
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Earth Sci., 17 January 2023
Sec. Paleontology
Volume 10 - 2022 | https://doi.org/10.3389/feart.2022.1074000
This article is part of the Research TopicCo-Evolution of Life and Environment During the Ediacaran-Cambrian TransitionView all 6 articles
Anabarella, a conspicuous taxon of early mollusc, is widely distributed in the early Cambrian strata and is considered an important link in the evolutionary lineage that reflects a transitional form from helcionelloids to bivalves. In South China, Anabarella has mainly been documented from Yunnan, Shaanxi, Sichuan, and Hubei provinces. However, the taxonomy of Anabarella is questionable, which has implications for the interpretation of the genus’ temporal and spatial distribution. New and abundant well-preserved specimens of the helcionelloid mollusc Anabarella were recovered from the Member 5 of the Yanjiahe Formation in the Three Gorges area. Through morphological study, these specimens can be definitely identified as A. plana. On the basis of this new material, the species of Anabarella previously reported in the literature from South China were taxonomically revised, and, with the exception of A. plana from the Yanjiahe Formation, other species should be assigned to Igorella. Therefore, at present, A. plana is the only valid species of the genus Anabarella in South China and is limited to Cambrian Stage 2. Study of the available specimens of A. plana reveal three types of microstructures: convex polygonal impressions, concave polygons, and lamello-fibrillar microstructure. In addition, the thicker shell of the sub-apical area and the three different structures of the sub-apical area provide more evidence that A. plana might have adapted a semi-infanual mode of life and indicate that Anabarella is a likely ancestor of Watsonella.
The abrupt appearance of small shelly fossils (SSFs) in the Cambrian strata is one of the most crucial evolutionary bio-events in Earth’s history (Shu, 2008; Maloof et al., 2010; Erwin et al., 2011). As an important component of SSFs, microscopic molluscs play an important role in deciphering the radiation and evolution of early animals (Qian, 1999; Parkhaev, 2000; Parkhaev, 2001; Parkhaev, 2002; Parkhaev, 2008). The fossil record of molluscs in the lower Cambrian of South China provides an important archive for studying their diversity and affinity (Luo et al., 1982; Xing et al., 1983; Yu, 1987; Parkhaev and Demidenko, 2010; Yang et al., 2014; Steiner et al., 2020). In addition, this abundant molluscan constituent is useful for the stratigraphic correlation of the lower Cambrian (Li et al., 2011; Guo et al., 2021). Nevertheless, due to brief morphological descriptions and low-resolution images (Parkhaev and Demidenko, 2010; Li et al., 2019), the taxonomy of molluscs in the Cambrian strata was over-split and has subsequently produced many junior synonyms. This has hampered the further study of their paleobiology and applications in the correlation of lower Cambrian strata. Taxonomic revision of the Cambrian molluscs is necessary for reconstructing molluscan evolutionary lineages (Qian et al., 2009).
Anabarella Vostokova, 1962, one of the earliest univalve mollusc fossils exhibiting a laterally compressed shell, is an important genus that is widely distributed in early Cambrian strata (Fortunian to Stage 4) of North China (He et al., 1984; Feng et al., 1994; Li et al., 2019), Siberia (Vostokova, 1962; Rozanov et al., 1969; Gubanov and Peel, 2003; Kouchinsky et al., 2017), Australia (Bengtson et al., 1990), Mongolia (Esakova and Zhegallo, 1996), Baltica (Isakar and Peel, 2007), Avalonia (Landing, 1989), and Germany (Elicki, 1994). However, it is uncommon in South China (Steiner et al., 2020; Guo et al., 2021). Anabarella has been suggested as being a transitional form from univalve helcionelloids to bivalves (Gubanov, 1998; Gubanov and Peel, 2003; Vendrasco et al., 2011a). In addition, the strongly laterally compressed shell of Anabarella led to it being considered as the first mollusc adapted to a semi-infaunal mode of life (Gubanov, 1998; Gubanov et al., 1999; Gubanov and Peel, 1999; 2003). Based on morphological variations, 16 nominal and several indeterminate species were described. However, the diagnostic characters of most species have been regarded as intraspecific variations of the type species, A. plana (Landing, 1989). Anabarella shares a laterally compressed shell with Mellopegma Runnegar and Jell, 1976 and Stenotheca Salter in Hicks, 1872, which makes correct determination of the materials difficult (Vendrasco et al., 2011b). Although the coiled degree and expansion ratio of the shells are different, several species have been imprecisely assigned to Anabarella, such as A. indecora (=Mellopegma indecora), A. drepanoida (=Stenotheca drepanoida), A. simesi (=M. simesi), and A. cheleta (=M. cheleta). Therefore, the classification of the laterally compressed molluscs and the taxonomic revision of Anabarella in the previous publications are confusing, and the species assigned to Anabarella have not been systematically investigated, especially those found in South China.
Recently, hundreds of specimens of Anabarella were collected from the Member 5 of the Yanjiahe Formation in the Three Gorges area, South China. These new collections allow us to describe these specimens in detail and to further revise the species of Anabarella previously reported in South China. Meanwhile, the microstructures and the morphology of A. plana are studied and the importance of Anabarella in the context of early molluscan evolution are here discussed.
The rock samples were collected from the Member 5 of the Yanjiahe Formation (Watsonella crosbyi assemblage zone) in the Gunziao and Yanjiahe sections, Yichang, Hubei Province (Figure 1). SSFs’ distribution and the stratigraphy of the Yanjiahe Formation has been systematically investigated in recent years (Guo et al., 2014; Guo et al., 2020; Steiner et al., 2020; Guo et al., 2021). More than 100 specimens of A. plana have been collected from the insoluble residues of siliceous-phosphatic, intraclastic limestone digested in 5%–10% acetic acid. All specimens were manually picked from the residues under a binocular microscope. Selected specimens were coated with gold and photographed using an FEI Quanta 650 Scanning Electron Microscope (SEM) at Chang’an University.
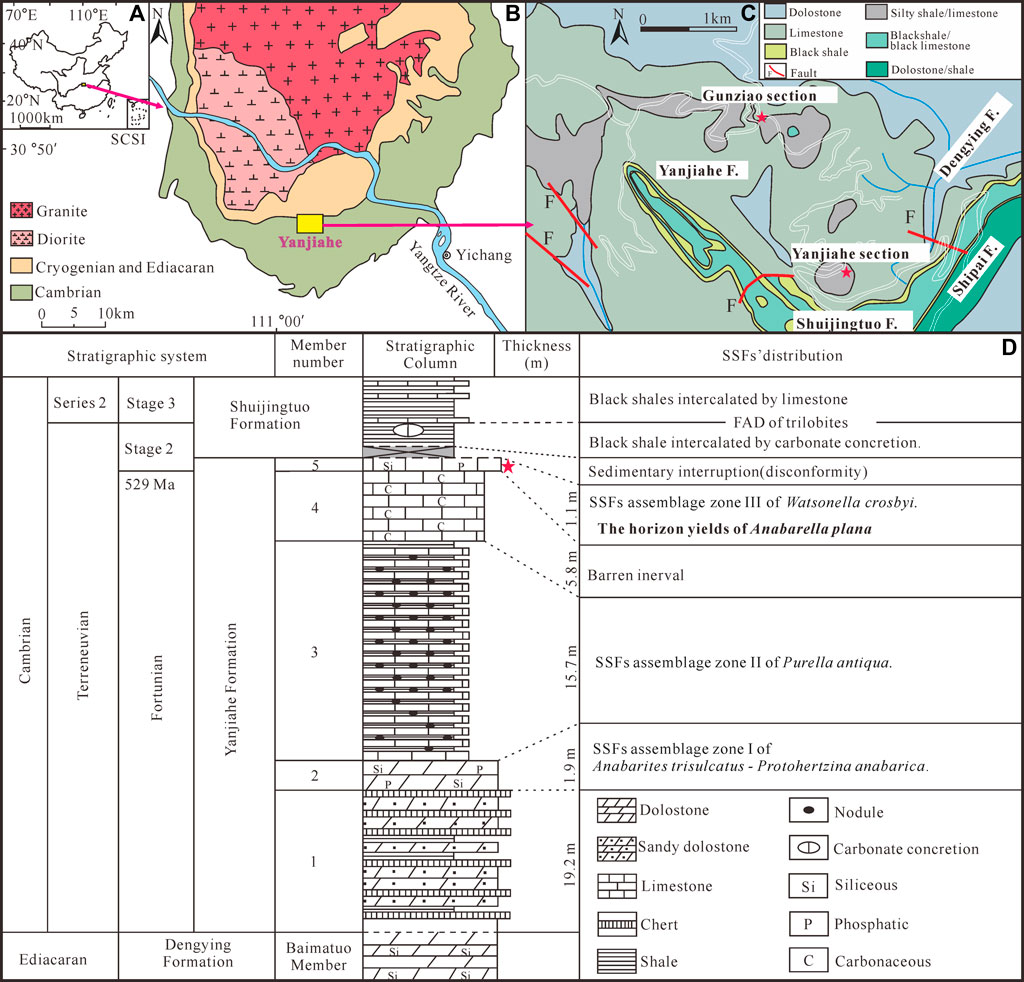
FIGURE 1. Location and stratigraphy of the Terreneuvian Yanjiahe Formation in the Three Gorges area, Hubei Province, China (modified from Guo et al., 2021). (A) Sketch map of the People’s Republic of China, the rose-red arrow showing the position of the collecting locality in Hubei Province. (B) Simplified geological map of the Three Gorges area, Hubei Province, showing the outcrops of the Cambrian strata, the rose-red arrow showing the position of the Yanjiahe area. (C) Detailed geological map of the Yanjiahe area, showing the outcrops of the Yanjiahe Formation, red stars showing the locations of the Yanjiahe Section and Gunziao Section. (D) Lithostratigraphic column and biostratigraphy of key SSFs from the Yanjiahe Formation in the Yanjiahe section, Three Gorges area, red star indicating the horizon where specimens of A. plana were collected.
