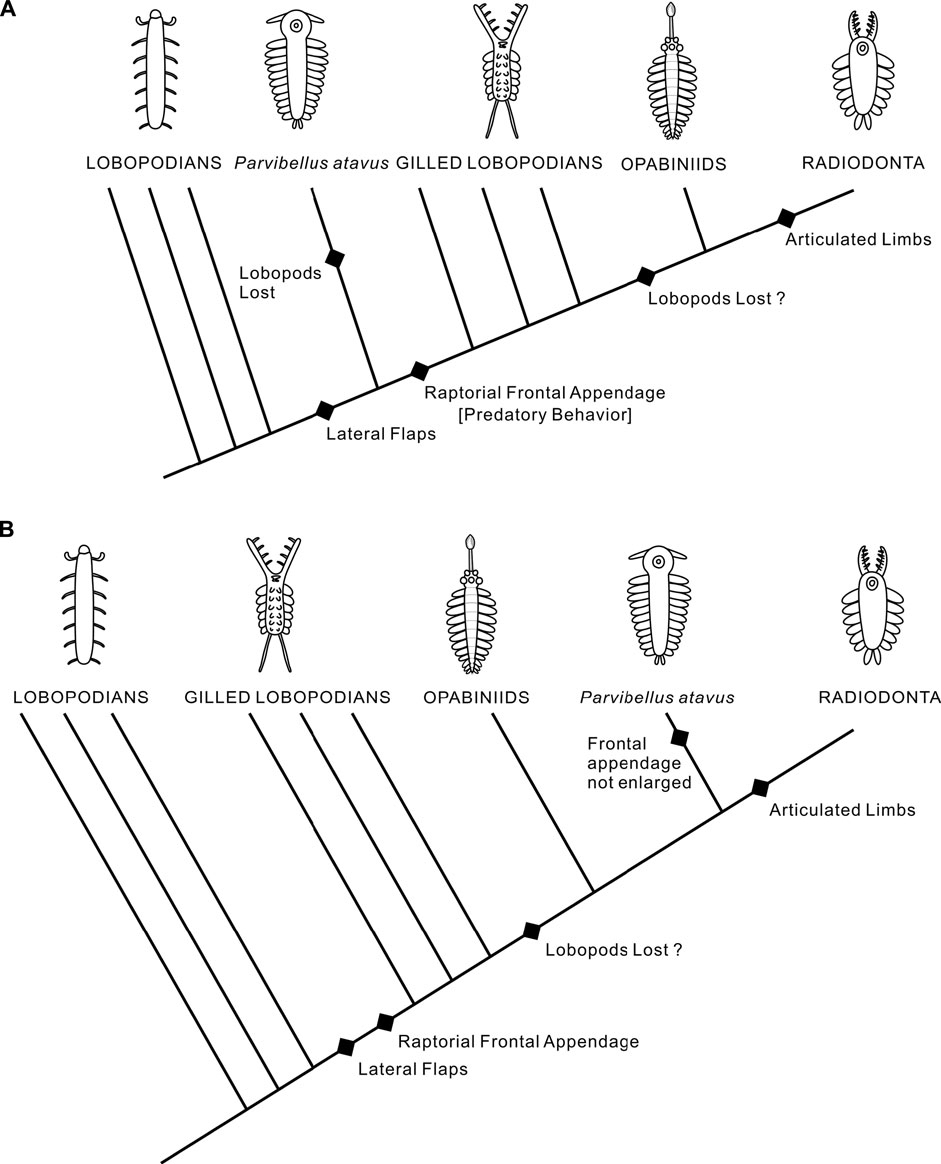
FIGURE 5. Two possible evolutionary scenarios for the position of P. atavus. (A) Sister-group to gilled lobopodians, opabiniids and radiodonts. (B) Sister-group to radiodonts only.
