
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Earth Sci. , 19 January 2021
Sec. Cryospheric Sciences
Volume 8 - 2020 | https://doi.org/10.3389/feart.2020.536036
This article is part of the Research Topic Impure Snow and Ice in Remote Areas: Arctic, Antarctica and High Mountains View all 11 articles
 Andrea Spolaor1*
Andrea Spolaor1* Beatrice Moroni2
Beatrice Moroni2 Bartłomiej Luks3
Bartłomiej Luks3 Adam Nawrot3,4
Adam Nawrot3,4 Marco Roman5
Marco Roman5 Catherine Larose6
Catherine Larose6 Łukasz Stachnik7
Łukasz Stachnik7 Federica Bruschi1,2
Federica Bruschi1,2 Krystyna Kozioł8
Krystyna Kozioł8 Filip Pawlak8
Filip Pawlak8 Clara Turetta1
Clara Turetta1 Elena Barbaro1
Elena Barbaro1 Jean-Charles Gallet9
Jean-Charles Gallet9 David Cappelletti2
David Cappelletti2We present a thorough evaluation of the water soluble fraction of the trace element composition (Ca, Sr, Mg, Na, K, Li, B, Rb, U, Ni, Co, As, Cs, Cd, Mo, Se, Eu, Ba, V, Ge, Ga, Cr, Cr, P, Ti, Mn, Zr, Ce, Zn, Fe, Gd, Y, Pb, Bi, Yb, Al, Nb, Er, Nd, Dy, Sm, Ho, Th, La, Lu, Tm, Pr, Tb, Fe, In, Tl) and their fluxes in the annual snowpack and the firn of the Hansbreen (a tidewater glacier terminating in the Hornsund fjord, southwest Spitsbergen). The trace element samples were obtained from a 3 m deep snow pit dug at the plateau of the glacier (450 m a.s.l.), and from a 2 m deep firn core collected from the bottom of the snow pit. The comparison of elemental fluxes and enrichment factors allowed us to constrain specific summer and wintertime deposition patterns of water soluble trace elements in the southern part of the Svalbard archipelago. Our results suggest that the chemical composition of the Hansbreen (and likely other glaciers where the summit is close to the equilibrium line) is mainly affected by summertime deposition of trace elements from local sources and some volatile elements, which may be transported into the Arctic when polar vortex is weak. The melting of the annual snowpack seems to have a minor influence on the overall chemical signature of the glacier ice.
The climate of the Svalbard archipelago is characterized by a marked thermal gap between summer and winter periods (Maturilli et al., 2013). As a consequence, high variability in snow accumulation between the coastline and the interior of the archipelago exists (Winther et al., 1998). The annual snowpack covers almost the entire archipelago between October and May, but the recent air temperature increase, estimated at 1.3°C per decade (Maturilli et al., 2013), is affecting its onset, evolution and duration. The snow season has been shortening from 250 to 220 days per year at Longyearbyen and Ny-Ålesund meteorological stations (www.mosj.no), with similar trends observed at Barentsburg (−4.6 days decade−1) and Hornsund stations (−10.0 days decade−1) (Osuch and Wawrzyniak, 2017). Both the warming and the change in snow season duration have direct consequences for glacier mass balance, which has registered a constant decrease over the past years (Kohler et al., 2007; Aas et al., 2016; Van Pelt et al., 2019), despite the recorded rise in the amount of precipitation (from 400 to 600 mm; www.mosj.no) and a potential increase in the annual snowpack thickness. The decrease in glacier mass balance is shifting the equilibrium line altitude upwards, i.e. the altitude above which winter snow survives summer melting and can contribute to positive mass balance (Möller and Kohler, 2018). Besides being crucial for glacier mass balance, the annual snowpack is a sink and a reservoir for a wide range of inorganic and organic elements naturally present and/or released by human activities. The presence of impurities in the annual snowpack, in the form of either insoluble dust particles (Barbante et al., 2017), soluble chemical species (Barbaro et al., 2017b) or organic compounds (Vecchiato et al., 2018), can be used to study the transport processes from polluted source areas at mid-latitudes and constrain the potential impact of human activities on the Arctic environment (Barbante et al., 2001; Ezerinskis et al., 2014; Nawrot et al., 2016).
The chemical composition of the annual snow depends on various factors including the source of air masses (local, regional, long-range) and impurities (natural, anthropogenic) (Barbante et al., 2017; Vecchiato et al., 2018; Conca et al., 2019). Considering the location of Svalbard, marine processes and oceanic emissions contribute markedly to the deposition flux of multiple elements, next to the crustal components (Spolaor et al., 2013; Barbaro et al., 2017a). However, the long-range transport of atmospheric dust and wildfire emissions from Eurasia can significantly impact the elemental composition of the annual snowpack (Moroni et al., 2016; Feltracco et al., 2020). A recent study investigating the lead isotope composition of aerosol samples suggests a predominant contribution of air masses from North Eurasia during spring, and the main influence during summer coming from North America (Barbaro et al., 2016; Zielinski et al., 2020). Furthermore, the anticipated shortening of the snow season, combined with glacier retreat, will increase the time and extent of soil exposure locally, thus likely enhancing the impact of dust on snowpack composition. The annual snowpack is a critical component of the cryosphere and interfaces with most environmental spheres (hydrosphere, atmosphere, pedosphere and ecosphere) in Svalbard (Gallet et al., 2019). For example, the impact of the release of elements and compounds during the melting season, in particular biologically active elements such as iron and phosphorus, on the surface oceanic water composition, highlights the importance of snowmelt on ecosystems (Stachnik et al., 2019). Therefore, investigating the chemical composition of snow and the underlying processes controlling it is fundamental for constraining the impact of snowpacks on down-stream ecosystems. Trace elements could be a good indicator of human contamination of remote areas. As previously reported by Siudek et al. (2015), the major sources of trace elements are associated with natural processes (rock weathering, mineralization, dust storm, volcanic eruption), although industrial activities have also been shown to influence their abundance in the environment. Industrial processes such as fossil (As, Cu, Co, Cr, V, Ni, Sb, Fe, Mn, Zn, Sn) and oil combustion (Mn, Pb, Fe, Ni), motor vehicle exhausts (Pb, Cu, Cr, Sn, Sb), smelting (Ni, Cu, As, Pb, Cd), iron/steel manufacturing (Cr, Mn, Ni, Co), waste incineration (Pb, Zn), and cement production lead to the release of trace elements to the atmosphere. The Svalbard archipelago can almost be considered as a pristine environment since industrial activities are mostly limited to coal extraction and oil combustion through ship and snow mobile traffic. Therefore, potential sources of trace elements could also be ascribed to long-range transport in addition to natural emissions.