All specimens are catalogued and deposited in Chang’an University (CU), Xi’an, China.
Phylum Mollusca Cuvier, 1797
Class Helcionelloida Peel, 1991
Order Helcionellida Geyer, 1994
Family Helcionellidae Wenz, 1938
Genus Anabarella Vostokova, 1962
1962 Anabarella Vostokova, p.56.
1969 Anabarella Vostokova; Rozanov et al., p. 144.
1976 Anabarella Vostokova; Runnegar and Jeel, p.130.
1990 Anabarella Vostokova; Bengtson et al., p. 244.
1994 Planutenia Elicki, p. 81.
1996 Anabarella Vostokova; Esakova and Zhegallo, p. 169.
2001 Anabarella Vostokova; Gravestock et al., p.184.
2003 Anabarella Vostokova; Gubanov and Peel, p. 1077.
2004 Anabarella Vostokova; Gubanov et al., p. 721.
2017 Anabarella Vostokova; Kouchinsky et al., p. 340.
2019 Anabarella Vostokova; Li et al., p. 30.
Type species—Anabarella plana Vostokova, 1962; lower Cambrian, Siberia.
Species included—See Table 1.
Diagnosis—See (Gubanov and Peel, 2003).
Remarks—Anabarella Vostokova, 1962 was firstly designated as a univalve mollusc—due to its strongly laterally compressed shell—from the early Cambrian. Anabarella, Mellopegma, Stenotheca, and Watsonella can easily be distinguished from most other univalve molluscs by their strongly laterally compressed shell. Even so, Stenotheca differs from Anabarella in its taller, loosely coiled, moderately expanding shell, and slightly curved aperture margin (Vendrasco et al., 2011b, Figure 15). Mellopegma differs from Anabarella in having a less coiled, longer shell, a pronounced sub-apical shelf, and a greater curvature of the aperture margin (Vendrasco et al., 2011b, Figures 5–8). Igorella is also similar to Anabarella in lateral view (Figures 2A,B) but differs from Anabarella in its slightly laterally compressed shell, elliptic aperture, and loosely coiled shell. Watsonella differs from Anabarella in its unremarkable apex, a greater curvature of the aperture margin, and short sub-apical area (Figures 2C,D; Guo et al., 2021). Planutenia Elicki, 1994 was established based on the internal molds from the upper Ludwigsdorf member in Germany (Elicki, 1994). This genus has been regarded as a junior synonym of Anabarella, according to its similar morphology (Gravestock et al., 2001). Hence, Planutenia flectata and P. inclinata have been assigned to A. australis (Parkhaev, 2004; Li et al., 2019). The taxonomic revision of other species is shown in Table 1.
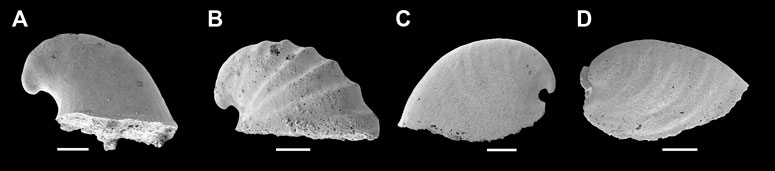
FIGURE 2. Representative members of Cambrian univalve molluscs from the Yanjiahe Formation. (A) Igorella emeiensis, internal mold, CUbar58-21. (B) I. maidipingensis, internal mold, CUBar119-4. (C) A. plana, internal mold, CUbar246-4. (D) Watsonella crosbyi, internal mold, CUBar186-6. (A,B,D) from the Member 5 of the Yanjiahe Formation in Yanjiahe section. (C) From the Member 5 of the Yanjiahe Formation in Gunziao section. Scale bars: 500 μm.
Distribution—Cambrian Fortunian-Stage 4, South China, North China, Siberia, Mongolia, Australia, Antarctica, Baltica, Avalonia.
Anabarella plana Vostokova, 1962
1962 Anabarella plana Vostokova, p.56, pl. 2, Figures 1.
1969 Anabarella plana Vostokova; Rozanov et al., p. 144, pl. 2, Figure 3; pl.6, Figures 4–6.
1982 Anabarella plana Vostokova; Zhegallo in Voronin et al., p. 45, pl. 1, Figures 6–7.
1982 Anabarella exigua Zhegallo in Voronin et al., p. 45, pl. 1, Figure 8.
1983 Anabarella plana Vostokova; Lendzion and Posti, p. 126, pl. 94, Figure 8.
1987 Anabarella plana Vostokova; Val’kov, p.121, pl. 16, Figures 2–3.
1989 Anabarella plana Vostokova; Landing, pp. 755–756, Figures 9.4, 10.11–12.
1995 Anabarella plana Vostokova; Easkova and Ermak in Pospelov et al., p. 204.
1996 Anabarella plana Vostokova; Zhegallo in Esakova and Zhegallo, p. 170, pl. 20, Figures 1–2.
1996 Anabarella exigua Zhegallo; Zhegallo in Esakova and Zhegallo, p. 170, pl. 20, Figure 3.
1999 Anabarella sp. cf. A. plana Vostokova; Gubanov in Vidal et al., p. 142, Figures 4–5.
2003 Anabarella plana Vostokova; Gubanov and Peel, p. 1077, pl. 1–3; text–Figure 3.
2007 Anabarella cf. A. plana Vostokova; Isakar and Peel, pp. 260–261, Figure 4.
2011a Anabarella plana Vostokova; Vendrasco et al., pl. 6, Figures 1–6.
2015 Anabarella plana Vostokova; Budd and Jackson, Figure 6e.
2017 Anabarella plana Vostokova; Kouchinsky et al., pp. 340–343, Figures 14, 15.
2020 Anabarella plana Vostokova; Steiner et al., Figures 5A, B, F.
2021 Anabarella plana Vostokova; Guo et al., Figures 3E, F.
Holotype—CNIGRM No. 8361–8 from the lower Cambrian of the Kenyada River, western tributary of the Olenek River, North Siberia.
Material—More than 100 specimens (internal molds) collected from the Yanjiahe and Gunziao sections, Yichang, Hubei Province; Member 5 of the Yanjiahe Formation.
Diagnosis—Small, cyrtoconic, rapidly expanding, strongly laterally compressed, bilaterally symmetrical univalve shell. Shell coiled to one whorl, sub-apical side concave and short, dorsal margin broadly rounded, lateral surface flat. Aperture curved, narrow. Apex hook-shaped, curved to the posterior aperture margin. Shell smooth or slightly ornamented with growth lines and regularly spaced comarginal rugae.
Description—Univalve shell, up to 3,000 μm in length and 2000 μm in height (Figure 3A), rapidly expanding from the apex to aperture (Figures 4A,B,C1,H1,I1), strongly laterally compressed, bilaterally symmetrical (Figures 4C2,D2,E2), isostrophically coiled about one whorl. Apex hook-shaped, tightly coiled (Figures 4H1,I1,J1). Sub-apical side very short, dorsal side long and round (Figures 4A,B,C1,D1,E1,H1,I1). Aperture narrow (Figures 4D3,E3,F4,H3,I2,J2), elongated oval with length/width ratio about 4:1 in planar view (Figure 3B), always wider anteriorly and narrower posteriorly (Figures 4H3,I2,J2). Apertural margin convex in lateral view (Figures 4C1,H1,I1). Lateral sides flat (Figures 4D2,E2,F3). Shell ornamented with growth lines. Internal mold smooth (Figures 4A,C1,G1) ornamented with shallow comarginal folds on the lateral side (Figures 4B,D1,F1,H1,I1,J1) or faint thin ribs parallel to dorsal margin (Figure 4F2). Dorsum of internal mold usually bears two shallow grooves (Figures 4G2,G3,H2). Sub-apical side short and open, almost circular in outline (Figure 4H1). Apex overhanging on the apertural margin or even projecting over it. Sinus below the apex on the internal mold (Figures 4B,C1,H1,I1,J1).
