- Paläontologisches Institut und Museum, Zürich, Switzerland
About half of all vertebrate species today are ray-finned fishes (Actinopterygii), and nearly all of them belong to the Neopterygii (modern ray-fins). The oldest unequivocal neopterygian fossils are known from the Early Triassic. They appear during a time when global fish faunas consisted of mostly cosmopolitan taxa, and contemporary bony fishes belonged mainly to non-neopterygian (“paleopterygian”) lineages. In the Middle Triassic (Pelsonian substage and later), less than 10 myrs (million years) after the Permian-Triassic boundary mass extinction event (PTBME), neopterygians were already species-rich and trophically diverse, and bony fish faunas were more regionally differentiated compared to the Early Triassic. Still little is known about the early evolution of neopterygians leading up to this first diversity peak. A major factor limiting our understanding of this “Triassic revolution” is an interval marked by a very poor fossil record, overlapping with the Spathian (late Olenekian, Early Triassic), Aegean (Early Anisian, Middle Triassic), and Bithynian (early Middle Anisian) substages. Here, I review the fossil record of Early and Middle Triassic marine bony fishes (Actinistia and Actinopterygii) at the substage-level in order to evaluate the impact of this hiatus–named herein the Spathian–Bithynian gap (SBG)–on our understanding of their diversification after the largest mass extinction event of the past. I propose three hypotheses: 1) the SSBE hypothesis, suggesting that most of the Middle Triassic diversity appeared in the aftermath of the Smithian-Spathian boundary extinction (SSBE; ∼2 myrs after the PTBME), 2) the Pelsonian explosion hypothesis, which states that most of the Middle Triassic ichthyodiversity is the result of a radiation event in the Pelsonian, and 3) the gradual replacement hypothesis, i.e. that the faunal turnover during the SBG was steady and bony fishes were not affected by extinction events subsequent to the PTBME. Based on current knowledge, hypothesis three is favored herein, but further studies are necessary to test alternative hypotheses. In light of the SBG, claims of a protracted diversification of bony fishes after the PTBME should be treated with caution.
Introduction
In the wake of the most severe mass extinction event at the Permian-Triassic boundary, ca. 252 Ma ago (Baresel et al., 2017), the Triassic witnessed the recovery of various metazoan groups, as is evidenced by their fossil record. During the last decades, a wealth of data has shed new light on the evolutionary changes of various clades during and after mass extinctions, including the Permian-Triassic boundary mass extinction event (PTBME). With regard to bony fishes (herein I only refer to the non-tetrapod members of Osteichthyes), low diversity during most of the Permian was followed by an impressive diversification in the aftermath of the PTBME, leading to a peak in taxic richness in the Middle Triassic, less than 10 myrs (million years) after the event (Tintori et al., 2014a; Friedman 2015; Romano et al., 2016a). While bony fishes became more diverse in the Triassic, it is worth noting that they seemingly remained unaffected by the PTBME (e.g. Romano et al., 2016a; Smithwick and Stubbs 2018). Therefore, rather than a recovery, the diversification of bony fishes after the PTBME should be considered an opportunistic radiation after the demise of other nektonic groups.
The timing and pattern of the diversification (or recovery) of different organismic groups following the PTBME has been controversially discussed in recent years. Whereas for some clades a slow or protracted increase in diversity is noted (e.g. Chen and Benton 2012), for others, species richness increased rapidly (e.g. Orchard 2007; Brayard et al., 2009). Some authors argued for a slow diversification of bony fishes after the PTBME, often citing the very diverse Middle Triassic assemblages from South China and the Southern Alps as examples of fully recovered ecosystems (e.g. Chen and Benton 2012; Benton et al., 2013; Tintori et al., 2014a). Regardless of the timing, the radiation of bony fishes at the onset of the Mesozoic led to the evolution of new clades, probably most importantly among the Neopterygii, the most species-rich clade of bony fishes today (>99.9%), and the most diverse group of all extant vertebrates (∼50% of species; Sallan 2014). Unambiguous members of Neopterygii first appear in the Early Triassic, although based on molecular clock studies (e.g. Near et al., 2012) a Paleozoic origin is suspected. A first diversity peak among neopterygians is evident in the Middle Triassic, both in terms of genus richness and trophic adaptations (Bürgin, 1996; Friedman, 2015). However, due to the inadequately known post-PTBME record of neopterygians and other bony fishes, the detailed pattern of their diversifications is not well understood. In particular, the Spathian (late Olenekian, Early Triassic; Tozer 1965), Aegean (Early Anisian, Middle Triassic; Balini et al., 2010), and Bithynian (early Middle Anisian) substages still suffer from a patchy fossil record. This hiatus, coined herein the “Spathian–Bithynian gap”, separates the better-known faunas from the Griesbachian, Dienerian, and Smithian intervals (Early Triassic; Tozer 1965) on the one hand from those of the later part of the Middle Triassic on the other hand.
Here, I review the fossil record of Early–Middle Triassic marine bony fishes, aiming to assess the implications of the Spathian–Bithynian gap on our understanding of their Triassic radiation. My analyses are based on substage-level occurrence data of both Actinistia (coelacanths) and Actinopterygii, which I compiled from the literature (see Romano et al., 2016a for references published until 2013; studies published thereafter are cited in the text). I exclude dipnoans from my analyses because their Triassic record is mostly restricted to the freshwater. Substage-level occurrence data for actinopterygian and actinistian taxa from the Middle Triassic site Monte San Giorgio are based on the literature and the collection data base of the Paleontological Institute and Museum, University of Zurich (PIMUZ). I focus primarily on marine and brackish occurrences due to the finer and usually more precise biostratigraphic age control compared to the freshwater record. Additionally, it has been inferred that freshwater environments served as refugia for taxa that disappeared in the marine realm earlier (e.g. Romano et al., 2012; Romano et al., 2016a). In agreement with this view, there is also evidence for a marine ancestry of present-day freshwater fishes and not the other way around (Betancur-R. et al., 2015). Late Early Triassic and early Middle Triassic freshwater fishes described from southern Gondwana and Laurasia are dominated by non-neopterygians and a few perleidiforms (see Romano et al., 2016a and references therein). Based on the above, the freshwater record is probably inadequate to compensate the lack of knowledge resulting from gaps in the marine record of bony fishes. Finally, I concentrate mainly on Early–Middle Triassic collecting sites that yield relatively complete bony fish fossils, as such specimens allow for more secure taxonomic and systematic interpretations.
The Early Triassic Record
Griesbachian
The Griesbachian (early Induan; Tozer 1965) is the first stage of the Early Triassic. This interval covers the immediate aftermath of the PTBME. Very little is known about marine Griesbachian Actinistia and Actinopterygii because our knowledge is based almost exclusively on fossils from sites in East Greenland. The only exception is a tooth plate of Bobasatrania from the Salt Range, Pakistan (Böttcher 2014). Fossils from East Greenland were collected mostly during the first half of the 20th century by Danish expeditions (e.g. Stensiö 1932; Nielsen 1936; Nielsen 1961; Brinkmann et al., 2010), but also during subsequent fieldtrips by other researchers (e.g. by R. Trümpy, material stored at PIMUZ).
The Early Triassic fishes of East Greenland originate from six horizons (Nielsen 1942; Nielsen 1949; Perch-Nielsen et al., 1974), the lower five of which (“fish-zones” I–V) are dated as early to late Griesbachian (Surlyk et al., 2017), whereas the sixth horizon is early Dienerian in age (see below). Biostratigraphic control comes from ammonoids. Although the ichthyofaunal compositions of these six horizons differ (Nielsen 1961), collectively they show that all of the typical Early Triassic taxa were already present in the Griesbachian: Australosomus, Birgeria, Bobasatrania, Boreosomus (Ptycholepidae), ?Errolichthys, Helmolepis (Platysiagidae), parasemionotids (Broughia, Ospia, ?Parasemionotus, ?Watsonulus) Pteronisculus, Saurichthys, ?Teffichthys (“Perleidus”; Marramà et al., 2017), Sassenia, and Whiteia (Stensiö 1932; Nielsen 1936; Nielsen 1942; Nielsen 1949; Nielsen 1952; Nielsen 1955; Nielsen 1961; Nybelin 1977; Mutter 2005; Kogan 2011; Argyriou et al., 2018). These taxa are part of Tintori et al. (2014a)'s “Triassic Early Fish Fauna” (TEFF).
In contrast to the Dienerian and Smithian (see below), the good preservation of the bony fish fossils in the Griesbachian of Greenland is not linked to widespread anoxia on marine shelves. Rather, it is hypothesized that the preservation of articulated and complete skeletons was favored by the globally low sea-level at the time (pers. comm. H. Bucher, 2020). The low sea level led to the formation of local silled basins with low O2 content, as was, for instance, the case in the Nanpanjiang basin in South China (Bagherpour et al., 2017).
Dienerian
The second stage of the Early Triassic is the Dienerian (late Induan; Tozer 1965). The Dienerian was characterized by the widespread distribution of anoxia on continental shelves (Ware et al., 2015) and elevated temperatures (Romano et al., 2013). Marine anoxia favored the preservation of skeletons of actinistian and actinopterygian fishes (cf. Tintori 1992). As a result, articulated and complete fossils of these animals can frequently be found in Dienerian aged marine deposits around the globe (Canada, China, Greenland, India, Madagascar, United States).
A long-known locality preserving marine Dienerian osteichthyans is northwest Madagascar (e.g. Lehman 1952; Nielsen 1955; Beltan 1968; Brinkmann et al., 2010; Marramà et al., 2017). The fishes occur in float concretions. A Dienerian to possibly earliest Smithian age is indicated by ammonoids that are occasionally preserved in the same nodules as the fishes (see Kogan and Romano 2016a). The fossiliferous layers have yielded taxa such as Australosomus, Birgeria, Bobasatrania, Boreosomus (Ptycholepidae), Ecrinesomus, Errolichthys, Helmolepis (Platysiagidae), Paracentrophorus, Pteronisculus, Saurichthys, Teffichthys (species were previously referred to Perleidus; Marramà et al., 2017), several parasemionotid genera, and actinistians (Piveteauia, Rhabdoderma, Whiteia). Many of these taxa are typical for the Early Triassic and belong therefore to the TEFF of Tintori et al. (2014a), though some genera, especially among parasemionotids and actinistians, were seemingly endemic. Localities in southwestern Madagascan also yield bony fishes (e.g. Lehman et al., 1959). These occurrences are herein treated as coeval to those from the northwestern part of the island due to the many shared taxa, but conclusive evidence from index fossils is still wanting for the southwestern localities (cf. Kogan and Romano 2016a).
In East Greenland, which is famous for Griesbachian aged fish fossils (see above), there is a horizon yielding Dienerian aged fishes (“stegocephalian-horizon” of Nielsen 1942; Nielsen 1949; “Zone VI” of Nielsen 1961; Surlyk et al., 2017). However, fossils appear to be few in these beds (Nielsen 1961). In addition, two western Canadian occurrences have been dated as Dienerian: a parasemionotid collected in Alberta (Davies et al., 1997) and a bobasatraniid reported from British Columbia (Wignall and Newton 2003). There are also occurrences of possibly Dienerian aged actinopterygians in Idaho, United States (Tanner 1936; Romano et al., 2012).
The Early Triassic bony fish record from China is relatively poor compared to the Middle Triassic one (see below). Isolated occurrences in the lower Yinkeng Formation (Zhejiang province) comprise Paraperleidus in addition to fragmented specimens (Dienerian according to Benton et al., 2013).
Two new localities yielding articulated Dienerian osteichthyan fishes were recently discovered in the Spiti subdistrict (Himachal Pradesh, India; Romano et al., 2016b) and in the Candelaria Hills (southwestern Nevada, United States; Romano et al., 2019). From Spiti, Saurichthys, a parasemionotid, an actinistian and additional, indeterminable actinopterygian remains have been described. The material was collected in situ together with ammonoids (Ware et al., 2018). Regarding the Candelaria Hills, a parasemionotid (Candelarialepis), a ?turseoid (Pteronisculus), a ptycholepid (Ardoreosomus), and several indeterminable actinopterygians were described. The collected material also contains several undescribed actinistian remains referable to Whiteia (pers. comm. R. Socha and P. Skrzycki, 2020). Although two genera from the Candelaria Hills appear to be endemic, they do belong to families that had a widespread distribution at the time of deposition. The families are therefore part of the TEFF (Tintori et al., 2014a).