An alternative hypothesis would place the new fossil higher in the tree, for example, as the sister-group to Radiodonta or perhaps to opabiniids and radiodonts if the opabiniids also lacked lobopod limbs (Figure 5B). This scenario could be reconciled with only a single loss of the lobopod limbs. However, it would require a reversal with respect to the enlargement and modification of the frontal appendages. Our phylogenetic analysis (Figure 6) favors the former scenario by recovering P. atavus gen. et sp. nov. as the sister-group to the clade encompassing the gilled lobopodians, opabiniids, and radiodonts. Further material may reveal additional characters, e.g., eyes, which could help refine its affinities further. In a broader context, lobopodians seem to have been a diverse grade of animals with walking appendages associated with several individual body plans. Our new fossil could imply that there may have been a similar nektonic grade of lower stem-group arthropods in the Early Cambrian whose diversities and relationships remain to be explored.
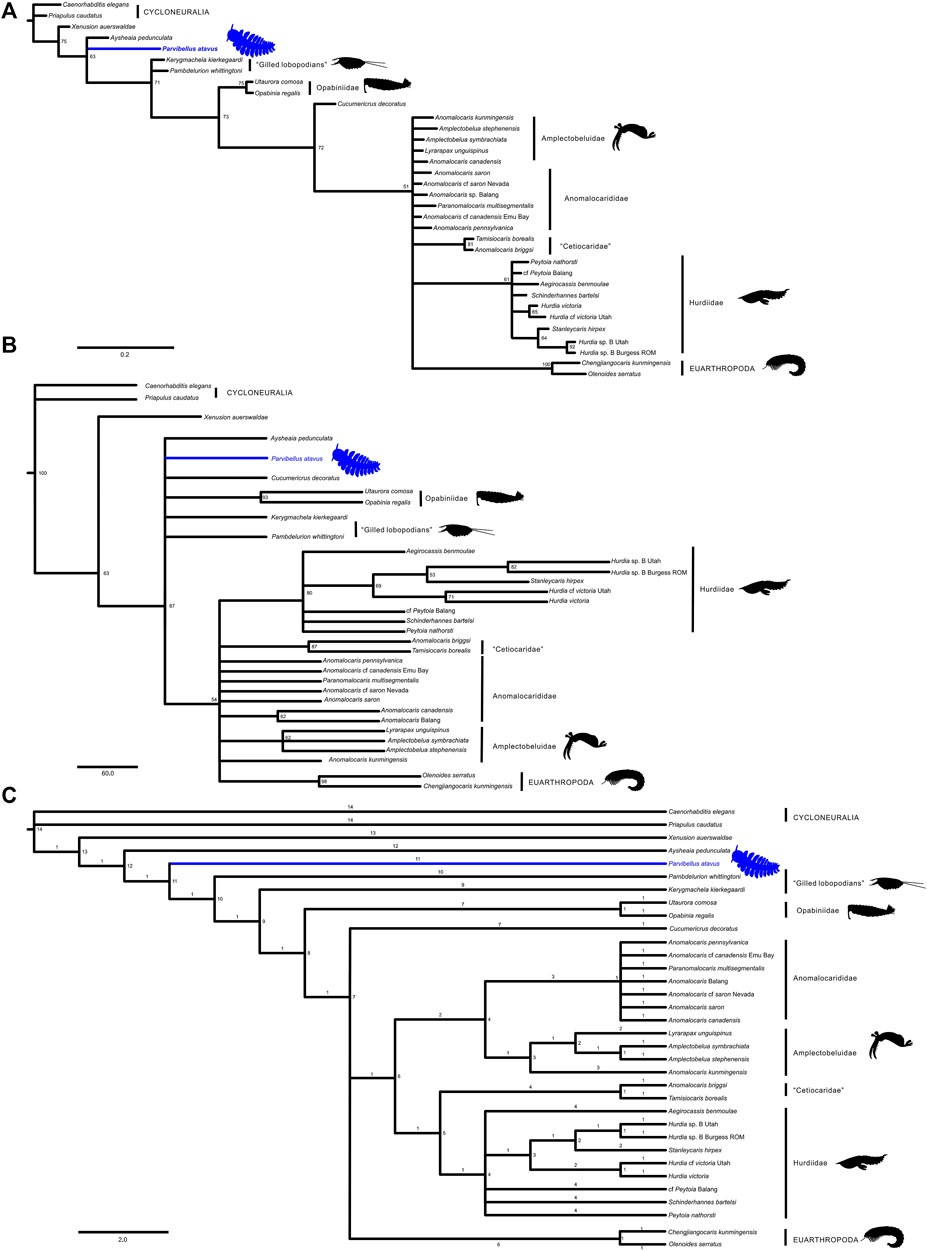
FIGURE 6. Full phylogenetic results for P. atavus: (A) Majority rule consensus tree retrieved with BI, the numbers above the nodes indicate posterior probabilities; (B) Bootstrap Majority Consensus tree retrieved with MP; (C) Strict Consensus tree retrieved with MP (147 MPTs, tree length: 124, consistency index: 0.629, and retention index: 0.836).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
JL and DS conceived the project and interpreted the fossil material. JL wrote the draft manuscript with input from JD and MS. All authors were involved in developing the observations, and discussed the results.
