
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Earth Sci. , 18 January 2023
Sec. Geochemistry
Volume 10 - 2022 | https://doi.org/10.3389/feart.2022.1060213
This article is part of the Research Topic Mechanisms and Applications of Chemical Techniques for Effective Development of Unconventional Reservoirs View all 13 articles
The matrix acidizing method has been widely employed in the petroleum industry to increase the output of oil and gas from reservoirs that have low permeability and are experiencing formation degradation as a result of drilling, stimulation, and completion activity (Mohsin et al., 2019). It is a method of dissolving carbonate reservoirs (calcite or dolomite) by injection of HCl, which reacts with minerals in the rock formation to increase permeability and production close to the wellbore (Buijse and Domelen, 1998). The new ‘wormhole’ channels are created by the introduction of the treatment fluid at a downhole injection pressure just below the fracturing pressure to bypass the damaged zone near the wellbore (Amro, 2002; Kiani et al., 2021). The wormholes are formed from the reaction of HCl with the CaCO3 and CaMg(CO3)2 minerals in the matrix, which avoids the possibility of formation damage (Chang et al., 2007).
In carbonate formations, the purpose of matrix acidizing is primarily to restore and enhance the formation’s productivity; but, it also reduces near-wellbore damage and establishes novel hydrocarbon flow channels (wormholes) (Ibrahim et al., 2020). By obstructing the path that the acid normally takes, these agents redirect it to the less permeable, untreated zones as shown in Figure 1 (Ghommem et al., 2015). The use of HCl alone for acidization, especially in higher temperature reservoirs, has suffered some signal failures. A large volume of acid is required because the rapid reaction of the acid with the carbonate minerals of the formation consumes acid at a far faster rate (Chavez, 2007). Due to the shorter residence time of the acid in the formation, especially at high temperatures, the wormholes created are less efficient hydrocarbon channels (Chacon and Pournik, 2022). Therefore, gelled acid, foamed acid, micro-emulsified acids, and other chemical agents have been formulated in an effort to circumvent the drawbacks of the fast acid-rock reaction by lowering H+ ion dissociation, reducing H+ ion mass transfer, and changing the wettability of the rock surface (Zhu et al., 2022).
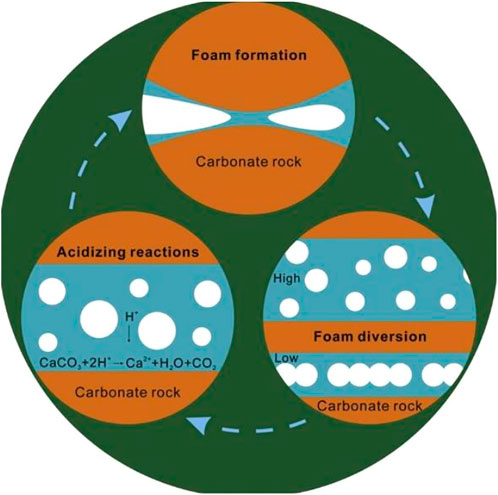
FIGURE 1. Induced acid diversion by foam-based acid (Yan et al., 2019).
The gelled acid fluid is comprised of water, a water-dispersible polymer, an acid, and a gelling agent (Chang et al., 2007; Ismail and Kweh, 2012). Two types of polymer-based acid have been used in the field for matrix acidization: gelled acid for acid retardation and in situ gelled acids for acid diversion. The in situ formulation includes polymers or VES, crosslinkers, and pH-controlled breakers to achieve dissolution by preventing the acid from passing through highly permeable zones and diverting it to less-permeable zones (Ratnakar et al., 2012; Gomaa and Nasr-El-Din, 2015). In matrix acidization, the gelled acid relies on the viscosity difference of the fluid to reduce the mass transfer of H+ ions and consequently the acid reaction rate. The use of polymers as viscosifiers poses a challenge, however, because of the buildup of residual chemicals in the formation, which can damage it (Chang et al., 2007).
The formulation of a micro-emulsion acid requires that the hydrochloric acid is the internal phase and that the hydrocarbons (crude oil and diesel) act as the external phase (Zakaria et al., 2015). The hydrocarbons reduce the diffusion rate of the HCl droplets and lower the dissolution rate of the rock surface, thus generating highly permeable wormholes (Carvalho et al., 2019). The use of micro-emulsified acid has an advantage over other matrix acidizing methods in that it creates more conductive channels and reduces the mobility or effective diffusivity of HCl without jeopardizing the formation productivity (Fredd et al., 2016). A transient skin factor is created in the high permeability zones, thus diverting the acid to the lower permeability zones (Liu et al., 2013). The reduction in contact time and area between the acid and the casing and tubing minimizes corrosion (Liu et al., 2013; Derendyaev et al., 2022).