Smithian
The third stage of the Early Triassic, the Smithian (early Olenekian; Tozer 1965), ended with an extinction event, the Smithian-Spathian boundary event (SSBE; Galfetti et al., 2007). This extinction event severely decimated ammonoids and conodonts, both being nekto-pelagic taxa (e.g. Orchard 2007; Brayard et al., 2009; Leu et al., 2019). To what extend bony fishes were affected by the SSBE is not well known, predominantly due to the patchy Spathian record (see below). The Smithian was marked by profound global change, as indicated by large perturbations in the carbon isotope and oxygen isotope records (e.g. Payne et al., 2004; Romano et al., 2013; Goudemand et al., 2019). These data suggest a cooler episode during the early Smithian, followed by a rapid warming leading to peak temperatures during the middle Smithian. The late Smithian, coinciding with the extinction event, was associated with a drop in global temperatures (Goudemand et al., 2019; Widmann et al., 2020).
A prolific collecting area for Smithian aged osteichthyans is the Arctic island of Spitsbergen, Svalbard archipelago, Norway (see e.g. Brinkmann et al., 2010). Although the occurrence of Early Triassic fishes in Spitsbergen was already discovered in the 19th century, taxonomic studies were performed mostly during the 1920’s, with very few taxonomic works since that time (e.g. Stensiö 1921; Kogan and Romano 2016b). Fossils are preserved in float concretions derived from the “fish horizon” (e.g. Stensiö 1921). Ammonoids and conodonts are frequently preserved with the fishes, but they are rarely mentioned in the literature. These index fossils point at an early late Smithian age (Wasatchites tardus ammonoid zone; cf. Kogan and Romano 2016b). Osteichthyan fossils were also collected from strata below or above the “fish horizon”, but for the most part these consist of isolated skeletal elements (cf. Kogan and Romano 2016b). Typical taxa from Spitsbergen include Birgeria, Bobasatrania, Helmolepis, Pteronisculus, Saurichthys, ?Teffichthys (“Perleidus”; Marramà et al., 2017), Ptycholepidae (Acrorhabdus, Boreosomus), and several actinistian genera (Axelia, Mylacanthus, Sassenia, Scleracanthus, Wimania).
Fossiliferous sites in western Canada (Alberta, British Columbia) have yielded numerous articulated bony fishes (e.g. Schaeffer and Mangus 1976; Neuman and Mutter 2005; Wendruff and Wilson 2012; Wendruff and Wilson 2013; Neuman 2015), most of which are probably of Smithian age (Orchard and Zonneveld 2009), though some have also been found in Dienerian strata (see above). Additional occurrences are known from the Canadian Arctic (Ellesmere Island, Nunavut; Schaeffer and Mangus 1976). Collectively, these Canadian sites have produced the following taxa: Albertonia, Australosomus, Birgeria, Bobasatrania, Boreosomus, Helmolepis, Saurichthys, as well as actinistians (Belemnocerca, Rebellatrix, Whiteia).
During the last decade, repeated fieldwork in the western United States has led to the discovery of articulated, Smithian aged bony fish fossils in Idaho and northeast Nevada (Romano et al., 2012; Romano et al., 2017). Described taxa encompass Birgeria, Saurichthys, as well as indeterminate, mostly disarticulated actinopterygian and actinistian remains.
Several, frequently complete osteichthyans have been recovered from the Lower Qinglong Formation and the Helongshan Formation in eastern China (Tong et al., 2006; Li 2009). The material from these two formations is Smithian in age (cf. Tong and Yin 2002; Ji et al., 2015; Liu et al., 2020). Collected specimens include Plesioperleidus, several genera of Parasemionotidae, an actinistian (Chaohuichthys), as well as other, indeterminate bony fish remains.
The good preservation of bony fish fossils in the abovementioned sites is related to widespread anoxic conditions in the oceans during the late Smithian (Hermann et al., 2011), which are known to enable preservation of fish fossils with fine details (Tintori 1992). Consequently, articulated and largely complete fossils are frequently to be found in late Smithian strata around the world. As for the Griesbachian and Dienerian localities, the Smithian localities produce taxa that had a widespread distribution in the aftermath of the PTBME, and which are typical for the TEFF (Tintori et al., 2014a), with relatively few endemic genera.
Spathian
With a duration of ca. 3 myrs, the Spathian (late Olenekian; Tozer 1965) represents the last and by far the longest substage of the Early Triassic epoch (Ovtcharova et al., 2006; Ovtcharova et al., 2015). Conversely, only very few occurrences of identifiable bony fish fossils are known. The reason for the scarcity of Spathian fishes is likely due to the absence of global episodes of black shale deposition on continental shelves, in contrast to the Dienerian and late Smithian (see above). Near the Spathian-Anisian boundary (Early Triassic–Middle Triassic boundary), a drop in global ammonoid diversity is evident (Bucher 1989), but the mechanisms and extent of this extinction event are not well studied.
Well-dated Spathian bony fish are known from China, Romania, and the western United States. From the upper member of the Nanlinghu Formation in eastern China (Anhui Province), a perleidid (Chaohuperleidus) and a saurichthyid have been described (Sun et al., 2013; Tintori et al., 2014b). From Romania, cranial remains of the actinistian Dobrogeria have been described (Cavin and Grădinaru 2014), and material of additional taxa is currently under study (work in progress). From northeast Nevada, an indeterminate actinopterygian postcranium (a ptycholepid) and a large, yet undescribed skull are known (Romano et al., 2017). Furthermore, complete specimens of Bobasatrania from Spathian deposits of Idaho (cf. Brayard et al., 2017) are currently under study by the author. Another probable Spathian occurrence is known from Spitsbergen (a specimen of Saurichthys; cf. Kogan and Romano 2016b).
The Gogolin beds (Upper Buntsandstein) of Upper Silesia, Poland, yield fossils of Saurichthys, Nephrotus, and actinistians (cf. Romano et al., 2017 for references). Here, I include the fishes from Gogolin in the Spathian substage (see Kowal-Linka and Bodzioch 2017).
Most actinopterygian taxa documented by fossil occurrences in Spathian strata are also known from pre-Spathian and Middle Triassic deposits. Their presence in the Spathian is therefore unsurprizing. These taxa are Saurichthys, Bobasatrania, and Pygopterus. In addition, the family Ptycholepidae comprises pre-Spathian (Acrorhabdus, Ardoreosomus, Boreosomus) and post-Spathian genera (Ptycholepis); it is known from a single fossil, indeterminable at the genus level, from the Spathian of Nevada. An exception are the perleidids. The Spathian Chaohuperleidus represents the earliest occurrence of this group in the fossil record (note that Griesbachian–Smithian perleidids were reallocated to Teffichthys; Marramà et al., 2017). Tintori et al. (2014a) include perleidids in the TMFF (“Triassic Middle Fish Fauna”).
The Middle Triassic Record
The Middle Triassic epoch contains two stages: Anisian and Ladinian (e.g. Balini et al., 2010). Each stage can be subdivided into substages. The Anisian is divided into the Aegean (Early Anisian), Bithynian (early Middle Anisian), Pelsonian (late Middle Anisian), and Illyrian (Late Anisian). The Ladinian comprises two substages, the Fassanian (Early Ladinian) and the Longobardian (Late Ladinian). The Longobardian occurrences have been omitted herein, because they do not add more information to the scope of the present study. Originally, the substages were used for the European Middle Triassic, but they are presently also applied to other Tethyan sections.
Aegean
To date, Early Anisian osteichtyan fishes are barely known. Fossils were collected from the Upper Buntsandstein in Alsace, France (Grès à Voltzia), and near Durlach, Baden-Württemberg, Germany, from where the following taxa have been described: Dipteronotus (=Praesemionotus), Dorsolepis, Pericentrophorus, Pygopterus, Saurichthys, and actinistians (Wilser 1923; Jörg 1969; Gall et al., 1974; Schultze and Kriwet 1999; Gall and Grauvogel-Stamm 2005). Except for Pygopterus and Saurichthys, these genera are not yet known from deposits older than Middle Triassic. Dorsolepis and Pericentrophorus are probably endemic. These taxa are grouped in the “Triassic Middle Fish Fauna” (TMFF; Tintori et al., 2014a). Although an Early Anisian age was assumed based on index fossils (Gall and Grauvogel-Stamm 2005), Werneburg et al. (2014) questioned this age assignment and suggested that they were younger (Bithynian or Pelsonian).
Bithynian
The Bithynian record of bony fishes is poor and restricted to a few occurrences in the United States, the Netherlands, Germany, and possibly Slovenia. The Fossil Hill Member of the Favret Formation in Nevada has yielded cranial material of Saurichthys together with other isolated bony fish remains (Sander et al., 1994; Rieppel et al., 1996). Specimens of Eosemionotus, Gyrolepis, Saurichthys and other actinopterygians have been collected from the lower beds of the Vossenveld Formation in the Netherlands, which are Bithynian in age (Oosterink and Poppe 1979; Oosterink 1986; Maxwell et al., 2016). From Germany, Werneburg et al. (2014) described small species of Saurichthys. These authors also mention the occurrence of Dipteronotus, cf. Peltoperleidus, and Eosemionotus. The Velika Planina Horizon (Slovenia), whose precise age is not known but is thought to be pre-Pelsonian, has produced specimens of Eosemionotus, Placopleurus, and Saurichthys (Hitij et al., 2010; Tintori et al., 2014a; Tintori et al., 2016). The Velika Planina Horizon is herein treated as Bithynian in age, although an Aegean age cannot currently be confidently excluded. Apart from Saurichthys, all other genera listed above have their first appearance datum in this interval. They are part of the TMFF of Tintori et al. (2014a).
Pelsonian
Localities in Europe (Italy, Slovenia) and China produce Pelsonian bony fishes, many of which have been studied intensively during recent years. The Strelovec Formation of Slovenia has yielded fossils of Eosemionotus, Habroichthys, Peltopleurus, Placopleurus, Marcopoloichthys, Perleidus, possibly Colobodus, and other actinopterygians (Hitij et al., 2010; Tintori et al., 2014a; Tintori et al., 2016). The Strelovec Formation has been treated as Pelsonian by several paleoichthyologists, and I provisionally follow this interpretation here; however, note that Miklavc et al. (2016) consider this formation as Illyrian aged because index fossils supporting a Pelsonian age are lacking.
In Italy, a new locality in the Dolomites (Prà della Vacca/Kühwiesenkopf) has produced specimens of Bobasatrania, Dipteronotus, Habroichthys, Placopleurus. Peltoperleidus, Ptycholepis, and Saurichthys (Tintori et al., 2016).
A rich ichthyofauna is known from two horizons in South China, which became famous as the Luoping Biota and Panxian Biota, respectively, both being almost the same age (Upper Member of the Guanling Formation; Benton et al., 2013). Sun et al. (2012) suggested a Pelsonian age for the Luoping fish beds and I herein follow these authors’ interpretation, although Tintori et al. (2016) argued for a younger (Illyrian?) age. Sun et al. (2016a) presented evidence for a Pelsonian age of the Panxian biota. The Panxian-Luoping fauna comprises the following actinopterygians: Altisolepis, Birgeria, Colobodus, Diandongperleidus, Eosemionotus, Ferroxichthys, Frodoichthys, Fuyuanichthys, Fuyuanperleidus, Gimlichthys, Gymnoichthys, Habroichthys, Kyphosichthys, Louwoichthys, Luopingichthys, Luopingperleidus, Luoxiongichthys, Marcopoloichthys, Panxianichthys, Perleidus, Placopleurus, Platysiagum, Pteronisculus, Robustichthys, Sangiorgioichthys, Saurichthys (including Sinosaurichthys), Subortichthys, Venusichthys, and Yelangichthys, as well as the actinistians Luopingcoelacanthus and Yunnancoelacanthus (e.g. Wu et al., 2011, Geng et al., 2012; Sun et al., 2012, Wen et al., 2012, Benton et al., 2013; Wen et al., 2013, Wu et al., 2013; Xu et al., 2014a, Xu et al., 2014b; Ma and Xu 2015, Xu and Shen 2015; Sun et al., 2016b; Xu and Zhao, 2016; Wu et al. 2018; Xu et al., 2018; Wen et al., 2019; Xu 2020a, Xu 2020c). These are mostly endemic taxa, suggesting incipient regionally contrasted ichthyofaunas in the eastern Tethys in the Pelsonian (as opposed to the cosmopolitan faunas of the Griesbachian–Smithian interval). The genera Eosemionotus, Marcopoloichthys, and Saurichthys are known from coeval European localities. Only Birgeria, Eosemionotus, Pteronisculus, and Saurichthys have pre-Pelsonian occurrences, whereas all other genera have their first occurrence in the Pelsonian. It is no wonder that Tintori et al. (2014a) uses the term “explosion” for the Pelsonian, although the authenticity of this rapid diversification requires critical testing (see below). Some of the abovementioned genera (e.g. Habroichthys) are also known from the Late Ladinian (Longobardian) Xingyi Biota (Sun et al., 2016a).