Few studies on the elemental composition of the Svalbard snowpack exist (Spolaor et al., 2013; Pedersen et al., 2015; López-Moreno et al., 2016; Nawrot et al., 2016; Spolaor et al., 2016; Barbaro et al., 2017a), and they are mostly geographically limited to the western side of the Archipelago. There is a general lack of data on trace element concentrations and their fluxes in the southern part of the Svalbard archipelago. Here, we present the first characterization of the water-soluble fraction of trace elements in the annual snowpack in the Hansbreen, a tidewater glacier terminating in the Hornsund fjord. We focused on the water-soluble fraction being that mostly affected by the melting episodes that occur in the Hansbreen. The element concentrations and deposition fluxes in both snow and ice were evaluated to better characterize their sources (marine, crustal or anthropogenic) and impacts. In addition, considering the moderate altitude of the summit of the Hansbreen (500 m a.s.l) and the glacier equilibrium line (370 m a.s.l., Laska et al., 2016) (Schuler et al., 2020), this study provides an important piece of information evaluating the effect of the annual snow pack chemical composition on the glacier firn trace element abundance in a low elevation Svalbard glacier.
The southern part of the Svalbard archipelago is characterized by higher winter snow accumulation and higher temperatures as compared to the Svalbard average and in particular to those measured in the northern territory of the archipelago. The Hansbreen covers an area of about 56 km2 with a length of approximately 15 km (Laska et al., 2016). The glacier terminates in the Hornsund fjord and its summit is located approximately at 500 m a.s.l in connection with Vrangpeisbreen (Figure 1). Due to the moderate altitude of the glacier summit and the calculated glacier equilibrium line (370 m a.s.l., Laska et al., 2016), the annual snowpack covering the Hansbreen is strongly affected by summer melting. Hansbreen lies over a mosaic of metamorphic rocks. The eastern flank of the glacier laps the Sofiekammen (mainly calcitic and dolomitic marbles) and the Sørkapp Land group (quartzite-dominated) formations (Birkenmajer, 1990): Sofiekammen (mainly carbonates: marbles and dolomites) and Sørkapp Land group (quartzite-dominated). The western side of the glacier (Czerny et al., 1993) is surrounded by the Deilegga and Eimfjellet groups. Deilegga is a formation built of metasedimentary rocks (phyllites, calcitic and dolomitic marbles). It contains pyrite, pyrrhotite, chalcopyrite, and subordinately, marcasite, galena, sphalerite, arsenopyrite, and chlorite accessory minerals. At Slyngfjellet (the mountain ridge to the side of the Hansbreen) siderite, ankerite and quartz veins have also been found (Kieres and Piestrzyński, 1992). This group can, thus, supply significant concentrations of major (Fe, S, Cu, Pb, Zn, As, Ca, Mg and Mn) and, likely, minor elements (e.g., Ni from chlorites) to the glacier. The Eimfjellet group, situated ∼3 km west from Hansbreen, is built of quartzites, schists, and amphibolites. These rock types include the minerals pyrite, chalcopyrite, pyrrhotite, hematite, galena, arsenopyrite, marcasite, sphalerite, and mackinawite. Native bismuth has also been found in these rocks. Thus, it can be a source, besides the aforementioned elements, of Ni and Bi in atmospheric dust of the area. Some of the listed minerals were also found to contain Cd and Co as vicariant elements of Zn and Fe, respectively (Kieres and Piestrzyński, 1992).
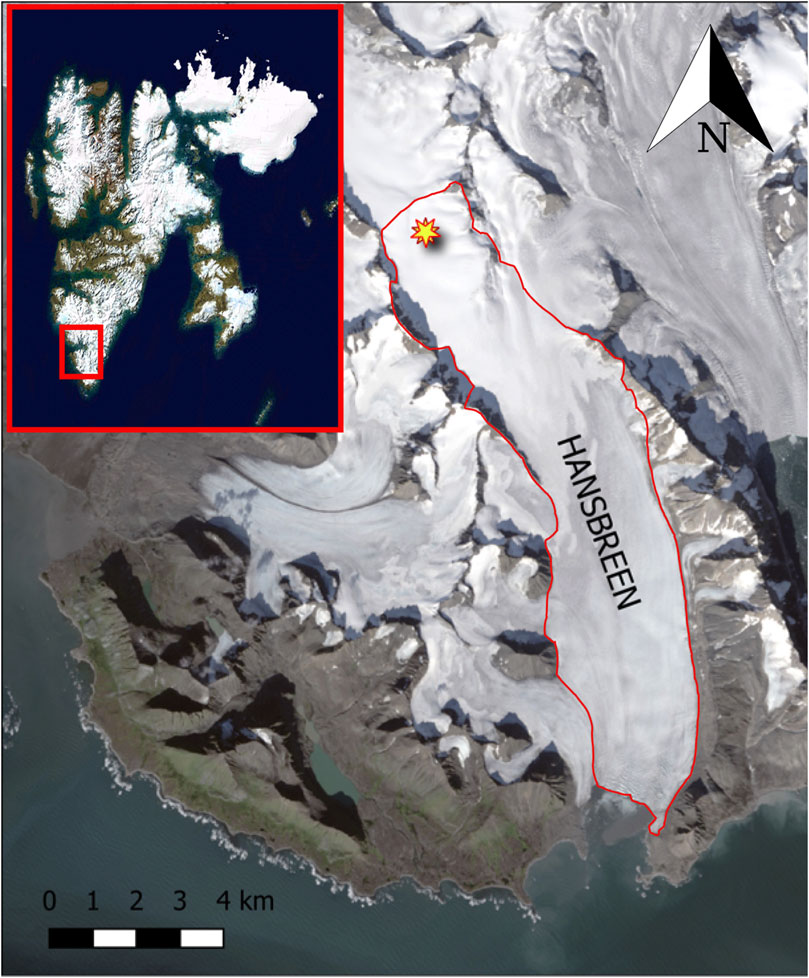
FIGURE 1. Location of the Hansbreen (inset panel) and the sampling site during the summer season (both marked with a star marker) (Norwegian Polar Institute/USGS Landsat). The red contour indicates the glacier basin (see https://toposvalbard.npolar.no/ for a detailed map).