Foamed acid is formed by mixing acid and surfactants and injecting gas (N2/CO2). Compared to traditional acidizing processes, foaming has the advantages of diversion, moderate speed, low water sensitivity, increased energy to assist discharge, and less damage to the formation; thus, foam diversion acidizing technology has attracted much attention in recent years (Motta et al., 2021; Yuan et al., 2021). Foamed acid may be used to uniformly treat the entire formation by temporarily limiting acid access to the higher permeability zones and channeling it to the less permeable zones (Letichevskiy et al., 2017; Yan et al., 2019). In comparison to typical acid treatments, foamed-VES formulations have shown excellent acid diversion performance and also pose little threat of formation damage because they contain no polymers (Chang et al., 2007; Yan et al., 2019). They also contain no solids that could impair permeability, and the gas expansion in the foam allows easy flow back during well cleaning (Chang et al., 2007). The retarding action of foaming slows down the speed with which the acid reacts with the rock, resulting in deep matrix acidification (Stringfellow et al., 2017; Zhang et al., 2021).
Due to the fact that carbonate reservoirs are heterogeneous with varied permeability zones and a high temperature environment, the selection criteria for choosing one of the three proposed matrix acidizing methods must be careful observed. Various researchers have reported that foam-based acids were superior to traditional acidizing methods. They provided effective diversion, reduced the acid reaction rate and water sensitivity, increased energy for discharge of acid into the formation, and caused less harm to the formation. Foaming lowered the effective acid diffusion coefficients into the formation, thus regulating convection and diffusion rate. Foamed acid remains in contact longer with the less permeable zones of the formation, and the process prevents the carbonate-acid reaction from happening too rapidly in the highly permeable zones. At low injection rates, the poor diffusivity of micro-emulsified acid produces narrow but deeper effective wormholes.
The gelled acid relies on viscosity differences in the fluid to reduce the mass transfer of H+ and the acid reaction rate. Despite its good volumetric efficiency and acid diversion tendency, the field application of gelled acid is limited by the buildup of residuals that impair the flow of oil back through the wormholes to the wellbore. The polymers used as viscosifiers can remain as residual chemicals in the formation and lead to damage; therefore, VES formulations are preferred.
CW: investigation and research, writing manuscript draft; LZ: resources and conceptualization; BH: modify analysis; XZ: typesetting; AM: supervision.
Author BH was employed by Zhenhua Oil Co. Ltd.
The authors declare that this study received funding from PetroChina “Fourteenth Five Year” Significant Programs (No. 2021DJ3203). The funder had the following involvement in the study: design, collection, analysis, interpretation of data, and the writing of this article.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amro, M. M. (2002). Laboratory study and field matching of matrix acidizing of petroleum reservoir rocks. J. King Saud Univ. Eng. Sci. 14 (1), 119–135. doi:10.1016/S1018-3639(18)30748-7
Buijse, M. A., and Domelen, M. S. (1998). “SPE 39583 novel application of emulsified acids to matrix stimulation of heterogeneous formations,” in SPE formation damage control conference (Lafayette, Louisiana: Society of Petroleum Engineers).
Carvalho, R. T. R., Oliveira, P. F., Palermo, L. C. M., Ferreira, A. A. G., and Mansur, C. R. E. (2019). Prospective acid microemulsions development for matrix acidizing petroleum reservoirs. Fuel 238, 75–85. doi:10.1016/j.fuel.2018.10.003
Chacon, O. G., and Pournik, M. (2022). Matrix acidizing in carbonate formations. Processes 10 (1), 174. doi:10.3390/pr10010174
Chang, F. F., Qiu, X., and Nasr-El-Din, H. A. (2007). “Chemical diversion techniques used for carbonate matrix acidizing: An overview and case histories,” in Proceedings - SPE international symposium on oilfield chemistry (Houston, Texas, U.S.A: Society of Petroleum Engineers), 574–579. doi:10.2118/106444-ms
Chavez, M. R. (2007). Evaluation and optimisation of combinatorial optimization heuristic algorithms. arXiv.