Illyrian
Illyrian bony fishes are known from Europe (Germany, the Netherlands, Italy, Switzerland). From the Middle Muschelkalk of Germany, several taxa were described, including articulated remains of Eosemionotus, as well as isolated bones of Saurichthys, and scales of Colobodus and Gyrolepis (e.g. Schultze and Möller 1986; Romano et al., 2012). Plesker (1995) examined articulated fossils of Colobodus, Dollopterus, and Gyrolepis (also see Stolley 1920; Schultze and Kriwet 1999). From early Illyrian beds in the Netherlands, Maxwell et al. (2016) described a small species of Saurichthys.
The famous collecting area of Monte San Giorgio and Besano, which extends across the border between Switzerland and Italy, has yielded a plethora of well-preserved, articulated vertebrate remains, including bony fishes. The fossils are mostly derived from the Illyrian–Fassanian Besano Formation; hence, the fossiliferous layers straddle the Anisian–Ladinian boundary (Stockar et al., 2012). From the Illyrian part of the Besano Formation, the following genera have been described: Aetheodontus, Altisolepis, Besania, Birgeria, Bobasatrania, Cephaloxenus, Colobodus, Crenilepis, Ctenognathichthys, Eoeugnathus, Eosemionotus, Gracilignathichthys, Gyrolepis, Habroichthys, ?Holophagus, Luganoia, Meridensia, Nannolepis, ?Ophiopsis, Peltoperleidus, Peltopleurus, Peripeltopleurus, Pholidopleurus, Placopleurus, Platysiagum, Ptycholepis, Saurichthys, Ticinocolobodus, and Ticinolepis (e.g. Brough 1939; Bürgin 1992, Bürgin 2004; López-Arbarello et al., 2016; Maxwell et al., 2015; Mutter 2002; Mutter and Herzog 2004; Romano and Brinkmann 2009; PIMUZ collection database). The ichthyofauna is diverse and contains endemic genera but also genera that are known from other Anisian localities (e.g. Birgeria, Eosemionotus, Gyrolepis, Placopleurus, Platysiagum, Ptycholepis, Saurichthys). The deep‐bodied Bobasatrania first appeared in the latest Permian, and the yougest complete body fossils are from the Illyrian (PIMUZ collection database). Tooth plates referrable to Bobasatrania from the Longobardian of Germany and France mark the last appearance of this genus in the fossil record (Böttcher 2014). Meanwhile, Luganoia has also been described from the Late Ladinian Xingyi Biota (Sun et al., 2016a; Xu 2020b), suggesting a longer temporal range than previously thought (Illyrian–Longobardian) for this taxon.
Fassanian
Identifiable remains of bony fishes from the Fassanian, the first substage of the Ladinian, are known from Europe (Switzerland, Italy, and Germany).
Early Ladinian bony fishes are known from the Prosanto Formation in southeast Switzerland (Graubünden). This formation has produced fossils of the actinopterygians Archaeosemionotus, Besania, Colobodus, Ctenognathichthys, Ducanichthys, Eoeugnathus, Eosemionotus, Habroichthys, ?Legnonotus, Ophiopsis, Peltoperleidus, Peltopleurus, Placopleurus, Prohalecites, Saurichthys, and Stoppania (Bürgin et al., 1991; Bürgin and Herzog 2002; Herzog 2003; Herzog and Bürgin 2005; Lombardo et al., 2008), as well as of the actinistians Foreyia and Ticinepomis (Cavin et al., 2013; Cavin et al., 2017).
In the classic collecting site Monte San Giorgio and Besano (Swiss-Italian borderland), several layers produce osteichthyan fossils of Fassanian age. These are restricted to the upper portion of the Besano Formation as well the lower part of the Meride Limestone (Cava inferiore, Cava superiore; Stockar 2010). The following taxa were recovered from the Fassanian aged beds of the Besano Formation: Altisolepis, Archaeosemionotus, Besania, Birgeria, Bobasatrania, Cephaloxenus, Colobodus, Crenilepis, Ctenognathichthys, Eoeugnathus, Eosemionotus, Gracilignathichthys, Gyrolepis, Habroichthys, Luganoia, Meridensia, Nannolepis, Peltoperleidus, Peltopleurus, Peripeltopleurus, Pholidopleurus, Placopleurus, Platysiagum, Ptycholepis (though nearly all specimens are from Illyrian beds), Saurichthys, Stoppania (“Dipteronotus”; see Lombardo et al., 2008), Ticinocolobodus, and Ticinolepis (e.g. Dames 1888; Mutter 2002; Mutter and Herzog 2004; Romano and Brinkmann 2009; Maxwell et al., 2015; López-Arbarello et al., 2016, López-Arbarello et al., 2019; PIMUZ collection database). From Cava inferiore and Cava superiore, the following taxa are known: Ctenognathichthys, Eosemionotus, Habroichthys, Placopleurus, Saurichthys, and Ticinolepis (e.g. Stockar et al., 2012; Tintori 2013; López-Arbarello et al., 2016, López-Arbarello et al., 2019; PIMUZ collection database).
Additional occurrences of Fassanian bony fishes are known from the Southern Alps of northern Italy. From the Perledo area, the following taxa have been documented Archaeosemionotus, Ctenognathichthys, Habroichthys, Heptanema, Placopleurus, Saurichthys, and Stoppania. (see e.g. Tintori 2013; López-Arbarello et al., 2014; Renesto and Stockar 2018).
From the uppermost Muschelkalk of Germany, specimens of the actinistians Garnbergia (Martin and Wenz 1984) and Hainbergia (Schweizer 1966), as well as the actinopterygian Gyrolepis (Stolley 1920; Plesker 1995) have been collected.
The taxonomic composition of Fassanian localities in Germany, Switzerland and Italy is relatively similar at the genus level due to either the close paleogeographic proximity of the sites or due to seaway connections at the time of deposition (Schultze and Kriwet 1999). Several of the genera listed above can also be found in Pelsonian (Upper Member of the Guanling Formation) and Longobardian (Zhuganpo Formation; Sun et al., 2016a) deposits in South China, suggesting that these taxa had a widespread distribution in the Tethys since at least the late Middle Anisian.
The Spathian–Bithynian: A Hiatus or the Base Between Two Waves?
The current review of the post-extinction fossil record of bony fishes (Griesbachian–Fassanian) highlights that the stratigraphic coverage of known localities is incomplete. Collectively, the fossiliferous layers belong to preservation windows mainly during the early aftermath after the PTBME (Griesbachian–Smithian), as well as during a Middle Triassic interval beginning in the late Middle Anisian (Pelsonian). Based on this study, it becomes evident that the so-called “Spathian Gap” (Romano et al., 2016a, Romano et al., 2017) extends into the early Middle Anisian, straddling the Spathian, Aegean, and Bithynian. This hiatus is therefore subsequently referred to as the “Spathian–Bithynian Gap” (SBG). The SBG, characterized by a patchy osteichthyan fossil record, had a duration of over 3 myrs (Ovtcharova et al., 2006, 2015).
Only a few localities preserve fossils from the SBG (Figure 1A). They mostly represent isolated occurrences, and the fossils often consist of disarticulated bones only, which are only rarely diagnostic and therefore often not collected in the field. Only a couple of occurrences of articulated fishes are known from this interval (e.g. Gall et al., 1974; Rieppel et al., 1996; Sun et al., 2013; Tintori et al., 2014a; Cavin and Grădinaru 2014; Romano et al., 2017).
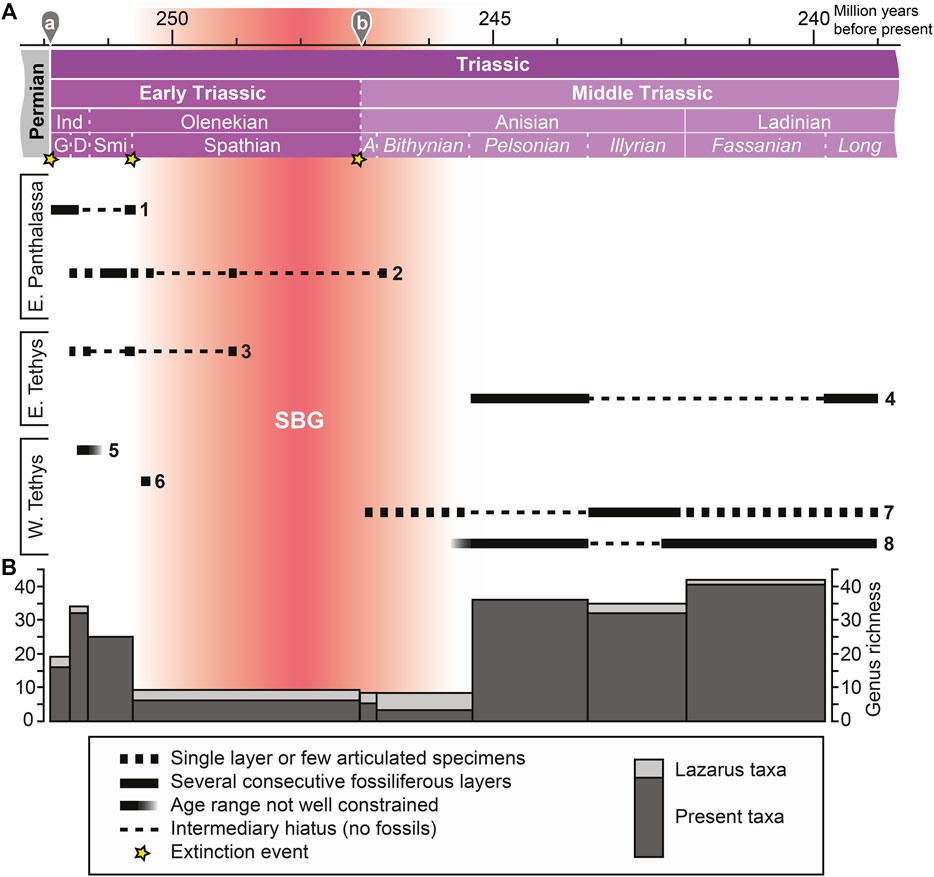
FIGURE 1. (A) Stratigraphic ranges of beds preserving bony fish fossils across the Spathian-Bithynian Gap (SBG), with localities grouped paleogeographically (East and West Tethys, East Panthalassa). 1. Arctic: Canada (Ellesmere Island, Nunavut), Greenland, Spitsbergen; 2. Western North America: Canada (Alberta, British Columbia), United States (Idaho, Nevada); 3. Eastern China (Anhui, Jiangsu, and Zhejiang provinces); 4. South China (Guizhou and Guangxi provinces); 5. India (Himachal Pradesh) and Madagascar; 6. Romania; 7. Buntsandstein and Muschelkalk (includes brackish taxa): France, Germany, Poland, The Netherlands; 8. Southern Alps: Italy, Slovenia, Switzerland. Absolute ages:a, Baresel et al., 2017; b, Ovtcharova et al., 2015. Abbreviated stratigraphic intervals: A, Aegean; D, Dienerian; G, Griesbachian; Ind, Induan; Long, Longobardian; Smi, Smithian. (B) Stacked diversity of present taxa and Lazarus taxa per time interval (number of bony fish genera).