Snow pack proprieties and methodological details on sample collection, preparation and analysis for the water-soluble fraction of the elements are detailed reported in the Supplementary Material.
In the Hansbreen snowpack, Na concentration was the highest (1,016 ng g−1 on average), followed by Mg (122 ng g−1 on average), K (44 ng g−1 on average) and P (14 ng g−1 on average). Compared to the elements of marine origin (Millero et al., 2008; Weller et al., 2008), crustal element (Gabrielli et al., 2005) concentrations were at least one order of magnitude lower in the snow pack and firn samples (Table 1). For example, average Al, Fe and Zn concentrations in the snow pack were 4.2, 2.6, and 2.0 ng g−1, respectively. Specific trace elements, that has already been associated with human activities such as Pb, Cr, Cd and As, showed concentrations in the snow pit of 0.052, 0.021, 0.039, and 0.014 ng g−1, respectively. Assuming that Na is primarily derived from sea spray aerosols (Rhodes et al., 2018) and using the average elemental concentration in seawater (Millero et al., 2008), we can distinguish the elements with predominant sea spray contribution (ssC) from those that are mainly of crustal origin (Table 1 and Supplementary Material). In the Hansbreen snowpack, Mg, Ca and Sr were completely derived from sea spray emissions, while the sea-spray contribution rates for Li, K, B, Rb and U were 80, 87, 58, 37, and 20%, respectively (Table 1). Ca and Sr can also have crustal sources (e.g. Sofiebogen Ridge, Slyngfjellet peak, Deillega Ridge), but due to the high sea spray emission and the short distance between the sampling site and coastline (15 km), those seem completely overwhelmed by the marine contribution. The firn samples reveal an overall high ssC for elements such as Na, Mg and K (Table 1), however, the lower ssC for Li, U, K and Rb, in the firn with respect to annual snow pack suggests a rather likely increased contribution of dust deposition during snow-free season.
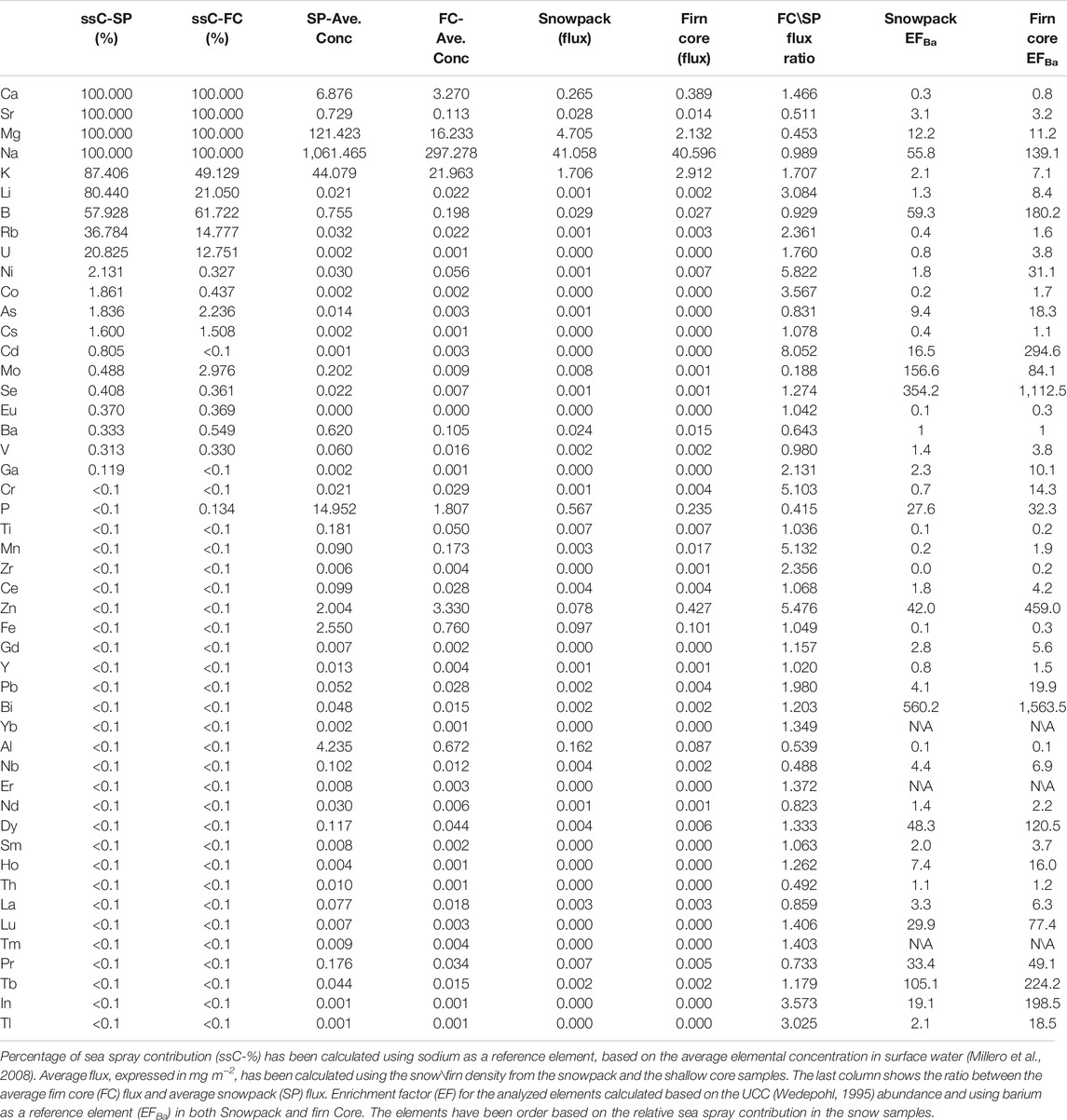
TABLE 1. Average elemental concentration order based on its sea spray contribution in the snowpack (SP Ave. Conc) and the firn core (FC. Ave Conc) expressed both as ng g−1.