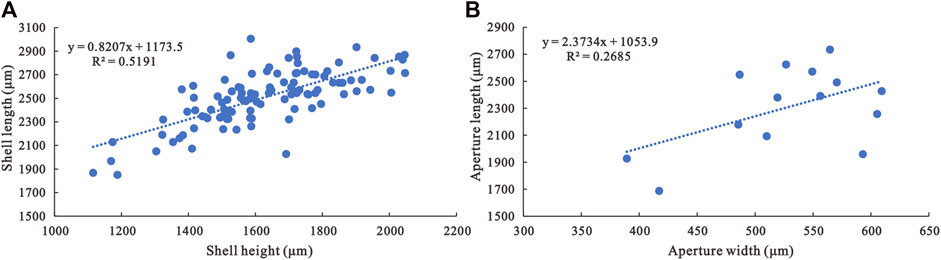
FIGURE 3. Morphological parameter analyses of A. plana. (A) Shell length/shell height ratio of A. plana (N=105). (B) Aperture length/aperture width ratio of A. plana (N=14).
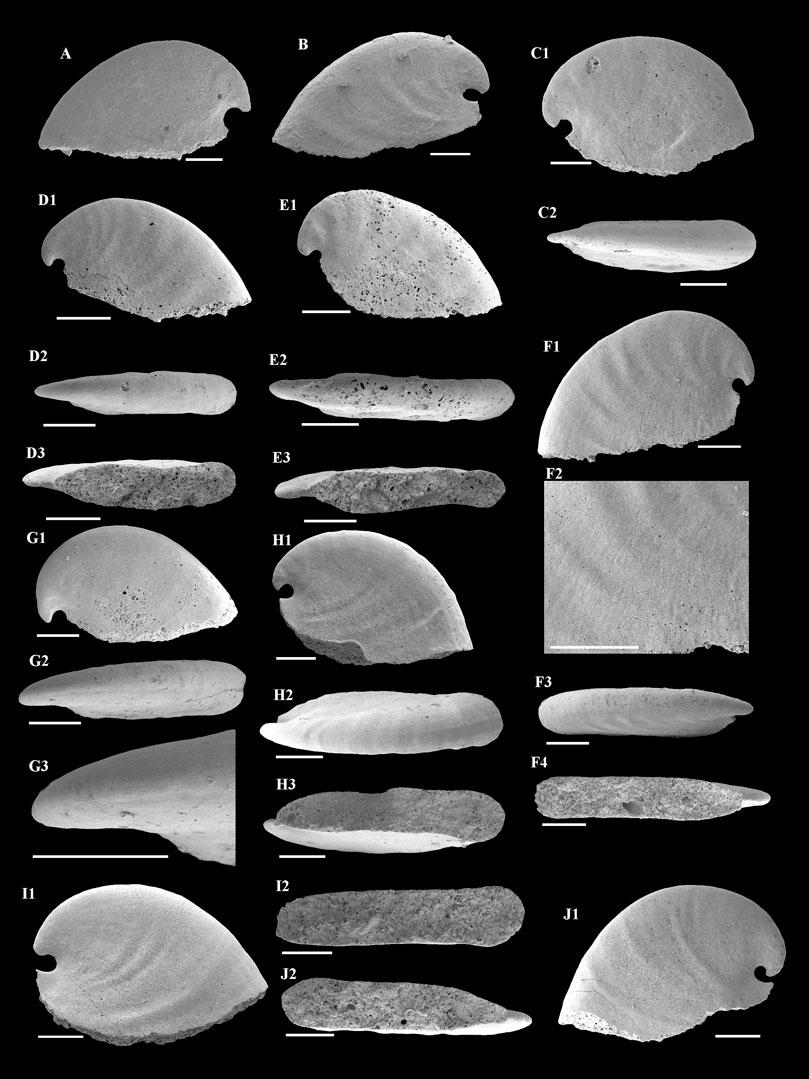
FIGURE 4. A. plana from the Member 5 of the Yanjiahe Formation. (A,B) Internal molds, lateral view, CUBar23-1, CUBar41-1. (C1) Internal mold, lateral view, CUBar235-1: (C2) dorsal view of (C1). (D1) Internal mold, lateral view, CUBar241-2: (D2) dorsal view of (D1), (D3) apertural view of (D1). (E1) Internal mold, lateral view, CUBar241-3: (E2) dorsal view of (E1), (E3) apertural view of (E1). (F1) Internal mold, lateral view, CUBar243-2: (F2) magnification of (F1) showing the faint thin ribs, (F3) dorsal view of (F1), (F4) apertural view of (F1). (G1) Internal mold, lateral view, CUBar241-5: (G2) dorsal view of (G1), (G3) magnification of (G2) showing the shallow grooves on the dorsum. (H1) Internal mold, lateral view, CUBar242-1: (H2) dorsal view of (H1), (H3) apertural view of (H1). (I1) Internal mold, lateral view, CUBar242-2: (I2) apertural view of (I1). (J1) Internal mold, lateral view, CUBar243-5: (J2) apertural view of (J1). (A,B) From the Yanjiahe Section, (C1–J1) from the Gunziao Section. Scale bars: 500 μm.
Three types of microstructure are present on the surface of internal molds: convex polygonal impressions, concave polygonal textures, and lamello-fibrillar microstructures. Convex polygonal impressions are restricted to the apical areas (Figures 5A1,A2,B1,B2,C1,C2), up to 40 μm in width. The convex polygons vary in shape and are separated by shallow grooves (Figure 5C2). The concave polygonal textures occur near the apertural and dorsal margins (Figures 5D1,D2,E3,E4), which is much clearer on the ridge near the aperture (Figures 5E1,E2). The width of these concave polygons is small, approximately 10 μm. Additionally, rarely preserved lamello-fibrillar microstructures appear on the apical and sub-apical areas (Figures 6A1,B1,C1,D1).
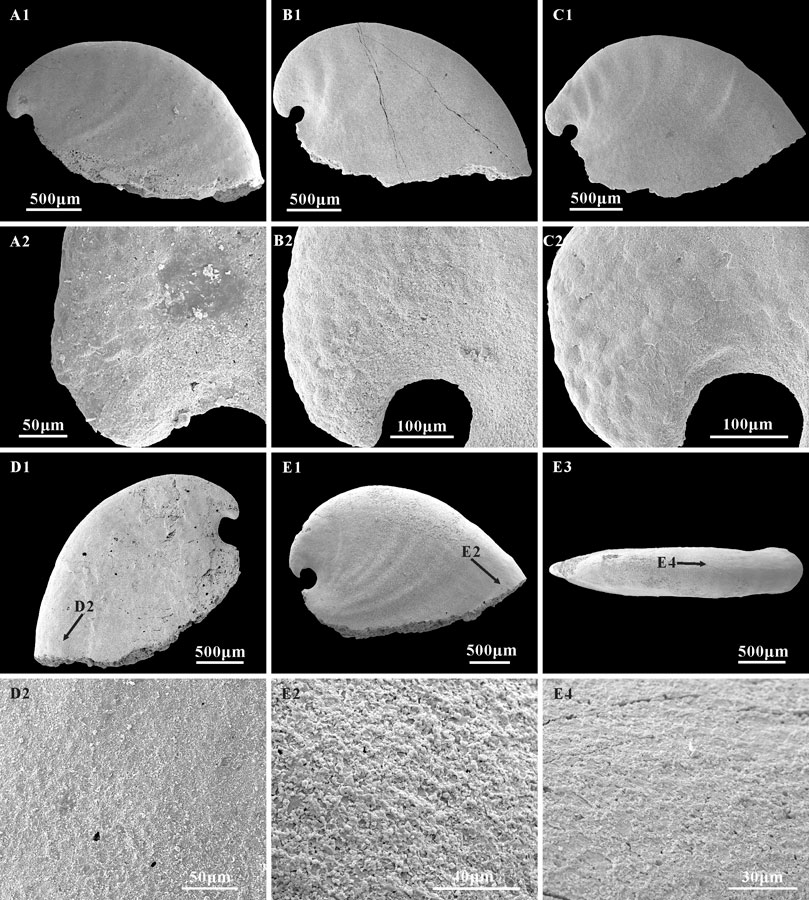
FIGURE 5. Polygonal impressions on the internal molds of A. plana. (A1,B1,C1) internal molds, lateral view, CUBar241-6, CUBar244-3, CUBar244-10, respectively; (A2,B2,C2) magnifications of (A1,B1,C1), showing the convex polygonal impressions on the apical area, represent the cell imprints of the outer mantle epithelium. (D1,E1) Internal molds, CUBar75-38, CUBar242-3; (D1,E1) lateral view, (E3), dorsal view of (E1); (D2,E2,E4), magnifications of (D1,E1,E3), showing the concave polygonal impressions on the apertural and dorsal areas, representing the inner ends of shell prisms. (A1,B1,C1,E1) from the Member 5 of the Yanjiahe Formation in Gunziao Section, (D1) from the Member 5 of the Yanjiahe Formation in Yanjiahe Section. Arrows show magnified locations.