The SBG likely represents a taphonomic megabias (Behrensmeyer et al., 2000). It is probably the result of extrinsic physical changes, such as the improved oxygenation on the shelves, cooler global temperatures and a change in oceanic chemistry after the Smithian substage (e.g. Zhang et al., 2015; Goudemand et al., 2019; Widmann et al., 2020). The lack of episodes of black shale deposition on shelves during the SBG contrasts with the iterative occurrences of such phases in the Griesbachian–Smithian (Hermann et al., 2011; Ware et al., 2015) and the Pelsonian–Longobardian (e.g. Tintori 1992; Benton et al., 2013). Consequently, fossil remains of Spathian, Aegean and Bithynian osteichthyans have only rarely been collected or were for the most part not studied taxonomically. Additionally, there are some uncertainties regarding the precise age of these assemblages (e.g. Werneburg et al., 2014; Miklavc et al., 2016).
The rarity of complete and articulated bony fishes in SBG deposits is reflected in the very low diversity during this interval (Figure 1B). Some of the collected fossils belong to long ranging taxa (Bobasatrania, Dipteronotus, Gyrolepis, Saurichthys), but there are also endemic genera (e.g. Dobrogeria, Dosolepis, Nephrotus, Pericentrophorus). Some of these taxa (e.g. Saurichthys) exhibit peculiar skeletal morphologies and can thus be easily identified even on the basis of isolated skeletal remains (Romano et al., 2012). The identified genera from the SBG comprise taxa of both the TEFF (Bobasatrania, Saurichthys) and the TMFF (e.g. Eosemionotus, Placopleurus, perleidiforms; Tintori et al., 2014a).
The scarcity of well-preserved bony fish fossils from the SBG is also mirrored in the elevated occurrences of Lazarus taxa (Figure 1B). These include mostly genera typical for both the TEFF and the TMFF, such as Birgeria, Bobasatrania, and Pteronisculus. Lazarus occurrences are also relatively high during the Illyrian due to the absence of fossiliferous strata in the East Tethys. Many of these taxa are found in Pelsonian and Ladinian deposits on either side of the Tethys (Europe, China).
In addition to the SBG, there is also a paleogeographic hiatus in the Early–Middle Triassic bony fish record (Figure 1A). While the Tethyan record comprises both Early Triassic and Middle Triassic occurrences, the East Panthalassan record vanishes after the Bithynian. Both the Tethyan and the Panthalassan record are affected by the SBG. Furthermore, Romano et al. (2016a) showed that the Early Triassic record is mostly covered by mid-paleolatitudinal localities, whereas the Middle Triassic record is heavily based on low-paleolatitudinal sites. No records of marine Triassic bony fishes exist from Antarctica, Australia, and South America (e.g. Romano et al., 2016a). The aforementioned gaps may potentially obscure geographically restricted early diversification events.
The SBG blurs an important phase during the post-PTBME diversification of bony fishes. The Griesbachian–Smithian faunas were homogenous, containing many cosmopolitan genera (high α-diversity, low β- and γ-diversity). Genera like Australosomus, Bobasatrania, Boreosomus, Helmolepis, or Pteronisculus dwelled on the shelf on opposite sides of the Pangean supercontinent within less than 2 myrs after the PTBME. By contrast, Middle Triassic faunas comprise more endemic taxa, resulting in higher γ-diversity (Romano et al., 2016a). How this transition took place, whether it was abrupt or gradual, remains currently unknown (see below). Similarly, how the Griesbachian–Smithian faunas arose is unclear due to the poor Changhsingian (latest Permian) record. Cosmopolitanism has also been documented in ammonoids during different intervals of the Early Triassic, which can be explained either due to the survival and dispersal of endemic genera, or an increase in the number of new cosmopolitan genera, or the selective extinction of endemic genera (Dai and Song 2020).
A TALE OF BOOMERS AND SURVIVORS
Based on their stratigraphic ranges, I divide the studied genera into three main groups: 1) long survivors, i.e. taxa that have their first appearance datum (FAD) in the Lopingian (late Permian) or Early Triassic and their last appearance datum (LAD) in the Middle Triassic or later, 2) early boomers, i.e. genera with FADs in the Paleozoic (with a long hiatus until the Early Triassic) or Early Triassic, and LADs in the Early Triassic, and 3) late boomers, i.e. genera that appear during the Middle Triassic. These groups are summarized in Figure 2.
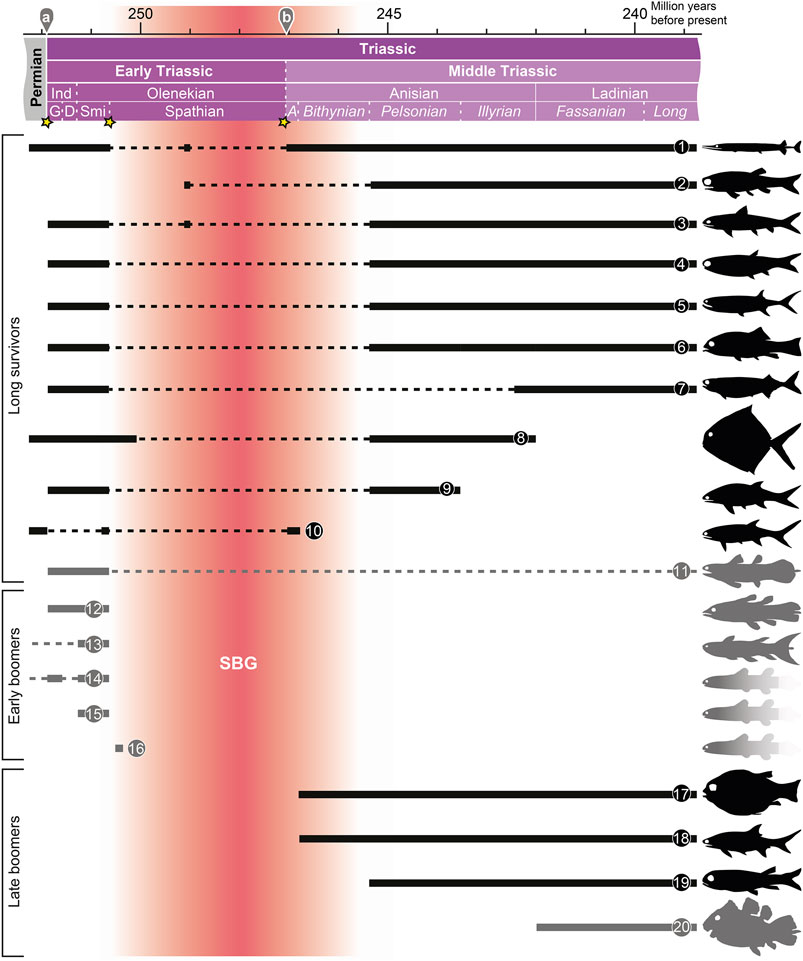
FIGURE 2. Stratigraphic ranges of various groups of bony fishes (Actinopterygii: black; Actinistia: grey; gradient: taxa based on incomplete specimens) during the Early and Middle Triassic (Griesbachian–Fassanian). 1. Saurichthyidae (Saurichthys); 2. Perleididae (Chaohuperleidus, Fuyuanperleidus, Perleidus); 3. Ptycholepidae (Acrorhabdus, Ardoreosomus, Boreosomus, Ptycholepis); 4. Platysiagidae (Helmolepis, Platysiagum); 5. Birgeriidae (Birgeria); 6. Halecomorphi (e.g. Ospia, Robustichthys); 7. Pholidopleuriformes (Australosomus, Pholidopleurus); 8. Bobasatraniidae (Bobasatrania); 9. Pteronisculus; 10. Pygopterus; 11. Laugiidae (Belemnocerca, Coccoderma, Laugia, Piveteauia); 12. Whiteia; 13. Rebellatrix (+ Hadronector); 14, Sasseniidae (Sassenia, Spermatodus); 15 Axelia + Wimania; 16. Dobrogeria; 17. Semionotiformes (e.g. Eosemionotus, Sangiorgioichthys); 18. Gyrolepis; 19. Peltopleuriformes (e.g. Peltopleurus); 20. Latimeriidae (e.g. Foreyia). For abbreviations for the absolute ages and stratigraphic ages: see caption of Figure 1. See text for details.
Long Survivors
The long survivors group includes, for instance, the Halecomorphi (Parasemionotidae, Ionoscopiformes; ?Early Triassic–Recent; e.g. López-Arbarello and Sferco 2018), Pholidopleuriformes (Australosomus, Pholidopleurus; Triassic; e.g. Bürgin 1992); Laugiidae (Belemnocerca, Coccoderma, Laugia, Piveteauia; Griesbachian–Jurassic; Cavin et al., 2017), Perleididae sensu stricto (Chaohuperleidus, Fuyuanperleidus, Perleidus; e.g. Sun et al., 2013), Platysiagidae (Helmolepis, Platysiagum; Griesbachian–Jurassic; e.g. Neuman and Mutter 2005), Ptycholepidae (Acrorhabdus, Ardoreosomus, Boreosomus, Ptycholepis; Griesbachian–Jurassic; e.g. Romano et al., 2019), Birgeria (Triassic; e.g. Romano et al., 2017), Bobasatrania (Griesbachian–Illyrian; e.g. Bürgin 1992), Pteronisculus (Griesbachian–Pelsonian; e.g. Xu et al., 2014b), Pygopterus (Lopingian–Aegean; e.g. Schultze and Kriwet 1999), and Saurichthys (Lopingian–Triassic; e.g. Romano et al., 2012). Most of these taxa are typical faunal components of Triassic aquatic ecosystems and had a cosmopolitan distribution during the Griesbachian–Smithian (e.g. Tintori et al., 2014a; Romano et al., 2016a). Many long survivors exhibit Lazarus occurrences during the SBG. Several fossils recovered from Spathian deposits belong to long survivor taxa. Therefore, these finds contribute little information about the diversification of bony fishes during the late Early Triassic and earliest Middle Triassic because we can already infer their presence during the hiatus.
Early Boomers
The early boomers comprise genera that are exclusively known from the Early Triassic (e.g. Whiteia), as well as genera that belong to Paleozoic clades that – after a long hiatus - made a reappearance in the fossil record during the Early Triassic, such as Sassenia (closely related with the Permian Spermatodus; Sasseniidae; Cavin et al., 2017) or Rebellatrix (probably closely related with the Carboniferous Hadronector), and Rhabdoderma (Carboniferous to Early Triassic; omitted in Figure 2; cf. Forey 1998; Cavin et al., 2017). The early boomers encompass several genera whose “family”-level interrelationships to other genera are not known, such as Errolichthys, Whiteia, and Dobrogeria (Lehman 1952; Nielsen 1955; Cavin et al., 2017). Noteworthy is the high representation of actinistians in the early boomer group. Actinistians have a patchy post-Devonian fossil record (e.g. Schultze 2004) and diversified during the Early Triassic (Cavin et al., 2013). The fate of several of these early booming actinistians is not known. They could have fallen victim to the SSBE, or potentially survived in environments with a poor fossil record, analogous to the Latimeriidae after the Cretaceous-Paleogene extinction event.
Late Boomers
The late boomers mostly correspond to Tintori et al. (2014a)'s TMFF. The taxa of this group have their FAD in the Anisian–Fassanian. With Ticinepomis and Foreyia, the late boomers also include the first members of the Latimeriidae, the family that includes the extant coelacanths Latimeria chalumnae and L. menadoensis (Cavin et al., 2017). A successful Triassic clade placed among the late boomers are the Peltopleuriformes (e.g. Habroichthys, Peltopleurus; Bürgin 1992). Also, Eosemionotus, which belongs to Semionotiformes (Tintori et al., 2014a; López-Arbarello et al., 2019), first appears in the Bithynian and thus represents the earliest semionotiform. Earlier genera that have been referred to Semionotiformes, such as the Lopingian Acentrophorus or the Dienerian Paracentrophorus, are pending restudy. Semionotiformes and therefore ginglymodians belong to the late boomer, unlike halecomorphs, the other main holostean clade, which belong to the long survivor group (see above). The Semionotiformes were a highly successful Mesozoic clade. Today, the Ginglymodi are represented only by gars (Sallan 2014). The teleosts, the group to which nearly all extant bony fish clades belong, have their origins in the Fassanian with Prohalecites (Arratia 2013; Tintori et al., 2014a; Romano et al., 2016a). Their relationship to the SBG is presently not known.