While the annual snowpack is representative of a single year, the firn is a result of several years of accumulation. In the Hansbreen, it is particularly difficult to date the firn, since percolation of liquid water might mix the layers and re-distribute the water-soluble fraction of chemical species across them. Therefore, in order to compare snow and firn, the elemental concentration in each snow and shallow core sample was multiplied by the water equivalent to obtain a deposition flux for each element expressed as mg m−2 (see Supplementary Material and Supplementary Table S1). This flux is representative of the mass deposition for each studied layer/sample (Table 1). We used the average flux per m2 calculated from each sample instead of the annual flux to avoid the inaccurate dating problem.
Based on the firn\snow flux ratio, three main groups of elements can be distinguished. Snow pit and firn profile of representative elements for each group are reported in Figure 2. The first group includes Mo, P, Mg, Th, Sr, Al, Ba and As. These elements showed an average deposition flux that is higher in the annual snowpack than in the firn (firn\snow ratio <1). The second group includes B, V, Na, Ti, Fe and Ce, whose average depositional fluxes in the two compartments were not different (firn\snow ratio ≈1). The third group included Bi, Se, Ca, K, U, Pb, Rb, Tl, Li, Co, In, Cr, Mn, Zn, Ni and Cd; which had a higher average depositional flux in the firn samples (firn\snow ratio >1) (Figure 3).
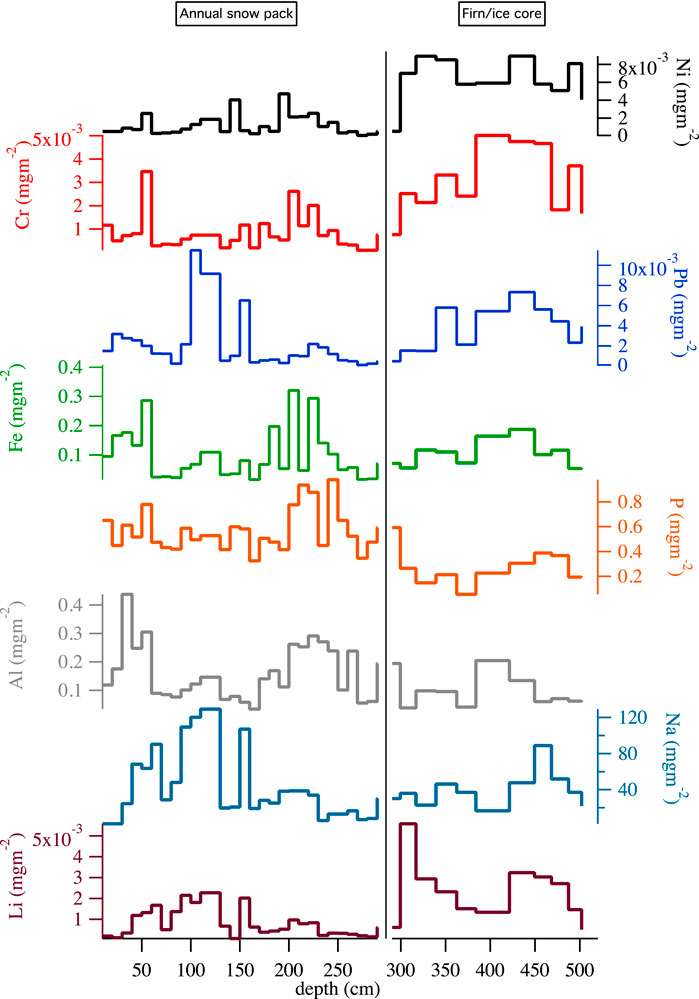
FIGURE 2. Snowpack sample flux (left panels) versus firn sample flux (right panels) of selected elements for the three groups. Lithium, Ni, Cr, and Pb are representative of the third group (Bi, Se, Ca, K, U, Pb, Rb, Tl, Li, Co, In, Cr, Mn, Zn, Ni, and Cd), Fe and Na of the second (B, V, Na, Ti, Fe, and Ce ) and Al and P of the third (Mo, P, Mg, Th, Sr, Al, Ba, and As). The left and right axis units are both mg m−2, lower axis represents the depth (in cm) from the annual snowpack surface.
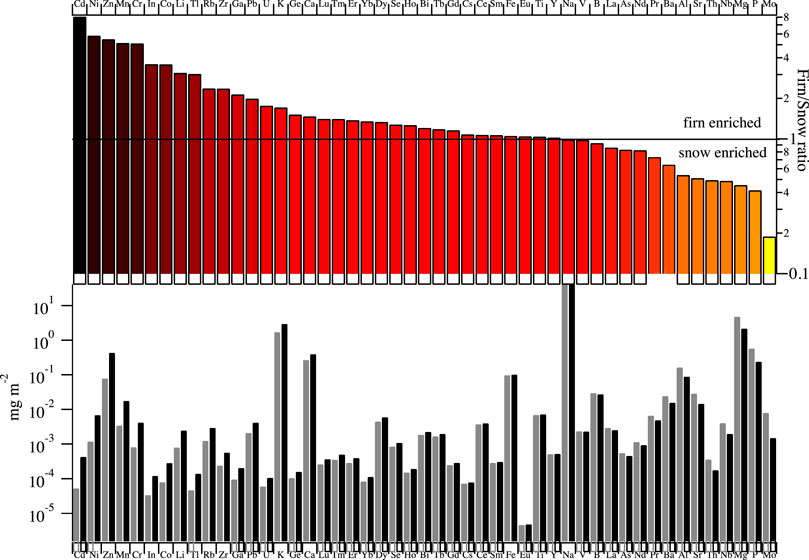
FIGURE 3. Firn and snow flux and their ratio for the analyzed elements. The upper panel shows the firn\snow ratio for each element. The enrichment of elements in firn is represented on a color scale from light\yellow (low) to the dark red (high). The lower panel shows the average flux in the annual snowpack (grey bars) and in the firn samples (black bars).