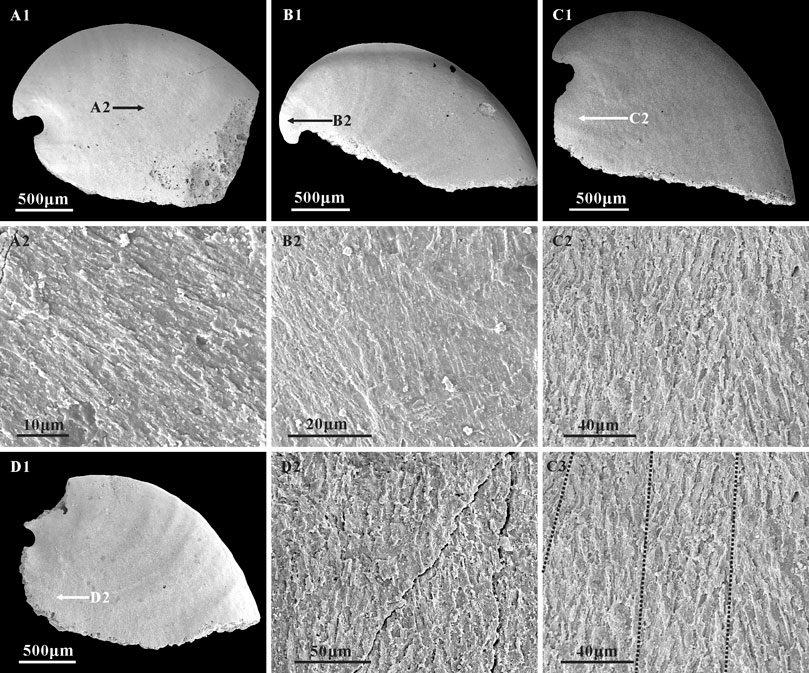
FIGURE 6. Lamello-fibrillar microstructure in the internal molds of A. plana. (A1,B1,C1,D1) Internal molds, lateral view, CUBar204-4, CUBar205-2, CUBar244-14, CUBar241-13, respectively. (A2,B2,D2) magnifications of (A1,B1,D1) respectively, (C2,C3) magnifications of (C1), showing the lamello-fibrillar microstructures. Black dotted lines on (C3) showing the convergence of different orientation fibers. Specimens from the Member 5 of the Yanjiahe Formation in Gunziao Section. Arrows indicate magnified locations.
Remarks—A. plana belongs to the univalve molluscs, with a stratigraphic range restricted to the Cambrian strata of upper Fortunian to Stage 2. Landing (1989) regarded the morphological differences of Anabarella as intraspecific variability and revised all species to A. plana. This opinion was not accepted by some researchers. According to Gubanov and Peel (2003), only A. exigua can be regarded as a junior synonym of A. plana. Among the five valid species, A. plana differs from A. australis Runnegar in Bengtson et al., 1990 by the rapidly expanding, tightly coiled shell and shorter sub-apical side (dorsal side length/sub-apical side length ratio 7:1–8:1 vs. 2:1–4:1 in A. australis); from A. tshitaensis Parkhaev, 2004 by the hook-shaped apex, rapidly expanding, tightly coiled shell, and the semicircular sinus under the apex; from Anabarella navaranae Peel, 2021 by the lower (shell length/shell height ratio ca. 1.5:1 vs. 1:1 in A. navaranae), tightly coiled, rapidly expanding shell; from A. applanta Jermak in Jermak and Pelman, 1986 by a larger shell (2,500 μm vs. 700 μm in A. applanta) and its hooked apex.
Occurrence—Upper Fortunian and lower part of Cambrian Stage 2; Siberia, western Mongolia, Baltica, South China, Spain, Avalonia.
In South China, the fossil records of Anabarella were mainly documented from Yunnan (Luo et al., 1982; Yu, 1987), Sichuan (Xing et al., 1983; Yu, 1987), Shaanxi (Xing et al., 1983), and Hubei provinces (Steiner et al., 2020; Guo et al., 2021). With the exception of the type species A. plana (Luo et al., 1982; Steiner et al., 2020; Guo et al., 2021), three nominal species of Anabarella were previously named from South China: A. emeiensis Yu, 1987, A. lentiformis Yue in Xing et al., 1983, and A. gypirhynchosa He in Xing et al., 1983 (Table 1; Xing et al., 1983; Yu, 1987).
Anabarella plana in South China was initially discovered by Luo et al. (1982) from the Dahai Member of the Zhujiaqing Formation in Huize, Yunnan Province, based on a single specimen. This specimen (Luo et al., 1982, plate 20, Figures 11, 11a), however, shows a small, cyrtoconic, cap-shaped, moderately high, slightly laterally compressed shell, with an obtuse apex and an elliptical aperture—features more consistent with the genus Igorella Missarzhevsky in Rozanov et al., 1969, and hence is herein identified as I. emeiensis Yu, 1987. In addition, A. plana was also reported without description from the Member 5 of the Yanjiahe Formation in the Three Gorges area of South China (Steiner et al., 2020; Guo et al., 2021). In this study, based on numerous specimens, the morphology, taxonomy, and stratigraphic distribution of A. plana in the Yanjiahe Formation are systematically analyzed.
Anabarella emeiensis Yu in Lu, 1979 (plate Ⅲ, Figures 12–15) was originally proposed based on specimens from the Maidiping Formation in the Emei region, Sichuan Province (Lu, 1979). However, this material was illustrated without associated descriptions of the taxon; hence, A. emeiensis is considered a nomen nudum. Yu (1987) formally described and reassigned the taxon as A. emeiensis Yu, 1987 based on the former specimens (plate 39, Figures 7–9) and other specimens (plate 40, Figures 7–9) from the Zhongyicun Member in Xundian, Yunnan Province. Subsequently, A. emeiensis is regarded as a junior synonym for Stenotheca emeiensis based on specimens collected from the Xinji Formation of Henan Province (Feng et al., 1994). However, based on the description and illustrations, the diagnostic features of “S. emeiensis” described by Feng et al. (1994) are more consistent with the diagnostic features of A. australis described by Bengtson et al. (1990). Hence, S. emeiensis should be assigned to A. australis. Nevertheless, the length/width ratio of the aperture in the specimens of A. emeiensis illustrated by Yu (1987) is approximately 2:1, much smaller than in other forms of Anabarella (ca. 4:1) and inconsistent with the character of a laterally compressed shell—a distinct diagnostic feature of Anabarella. Additionally, the taller and less coiled shell is much closer to Igorella. Therefore, we agree with Parkhaev and Demidenko (2010) that A. emeiensis should be assigned to I. emeiensis.
Anabarella lentiformis Yue in Xing et al., 1983 (plate 26, Figures 24–26) was discovered in the upper Kuanchuanpu Formation in the Ningqiang country of Shaanxi Province. The internal molds shown in the original illustration are small, recurved, cyrtoconic, coiled less than one whorl, and slightly laterally compressed. The apex is hook-shaped, projecting over the apertural margin. The aperture is lenticular, with a length/width ratio of about 2.5:1. The coarser comarginal rugae occur near the dorsal areas. These features were previously regarded as intraspecific variability of A. plana (Landing, 1989). However, compared to the diagnostic features of Anabarella, the less coiled, slightly laterally compressed shell is more similar to Igorella. Hence, A. lentiformis has been regarded as a junior synonym of I. maidipingensis Yu, 1974 (Parkhaev and Demidenko, 2010).
Anabarella gypirhynchosa He in Xing et al., 1983 (plate 13, Figure 7) was established based on a broken specimen from the Niuniuzhai Member (=Maidiping Formation) of the Hongchunping Formation, Sichuan Province. The specimen is semicircular in lateral view. A hook-shaped apex curves to the aperture margin. The aperture shape is unknown. Irregular comarginal folds are present on the internal mold surface. According to these features, we follow Parkhaev and Demidenko (2010) and assign A. gypirhynchosa to I. maidipingensis.
After revisions, the valid identification of A. plana in South China is only from the Member 5 of the Yanjiahe Formation. Given the co-occurrence of Aldanella attleborensis and W. crosbyi in the Member 5 of the Yanjiahe Formation, the stratigraphic range of A. plana in South China is limited to the Cambrian Stage 2. This changes the traditional understanding that A. plana appeared earlier than W. crosbyi in South China (Gubanov and Peel, 2003). A. plana also appeared in the W. crosbyi assemblage zone (Cambrian Stage 2) of Avalonia (Landing, 1989) and Estonia (Mens and Isakar, 1999; Isakar and Peel, 2007), but the earliest record of it was discovered from the upper Purella antiqua assemblage zones (Fortunian) of Siberia (Khomentovsky and Karlova, 1993; Gubanov and Peel, 2003; Kouchinsky et al., 2017), Mongolia (Esakova and Zhegallo, 1996), and Spain (Gubanov and Peel, 2003).