IMPLICATIONS FOR THE DIVERSIFICATION OF BONY FISHES AFTER THE PTBME
Current data indicates elevated diversity of marine actinistians and actinopterygians in the aftermath of the end-Permian mass extinction, and relatively higher diversity during the Middle Triassic (Romano et al., 2016a). However, the Early Triassic and Middle Triassic peaks in genus richness are separated by an interval of very low diversity during the Spathian, Aegean, and Bithynian, resulting from sparse occurrences and limited quality of preservation of fossils. The quality of the fossil record is uneven due to taphonomic biases (Behrensmeyer et al., 2000). Whenever the radiation of a major lineage falls between two preservation windows our understanding of the origins and early diversity of said clade becomes elusive. Several gaps during important evolutionary events have been noted, such as “Talimaa’s Gap” (Turner et al., 2004), “Romer’s Gap” (Coates and Clack 1995) and “Olson’s Gap” (Lucas 2004).
“Talimaa’s Gap” during the Rhuddanian (earliest Silurian) had a duration of 3 myrs and coincides with the early evolution of vertebrates, potentially blurring the diversifications of thelodonts, pteraspidomorphs and acanthodians after the Hirnantian (latest Ordovician) glaciation (Turner et al., 2004; Žigaitė and Blieck 2013). “Romer’s Gap” originally referred to a ∼20 myrs long hiatus in the non-marine vertebrate record during the Tournaisian (earliest Carboniferous). However, through intensified research, this gap could be shortened (Otoo et al., 2017). The paucity of fossils from this interval has obscured potential cladogenetic events after the end-Devonian Hangenberg event (Sallan and Coates 2010). “Olson’s Gap” refers to an interval during the latest Kungurian (late Cisuralian/early Permian) and Roadian (early Guadalupian/middle Permian) that is marked by a scarcity of terrestrial tetrapod remains (e.g. Blieck 2011).
Similarly, the SBG obscures the early evolution of important Mesozoic and modern bony fish clades, and that of the Neopterygii (Holostei and Teleostei) in particular. The Early Triassic (pre-Spathian) and Middle Triassic (post-Bithynian) fish faunas differ in taxic composition and dominance, as well as in their body size (both distribution and median; Romano et al., 2016a). Deecke (1927) – given his limited knowledge at the time – already noticed the gradual change in composition of fish faunas during the Triassic, in which Paleozoic groups (“paleopterygians” or “paleoniscoids”), still frequently present in the beginning of the Triassic, were replaced by more modern taxa during this period. Tintori et al. (2014a) classified Triassic actinopterygians into three groups, the Triassic Early Fish Fauna (TEFF), the Triassic Middle Fish Fauna (TMFF), and the Triassic Late Fish Fauna (TLFF). Taking the fossil record at face value, they postulated that the Smithian-Spathian boundary probably marks the limit between the TEFF and the TMFF (implying that TEFF taxa fell victim to the SSBE) and that a diversification followed in the Pelsonian.
Based on the limited data currently available, I suggest the following three hypotheses for the Early–Middle Triassic turnover of bony fish faunas (summarized in Figure 3):
(1) SSBE hypothesis: extinction of the TEFF at the end of the Smithian and diversification of the TMFF during the post-extinction recovery, coinciding with the SBG. This hypothesis postulates two radiations within less than 2 myrs after the PTBME.
(2) Pelsonian explosion hypothesis: rapid diversification of the TMFF in the Pelsonian. The TEFF either disappeared during a prior extinction event or slowly faded away during the SBG. This hypothesis suggests two radiations after the PTBME, but further apart temporally than in hypothesis 1.
(3) Gradual replacement hypothesis: the TEFF was replaced by the TMFF over time during the SBG. Hence, this hypothesis suggests that osteichthyans were not affected by Early Triassic extinction events much like they were also not harmed by the PTBME.
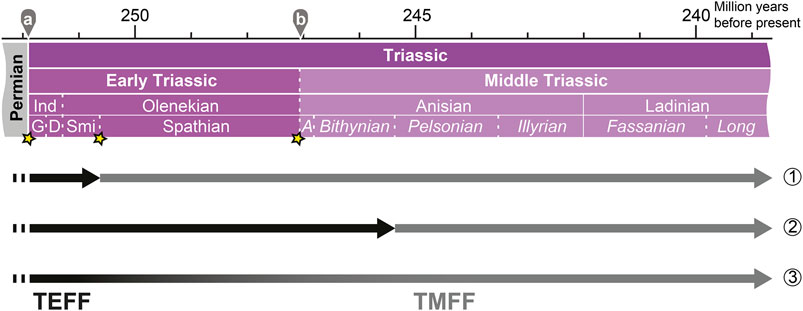
FIGURE 3. Hypotheses for the replacement of the TEFF (Triassic Early Fish Fauna; Tintori et al., 2014a) by the TMFF (Triassic Middle Fish Fauna) during the diversification of Osteichthyes in the Early–Middle Triassic: (1) the SSBE hypothesis, (2) the Pelsonian explosion hypothesis, and (3) the gradual replacement hypothesis. For abbreviations for the absolute ages and stratigraphic ages: see caption of Figure 1. See text for details.
None of the three hypotheses can be rejected based on the current knowledge derived from the fossil record. Hypothesis three is best supported by the current data, given that several lineages bridge the SBG. More studies on bony fishes from the SBG and further analyses are necessary to test for potential impacts of Early–Middle Triassic extinction events on bony fish diversity and the alleged Pelsonian radiation. Finally, the beginning of the TEFF is also not well constrained. Although most TEFF taxa appear in the Early Triassic, a few are known to have originated in the latest Permian (Bobasatrania, Saurichthys).
Conclusion
The Spathian–Bithynian Gap (SBG) is the only post-Paleozoic hiatus thus far recorded in the vertebrate fossil record. It overlaps with the Triassic diversification of bony fishes, which entailed the first neopterygian radiation. The Neopterygii comprise the vast majority of bony fish diversity today, and ca. 50% of the total vertebrate diversity (Sallan 2014). The SBG can be classified as a taphonomic megabias (Behrensmeyer et al., 2000). It is marked by 1) a small number of fossil sites, 2) relatively low taxic diversity, and 3) elevated Lazarus occurrences for actinopterygians and actinistians. Because of the SBG, the pattern of diversification of bony fishes following the PTBME is not well understood. This also concerns the impact of subsequent extinction events on bony fishes, such as the SSBE (Galfetti et al., 2007; Romano et al., 2016a), which severely affected conodonts (Orchard 2007; Leu et al., 2019) and ammonoids (Brayard et al., 2009).
Mass extinction events and their aftermaths reset the rules abruptly and thereby redirect the course of evolution. Whenever such fateful events fall into an interval of unfavorable fossilization conditions, a gap of knowledge is left behind, obscuring the evolution of the clades that thrived after the extinction. The SBG separates the diverse, cosmopolitan Griesbachian–Smithian faunas (TEFF; Tintori et al., 2014a) from the more diverse and regionally more contrasted post-Bithynian faunas (TMFF). Claims of a protracted recovery of bony fishes after the PTBME partly rest on the poor fossil record during the Spathian–Bithynian, an interval that comprises more than 3 myrs. This begs the question whether there was an “explosion” (Tintori et al., 2014a) in bony fish diversity in the Pelsonian or whether we are just observing the beginning of a new taphonomic window. Such claims should be treated with caution. Based on the current data, I propose three hypotheses for the diversification of bony fishes after the PTBME: 1) the SSBE hypothesis, 2) the Pelsonian explosion hypothesis, and 3) the gradual replacement hypothesis. Although hypothesis three appears to be the most probable one given the current data, further studies are necessary to test alternative hypotheses in order to improve our current understanding of the post-PTBME radiation of modern bony fishes.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
Swiss National Science Foundation project numbers 120311/135075 and 144462 to Winand Brinkmann (formerly PIMUZ).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work greatly benefitted from critical, constructive comments by L. Cavin (Muséum d’Histoire Naturelle, Geneva) and S. G. Lucas (New Mexico Museum of Natural History and Science, Albuquerque). I thank T. Argyriou (Muséum National d’Histoire Naturelle, Paris), M. S. Torres Ladeira, H. Bucher (both PIMUZ), W. Brinkmann (formerly PIMUZ), R. Socha and P. Skrzycki (both Krakow), D. Ware (Museum für Naturkunde, Berlin), and H. Diependaal (Department of Earth Sciences, Utrecht University) for information, valuable discussions and help during preparation of this study. I am thankful to I. R. Frick for her continuing support. The author appreciates support by the Swiss National Science Foundation (project numbers 120311/135075 and 144462 to Winand Brinkmann, PIMUZ) during compilation of most of the data used in the present study.
References
Argyriou, T., Giles, S., Friedman, M., Romano, C., Kogan, I., and Sánchez-Villagra, M. R. (2018). Internal cranial anatomy of Early Triassic species of †Saurichthys (Actinopterygii: †Saurichthyiformes): implications for the phylogenetic placement of †saurichthyiforms. BMC Evol. Biol. 218, 161. doi:10.1186/s12862-018-1264-4
Arratia, G. (2013). Morphology, taxonomy, and phylogeny of Triassic pholidophorid fishes (Actinopterygii, Teleostei). J. Vertebr. Paleontol. 33, 1–138. doi:10.1080/02724634.2013.835642
Bagherpour, B., Bucher, H., Baud, A., Brosse, M., Vennemann, T., Martini, R., et al. (2017). Onset, development, and cessation of basal Early Triassic microbialites (BETM) in the Nanpanjiang pull-apart basin, South China block. Gondwana Res. 44, 178–204. doi:10.1016/j.gr.2016.11.013
Balini, M., Lucas, S. G., Jenks, J. F., and Spielmann, J. A. (2010). Triassic ammonoid biostratigraphy: an overview. London. Geol. Soc. Spec. Publ. 334, 221–262. doi:10.1144/SP334.10
Baresel, B., Bucher, H., Bagherpour, B., Brosse, M., Guodun, K., and Schaltegger, U. (2017). Timing of global regression and microbial bloom linked with the Permian-Triassic boundary mass extinction: implications for driving mechanisms. Sci. Rep. 7, 43630.
Behrensmeyer, A. K., Kidwell, S. M., and Gastaldo, R. A. (2000). Taphonomy and paleobiology. Paleobiology. 26, 103–147. doi:10.1017/S0094837300026907
Beltan, L. (1968). La Faune Ichthyologique de l’Eotrias du N.W. de Madagascar: le Neurocrâne. Paris: Cahiers de Paléontologie CNRS
Benton, M. J., Zhang, Q., Hu, S., Chen, Z.-Q., Wen, W., Liu, J., et al. (2013). Exceptional vertebrate biotas from the Triassic of China, and the expansion of marine ecosystems after the Permo-Triassic mass extinction. Earth Sci. Rev. 125, 199–243. doi:10.1016/j.earscirev.2013.05.014
Betancur-R., R., Ortí, G., and Pyron, R. A. (2015). Fossil-based comparative analyses reveal ancient marine ancestry erased by extinction in ray-finned fishes. Ecol. Lett. 18, 441–450. doi:10.1111/ele.12423
Blieck, A. (2011). From adaptive radiations to biotic crises in Palaeozoic vertebrates: a geobiological approach. Geol. Belg. 14, 203–227
Böttcher, R. (2014). Phyllodont tooth plates of Bobasatrania scutata (Gervais, 1852) (Actinoperygii, Bobasatraniiformes) from the Middle Triassic (Longobardian) Grenzbonebed of southern Germany and eastern France, with an overview of Triassic and Palaeozoic phyllodont tooth plates. N. Jb. Geol. Paläont. Abh. 274, 291–311. doi:10.1127/njgpa/2014/0454
Brayard, A., Escarguel, G., Bucher, H., Monnet, C., Brühwiler, T., Goudemand, N., et al. (2009). Good genes and good luck: ammonoid diversity and the end-Permian mass extinction. Science. 325, 1118–1121. doi:10.1126/science.1174638
Brayard, A., Krumenacker, L. J., Botting, J. P., Jenks, J. F., Bylund, K. G., Fara, E., et al. (2017). Unexpected Early Triassic marine ecosystem and the rise of the Modern evolutionary fauna. Sci. Adv. 3, e1602159. doi:10.1126/sciadv.1602159
Brinkmann, W., Romano, C., Bucher, H., Ware, D., and Jenks, J. (2010). Palaeobiogeography and stratigraphy of advanced gnathostomian fishes (chondrichthyes and Osteichthyes) in the Early Triassic and from selected Anisian localities (report 1863–2009). Zbl. Geol. Paläontol. Teil II 2009, 765–812
Brough, J. (1939). The Triassic Fishes of Besano, Lombardy. London, United Kingdom: British Museum (Natural History)
Bucher, H. (1989). Lower Anisian ammonoids from the northern Humboldt range (northwestern Nevada, United States) and their bearing upon the Lower–Middle Triassic boundary. Eclogae Geol. Helv. 82, 945–1002
Bürgin, T. (1996). “Diversity in the feeding apparatus of perleidid fishes (Actinopterygii) from the Middle Triassic of Monte San Giorgio (Switzerland),” in Mesozoic fishes. Systematics and paleoecology. Editors G. Arratia and G. Viohl (München: Dr. Friedrich Pfeil), 114, 555–565.