Funding
This research was supported by the National Natural Science Foundation of China (42130206, 41890845, and 41621003), the 111 Project, Key Scientific and Technological Innovation Team Project in Shaanxi Province and the Ministry of Education of China for Changjiang Scholars to JL.
Acknowledgments
We thank Stephen Pates and a reviewer for valuable comments on an earlier version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2022.861934/full#supplementary-material
Supplementary Figure S1 | The 3D-Reconstruction of life style for P. atavus.
Supplementary Data S2 | The character matrix for phylogeny analysis.
Supplementary Video S3 | Micro-CT analyses of P. atavus.
References
Aria, C. (2022). The origin and early evolution of arthropods. PaleorXiv, 4zmey, ver. 4. peer-reviewed by PCI Paleo. doi:10.31233/osf.io/4zmey
Budd, G. E., and Daley, A. C. (2012). The lobes and lobopods of Opabinia regalis from the middle Cambrian Burgess Shale. Lethaia 45, 83–95. doi:10.1111/j.1502-3931.2011.00264.x
Budd, G. E. (1997). “Stem group arthropods from the lower cambrian Sirius Passet fauna of North Greenland,” in Arthropod relationships. Editors R. A. Fortey, and R. H. Thomas (London: Chapman & Hall), 125–138.
Budd, G. E. (1999). The morphology and phylogenetic significance of Kerygmachela kierkegaardiBudd. Trans. R. Soc. Edinburgh: Earth Sci. 89, 249–290.
Budd, G. E. (2001). Tardigrades as 'stem-group Arthropods': the evidence from the cambrian fauna. Zool. Anzeiger - A J. Comp. Zool. 240, 265–279. doi:10.1078/0044-5231-00034
Budd, G. E. (2021). The origin and evolution of the euarthropod labrum. Arthropod. Struct. Dev. 62, 101048. doi:10.1016/j.asd.2021.101048
Cong, P., Daley, A. C., Edgecombe, G. D., Hou, X., and Chen, A. (2016). Morphology of the radiodontan Lyrarapax from the early Cambrian Chengjiang biota. J. Paleontol. 90, 663–671. doi:10.1017/jpa.2016.67
Cong, P., Ma, X., Hou, X., Edgecombe, G. D., and Strausfeld, N. J. (2014). Brain structure resolves the segmental affinity of anomalocaridid appendages. Nature 513, 538–542.