Study of the microstructure of Cambrian fossils provides significant characteristics that could be used to better understand the degree of diversification, phylogeny, and shell strength of the early molluscs (Vendrasco et al., 2010). To date, extensive work has been done based on Cambrian molluscan microstructure (Runnegar, 1985; Kouchinsky, 1999; Kouchinsky, 2000; Vendrasco et al., 2010; Vendrasco et al., 2011a; Li et al., 2022). Three types of microstructure (i.e., polygonal impressions, stepwise texture, and lamello-fibrillar microstructure) have been studied on the laterally compressed internal molds of A. plana and W. crosbyi to reveal their phylogenetic relationships (Kouchinsky, 1999; Vendrasco et al., 2011a; Li et al., 2011; Guo et al., 2021). These microstructures, observed on the internal molds of A. plana from the Yanjiahe Formation, are similar to those of Anabarella and Watsonella (Bengtson et al., 1990; Kouchinsky, 1999; Vendrasco et al., 2011a; Li et al., 2019), such as an outer layer of polygonal impressions and inner layer lamello-fibrillar microstructure, but no sign of stepwise texture. The polygonal impressions are common microstructures that appear on the internal molds of A. plana, W. crosbyi, Pojetaia runnegari, and other Cambrian molluscs (Kouchinsky, 1999; Vendrasco et al., 2011a; Li et al., 2011; Guo et al., 2021). Several interpretations of the polygonal impressions on Cambrian molluscs have been made, such as muscle scars (Parkhaev, 2006), imprints of shell prisms (Kouchinsky, 1999; Vendrasco et al., 2010; Li et al., 2011; Guo et al., 2021), or cell imprints of the outer mantle epithelium (Ushatinskaya and Parkhaev, 2005; Parkhaev and Karlova, 2011). Herein, the polygonal impressions on A. plana appear as convex (Figures 5A2,B2,C2) and concave structures (Figures 5D2,E3,E4). On the new material of Anabarella, the convex polygons vary in shape: mainly circular or nearly circular, up to 40 μm in width, and separated by shallow grooves (Figure 5C2). Unlike the positive relief of A. plana described by Kouchinsky (1999, Figure 2B), the convex polygons illustrated herein are restricted to the apical areas (Figures 5A2,B2,C2) and no tubercles in the polygons. In addition, a convex polygon with such a large diameter has never been found in the apical area of Anabarella. Similar convex polygonal impressions have been described on the apex of Aldanella, Oelandiella, and Securiconus, and are mostly interpreted as imprints of shell prisms (Isakar and Peel, 2007; Vendrasco et al., 2010). However, the much larger diameter and specific position of such polygons are different from prismatic imprints. Therefore, we agree that the convex polygons could be interpreted as the impressions of cells of the outer epithelium (Ushatinskaya and Parkhaev, 2005; Parkhaev and Karlova, 2011). The concave polygonal texture is more common on the internal molds of Anabarella and appears on the apical and dorsal areas of A. plana (Kouchinsky, 1999) and sub-apical area of A. australis (Bengtson et al., 1990). The concave polygonal textures on the internal mold of A. plana from the Yanjiahe Formation are small—approximately 10 μm in diameter. They appear near the apertural and dorsal margin (Figures 5D1,D2,E3,E4) and are much clearer on the ridge near the aperture (Figures 5E1,E2). Compared to the concave polygons of W. crosbyi (Li et al., 2011; Guo et al., 2021), A. plana (Kouchinsky, 1999), and A. australis (Bengtson et al., 1990), the concave polygons here are less pronounced and frequent, which might be correlated with preservational bias. Concave polygonal textures have been interpreted as inner ends of the prismatic shell layer (Kouchinsky, 1999; Li et al., 2011; Guo et al., 2021).
Although inner layer lamello-fibrillar microstructures are common in early Cambrian molluscs such as W. crosbyi (Guo et al., 2021) and A. australis (Li et al., 2019), this structure is rarely reported on the internal molds of A. plana. In the specimens of the Yanjiahe Formation, lamello-fibrillar textures consisting of bundles of fibers occur on the lateral fields, and the apical and sub-apical areas (Figures 6A2,C2,D2). Fibers are arranged at the same orientation (Figure 6A2) or intersect at low angles (Figures 6C2,C3) but belong to different layers (Figure 6B2).
The similarities in microstructures between Anabarella and Watsonella—such as polygonal impressions, stepwise texture and lamello-fibrillar microstructure—taken together with their shell form, were the important evidence for Anabarella being a likely ancestor of Watsonella (Kouchinsky, 1999; Vendrasco et al., 2011a).
The preservation forms of SSFs strongly depend on facies, diagenesis, and the methods of fossil extraction in the lab (Gubanov and Peel, 2003; Jacquet et al., 2019; Li et al., 2019). Among SSFs, molluscs are usually extracted as secondarily replaced shells, internal molds, external molds, or external coatings in phosphatized calcareous rocks treated with acetic acid. However, it is widely known that the morphology of molluscs preserved as listed above show varying fidelity to their characteristic features, depending on the mode of preservation (Bengtson et al., 1990; Skovsted, 2004). Morphological variation between external coatings and internal molds is a common in univalve SSFs, particularly in Anabarella. For instance, the internal molds of Anabarella are always smooth and ornamented with gentle rugae, but the external coatings are only ornamented with growth lines. In addition, the main difference between internal molds and external coatings focuses on the shape and size of the shell sub-apical areas, and has been ascribed to preservational artifacts—a heavily phosphatized shell and degree of recrystallization (Gubanov and Peel, 2003; Li et al., 2019). However, the observed morphological variation is not necessarily caused by preservational bias. The new materials of A. plana in the Yanjiahe Formation may reveal another interpretation of the cause of morphological variation between external coating and internal mold.
Thin phosphatic coatings are preserved on some specimens of A. plana from the Member 5 of the Yanjiahe Formation (Figures 7B2,C) which replicate the external shell morphology well. The morphology of internal molds is extremely different to external phosphatic coatings, especially on the sub-apical area (Figures 7A1–A3,B1,B2,C). In contrast to the Spanish specimens (from the lower Cambrian of Sant Lorenzo de Calatrava, Sierra Morena) that have heavily phosphatized shells (Gubanov and Peel, 2003, plate 3), the material studied here is lightly phosphatized. The well-preserved growth lines in the thin external coating (Figures 7A2,C) and the imprints of the shell microstructures (Figures 5, 6) in the internal mold surfaces suggest that the free space between the external coating and internal mold (Figures 7D,E; Li et al., 2019) represents the morphology of the original shell, and do conform to being phosphatization overgrowths around the original shell. It is worth noting that the sub-apical shell of Anabarella is thicker than the rest of the shell (Figure 7A3; Li et al., 2019, Figures 3H,I). This might be related to the adaptive strategy of Anabarella (Gubanov, 1998; Figure 2). As possibly the first molluscan taxon to adapt the semi-infaunal mode of life, it has been suggested Anabarella burrowed to softer substrates for food or to avoid predators (Gubanov, 1998; Gubanov et al., 1999). In this mode of life, the posterior of the shell would always be exposed above the substrate and be easily damaged by predators in the sediment. Mellopegma, another mollusc interpreted to have adapted to a burrowing lifestyle, shows concentrated damage in the sub-apical area (Vendrasco et al., 2011b), which indicates that this portion of the shell is more susceptible to damage or attack. Although the scar is rarely preserved on the internal molds, a possible scar imprint appeared on the sub-apical area of A. plana (Figure 8G1,G2). Hence, the thicker original shell in sub-apical area of Anabarella might be adapted to resist the attacks of predators and sediments. Hence, the morphological differences between the external coatings and internal molds of Anabarella, especially of the sub-apical area, were caused by the variation in thickness of the original shell, not merely by preservational bias.