Bürgin, T., Eichenberger, U., Furrer, H., and Tschanz, K. (1991). Die Prosanto Formation – eine fischreiche Fossil-Lagerstätte in der Mitteltrias der Silvretta-Decke (Kanton Graubünden, Schweiz). Eclogae Geologicae Helvetiae. 84, 921–990
Bürgin, T. (1992). Basal ray-finned fishes (Osteichthyes; Actinopterygii) from the Middle Triassic of Monte San Giorgio (Canton Tessin, Switzerland). Systematic palaeontology with notes on functional morphology and palaeoecology. Schweizerische Paläontologische Abhandlungen. 114, 1–164
Bürgin, T., and Herzog, A. (2002). Die Gattung Ctenognathichthys (Actinopterygii; Perleidiformes) aus der Prosanto-Formation (Ladin, Mitteltrias) Graubündens (Schweiz), mit der Beschreibung einer neuen Art, C. hattichi sp. nov. Eclogae Geol. Helv. 95, 461–469.
Bürgin, T. (2004). †Eosemionotus ceresiensis sp. nov., a new semionotiform fish (Actinopterygii, halecostomi) from the Middle Triassic of Monte San Giorgio (southern Switzerland),” in Mesozoic fishes 3. Systematics, paleoenvironments and biodiversity. Editors G. Arratia, and A. Tintori (München, Germany: Dr. Friedrich Pfeil), 239–251
Cavin, L., Mennecart, B., Obrist, C., Costeur, L., and Furrer, H. (2017). Heterochronic evolution explains novel body shape in a Triassic coelacanth from Switzerland. Sci. Rep. 7, 13695. doi:10.1038/s41598-017-13796-0
Cavin, L., Furrer, H., and Obrist, C. (2013). New coelacanth material from the Middle Triassic of eastern Switzerland, and comments on the taxic diversity of actinistians. Swiss J. Geosci. 106, 161–177. doi:10.1007/s00015-013-0143-7
Cavin, L., and Grădinaru, E. (2014). Dobrogeria aegyssensis, a new early Spathian (Early Triassic) coelacanth from north Dobrogea (Romania). Acta Geol. Pol. 64, 161–187. doi:10.2478/agp-2014-0010
Chen, Z.-Q., and Benton, M. J. (2012). The timing and pattern of biotic recovery following the end-Permian mass extinction. Nat. Geosci. 5, 375–383. https://doi.org/10.1038/ngeo1475
Coates, M. I., and Clack, J. A. (1995). Romer's Gap: tetrapod origins and terrestriality. Bulletin du Muséum national d'Histoire naturelle, 4ème série, section C, Sciences de la Terre, Paléontologie, Géologie, Minéralogie 17, 373–388
Dai, X., and Song, H. (2020). Toward an understanding of cosmopolitanism in deep time: a case study of ammonoids from the middle Permian to the Middle Triassic. Paleobiology. 46, 533–549. doi:10.1017/pab.2020.40
Dames, W. (1888). Die Ganoiden des deutschen Muschelkalks. Paläontologische Abhandlungen. 4, 133–180
Davies, G. R., Moslow, T. F., and Sherwin, M. D. (1997). Ganoid fish Albertonia sp. from the Lower Triassic Montney Formation, western Canada sedimentary basin. Bull. Can. Petrol. Geol. 45, 715–718
Friedman, M. (2015). The early evolution of ray-finned fishes. Palaeontology. 58, 213–228. doi:10.1111/pala.12150
Galfetti, T., Hochuli, P. A., Brayard, A., Bucher, H., Weissert, H., and Vigran, J. O. (2007). Smithian–Spathian boundary event: evidence for global climatic change in the wake of the end-Permian biotic crisis. Geology. 35, 291–294. doi:10.1130/G23117A.1
Gall, J.-C., Grauvogel, L., and Lehman, J.-P. (1974). Faune du Buntsandstein, V. Les Poissons fossiles de la collection Grauvogel-Gall. Ann. Paleontol. 60, 129–147
Gall, J.-C., and Grauvogel-Stamm, L. (2005). The early Middle Triassic ‘Grès à Voltzia’ Formation of eastern France: a model of environmental refugium. Comptes Rendus Palevol. 4, 637–652. doi:10.1016/j.crpv.2005.04.007
Geng, B.-H., Jin, F., Wu, F.-X., and Wang, Q. (2012). New perleidid fishes from the Middle Triassic strata of yunnan province. Geol. Bull. China. 31, 915–927.
Goudemand, N., Romano, C., Leu, M., Bucher, H., Trotter, J. A., and Williams, I. S. (2019). Dynamic interplay between climate and marine biodiversity upheavals during the Early Triassic Smithian-Spathian biotic crisis. Earth Sci. Rev. 195, 169–178. doi:10.1016/j.earscirev.2019.01.013
Hermann, E., Hochuli, P. A., Méhay, S., Bucher, H., Brühwiler, T., Ware, D., et al. (2011). Organic matter and palaeoenvironmental signals during the Early Triassic biotic recovery: the Salt Range and Surghar Range records. Sediment. Geol. 234, 19–41. doi:10.1016/j.sedgeo.2010.11.003
Herzog, A., and Bürgin, T. (2005). A new species of the genus Besania Brough 1939 from the Middle Triassic of Canton Grisons (Switzerland) with a discussion of the phylogenetic status of the taxon. Eclogae Geol. Helv. 98, 113–122. doi:10.1007/s00015-005-1153-x
Herzog, A. (2003). Eine Neubeschreibung der Gattung Eoeugnathus Brough, 1939 (Actinopterygii; Halecomorphi) aus der alpinen Mitteltrias Graubündens (Schweiz). Paläontol. Z. 77, 223–240. doi:10.1007/BF03004570
Hitij, T., Tintori, A., Žalohar, J., Renesto, S., Celarc, B., Križnar, M., et al. (2010). “New fossil sites with Triassic vertebrate fauna from the Kamnik-Savinja Alps, Slovenia,” in Program and abstract (Beijing: international symposium on Triassic and later marine vertebrate faunas), Beijing, China, August 29, 2010 (Beijing, China: Peking University), 42–46
Ji, C., Zhang, C., Jiang, D.-Y., Bucher, H., Motani, R., and Tintori, A. (2015). Ammonoid age control of the Early Triassic marine reptiles from Chaohu (South China). Palaeoworld. 24, 277–282. doi:10.1016/j.palwor.2014.11.009
Jörg, E. (1969). Eine Fischfauna aus dem Oberen Buntsandstein (Unter-Trias) von Karlsruhe-Durlach (Nordbaden). Beiträge zur Naturkundlichen Forschung in Südwestdeutschland. 28, 87–102
Kogan, I. (2011). Remains of Saurichthys (Pisces, Actinopterygii) from the Early Triassic Wordie Creek Formation of east Greenland. Bull. Geol. Soc. Den. 59, 93–100.
Kogan, I., and Romano, C. (2016a). Redescription of Saurichthys madagascariensis Piveteau, 1945 (Actinopterygii, Early Triassic), with implications for the early saurichthyid morphotype. J. Vertebr. Paleontol. 36, e1151886. doi:10.1080/02724634.2016.1151886
Kogan, I., and Romano, C. (2016b). A new postcranium of Saurichthys from the Early Triassic of Spitsbergen. Paläontologie, Stratigraphie, Fazies 23. Freiberger Forschungshefte-A C. 550, 205–221. doi:10.5167/uzh-136748
Kowal-Linka, M., and Bodzioch, A. (2017). Genesis of the Lower Triassic bonebeds from Gogolin (S Poland): the impact of microbial mats on trapping of vertebrate remains. Palaeogeogr. Palaeoclimatol. Palaeoecol. 466, 38–58. doi:10.1016/j.palaeo.2016.11.010
Lehman, J.-P., Château, C., Laurain, M., and Nauche, M. (1959). Paléontologie de Madagascar 27. Les poissons de la Sakamena moyenne. Ann. Paleontol. 45, 175–219.
Lehman, J.-P. (1952). Etude complémentaire des poissons de l’Eotrias de Madagascar. Kungliga Svenska Vetenskapsakademiens Handlingar. Fjärde Serien. 2, 1–201.