Elements in the first and second group seem mainly related to sea spray aerosols and more water-soluble since they are mainly present in the salt form. However in this two groups exception are presence since Al, V, Ti and Mo are mainly crustal origin elements (Gabrielli et al., 2005). Snowmelt has an effect on the concentration of soluble elements (Brimblecombe et al., 1987; Spolaor et al., 2016) such as Na, P, Mg, Ca, K, Ba and B, by washing them out of the snowpack. For Mg, P and Ba, in particular, the lower average flux in the firn suggests a strong wash out from snowpack and likely a subsequent glacier surface run-off during melting season. The fact that Na and Ca do not show differences in the average deposition flux between firn and snow possibly indicates constant/similar deposition throughout the entire year. Molybdenum shows a significantly higher flux in the snowpack as compared to the firn (Figure 3). The natural presence of Mo is commonly associated with the occurrence of the mineral molybdenite (MoS2). This latter mineral is typical of granitoid rocks outcropping in different Arctic regions (e.g, N-Norway and E-Greenland), and only small outcrops of these rocks were found in the northernmost Svalbard (Johansson et al., 2002). In coal mining regions, however, Mo is usually present in trace amounts in ore deposits, but it can be enriched due to exploitation (Frascoli and Hudson-Edwards, 2018). Also, Mo associated with over bank deposits from Triassic rocks has low content in sediments. In this respect, the lower average depositional flux of Mo in the firn compared to the snowpack suggests a medium to long-range transport of molybdenite to Svalbard. Mo can also be derived from coal combustion (Harkness et al., 2017). Therefore, its presence in the snowpack might be related to long-range or regional transport, since coal is used in the Svalbard archipelago for energy production since its marine contribution is less than 1% in the snow-samples (Table 1). Aluminum is a crustal element and the sea spray contribution is relatively low. Aluminum has a poor solubility, as supported by the EF << 1, and its total abundance could be determined only after complete mineralization of the particulate fraction. The lower amount of water-soluble fraction in the firn core compared to the snow pit (FC\SP < 1) could be the result of removing the soluble fraction during snow pack melting. The late spring melting could remove the soluble Al fraction (Stachnik et al., 2019), leaving the insoluble Al fraction trapped in the ice. This might also occur for Fe, which is abundant in the mineral particle lattice.
The third and largest group of elements (Table 1), suggests the occurrence of different specific processes which can strongly influence their concentrations in the firn as compared to the snowpack, such as 1) the retention of dust particles in the annual accumulated firn, 2) their increase weathering due to water presence during summer 3) the contribution of human activities, 4) the change in dust transport pathways, and 5) the activation of different, local sources during summer. The retention (hypothesis a) of the insoluble dust particles in firn due to snow mass reduction during summer can play an important role. If this were the main process contributing to elemental load, we would expect that their abundance should be similar to that of the snowpack. However as observed in Table 1 and Figure 3, this is not the case, therefore the reduction of snow mass during the melting season cannot explain the observed difference alone. We should note that we are considering the water-soluble fraction of the investigated elements, and not the whole composition of insoluble dust particles. We cannot exclude that summer weathering process (hypothesis b) could act diversely for each element and partially contributing to the difference observed but crustal elements are present in the mineral lattice and only a minor fraction could be released\converted in the water-soluble fraction. Human activities can also impact elemental deposition in the Arctic/Svalbard region. As a first approximation, and with caution (Reimann et al., 2005), we used the Enrichment Factor (EF) approach to disentangle possible anthropogenic contributions from natural ones. We calculated the EFs using barium as crustal tracer (EFBa) for its solubility, abundance and the negligible effect of sea spray emissions on its concentration. The enrichment factor was calculated based on the chemical element abundance in the Upper Continental Crust (UCC) as reported in (Wedepohl, 1995). Considering the low contribution of sea spray for almost all the crustal elements (no sea spray corrections were applied for the elements in this group), the EF was calculated for the average deposition flux in the snow pit and the firn samples (Table 1). Since only the soluble fraction of elements was determined, these values of EF might actually result from a combination of: 1) specific composition of the incoming material; 2) different in situ distribution between particulate and dissolved fraction due to different solubility; and 3) different mobility of the elements also due to their different solubility. Given that these processes cannot be disentangled with the available data, particular care must be taken in the interpretation of EFs. However, useful indications on the elemental sources can be extracted even so.
As a rule of thumb, an element is considered enriched when EF > 100. The enrichment factor is a method frequently adopted to evaluate possible extra sources, in addition to natural ones, that contribute to the deposition of a specific element. In this respect, Bismuth (Bi) is the most enriched element with values of 560 and ∼1,560 in the annual snowpack and the firn samples, respectively. The higher EFBa of Bi is difficult to assess and additional investigation must be considered but, in the surrounding area of the Hansbreen, pegmatite outcrops might contribute to the snow\ice load (Majka and Kośmińska, 2017) and native bismuth was also found in mountain peaks (Kieres and Piestrzyński, 1992). However, the presence of such rocks might not explain fully the high EFBa determined. Bismuth might also have an anthropogenic contribution from coal and oil combustion, as well as from aluminum production (Ferrari et al., 2000). A higher enrichment was also found for Se, probably resulting from marine (Amouroux et al., 2001) and terrestrial (Aastrup et al., 2000) biological emissions, although anthropogenic influences cannot be excluded (Tan et al., 2016). Lead had an EF of 19.9 in the firn samples and 4.1 in the snow. The increase in EFBa for several elements in firn might suggest that during the summer period, the extent of air mass transport from lower latitudes increases due to the breakdown of the polar vortex. Another hypothesis is that the local ship emissions contribute significantly to the amount of these specific trace elements in the aerosol. However, we sampled close to the equilibrium line altitude that is surrounded by snow-free mountain peak in summer (e.g. Skilryggen, Strypegga), which contain galena (PbS) bearing rocks (Kieres and Piestrzyński, 1992; Czerny et al., 1993), therefore local dust might also be a non-negligible source and might become the most important source for impurities in the glacier. This last hypothesis is supported by the REEs relative abundance and specific ratios that do not show significant differences between snow and firn samples (Supplementary Figure S1) suggesting a negligible change of the long range transport mainly affecting this group of elements. Zinc and In both had a relatively high flux and EFBa in the firn as compared to the snowpack (five and three times higher, respectively). This increased depositional flux in the firn can be associated with the common presence of sphalerite (ZnS) in the metamorphic basement outcropping in the Hornsund region (Kieres and Piestrzyński, 1992; Czerny et al., 1993). Nickel, Cr and Co, which had higher firn/snow flux ratios (Figure 3; Table 1), are frequently associated with minerals such as iron sulphides, sphalerite, magnetite found in Svalbard bedrock, including the study area (Kieres and Piestrzyński, 1992). This could suggest that the activation of local rock dust sources during summer significantly impacts the glacier firn elemental concentrations. The other elements with a positive firn/snow ratio are associated with several minerals that are widespread in Svalbard and other geological regions, thus rendering the identification of specific source regions difficult. However, out of fifteen elements in this third type of EF behavior, seven were found in rocks in the direct vicinity of Hansbreen, and further five could be sourced from rock erosion within 15 km radius from there (Kozak et al., 2015; Kosek et al., 2019a; Kosek et al., 2019b).