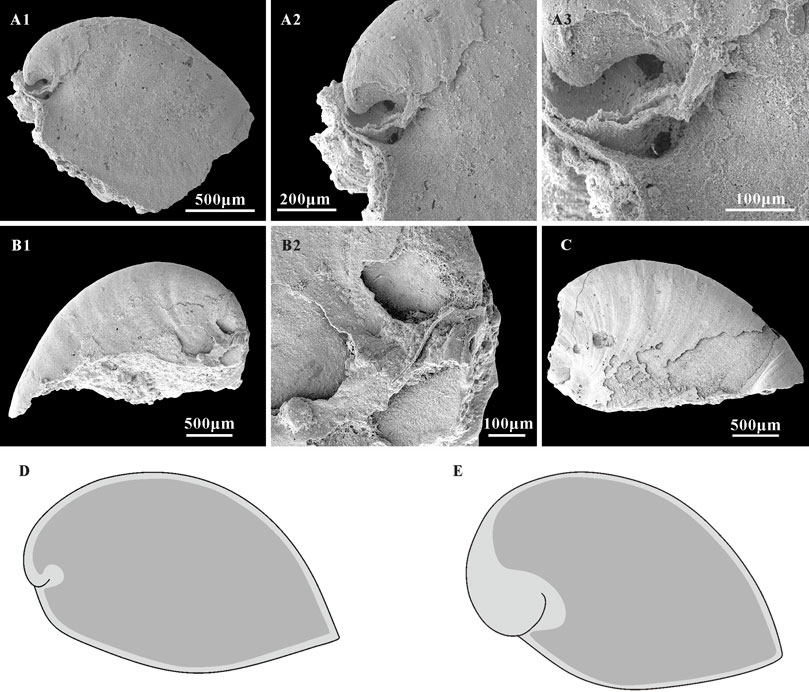
FIGURE 7. A. plana with external coating and associated schematic of shells and internal mold extent in Anabarella. (A1,B1,C) Internal molds, lateral view, CUBar128-24, CUBar243-6, CUBar246-15, respectively. (A2,A3) magnifications of (A1) showing the morphological variation of external coating and internal mold on the apical areas of A. plana. (B2) Magnification of (B1), showing the shell morphology of A. plana on apical area. (D) Sketch of A. plana. (E) Sketch of A. australis, according the specimens showed in Li et al., 2019 (Figures 3I,H). Dark gray, morphology of internal mold; light gray, morphology of shell. (A1) From the Member 5 of the Yanjiahe Formation in Yanjiahe Section, (B1,C) from the Member 5 of the Yanjiahe Formation in Gunziao Section.
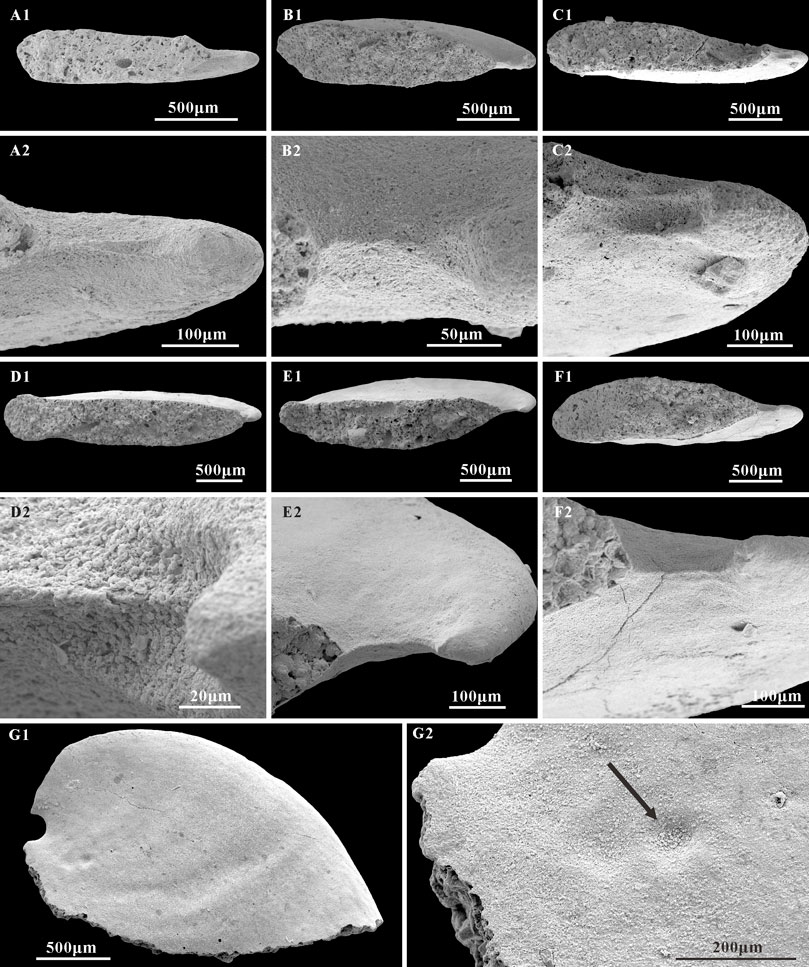
FIGURE 8. Structures on the sub-apical area of A. plana and possible imprint of scar. (A1,B1,C1,D1,E1,F1) Internal molds, apertural view, CUBar217-7, CUBar241-7, CUBar241-1, CUBar242-3, CUBar241-4, CUBar241-10, respectively. (A2,B2,C2,D2,E2,F2) magnifications of (A1,B1,C1,D1,E1,F1), showing the structures of sub-apical side. (G1) internal mold, lateral view, CUBar242-7. (G2) magnification of (G1), showing the possible imprint of scar. Arrow shows the location of scar. Specimens from the Member 5 of the Yanjiahe Formation in Gunziao Section.
Based on the stratigraphic distribution, morphology, and shell microstructures, a hypothetical evolutionary lineage from the uppermost Fortunian Oelandiella via Anabarella to the Stage 2 Watsonella, and even to the Stage 3 earliest bivalves Pojetaia and Fordilla has been suggested (Runnegar and Pojeta, 1974; Gubanov, 1998; Gubanov et al., 1999; Kouchinsky, 1999; Vidal et al., 1999; Gubanov and Peel, 2000; Gubanov and Peel, 2003; Vendrasco et al., 2011a; Vendrasco et al., 2011b; Li et al., 2011; Guo et al., 2021). The evolutionary lineage from Oelandiella via Anabarella to Watsonella is accompanied by a loss of the strong comarginal ornamentation and increased lateral compression (Gubanov and Peel, 1999). In addition to these evolutionary trends, the sub-apical structures of A. plana in the new materials of the Yanjiahe Formation also appear transitional.
Unlike most helcionelloids with a gentle sub-apical area, the well-preserved sub-apical areas of A. plana show three different types of structures in internal molds: a V-shaped convergence (Figures 8A1,A2,B1,B2), two sinuses separated by a ridge (Figures 8C1,C2,D1,D2), and a raised ridge (Figures 8E1,E2,F1,F2). These structures may indicate that the shell sub-apical areas of A. plana have a tendency to separate in order to extend the aperture, which is consistent with W. crosbyi having a relatively short sub-apical field and a strong upward posterior aperture. Compared to the wide aperture of Oelandiella, that of Anabarella and Watsonella is narrow but more curved in lateral view, which elongates the aperture’s length and improves efficiency of movement and foraging. This is accompanied by the gradual disappearance of the apex. In addition, the narrower and longer aperture of Anabarella and Watsonella makes it easier to burrow into the softer substrate. Hence, we suggest that the dorsal elongate structures of Anabarella (Figures 4G2,G3,H2) are analogous to the median furrow of Watsonella (Gubanov et al., 1999), which might be the ligament precursor of bivalves (Li et al., 2011). From a functional perspective, the morphological variation observed in Oelandiella, Anabarella, and Watsonella may reflect the change of adaptive life strategy—for example, epifaunal, semi-infaunal, and infaunal. The similarities in morphology, as well as the earlier occurrence of A. plana (upper Fortunian) in Cambrian strata, indicate the possibility that W. crosbyi is the descendent of Anabarella, although the systemic position of W. crosbyi is uncertain. In summary, the similarity in morphology and shell microstructure between A. plana and W. crosbyi implies their close relationship and supports the hypothesis of an evolutionary lineage from Oelandiella (uppermost Fortunian) through Anabarella (uppermost Fortunian-Stage 2) and Watsonella (Stage 2) to Pojetaia and Fordilla (Stage 3) (Vendrasco et al., 2011b). A. plana from the Member 5 of the Yanjiahe Formation provides important information about the intermediate morphological transition during the evolutionary process between helcionelloids and bivalves.
This taxonomic study of Anabarella in South China was performed on the basis of abundant new materials. Investigation shows that the only reliable species of Anabarella in South China is A. plana, which is only recognized in the Yanjiahe Formation of western Hubei, and its age is limited to Cambrian Stage 2. The studied specimens provide important information about the morphology and microstructures of A. plana. The well-preserved growth lines on the external coating and microstructures on the internal mold indicate that the phosphatization around the original shell is slight, and the morphological variations between shells and internal molds of A. plana may be caused by the variation in original shell thickness, not just by preservational bias. Three types of microstructure are identified on the internal mold of A. plana: convex polygonal impressions, concave polygons, and a lamello-fibrillar microstructure. The convex polygonal impressions on the apical area are interpreted as the cast of the cellular epithelium of the mollusc, but the concave polygons may correlate with the inner ends of shell prisms. Lamello-fibrillar microstructures consisted of fibers mostly related to the inner layer. The comparability of the microstructures and morphology of A. plana with W. crosbyi provides important evidence that supports a hypothetical evolutionary lineage from Anabarella to Watsonella.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Conceptualization: JG and YQ, writing-original draft: YQ; writing-review and editing: JG, GL, and JH; sample collection: YQ, JP, JS, ZS, and XZ.
This research was funded by the Natural Science Foundation of China (Nos. 42172016 to JG; 41890844 to JG; 41621003 to JH), Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB26000000 to JH and JG), and Key Scientific and Technological Innovation Team Project in Shaanxi Province, State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) (Nos. 203106 to JG; 163107 to JH).