Derendyaev, R. A., Novikov, V. A., Martyushev, D. A., Liu, Z., and Yang, Y. (2022). Acid treatment of carbonate reservoir with a new dual action microemulsion: Selection of optimal application conditions. J. Petroleum Sci. Eng. 216, 110809. doi:10.1016/j.petrol.2022.110809
Fredd, C. N., Hoefner, M. L., Fredd, C. N., Hoefner, M. L., Fogler, H. S., and Fogler, H. S. (2016). Microemulsion applications applications in microemulsion in carbonate carbonate reservoir reservoir stimulation stimulation. London: IntechOpen. doi:10.5772/65973
Ghommem, M., Zhao, W., Dyer, S., Qiu, X., and Brady, D. (2015). Carbonate acidizing: Modeling, analysis, and characterization of wormhole formation and propagation. J. Petroleum Sci. Eng. 131, 18–33. doi:10.1016/j.petrol.2015.04.021
Gomaa, A. M., and Nasr-El-Din, H. A. (2015). Effect of elastic properties on the propagation of gelled and in-situ gelled acids in carbonate cores. J. Petroleum Sci. Eng. 127, 101–108. doi:10.1016/j.petrol.2015.01.031
Ibrahim, A. F., Nasr-El-Din, H., and Jiang, L. (2020). “HP/HT matrix acidizing treatments of carbonate rocks using a new retarded HCl acid system,” in International petroleum technology conference 2020 IPTC (Dhahran, Kingdom of Saudi Arabia: Society of Petroleum Engineers), 1–14. doi:10.2523/iptc-19646-ms
Ismail, I., and Kweh, W. L. (2012). Matrix acidizing with gelled acid. J. Teknol. 38, 13–24. doi:10.11113/jt.v38.505
Kiani, S., Jafari, S., Apourvari, S. N., and Mehrjoo, H. (2021). Simulation study of wormhole formation and propagation during matrix acidizing of carbonate reservoirs using a novel in-situ generated hydrochloric acid. Adv. Geo-Energy Res. 5 (1), 64–74. doi:10.46690/ager.2021.01.07
Letichevskiy, A., Nikitin, A., Parfenov, A., Makarenko, V., Lavrov, I., and Samaraneftegaz, J. S. C. (2017). “SPE-187844-MS foam acid treatment - the key to stimulation of carbonate reservoirs in depleted oil fields of the samara region,” in SPE Russian petroleum technology conference (Moscow, Russia: Society of Petroleum Engineers).
Liu, M., Zhang, S., Mou, J., Zhou, F., and Shi, Y. (2013). Diverting mechanism of viscoelastic surfactant-based self-diverting acid and its simulation. J. Petroleum Sci. Eng. 105, 91–99. doi:10.1016/j.petrol.2013.03.001
Mohsin, M., Mysara, Y., Mohyaldinn, E., Muhammad, E., Abdalla, M., Amir, A., et al. (2019). Synthesis and evaluation of Jatropha oil-based emulsified acids for matrix acidizing of carbonate rocks. J. Pet. Explor. Prod. Technol. 9 (2), 1119–1133. doi:10.1007/s13202-018-0530-8
Motta, A. B. G., Thompson, R. L., Favero, J. L., Dias, R. A. C., Silva, L. F. L. R., Costa, F. G., et al. (2021). Rheological effects on the acidizing process in carbonate reservoirs. J. Petroleum Sci. Eng. 207, 109122. doi:10.1016/j.petrol.2021.109122
Ratnakar, R. R., Kalia, N., and Balakotaiah, V. (2012). Carbonate matrix acidizing with gelled acids: An experiment-based modeling study. SPE Prod. Operations Symposium, Proc. 1, 207–222. doi:10.2118/154936-ms
Stringfellow, W. T., Camarillo, M. K., Domen, J. K., Sandelin, W. L., Varadharajan, C., Jordan, P. D., et al. (2017). Identifying chemicals of concern in hydraulic fracturing fluids used for oil production. Environ. Pollut. 220, 413–420. doi:10.1016/j.envpol.2016.09.082
Yan, Y. li, Xi, Q., Unachibuike, C. B. W., Dou, L., and Wu, C. s. (2019). A novel acidizing technology in carbonate reservoir: In-situ formation of CO2 foamed acid and its self-diversion. Colloids Surfaces A Physicochem. Eng. Aspects 580, 123787. doi:10.1016/j.colsurfa.2019.123787
Yuan, H., Chen, X., Li, N., Zhou, H., Gong, Y., and Wang, Y. (2021). Numerical simulation of foam diversion acidizing in heterogeneous reservoirs. Petroleum. doi:10.1016/j.petlm.2021.01.006
Zakaria, A. S., Nasr-El-Din, H. A., and Ziauddin, M. (2015). Flow of emulsified acid in carbonate rocks. Ind. Eng. Chem. Res. 54 (16), 4190–4202. doi:10.1021/ie504167y
Zhang, L., He, J., Wang, H., Li, Z., Zhou, F., and Mou, J. (2021). Experimental investigation on wormhole propagation during foamed-VES acidizing. J. Petroleum Sci. Eng. 198, 108139. doi:10.1016/j.petrol.2020.108139
Keywords: tight carbonate reservoirs, matrix acidizing, gelled acids, foamed acidizing fluid, micro-emulsified acid
Citation: Wang C, Zhao L, He B, Zhang X and Mmbuji AO (2023) Comparative analysis of matrix-retarded acidizing methods for tight carbonate reservoirs: Gelled acid, micro-emulsified acid, and foamed acid. Front. Earth Sci. 10:1060213. doi: 10.3389/feart.2022.1060213
Received: 03 October 2022; Accepted: 31 October 2022;
Published: 18 January 2023.
Edited by:
Zhilin Cheng, Xi’an Shiyou University, ChinaReviewed by:
Yang Wang, Xi’an Shiyou University, ChinaCopyright © 2023 Wang, Zhao, He, Zhang and Mmbuji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Athumani Omari Mmbuji, NTkzNDUxMDQ0QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.