The highest difference between firn and annual snowpack depositional flux was determined for Cd, with firn values eight times higher than in the snow. Although we cannot exclude a local contribution (e.g. in range of 0.3–3% in sphalerite), Cd is generally considered a marker for long-range dust transport, since it is mainly anthropogenically produced from the smelting of non-ferrous metals (Łokas et al., 2019). Its increase in the firn samples might be associated with transport from North America and Siberia during the summer, following the breakdown of the polar vortex.
The results obtained in the present work suggest that the Hansbreen snowpack is strongly influenced by marine emissions with a higher depositional flux of marine-related elements as compared to crustal elements. Significant differences between the composition of the annual snowpack and the firn samples were observed. In particular, elements present in the sediment\rocks in the Hornsund region and characterizing the Svalbard geology showed a higher depositional flux in the firn as compared to the annual snowpack.
The annual snowpack showed higher depositional flux for a few elements (for instance, Mo) not abundant in the mineralogy of the area and might associate with local human activities or long-range transport. The higher depositional flux for a large number of crustal elements in the firn and, in particular, for Ni, Cr and Co are likely associated with minerals and rocks abundant in the Hornsund region. The higher abundance of the latter elements suggests that, during the summer periods, the local dust sources are predominant, although some anthropogenic contamination (such as for Cd), derived from long-range transport, might influence the total deposition.
The results presented suggest that Svalbard glaciers, like Hansbreen, whose summit is close to the equilibrium line, are prone to predominant local dust deposition during summer (late) when some freezing is possible after summer melting. Local dust deposition may, thus, affect the chemical composition of the glacier ice. On the other hand, elements released from annual snowpack during the melt season seem to have minor influence on the overall chemical signature. Quantitative evaluation of the whole composition compared to the water-soluble fraction is necessary to obtain a clear picture of deposition versus melting effects. In addition, the impact of cryoconite as a trap for dust and on the efficiency of dust particle transfer from surface to the glacier ice should also be considered in future studies.
The datasets generated for this study are available on request to the corresponding author.
AS, BL, AN, CL, KK, FP, and DC conceived the experiment and collected the samples, AS and MR. measured the samples, AS, BM, CL, and DC: wrote the paper with inputs from CT, EB, J-CG, LS, BL, AN, and KK.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The field activities of AS, CL, and DC at the Polish Polar Station in Hornsund were supported by the INTERACT Transnational Access (H2020, BC-HOR project, Grant Agreement No. 730938); of KK and FP-by the National Science Centre of Poland, grant no. 2017/26/D/ST10/00630. We thank also the Norwegian Research Council which supported BL’s and AN’s field activities through the Svalbard Strategic Grant BC-3D “Spatial Distributions of Black Carbon and Mineral Dust in Air and Snow Surface Layers upon Svalbard Glaciers”. This project has also received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 689443 via project iCUPE (Integrative and Comprehensive Understanding on Polar Environments). AN and BL were also supported by the Institute of Geophysics, Polish Academy of Sciences within statutory activities no. 3841/E-41/S/2020 of the Ministry of Science and Higher Education of Poland.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2020.536036/full#supplementary-material.
Aas, K. S., Dunse, T., Collier, E., Schuler, T. V., Berntsen, T. K., Kohler, J., et al. (2016). The climatic mass balance of svalbard glaciers: a 10-year simulation with a coupled atmosphere–glacier mass balance model. The Cryosphere 10, 1089–1104. 10.5194/tc-10-1089-2016
Aastrup, P., Riget, F., Dietz, R., and Asmund, G. (2000). Lead, zinc, cadmium, mercury, selenium and copper in Greenland caribou and reindeer (rangifer tarandus). Sci. Total Environ. 245, 149–159. doi:10.1016/s0048-9697(99)00440-4
Amouroux, D., Liss, P. S., Tessier, E., Hamren-Larsson, M., and Donard, O. F. X. (2001). Role of oceans as biogenic sources of selenium. Earth Planet Sci. Lett. 189, 277–283. doi:10.1016/S0012-821X(01)00370-3
Barbante, C., Veysseyre, A., Ferrari, C., Van De Velde, K., Morel, C., Capodaglio, G., et al. (2001). Greenland snow evidence of large scale Atmospheric contamination for platinum, palladium, and rhodium. Environ. Sci. Technol. 35, 835–839. doi:10.1021/es000146y
Barbante, C., Spolaor, A., Cairns, W. R. L., and Boutron, C. (2017). Man’s footprint on the Arctic environment as revealed by analysis of ice and snow. Earth Sci. Rev. 168, 218–231. doi:10.1016/j.earscirev.2017.02.010
Barbaro, E., Spolaor, A., Karroca, O., Park, K. T., Martma, T., Isaksson, E., et al. (2017a). Free amino acids in the Arctic snow and ice core samples: potential markers for paleoclimatic studies. Sci. Total Environ. 607-608, 454–462. doi:10.1016/j.scitotenv.2017.07.041
Barbaro, E., Zangrando, R., Padoan, S., Karroca, O., Toscano, G., Cairns, W. R. L., et al. (2017b). Aerosol and snow transfer processes: an investigation on the behavior of water-soluble organic compounds and ionic species. Chemosphere 183, 132–138. doi:10.1016/j.chemosphere.2017.05.098
Barbaro, E., Zangrando, R., Kirchgeorg, T., Bazzano, A., Illuminati, S., Annibaldi, A., et al. (2016). An integrated study of the chemical composition of Antarctic aerosol to investigate natural and anthropogenic sources. Environ. Chem. 13 (5) 867–876. doi:10.1071/EN16056
Birkenmajer, K. (1990). Hornsund Spitsbergen 1:75 000 Geology. Katowice, Poland: University of Silesia
Brimblecombe, P., Clegg, S. L., Davies, T. D., Shooter, D., and Tranter, M. (1987). Observations of the preferential loss of major ions from melting snow and laboratory ice. Water Res. 21, 1279–1286. doi:10.1016/0043-1354(87)90181-3
Conca, E., Abollino, O., Giacomino, A., Buoso, S., Traversi, R., Becagli, S., et al. (2019). Source identification and temporal evolution of trace elements in PM10 collected near to Ny-Ålesund (Norwegian Arctic). Atmos. Environ. 203, 153–165. doi:10.1016/j.atmosenv.2019.02.001
Czerny, J., Kieres, A., Manecki, M., and Rajchel, J. (1993). “Geological map of the SW part of wedel-jarlsberg land, spitsbergen,” in Institute of geology and mineral deposits. Editor A. Manecki (Kraków, Poland: University of Mining and Metallurgy).