We thank Luoyang Li (Ocean University of China) for many useful suggestions. We also thank reviewers and editor for their comments and suggestions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bengtson, S., Conway Morris, S., Cooper, B. J., Jell, P. A., and Runnegar, B. N. (1990). Early cambrian fossils from SouthSouth Australia. Memoirs Assoc. Aust. Palaeontol. 9, 1–364.
Budd, G. E., and Jackson, I. S. C. (2015). Ecological innovations in the Cambrian and the origins of the crown group phyla. Philosophical Trans. R. Soc. B Biol. Sci. 371, 20150287. doi:10.1098/rstb.2015.0287
Elicki, O. (1994). Lower cambrian carbonates from eastern Germany: Palaeontology, stratigraphy and palaeogeography. Neues Jahrb. für Geol. Paläontologie, Abh. 191, 69–93.
Erwin, D. H., Laflamme, M., Tweedt, S. M., Sperling, E. A., Pisani, D., and Peterson, K. J. (2011). The cambrian conundrum: Early divergence and later ecological success in the early history of animals. Science 334, 1091–1097. doi:10.1126/science.1206375
Esakova, N. V., and Zhegallo, E. A. (1996). Biostratigraphy and fauna of the lower Cambrian of Mongolia. Russia: Sovmestnaya Rossiysko-Mongol’skaya Paleontologicheskaya Ekspeditsiya, 216. (in Russian).
Feng, W. M., Qian, Y., and Rong, Z. Q. (1994). Study of monoplacophora and gastropoda form lower cambrian Xinji Formation in yexian, henan. Acta Micropalaeontologica Sin. 11, 1–19. (in Chinese with English abstract).
Geyer, G. (1994). Middle Cambrian molluscs from Idaho and early conchiferan evolution. N. Y. State Mus. Bull. 481, 69–86.
Gravestock, D. I., Alexander, E. M., Demidenko, Y. E., Esakova, N. V., Holmer, L. E., Jago, J. B., et al. (2001). The cambrian biostratigraphy of the stansbury basin, South Australia. Berlin, Germany: Transactions of the Palaeontological Institute, Russian Academy of Sciences, 1–344.
Gubanov, A. P., Kouchinsky, A. V., and Peel, J. S. (1999). The first evolutionary-adaptive lineage within fossil molluscs. Lethaia 32, 155–157. doi:10.1111/j.1502-3931.1999.tb00534.x
Gubanov, A. P., and Peel, J. S. (2000). Cambrian monoplacophoran molluscs (class Helcionelloida). Am. Malacol. Bull. 15, 139–145.
Gubanov, A. P., and Peel, J. S. (1999). Oelandiella, the earliest cambrian helcionelloid mollusc from Siberia. Palaeontology 42, 211–222. doi:10.1111/1475-4983.00070
Gubanov, A. P., and Peel, J. S. (2003). The early cambrian helcionelloid mollusc Anabarella vostokova. Palaeontology 46, 1073–1087. doi:10.1111/1475-4983.00334
Gubanov, A. P., Skovsted, C. B., and Peel, J. S. (2004). Anabarella australis (Mollusca, Helcionelloida) from the lower cambrian of Greenland. Geobios 37, 719–724. doi:10.1016/j.geobios.2003.05.009
Gubanov, A. P. (1998). The Early Cambrian molluscan evolution and its palaeogeographic implications. Acta Univ. Carolinae-Geologica 42, 419–422.
Guo, J. F., Han, J., Van Iten, H., Wang, X., Qiang, Y. Q., Song, Z. C., et al. (2020). A fourteen-faced hexangulaconulariid from the early cambrian (stage 2) Yanjiahe Formation, South China. J. Paleontology 94, 45–55. doi:10.1017/jpa.2019.56
Guo, J. F., Li, G. X., Qiang, Y. Q., Song, Z. C., Zhang, Z. F., Han, J., et al. (2021). Watsonella crosbyi from the lower cambrian (terreneuvian, stage 2) Yanjiahe Formation in three Gorges area, South China. Palaeoworld 30, 1–19. doi:10.1016/j.palwor.2020.04.006
Guo, J. F., Li, Y., and Li, G. X. (2014). Small shelly fossils from the early cambrian Yanjiahe Formation, Yichang, Hubei, China. Gondwana Res. 25, 999–1007. doi:10.1016/j.gr.2013.03.007
He, T. G., Pei, F., and Fu, G. H. (1984). Some small shelly fossils from the lower cambrian Xinji Formation in fangcheng county, henan province. Acta Palaeontol. Sin. 23, 350–357. (in Chinese with English abstract).
Hicks, H. (1872). On some undescribed fossils from the Menevian Group of Wales. Q. J. Geol. Soc. Lond. 27, 41–42. doi:10.1144/gsl.jgs.1872.028.01-02.17
Isakar, M., and Peel, J. S. (2007). Lower Cambrian helcionelloid molluscs from Estonia. GFF 129, 255–262. doi:10.1080/11035890701293255
Jacquet, S. M., Betts, M. J., Huntley, J. W., and Brock, G. A. (2019). Facies, phosphate, and fossil preservation potential across a Lower Cambrian carbonate shelf, Arrowie Basin, South Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 533, 109200. doi:10.1016/j.palaeo.2019.05.022
Jermak, V. V., and Pelman, Y. L. (1986). “Some Cambrian molluscs and brachiopods of northern Kharaulakh,” in Cambrian biostratigraphy and palaeontology of northern asia. Editor L. T. Zhuravleva (Russia: Trudy, Institut geologii i geofiziki, Sibirskoe otdelenie, Akademiya nauk SSSR), 188–200. (in Russian).
Khomentovsky, V. V., and Karlova, G. A. (1993). Biostratigraphy of the vendian-cambrian beds and the lower cambrian boundary in Siberia. Geol. Mag. 130, 29–45. doi:10.1017/s0016756800023700
Kouchinsky, A. V., Bengtson, S., Landing, E., Steiner, M., Vendrasco, M. J., and Ziegler, K. (2017). Terreneuvian stratigraphy and faunas from the anabar uplift, Siberia. Acta Palaeontol. Pol. 62, 311–440. doi:10.4202/app.00289.2016
Kouchinsky, A. V. (2000). Shell microstructures in early cambrian molluscs. Acta Palaeontol. Pol. 45, 119–150.
Kouchinsky, A. V. (1999). Shell microstructures of the early cambrian Anabarella and Watsonella as new evidence on the origin of the rostroconchia. Lethaia 32, 173–180. doi:10.1111/j.1502-3931.1999.tb00537.x
Landing, E. (1989). Paleoecology and distribution of the early cambrian rostroconch Watsonella crosbyi grabau. J. Paleontology 63, 566–573. doi:10.1017/s0022336000041196
Lendzion, K., and Posti, E. (1983). “Gastropoda,” in Upper precambrian and cambrian palaeontology of the east-European platform. Editors A. Urbanek, and A. Y. Rozanov (Warsaw: Wadawnictwa Geologiczne Publishing House), 158.
Li, G. X., Zhao, X., Gubanov, A. P., Zhu, M. Y., and Na, L. (2011). Early cambrian mollusc Watsonella crosbyi: A potential GSSP index fossil for the base of the cambrian stage 2. Acta Geol. Sinica-English Ed. 85, 309–319. doi:10.1111/j.1755-6724.2011.00400.x
Li, L. Y., Skovsted, C. B., and Topper, T. P. (2022). Deep origin of the crossed-lamellar microstructure in early Cambrian molluscs. Palaeontology 65, 1–14. doi:10.1111/pala.12620
Li, L. Y., Zhang, X. L., Skovsted, C. B., Yun, H., Li, G. X., and Pan, B. (2019). Shell microstructures of the helcionelloid mollusc Anabarella australis from the lower cambrian (series 2) Xinji Formation of North China. J. Syst. Palaeontol. 17, 1699–1709. doi:10.1080/14772019.2018.1546236
Lu, Y. H. (1979). Cambrian mineral deposits in China and the bio-environmental control hypothesis. Beijing: Geological Publishing House, 75. (in Chinese).
Luo, H. L., Jiang, Z. W., Wu, X. C., Song, X. L., and Ouyang, L. (1982). The sinian–cambrian boundary in eastern yunnan, China. Kunming: The Yunnan People’s Publishing House, 265. (in Chinese with English abstract).
Mackinnon, D. I. (1985). New Zealand late Middle Cambrian molluscs and the origin of Rostroconchia and Bivalvia. Alcheringa 9, 65–81. doi:10.1080/03115518508618959
Maloof, A. C., Porter, S. M., Moore, J. L., Dudás, F. Ö., Bowring, S. A., Hoggins, J. A., et al. (2010). The earliest Cambrian record of animals and ocean geochemical change. Geol. Soc. Am. Bull. 122, 1731–1774. doi:10.1130/b30346.1
Mens, K., and Isakar, M. (1999). Facies distribution of early Cambrian molluscs in Estonia. Proc. Est. Acad. Sci. Geol. 48, 110. doi:10.3176/geol.1999.2.04
Missarzhevsky, V. V. (1989). The oldest skeletal fossils and stratigraphy of the Precambrian–Cambrian boundary beds. Trudy Geol. Instituta SSSR 443, 1–237. (in Russian).