Leu, M., Bucher, H., and Goudemand, N. (2019). Clade-dependent size response of conodonts to environmental changes during the late Smithian extinction. Earth Sci. Rev. 195, 52–67. doi:10.1016/j.earscirev.2018.11.003
Li, Q. (2009). A new parasemionotid-like fish from the Lower Triassic of jurong, Jiangsu province, South China. Palaeontology. 52, 369–384. doi:10.1111/j.1475-4983.2009.00848.x
Liu, S., Sun, Z., Ji, C., Zhou, M., and Jiang, D. (2020). Conodont biostratigraphy and age of the Early Triassic fish-bearing-nodule levels from Nanjing and Jurong, Jiangsu province, South China. J. Earth Sci. 31, 9–22. doi:10.1007/s12583-019-1232-y
Lombardo, C., Rusconi, M., and Tintori, A. (2008). New perleidiform from the Ladinian (Middle Triassic) of the northern Grigna (northern Italy). Riv. Ital. Paleontol. Stratigr. 114, 263–272. doi:10.13130/2039-4942/5901
López-Arbarello, A., Bürgin, T., Furrer, H., and Stockar, R. (2016). New holostean fishes (Actinopterygii: Neopterygii) from the Middle Triassic of the Monte San Giorgio (Canton Ticino, Switzerland). PeerJ. 4, e2234. doi:10.7717/peerj.2234
López-Arbarello, A., Bürgin, T., Furrer, H., and Stockar, R. (2019). Taxonomy and phylogeny of Eosemionotus Stolley, 1920 (Neopterygii: Ginglymodi) from the Middle Triassic of Europe. Palaeontol. Electron. 22 (1), 1–64. doi:10.26879/904
López-Arbarello, A., and Sferco, E. (2018). Neopterygian phylogeny: the merger assay. Royal Society Open Science. 5, 172337. doi:10.1098/rsos.172337
López-Arbarello, A., Stockar, R., and Bürgin, T. (2014). Phylogenetic relationships of the Triassic Archaeosemionotus Deecke (Halecomorphi, Ionoscopiformes) from the “Perledo fauna”. PloS One. 9, e108665. doi:10.1371/journal.pone.0108665
Lucas, S. G. (2004). A global hiatus in the Middle Permian tetrapod fossil record. Stratigraphy. 1, 47–64
Ma, X.-Y., and Xu, G.-H. (2015). A new ionoscopiform fish (holostei: Halecomorphi) from the Middle Triassic (Anisian) of Yunnan, China. Vertebr. Palasiat. 55, 1–5
Marramà, G., Lombardo, C., Tintori, A., and Carnevale, G. (2017). Redescription of ‘Perleidus’ (Osteichthyes, Actinopterygii) from the Early Triassic of northwestern Madagascar. Riv. Ital. Paleontol. Stratigr. 123, 219–242. doi:10.13130/2039-4942/8328
Martin, M., and Wenz, S. (1984). Découverte d’un nouveau Coelacanthidé, Garnbergia ommata n. g., n. sp., dans le Muschelkalk supérieur du Baden-Württemberg. Stuttg. Beiträge Naturkd. - Ser. B 105, 1–17
Maxwell, E. E., Diependaal, H., Winkelhorst, H., Goris, G., and Klein, N. (2016). A new species of Saurichthys (Actinopterygii: Saurichthyidae) from the Middle Triassic of Winterswijk, The Netherlands. Neues Jahrbuch Geol. Palaontol. Abhand. 280, 119–134. doi:10.1127/njgpa/2016/0569
Maxwell, E. E., Romano, C., Wu, F., and Furrer, H. (2015). Two new species of Saurichthys (Actinopterygii: Saurichthyidae) from the Middle Triassic of Monte san Giorgio, Switzerland, with implications for character evolution in the genus. Zool. J. Linn. Soc. 173, 887–912. doi:10.1111/zoj.12224
Miklavc, P., Celarc, B., and Šmuc, A. (2016). Anisian Strelovec Formation in the Robanov kot, Savinja Alps (northern Slovenia). Geologija. 59, 23–34. doi:10.5474/geologija.2016.002
Mutter, R., Cartanyà, J., and Basaraba, S. A. U. (2008). “New evidence of Saurichthys from the Lower Triassic with an evaluation of early saurichthyid diversity,” in Mesozoic fishes 4. Homology and phylogeny. Editors G. Arratia, H.-P. Schultze, and M. V. H. Wilson (München: Dr. Friedrich Pfeil), 103–127
Mutter, R. J. (2005). Re-assessment of the genus Helmolepis Stensiö 1932 (Actinopterygii: Platysiagidae) and the evolution of platysiagids in the Early-Middle Triassic. Eclogae Geol. Helv. 98, 271–280. doi:10.1007/S00015-005-1164-7
Mutter, R. J. (2002). Revision of the Triassic family Colobodontidae sensu Andersson 1916 (emended) with a tentative assessment of perleidiform interrelationships (Actinopterygii: Perleidiformes). PhD thesis. Zürich (Switzerland): University of Zurich
Mutter, R. J., and Herzog, A. (2004). A new genus of Triassic actinopterygian with an evaluation of deepened flank scales in fusiform fossil fishes. J. Vertebr. Paleontol. 24, 794–801. doi:10.1671/0272-4634(2004)024[0794:ANGOTA]2.0.CO;2
Near, T. J., Eytan, R. I., Dornburg, A., Kuhn, K. L., Moore, J. A., Davis, M. P., et al. (2012). Resolution of ray-finned fish phylogeny and timing of diversification. Proc. Natl. Acad. Sci. United States. 109, 13698–13703. doi:10.1073/pnas.1206625109
Neuman, A. G. (2015). Fishes from the Lower Triassic portion of the Sulphur Mountain Formation in Alberta, Canada: geological context and taxonomic composition. Can. J. Earth Sci. 52, 557–568. doi:10.1139/cjes-2014-0165
Neuman, A. G., and Mutter, R. J. (2005). Helmolepis cyphognathus, sp. nov., a new platysiagid actinopterygian from the Lower Triassic Sulphur Mountain Formation (British Columbia, Canada). Can. J. Earth Sci. 42, 25–36. doi:10.1139/e04-096
Nielsen, E. (1952). A preliminary note on Bobasatrania groenlandica. Meddelelser fra Dansk Geologisk Förening. 12 (1952), 197–204
Nielsen, E. (1955). Notes on Triassic fishes from Madagascar. Meddelelser fra Dansk Geologisk Förening. 12 (1955), 563–578
Nielsen, E. (1961). “On the Eotriassic fish faunas of central east Greenland,” in Geology of the arctic 1, proceedings of the first international symposium on arctic geology. Editor G. O. Raasch (Toronto: University Press), 255–257
Nielsen, E. (1936). Some few preliminary remarks on Triassic fishes from East Greenland. Meddelelser om Grønland. 112 (3), 1–55
Nielsen, E. (1942). Studies on Triassic fishes from east Greenland 1. Glaucolepis and Boreosomus. Palaeozoologica Groenlandica. 1, 1–403
Nielsen, E. (1949). Studies on Triassic fishes from east Greenland 2. Australosomus and Birgeria. Palaeozoologica Groenlandica. 3, 1–309
Nybelin, O. (1977). Studies on Triassic fishes from east Greenland III—on Helmolepis gracilis Stensiö. Meddelelser om Grønland. 200 (2), 1–14
Oosterink, H. W., and Poppe, W. (1979). Vissen en visresten uit de Onder-Muschelkalk van Winterswijk. Grondboor Hamer. 33, 95–112
Oosterink, H. W. (1986). Winterswijk, geologie 2. De trias-periode (geologie, mineralen en fossielen). Wet. Meded. K. Ned. Natuurhistorische Ver. 178, 1–120
Orchard, M. J. (2007). Conodont diversity and evolution through the latest Permian and Early Triassic upheavals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 252, 93–117. doi:10.1016/j.palaeo.2006.11.037
Orchard, M. J., and Zonneveld, J.-P. (2009). The Lower Triassic Sulphur Mountain Formation in the Wapiti Lake area: lithostratigraphy, conodont biostratigraphy, and a new biozonation for the lower Olenekian (Smithian). Can. J. Earth Sci. 46, 757–790. doi:10.1139/E09-051
Otoo, B. K. A., Clack, J. A., Smithson, T. R., Bennett, C. E., Kearsey, T. I., and Coates, M. I. (2017). A fish and tetrapod fauna from Romer’s Gap preserved in Scottish Tournaisian floodplain deposits. Palaeontology. 62, 225–253. doi:10.1111/pala.12395
Ovtcharova, M., Bucher, H., Schaltegger, U., Galfetti, T., Brayard, A., and Guex, J. (2006). New Early to Middle Triassic U–Pb ages from South China: calibration with ammonoid biochronozones and implications for the timing of the Triassic biotic recovery. Earth Planet Sci. Lett. 243, 463–475. doi:10.1016/j.epsl.2006.01.042
Ovtcharova, M., Goudemand, N., Hammer, Ø., Guodun, K., Cordey, F., Galfetti, T., et al. (2015). Developing a strategy for accurate definition of a geological boundary through radio-isotopic and biochronological dating: the Early-Middle Triassic boundary (South China). Earth Sci. Rev. 146, 65–76. doi:10.1016/j.earscirev.2015.03.006
Payne, J. L., Lehrmann, D. J., Wei, J., Orchard, M. J., Schrag, D. P., and Knoll, A. H. (2004). Large perturbations of the carbon cycle during recovery from the end-Permian extinction. Science. 305, 506–509. doi:10.1126/science.1097023
Perch-Nielsen, K., Birkenmajer, K., Birkelund, T., and Aellen, M. (1974). Revision of Triassic stratigraphy of the Scoresby Land and Jameson Land region, east Greenland. Meddelelser om Grønland. 193, 1–51
Plesker, M. (1995). Neue Funde einiger Knochenfische (Osteichthyes) aus dem Oberen Muschelkalk (MO2) des Weserberglandes. Lippische Mitteilungen aus Geschichte und Landkunde. 64, 303–322
Renesto, S., and Stockar, R. (2018). First record of a coelacanth fish from the Middle Triassic Meride Limestone of Monte San Giorgio (Canton Ticino, Switzerland). Riv. Ital. Paleontol. Stratigr. 124, 639–653. doi:10.13130/2039-4942/10771
Rieppel, O., Kindlimann, R., and Bucher, H. (1996). “A new fossil fish fauna from the Middle Triassic (Anisian) of north-western Nevada,” in Mesozoic fishes, systematics and paleoecology. Editors G. Arratia, and G. Viohl (München: Dr. Friedrich Pfeil), 501–512
Romano, C., Koot, M. B., Kogan, I., Brayard, A., Minikh, A. V., Brinkmann, W., et al. (2016a). Permian-Triassic Osteichthyes (bony fishes): diversity dynamics and body size evolution. Biol. Rev. Camb. Phil. Soc. 91, 106–147. doi:10.1111/brv.12161
Romano, C., Ware, D., Brühwiler, T., Bucher, H., and Brinkmann, W. (2016b). Marine Early Triassic Osteichthyes from Spiti, Indian himalayas. Swiss Journal of Palaeontology. 135, 275–294. doi:10.1007/s13358-015-0098-6
Romano, C., and Brinkmann, W. (2009). Reappraisal of the lower actinopterygian Birgeria stensioei Aldinger, 1931 (Osteichthyes; birgeriidae) from the Middle Triassic of Monte San Giorgio (Switzerland) and Besano (Italy). Neues Jahrbuch Geol. Palaontol. Abhand. 252, 17–31. doi:10.1127/0077-7749/2009/0252-0017
Romano, C., Goudemand, N., Vennemann, T. W., Ware, D., Schneebeli-Hermann, E., Hochuli, P. A., et al. (2013). Climatic and biotic upheavals following the end-Permian mass extinction. Nat. Geosci. 6, 57–60. doi:10.1038/ngeo1667
Romano, C., Jenks, J. F., Jattiot, R., Scheyer, T. M., Bylund, K. G., and Bucher, H. (2017). Marine Early Triassic Actinopterygii from Elko County (Nevada, United States): implications for the Smithian equatorial vertebrate eclipse. J. Paleontol. 91, 1025–1046. doi:10.1017/jpa.2017.36
Romano, C., Kogan, I., Jenks, J., Jerjen, I., and Brinkmann, W. (2012). Saurichthys and other fossil fishes from the late Smithian (Early Triassic) of Bear Lake County (Idaho, United States), with a discussion of saurichthyid palaeogeography and evolution. Bull. Geosci. 87, 543–570. doi:10.3140/bull.geosci.1337
Romano, C., López-Arbarello, A., Ware, D., Jenks, J. F., and Brinkmann, W. (2019). Marine Early Triassic Actinopterygii from the Candelaria Hills (Esmeralda County, Nevada, United States). J. Paleontol. 93, 971–1000. doi:10.1017/jpa.2019.18
Sallan, L. C., and Coates, M. I. (2010). End-Devonian extinction and a bottleneck in the early evolution of modern jawed vertebrates. Proc. Natl. Acad. Sci. U.S.A. 107, 10131–10135. doi:10.1073/pnas.0914000107
Sallan, L. C. (2014). Major issues in the origins of ray-finned fish (Actinopterygii) biodiversity. Biol. Rev. Camb. Phil. Soc. 89, 950–971. doi:10.1111/brv.12086
Sander, P. M., Rieppel, O. C., and Bucher, H. (1994). New marine vertebrate fauna from the Middle Triassic of Nevada. J. Paleontol. 68, 676–680
Schaeffer, B., and Mangus, M. (1976). An Early Triassic fish assemblage from British Columbia. Bull. Am. Mus. Nat. Hist. 156, 127–216
Schultze, H.-P. (2004). “Mesozoic sarcopterygians,” in Mesozoic fishes 3. Systematics, paleoenvironments and biodiversity. Editors G. Arratia, and A. Tintori (München: Dr. Friedrich Pfeil), 463–492
Schultze, H.-P., and Kriwet, J. (1999). “Die Fische der Germanischen Trias,” in Trias–eine ganz andere Welt. Editors N. Hauschke, and V. Wilde (München: Dr. Friedrich Pfeil), 239–250.