Ezerinskis, Z., Spolaor, A., Kirchgeorg, T., Cozzi, G., Vallelonga, P., Kjær, H. A., et al. (2014). Determination of 129I in Arctic snow by a novel analytical approach using IC-ICP-SFMS. J. Anal. At. Spectrom. 29, 1827–1834. 10.1039/C4JA00179F
Feltracco, M., Barbaro, E., Tedeschi, S., Spolaor, A., Turetta, C., Vecchiato, M., et al. (2020). Interannual variability of sugars in arctic aerosol: biomass burning and biogenic inputs. Sci. Total Environ. 706, 136089. doi:10.1016/j.scitotenv.2019.136089
Ferrari, C. P., Hong, S., Van de Velde, K., Boutron, C. F., Rudniev, S. N., Bolshov, M., et al. (2000). Natural and anthropogenic bismuth in central greenland. Atmos. Environ. 34, 941–948. doi:10.1016/S1352-2310(99)00257-5
Frascoli, F., and Hudson-Edwards, K. A. (2018). Geochemistry, mineralogy and microbiology of molybdenum in mining-affected environments. Minerals 8 (4), 42. 10.3390/min8020042
Gabrielli, P., Planchon, F. A. M., Hong, S., Lee, K. H., Hur, S. D., Barbante, C., et al. (2005). Trace elements in Vostok Antarctic ice during the last four climatic cycles. Earth Planet Sci. Lett. 234, 249–259. 10.1016/j.epsl.2005.03.001
Gallet, J.-C., Björkman, M., Borstad, C. P., Hodson, A. J., Jacobi, H.-W., Larose, C., et al. (2019). “Snow research in Svalbard: current status and knowledge gaps,” in SESS report 2018. Editors E. Orr, G. Hansen, H. Lappalainen, C. Hübner, and H. Lihavainen (Longyearbyen, Norway: Svalbard Integrated Arctic Earth Observing System), 82–107.
Harkness, J. S., Darrah, T. H., Moore, M. T., Whyte, C. J., Mathewson, P. D., Cook, T., et al. (2017). Naturally occurring versus anthropogenic sources of elevated molybdenum in groundwater: evidence for geogenic contamination from southeast Wisconsin, United States. Environ. Sci. Technol. 51, 12190–12199. doi:10.1021/acs.est.7b03716
Johansson, Å., Larionov, A. N., Tebenkov, A. M., Ohta, Y., and Gee, D. G. (2002). Caledonian granites of western and central Nordaustlandet, northeast Svalbard. GFF 124 (3), 135–148. doi:10.1080/11035890201243135
Kieres, A., and Piestrzyński, A. (1992). Ore-mineralization of the hecla hoek succession (precambrian) around werenskioldbreen, South Spitsbergen. Stud. Geol. Pol. 98, 115–151.
Kohler, J., James, T. D., Murray, T., Nuth, C., Brandt, O., Barrand, N. E., et al. (2007). Acceleration in thinning rate on western Svalbard glaciers. Geophys. Res. Lett. 34, L18502. doi:10.1029/2007GL030681
Kosek, K., Luczkiewicz, A., Kozioł, K., Jankowska, K., Ruman, M., and Polkowska, Ż. (2019a). Environmental characteristics of a tundra river system in Svalbard. Part 1: bacterial abundance, community structure and nutrient levels. Sci. Total Environ. 653, 1571–1584. doi:10.1016/j.scitotenv.2018.11.378
Kosek, K., Kozioł, K., Luczkiewicz, A., Jankowska, K., Chmiel, S., and Polkowska, Ż. (2019b). Environmental characteristics of a tundra river system in Svalbard. Part 2: chemical stress factors. Sci. Total Environ. 653, 1585–1596. doi:10.1016/j.scitotenv.2018.11.012
Kozak, K., Kozioł, K., Luks, B., Chmiel, S., Ruman, M., Marć, M., et al. (2015). The role of atmospheric precipitation in introducing contaminants to the surface waters of the Fuglebekken catchment. Spitsbergen. Polar Res. 34, 24207. doi:10.3402/polar.v34.24207
Laska, M., Luks, B., and Budzik, T. (2016). Influence of snowpack internal structure on snow metamorphism and melting intensity on Hansbreen. Svalbard, Polish Polar Research 37 (2), 193–218. doi:10.1515/popore-2016-0012
Łokas, E., Zaborska, A., Sobota, I., Gaca, P., Milton, J., Kocurek, P., et al. (2019). Airborne radionuclides and heavy metals in high Arctic terrestrial environment as the indicators of sources and transfers of contamination. The Cryosphere 13, 2075–2086. doi:10.5194/tc-2019-34
López-Moreno, J. I., Boike, J., Sanchez-Lorenzo, A., and Pomeroy, J. W. (2016). Impact of climate warming on snow processes in Ny-Ålesund, a polar maritime site at Svalbard. Global Planet. Change 146, 10–21. doi:10.1016/j.gloplacha.2016.09.006
Majka, J., and Kośmińska, K. (2017). Magmatic and metamorphic events recorded within the southwestern basement province of svalbard. Arktos 3 (5), 1–7. doi:10.1007/s41063-017-0034-7
Maturilli, M., Herber, A., and König-Langlo, G. (2013). Climatology and time series of surface meteorology in Ny-Älesund, Svalbard. Earth Syst. Sci. Data 5, 155–163. doi:10.5194/essd-5-155-2013
Millero, F. J., Feistel, R., Wright, D. G., and McDougall, T. J. (2008). The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res. Oceanogr. Res. Pap. 55, 50–72. doi:10.1016/j.dsr.2007.10.001
Möller, M., and Kohler, J. (2018). Differing climatic mass balance evolution across Svalbard glacier regions over 1900–2010. Front. Earth Sci. 6, 128. doi:10.3389/feart.2018.00128