Parkhaev, P. Y., and Demidenko, Y. E. (2010). Zooproblematica and mollusca from the lower cambrian meishucun section (yunnan, China) and taxonomy and systematics of the cambrian small shelly fossils of China. Paleontological J. 44, 883–1161. doi:10.1134/s0031030110080010
Parkhaev, P. Y., and Karlova, G. A. (2011). Taxonomic revision and evolution of cambrian mollusks of the genus Aldanella vostokova, 1962 (gastropoda: Archaeobranchia). Paleontological J. 45, 1145–1205. doi:10.1134/s0031030111100030
Parkhaev, P. Y. (2004). Malacofauna of the lower cambrian bystraya Formation of eastern transbaikalia. Paleontological J. 38, 590–608.
Parkhaev, P. Y. (2006). New data on the morphology of ancient gastropods of the genus Aldanella vostokova, 1962 (archaeobranchia, pelagielliformes). Paleontological J. 40, 244–252. doi:10.1134/s0031030106030038
Parkhaev, P. Y. (2002). Phylogenesis and the system of the cambrian univalved mollusks. Paleontological J. 36, 25–36.
Parkhaev, P. Y. (2008). “The early cambrian radiation of Mollusca,” in Phylogeny and evolution of the Mollusca. Editors W. F. Ponder, and D. R. Lindberg (Berkeley: University California Press), 33–69.
Parkhaev, P. Y. (2000). The functional morphology of the cambrian univalved mollusks–helcionellids 1. Paleontological J. 34, 32–38.
Parkhaev, P. Y. (2001). The functional morphology of the cambrian univalved mollusks–helcionellids 2. Paleontological J. 35, 470–475.
Peel, J. S. (2021). An outer shelf shelly fauna from cambrian series 2 (stage 4) of North Greenland (laurentia). J. Paleontology 95, 1–41. doi:10.1017/jpa.2020.112
Peel, J. S. (1991). “Functional morphology of the Class Helcionelloida nov., and the early evolution of the Mollusca,” in The early evolution of metazoa and the significance of problematic taxa. Editors A. Simonetta, and S. Conway Morris (Cambridge: Cambridge University Press and University of Camerino), 157–177.
Peel, J. S., Streng, M., Geyer, G., Kouchinsky, A., and Skovsted, C. B. (2016). Ovatoryctocara granulata assemblage (cambrian series 2-series 3 boundary) of løndal, North Greenland. Australas. Palaeontol. Memoirs 49, 241–282.
Pospelov, A. G., Pelman, Y. L., Zhuravleva, I. T., Luchinina, V. A., Kuznetsova, V. G., Esakova, N. V., et al. (1995). Biostratigraphy of the kiya river section. Ann. Paléontologie 81, 169–246.
Qian, Y., Feng, W. M., Li, G. X., Yang, A. H., Feng, M., Zhao, X., et al. (2009). Taxonomy and biostratigraphy of the early Cambrian univalved mollusc fossils from Xinjiang. Acta Micropalaeontologica Sin. 26, 193–210. (in Chinese with English abstract).
Qian, Y. (1999). Taxonomy and biostratigraphy of small shelly fossils in China. Beijing: Science Press, 247. (in Chinese with English abstract).
Rozanov, A. Y., Missarzhevsky, V. V., Volkova, N. A., Voronova, L. G., Krylov, I. N., keller, B. M., et al. (1969). The Tommotian Stage and the Cambrian lower boundary problem. Tr. Geol. Instituta Akad. Nauk. SSSR 206, 1–380. (in Russian).
Runnegar, B., and Jell, P. A. (1976). Australian Middle Cambrian molluscs and their bearing on early molluscan evolution. Alcheringa 1, 109–138. doi:10.1080/03115517608619064
Runnegar, B., and Pojeta, J. (1974). Molluscan phylogeny: The paleontological viewpoint. Science 186, 311–317. doi:10.1126/science.186.4161.311
Runnegar, B. (1985). Shell microstructures of Cambrian molluscs replicated by phosphate. Alcheringa 9, 245–257. doi:10.1080/03115518508618971
Shu, D. G. (2008). Cambrian explosion: Birth of tree of animals. Gondwana Res. 14, 219–240. doi:10.1016/j.gr.2007.08.004
Skovsted, C. B. (2004). Mollusc fauna of the early cambrian bastion Formation of north-east Greenland. Bull. Geol. Soc. Den. 51, 11–37. doi:10.37570/bgsd-2004-51-02
Skovsted, C. B. (2006). Small shelly fossils from the basal emigrant formation (cambrian, uppermost dyeran stage) of split mountain, Nevada. Can. J. Earth Sci. 43, 487–496. doi:10.1139/e05-119
Steiner, M., Yang, B., Hohl, S., Zhang, L., and Chang, S. (2020). Cambrian small skeletal fossil and carbon isotope records of the southern Huangling Anticline, Hubei (China) and implications for chemostratigraphy of the Yangtze Platform. Palaeogeogr. Palaeoclimatol. Palaeoecol. 554, 109817. doi:10.1016/j.palaeo.2020.109817
Ushatinskaya, G. T., and Parkhaev, P. Y. (2005). Preservation of imprints and casts of cells of the outer mantle epithelium in the shells of Cambrian brachiopods, mollusks, and problematics. Paleontological J. 39, 251–263.
Val’kov, A. K. (1987). The lower Cambrian biostratigraphy of the eastern part of the Siberian Platform, the Yudomo-Olenek region. Moscow: Nauka, 135.
Vendrasco, M. J., Checa, A. G., and Kouchinsky, A. V. (2011a). Shell microstructure of the early bivalve pojetaia and the independent origin of nacre within the mollusca. Palaeontology 54, 825–850. doi:10.1111/j.1475-4983.2011.01056.x
Vendrasco, M. J., Kouchinsky, A. V., Porter, S. M., and Fernandez, C. Z. (2011b). Phylogeny and escalation in Mellopegma and other Cambrian molluscs. Palaeontol. Electron. 14, 199–215.
Vendrasco, M. J., Porter, S. M., Kouchinsky, A. V., Li, G. X., and Fernandez, C. Z. (2010). Shell microstructures in early mollusks. Festivus 10, 43–54.
Vidal, G., Palacios, T., Moczydłowska, M., and Gubanov, A. P. (1999). Age constraints from small shelly fossils on the early Cambrian terminal Cadomian Phase in Iberia. GFF 121, 137–143. doi:10.1080/11035899901212137
Voronin, Y. L., Voronova, L. G., Grigorieva, N. V., Drosdova, N. A., Zhegallo, E. A., Zhuravlev, A. Y., et al. (1982). The precambrian/cambrian boundary in the geosynclinal areas (the reference section of salany-gol, MRP). Tr. Sovmest. Sovetsko-Mongol'skaya Paleontol. ekspeditsiya 18, 1–152. (in Russian).
Vostokova, V. A. (1962). The cambrian gastropods from Siberia and taimyr. Tr. Naučno-Issledovatel’skogo Instituta Geol. Arktiki 28, 51–74. (In Russian).
Wenz, W. (1938). “Gastropoda. Allgemeiner Teil und Prosobranchia,” in Handbuch der Palaeozoologie. Band 6. Editor O. H. Schindewolf (Berlin: Borntraeger), 1–720.
Xing, Y. S., Ding, Q. X., Luo, H. L., He, T. G., Wang, Y. G., et al. (1983). The Sinian-Cambrian boundary of China. Beijing: Bulletin of the Institute of Geology, Chinese Academy of Geological Sciences, 262. (in Chinese with English abstract).
Yang, B., Steiner, M., Li, G. X., and Keupp, H. (2014). Terreneuvian small shelly faunas of East Yunnan (South China) and their biostratigraphic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 398, 28–58. doi:10.1016/j.palaeo.2013.07.003
Yu, W. (1974). “Cambrian gastropoda,” in Handbook of the stratigraphy and Paleontology of southwest China (Beijing: Sci. Press), 113. (in Chinese).
Keywords: Anabarella plana, taxonomic revision, microstructures, Yanjiahe Formation, Cambrian Stage 2, South China
Citation: Qiang Y, Peng J, Song Z, Sun J, Zhao X, Li G, Han J and Guo J (2023) Early Cambrian Anabarella plana from Three Gorges area, South China. Front. Earth Sci. 10:1074000. doi: 10.3389/feart.2022.1074000
Received: 19 October 2022; Accepted: 28 December 2022;
Published: 17 January 2023.
Edited by:
Ben Yang, Chinese Academy of Geological Sciences (CAGS), ChinaReviewed by:
Andrej Ernst, University of Hamburg, GermanyCopyright © 2023 Qiang, Peng, Song, Sun, Zhao, Li, Han and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junfeng Guo, anVuZmVuZ2dAY2hkLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.