Schultze, H.-P., and Möller, H. (1986). Wirbeltierreste aus dem Mittleren Muschelkalk (Trias) von Göttingen, West-Deutschland. Paläontol. Z. 60, 109–129
Schweizer, R. (1966). Ein Coelacanthide aus dem Oberen Muschelkalk Göttingens. Neues Jahrbuch Geol. Palaontol. Abhand. 125, 216–226
Smithwick, F. M., and Stubbs, T. L. (2018). Phanerozoic survivors: actinopterygian evolution through the Permo-Triassic and Triassic-Jurassic mass extinction events. Evolution. 72, 348–362. doi:10.1111/evo.13421
Stockar, R., Baumgartner, P. O., and Condon, D. (2012). Integrated Ladinian bio-chronostratigraphy and geochrononology of Monte San Giorgio (southern Alps, Switzerland). Swiss J. Geosci. 105, 85–108. doi:10.1007/s00015-012-0093-5
Stockar, R. (2010). Facies, depositional environment, and palaeoecology of the Middle Triassic Cassina beds (Meride Limestone, Monte San Giorgio, Switzerland). Swiss J. Geosci. 103, 101–119. doi:10.1007/s00015-010-0008-2
Stolley, E. (1920). Beiträge zur Kenntnis der Ganoiden des deutschen Muschelkalks. Palaeontographica. 63, 25–86
Sun, Z.-Y., Lombardo, C., Tintori, A., Jiang, D.-Y., Hao, W.-C., Sun, Y.-L., et al. (2012). Fuyuanperleidus dengi Geng et al., 2012 (Osteichthyes, Actinopterygii) from the Middle Triassic of Yunnan province, South China. Riv. Ital. Paleontol. Stratigr. 118, 359–373. doi:10.13130/2039-4942/6011
Sun, Z., Jiang, D., Ji, C., and Hao, W. (2016a). Integrated biochronology for Triassic marine vertebrate faunas of Guizhou province, South China. J. Asian Earth Sci. 118, 101–110. doi:10.1016/j.jseaes.2016.01.004
Sun, Z.-Y., Tintori, A., Lombardo, C., and Jiang, D.-Y. (2016b). New miniature neopterygians from the Middle Triassic of Yunnan province, South China. Neues Jahrbuch Geol. Palaontol. Abhand. 282, 135–156. doi:10.1127/njgpa/2016/0610
Sun, Z., Tintori, A., Jiang, D., and Motani, R. (2013). A new perleidid from the Spathian (Olenekian, Early Triassic) of Chaohu, Anhui province, China. Riv. Ital. Paleontol. Stratigr. 119, 275–285. doi:10.13130/2039-4942/6040
Surlyk, F., Bjerager, M., Piasecki, S., and Stemmerik, L. (2017). Stratigraphy of the marine Lower Triassic succession at Kap Stosch, Hold With Hope, north-east Greenland. Bull. Geol. Soc. Den. 65, 87–123.
Tanner, V. M. (1936). A study of Utah fossil fishes with the description of a new genus and species. Proc. Utah Acad. Sci. Arts Lett. 13, 81–89
Tintori, A., Hitij, T., Jiang, D., Lombardo, C., and Sun, Z. (2014a). Triassic actinopterygian fishes: the recovery after the end-Permian crisis. Integr. Zool. 9, 394–411. doi:10.1111/1749-4877.12077
Tintori, A., Huang, J.-D., Jiang, D.-Y., Sun, Z.-Y., Motani, R., and Chen, G. (2014b). A new Saurichthys (Actinopterygii) from the Spathian (Early Triassic) of Chaohu (Anhui province, China). Riv. Ital. Paleontol. Stratigr. 120, 157–164. doi:10.13130/2039-4942/6057
Tintori, A. (2013). A new species of Saurichthys (Actinopterygii) from the Middle Triassic (early Ladinian) of the northern Grigna mountain (Lombardy, Italy). Riv. Ital. Paleontol. Stratigr. 119, 287–302. doi:10.13130/2039-4942/6041
Tintori, A. (1992). Fish taphonomy and Triassic anoxic basins from the Alps: a case history. Riv. Ital. Paleontol. Stratigr. 97, 393–408
Tintori, A., Lombardo, C., and Kustatscher, E. (2016). The Pelsonian (Anisian, Middle Triassic) fish assemblage from Monte Prà della Vacca/Kühwiesenkopf (Braies Dolomites, Italy). Neues Jahrbuch Geol. Palaontol. Abhand. 282, 181–200. doi:10.1127/njgpa/2016/0612
Tong, J., and Yin, H. (2002). The Lower Triassic of South China. J. Asian Earth Sci. 20, 803–815. doi:10.1016/S1367-9120(01)00058-X
Tong, J., Zhou, X., Erwin, D. H., Zuo, J., and Zhao, L. (2006). Fossil fishes from the Lower Triassic of Majiashan, Chaohu, Anhui province, China. J. Paleontol. 80, 146–161. doi:10.1666/0022-3360(2006)080[0146:FFFTLT]2.0.CO;2
Tozer, E. T. (1965). Lower Triassic stages and ammonoid zones of Arctic Canada. Pap. Geol. Surv. Can. 65, 1–14
Turner, S., Blieck, A., and Nowlan, G. S. (2004). “Vertebrates (agnathans and gnathostomes),” in The great ordovician biodiversification event. Editors B. D. Webby, F. Paris, M. L. Droser, and I. G. Percival (New York, NY: Columbia University Press), 327–335
Ware, D., Bucher, H., Brayard, A., Schneebeli-Hermann, E., and Brühwiler, T. (2015). High-resolution biochronology and diversity dynamics of the Early Triassic ammonoid recovery: the Dienerian faunas of the Northern Indian Margin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 440, 363–373. doi:10.1016/j.palaeo.2015.09.013
Ware, D., Bucher, H., Brühwiler, T., and Krystyn, L. (2018). Dienerian (Early Triassic) ammonoids from Spiti (Himachal Pradesh, India). Fossils Strata. 63, 177–241. doi:10.1002/9781119522812.ch3
Wen, W., Hu, S. X., Zhang, Q. Y., Benton, M. J., Kriwet, J., Chen, Z. Q., et al. (2019). A new species of Platysiagum from the Luoping Biota (Anisian, Middle Triassic, Yunnan, South China) reveals the relationship between Platysiagidae and Neopterygii. Geol. Mag. 156, 669–682. doi:10.1017/S0016756818000079
Wen, W., Zhang, Q. Y., Hu, S. X., Zhou, C. Y., Xie, T., Huang, J. Y., et al. (2012). A new basal actinopterygian fish from the Anisian (Middle Triassic) of Luoping, Yunnan province, southwest China. Acta Palaeontol. Pol. 57, 149–160. doi:10.1017/S0016756818000079
Wen, W., Zhang, Q.−Y., Hu, S.−X., Benton, M. J., Zhou, C.−Y., Tao, X., et al. (2013). Coelacanths from the Middle Triassic Luoping Biota, Yunnan, South China, with the earliest evidence of ovoviviparity. Acta Palaeontol. Pol. 58, 175–193. doi:10.4202/app.2011.0066
Wendruff, A. J., and Wilson, M. V. H. (2012). A fork-tailed coelacanth, Rebellatrix divaricerca, gen. et sp. nov. (Actinistia, Rebellatricidae, fam. nov.), from the Lower Triassic of Western Canada. J. Vertebr. Paleontol. 32, 499–511. doi:10.1080/02724634.2012.657317
Wendruff, A. J., and Wilson, M. V. H. (2013). New Early Triassic coelacanth in the family Laugiidae (Sarcopterygii: Actinistia) from the Sulphur Mountain Formation near Wapiti Lake, British Columbia, Canada. Can. J. Earth Sci. 50, 904–910. doi:10.1139/cjes-2013-0010
Werneburg, R., Kogan, I., and Sell, J. (2014). Saurichthys (Pisces: Actinopterygii) aus dem Buntsandstein des Germanischen Beckens. Semana. 29, 3–35.
Widmann, P., Bucher, H., Leu, M., Vennemann, T., Bagherpour, B., Schneebeli-Hermann, E., et al. (2020). Dynamics of the largest carbon isotope excursion during the Early Triassic biotic recovery. Front. Earth Sci. 8 (196), 1–16. doi:10.3389/feart.2020.00196
Wignall, P. B., and Newton, R. (2003). Contrasting deep-water records from the upper Permian and Lower Triassic of South Tibet and British Columbia: evidence for a diachronous mass extinction. Palaios. 18, 153–167. doi:10.1669/0883-1351(2003)18<153:CDRFTU>2.0.CO;2
Wilser, J. L. (1923). Pygopterus crecelii n. sp. aus dem Oberen Buntsandstein bei Karlsruhe i. B. Berichte der Naturforschenden Gesellschaft zu Freiburg i. Br. 23, 68–78
Wu, F., Chang, M. M., Sun, Y., and Xu, G. (2013). A new saurichthyiform (Actinopterygii) with a crushing feeding mechanism from the Middle Triassic of Guizhou (China). PloS One. 8, e81010, doi:10.1371/journal.pone.0081010
Wu, F.-X., Sun, Y.-L., and Fang, G.-Y. (2018). A new species of Saurichthys from the Middle Triassic (Anisian) of southwestern China. Vertebr. PalAsiat. 56, 273�294. doi:10.19615/j.cnki.1000-3118.071023
Wu, F. X., Sun, Y. L., Xu, G. H., Hao, W. C., Jiang, D. Y., and Sun, Z. Y. (2011). New saurichthyid actinopterygian fishes from the Anisian (Middle Triassic) of southwestern China. Acta Palaeontol. Pol. 56, 581–614. doi:10.4202/app.2010.0007
Xu, G., Ma, X., and Ren, Y. (2020). Fuyuanichthys wangi gen. et sp. nov. from the Middle Triassic (Ladinian) of China highlights the early diversification of ginglymodian fishes. PeerJ 6, e6054. doi:10.7717/peerj.6054
Xu, G.-H. (2020a). A new stem-neopterygian fish from the Middle Triassic (Anisian) of Yunnan, China, with a reassessment of the relationships of early neopterygian clades. Zool. J. Linn. Soc. 1–20. doi:10.1093/zoolinnean/zlaa053
Xu, G.-H. (2020b). A new species of Luganoia (Luganoiidae, Neopterygii) from the Middle Triassic Xingyi Biota, Guizhou, China. Vertebr. Palasiat. 58, 267–282. doi:10.19615/j.cnki.1000-3118.200624
Xu, G. H. (2020c). Feroxichthys yunnanensis gen. et sp. nov. (Colobodontidae, Neopterygii), a large durophagous predator from the Middle Triassic (Anisian) Luoping Biota, eastern Yunnan, China. PeerJ. 8, e10229. doi:10.7717/peerj.10229
Xu, G.-H., and Shen, C.-C. (2015). Panxianichthys imparilis gen. et sp. nov., a new ionoscopiform (Halecomorphi) from the Middle Triassic of Guizhou, China. Vertebr. Palasiat. 53, 1–15
Xu, G. H., Zhao, L. J., and Coates, M. I. (2014a). The oldest ionoscopiform from China sheds new light on the early evolution of halecomorph fishes. Biol. Lett. 10, 20140204. doi:10.1098/rsbl.2014.0204
Xu, G.-H., Shen, C.-C., and Zhao, L. J. (2014b). Pteronisculus nielseni sp. nov., a new stem-actinopteran fish from the Middle Triassic of Luoping, Yunnan province, China. Vertebr. Palasiat. 52, 364–380.
Xu, G.-H., and Zhao, L.-J. (2016). A Middle Triassic stem-neopterygian fish from China shows remarkable secondary sexual characteristics. Sci. Bull. 61, 338–344. doi:10.1007/s11434-016-1007-0
Zhang, L., Zhao, L., Chen, Z.-Q., Algeo, T. J., Li, Y., and Cao, L. (2015). Amelioration of marine environments at the Smithian-Spathian boundary, Early Triassic. Biogeosciences. 12, 1597–1613. doi:10.5194/bg-12-1597-2015
Keywords: actinopterygii, triassic, biotic recovery, biodiversity, diversification, actinistia (coelacanthiformes), osteichthyes (bony fish)
Citation: Romano C (2021) A Hiatus Obscures the Early Evolution of Modern Lineages of Bony Fishes. Front. Earth Sci. 8:618853. doi: 10.3389/feart.2020.618853
Received: 18 October 2020; Accepted: 03 December 2020;
Published: 27 January 2021.
Edited by:
Robert Reisz, University of Toronto Mississauga, CanadaReviewed by:
Lionel Cavin, Natural History Museum of Geneva, SwitzerlandSpencer G. Lucas, New Mexico Museum of Natural History and Science, United States
Copyright © 2021 Romano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Romano, Y2FybG8ucm9tYW5vQHBpbS51emguY2g=
 Carlo Romano
Carlo Romano