Cong, P.-Y., Edgecombe, G. D., Daley, A. C., Guo, J., Pates, S., Hou, X.-G., et al. (2018). New radiodonts with gnathobase-like structures from the Cambrian Chengjiang biota and implications for the systematics of Radiodonta. Pap. Palaeontol. 4, 605–621. doi:10.1002/spp2.1219
Daley, A. C., and Bergström, J. (2012). The oral cone of Anomalocaris is not a classic ''peytoia''. Naturwissenschaften 99 (6), 501–504. doi:10.1007/s00114-012-0910-8
Daley, A. C., and Edgecombe, G. D. (2014). Morphology of Anomalocaris canadensis from the Burgess Shale. J. Paleontol. 88 (1), 68–91. doi:10.1666/13-067
Daley, A. C., Budd, G. E., Caron, J.-B., Edgecombe, G. D., and Collins, D. (2009). The Burgess Shale anomalocaridid Hurdia and its significance for early euarthropod evolution. Science 323, 1597–1600. doi:10.1126/science.1169514
Daley, A. C., Antcliffe, J. B., Drage, H. B., and Pates, S. (2018). Early fossil record of euarthropoda and the cambrian explosion. Proc. Natl. Acad. Sci. U.S.A. 115, 5323–5331. doi:10.1073/pnas.1719962115
Edgecombe, G. D. (2015). Palaeontology: In a flap about flaps. Curr. Biol. 25, R503–R506. doi:10.1016/j.cub.2015.04.029
Edgecombe, G. D. (2020). Arthropod origins: Integrating paleontological and molecular evidence. Annu. Rev. Ecol. Evol. Syst. 51, 1–25. doi:10.1146/annurev-ecolsys-011720-124437
Fu, D., Legg, D. A., Daley, A. C., Budd, G. E., Wu, Y., and Zhang, X. (2022). The evolution of biramous appendages revealed by a carapace-bearing Cambrian arthropod. Philos. Trans. R. Soc. B 377, 20210034. doi:10.1098/rstb.2021.0034
Legg, D. A., Sutton, M. D., and Edgecombe, G. D. (2013). Early fossil record of Euarthropoda and the Cambrian Explosion. Nat. Commun. 4, 2485. doi:10.1038/ncomms3485
Lerosey-Aubril, R., and Pates, S. (2018). New suspension-feeding radiodont suggests evolution of microplanktivory in Cambrian macronekton. Nat. Commun. 9 (1), 3774–3779. doi:10.1038/s41467-018-06229-7
Liu, J., and Dunlop, J. A. (2014). Cambrian lobopodians: A review of recent progress in our understanding of their morphology and evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 398, 4–15. doi:10.1016/j.palaeo.2013.06.008
Liu, J., Lerosey-Aubril, R., Steiner, M., Dunlop, J. A., Shu, D., and Paterson, J. R. (2018). Origin of raptorial feeding in juvenile euarthropods revealed by a Cambrian radiodontan. Natl. Sci. Rev. 5, 863–869. doi:10.1093/nsr/nwy057
Moysiuk, J., and Caron, J.-B. (2019). A new hurdiid radiodont from the Burgess Shale evinces the exploitation of Cambrian infaunal food sources. Proc. R. Soc. B 286, 20191079. doi:10.1098/rspb.2019.1079
Moysiuk, J., and Caron, J.-B. (2021). Exceptional multifunctionality in the feeding apparatus of a mid-Cambrian radiodont. Paleobiology 47, 704–724. doi:10.1017/pab.2021.19
Nielsen, C. (1995). Animal Evolution: Interrelationships of the Living Phyla. Oxford: Oxford University Press.
Ortega-Hernández, J. (2016). Making sense of ‘lower’ and ‘upper’ stem-group Euarthropoda, with comments on the strict use of the name Arthropoda von Siebold, 1848. Biol. Rev. 91, 255–273. doi:10.1111/brv.12168
Ou, Q., Liu, J., Shu, D., Han, J., Zhang, Z., Wan, X., et al. (2011). A rare onychophoran-like lobopodian from the Lower Cambrian Chengjiang Lagerstätte, southwestern China, and its phylogenetic implications. J. Paleontol. 85, 587–594. doi:10.1666/09-147r2.1
Park, T. S., Kihm, J. H., Woo, J., Park, C., Lee, W. Y., Smith, M. P., et al. (2018). Brain and eyes of Kerygmachela reveal protocerebral ancestry of the panarthropod head. Nat. Commun. 9 (1), 1–7. doi:10.1038/s41467-018-03464-w
Pates, S., Botting, J. P., McCobb, L. M. E., and Muir, L. A. (2020). A miniature Ordovician hurdiid from Wales demonstrates the adaptability of Radiodonta. R. Soc. open Sci. 7 (6), 200459. doi:10.1098/rsos.200459
Pates, S., Wolfe, J. M., Lerosey-Aubril, R., Daley, A. C., and Ortega-Hernández, J. (2022). New opabiniid diversifies the weirdest wonders of the euarthropod stem group. Proc. Biol. Sci. 289 (1968), 20212093. doi:10.1098/rspb.2021.2093
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., and Suchard, M. A. (2018). Posterior summarization in bayesian phylogenetics using tracer 1.7. Syst. Biol. 67 (5), 901–904. doi:10.1093/sysbio/syy032
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi:10.1093/sysbio/sys029
Smith, M. R., and Ortega-Hernández, J. (2014). Hallucigenia's onychophoran-like claws and the case for Tactopoda. Nature 514, 363–366. doi:10.1038/nature13576
Swofford, D. L. (2002). PAUP∗. Phylogenetic analysis using parsimony (∗and other methods). Sunderland: Sinauer. Version 4.0b10.
Usami, Y. (2006). Theoretical study on the body form and swimming pattern of Anomalocaris based on hydrodynamic simulation. J. Theor. Biol. 238, 11–17. doi:10.1016/j.jtbi.2005.05.008
Van Roy, P., Daley, A. C., and Briggs, D. E. G. (2015). Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522, 77–80. doi:10.1038/nature14256
Vinther, J., Stein, M., Longrich, N. R., and Harper, D. A. T. (2014). A suspension-feeding anomalocarid from the Early Cambrian. Nature 507, 496–499. doi:10.1038/nature13010
Vinther, J., Porras, L., Young, F. J., Budd, G. E., and Edgecombe, G. D. (2016). The mouth apparatus of the Cambrian gilled lobopodian Pambdelurion whittingtoni. Palaeontology 59, 841–849. doi:10.1111/pala.12256
Von Siebold, C. T. (1848). “Lehrbuch der vergleichenden Anatomie der Wirbellosen Thiere. Erster Theil,” in Lehrbuch der Vergleichenden anatomie. Editors C. T. Von Siebold, and H. Stannius (Berlin: Verlag von Veit & Company), 1–679.
Walcott, C. D. (1911). Cambrian Geology and Paleontology. II. Middle Cambrian annelids. Smithson. misc. Collns. 57, 109–144.
Walcott, C. D. (1912). Middle Cambrian Branchiopoda, Malacostraca, Trilobita and Merostomata. Cambrian geology and paleontology, II. Smithson. misc. Collns. 57, 146–228.
Whiteaves, J. F. (1892). Description of a new genus and species of phyllocaridCrustacea from the Middle Cambrian of Mount Stephen, BC. Can. Rec. Sci. 5 (4), 205–208.
Whittington, H. B., and Briggs, D. E. G. (1985). The largest Cambrian animal, Anomalocaris, Burgess Shale, British columbia. Philos. Trans. R. Soc. Lond. B 309, 569–609.
Whittington, H. B. (1975). ). The enigmatic animal Opabinia regalis, Middle Cambrian Burgess Shale, British columbia. Philos. Trans. R. Soc. Lond. B 271, 1–43.
Whittington, H. B. (1978). The lobopodian animal Aysheaia pedunculata Walcott, Middle Cambrian Burgess Shale, British columbia. Philos. Trans. R. Soc. Lond. B 284, 165–197.
Keywords: lobopodians, radiodont, Chengjiang fauna, Cambrian, the origin of arthropods
Citation: Liu J, Dunlop JA, Steiner M and Shu D (2022) A Cambrian fossil from the Chengjiang fauna sharing characteristics with gilled lobopodians, opabiniids and radiodonts. Front. Earth Sci. 10:861934. doi: 10.3389/feart.2022.861934
Received: 25 January 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Emily G. Mitchell, University of Cambridge, United KingdomReviewed by:
Stephen Pates, University of Cambridge, United KingdomBruce S. Lieberman, University of Kansas, United States
Copyright © 2022 Liu, Dunlop, Steiner and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianni Liu, ZWxpbGpuQG53dS5lZHUuY24=
 Jianni Liu
Jianni Liu Jason A. Dunlop
Jason A. Dunlop Michael Steiner
Michael Steiner Degan Shu
Degan Shu