Moroni, B., Cappelletti, D., Ferrero, L., Crocchianti, S., Busetto, M., Mazzola, M., et al. (2016). Local vs. long-range sources of aerosol particles upon Ny-Ålesund (Svalbard Islands): mineral chemistry and geochemical records. Rendiconti Lincei. 27, 115–127. doi:10.1007/s12210-016-0533-7
Nawrot, A. P., Migała, K., Luks, B., Pakszys, P., and Głowacki, P. (2016). Chemistry of snow cover and acidic snowfall during a season with a high level of air pollution on the Hans Glacier, Spitsbergen. Polar Sci. 1 (3), 249–261. doi:10.1016/j.polar.2016.06.003
Osuch, M., and Wawrzyniak, T. (2017). Variations and changes in snow depth at meteorological stations Barentsburg and Hornsund (Spitsbergen). Ann. Glaciol. 58 (75), 11–20. doi:10.1017/aog.2017.20
Pedersen, C. A., Gallet, J. C., Ström, J., Gerland, S., Hudson, S. R., Forsström, S., et al. (2015). In situ observations of black carbon in snow and the corresponding spectral surface albedo reduction. J. Geophys. Res.: Atmosphere 120, 1476–1489. doi:10.1002/2014JD022407
Reimann, C., and de Caritat, P. (2005). Distinguishing between natural and anthropogenic sources for elements in the environment: regional geochemical surveys versus enrichment factors. Sci. Total Environ. 337, 91–107. doi:10.1016/j.scitotenv.2004.06.011
Rhodes, R. H., Yang, X., and Wolff, E. W. (2018). Sea ice versus storms: what controls sea salt in arctic ice cores?. Geophys. Res. Lett. 45, 5572–5580. doi:10.1029/2018GL077403
Schuler, T. V., Glazovsky, A., Hagen, J. O., Hodson, A., Jania, J., Kääb, A., et al. (2020). “New data, new techniques and new challenges for updating the state of Svalbard glaciers (SvalGlac),” in SESS report 2019. Editors F. van den Heuvel, C. Hübner, M. Błaszczyk, M. Heimann, and H. Lihavainen (Longyearbyen, Norway: Svalbard Integrated Arctic Earth Observing System), 108–134.
Siudek, P., Frankowski, M., and Siepak, J. (2015). Trace element distribution in the snow cover from an urban area in central Poland. Environ. Monit. Assess. 187, 225. doi:10.1007/s10661-015-4446-1
Spolaor, A., Barbaro, E., Christille, J. M., Kirchgeorg, T., Giardi, F., Cappelletti, D., et al. (2016). Evolution of the Svalbard annual snow layer during the melting phase. Rendiconti Lincei. 2016, 1–8. doi:10.1007/s12210-015-0500-8
Spolaor, A., Gabrieli, J., Martma, T., Kohler, J., Björkman, M. B., Isaksson, E., et al. (2013). Sea ice dynamics influence halogen deposition to Svalbard. The Cryosphere 7, 1645–1658. doi:10.5194/tcd-7-1075-2013
Stachnik, Ł., Yde, J. C., Nawrot, A., Uzarowicz, Ł., Łepkowska, E., and Kozak, K. (2019). Aluminium in glacial meltwater demonstrates an association with nutrient export (Werenskiöldbreen, Svalbard). Hydrol. Process. 33, 1638–1657. doi:10.1002/hyp.13426
Tan, L. C., Nancharaiah, Y. V., van Hullebusch, E. D., and Lens, P. N. L. (2016). Selenium: environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 34, 886–907. doi:10.1016/j.biotechadv.2016.05.005
Van Pelt, W., Pohjola, V., Pettersson, R., Marchenko, S., Kohler, J., Luks, B., et al. (2019). A long-term dataset of climatic mass balance, snow conditions and runoff in Svalbard (1957–2018). The Cryosphere 13, 2259–2280. doi:10.5194/tc-13-2259-2019
Vecchiato, M., Barbaro, E., Spolaor, A., Burgay, F., Barbante, C., Piazza, R., et al. (2018). Fragrances and PAHs in snow and seawater of Ny-Ålesund (Svalbard): local and long-range contamination. Environ. Pollut. 242, 1740–1747. doi:10.1016/j.envpol.2018.07.095
Wedepohl, K. H. (1995). The composition of the continental crust. Geochem. Cosmochim. Acta 59, 1217–1232. doi:10.1016/0016-7037(95)00038-2
Weller, R., Wöltjen, J., Piel, C., Resenberg, R., Wagenbach, D., König-Langlo, G., et al. (2008). Seasonal variability of crustal and marine trace elements in the aerosol at Neumayer station, Antarctica. Tellus B. 60, 742–752. doi:10.1111/j.1600-0889.2008.00372.x
Winther, J. G., Bruland, O., Sand, K., Killingtveit, Å., and Marechal, D. (1998). Snow accumulation distribution on spitsbergen, svalbard, in 1997. Polar Res. 17, 155–164. doi:10.1111/j.1751-8369.1998.tb00269.x
Keywords: svalbard, snowpack, firn, trace element, transport
Citation: Spolaor A, Moroni B, Luks B, Nawrot A, Roman M, Larose C, Stachnik Ł, Bruschi F, Kozioł K, Pawlak F, Turetta C, Barbaro E, Gallet J-C and Cappelletti D (2021) Investigation on the Sources and Impact of Trace Elements in the Annual Snowpack and the Firn in the Hansbreen (Southwest Spitsbergen). Front. Earth Sci. 8:536036. doi: 10.3389/feart.2020.536036
Received: 18 February 2020; Accepted: 30 November 2020;
Published: 19 January 2021.
Edited by:
Jing Ming, Independent researcher, Melbourne, AustraliaReviewed by:
Zhiwen Dong, Chinese Academy of Sciences, ChinaCopyright © 2021 Spolaor, Moroni, Luks, Nawrot, Roman, Larose, Stachnik, Bruschi, Kozioł, Pawlak, Turetta, Barbaro, Gallet and Cappelletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Spolaor, YW5kcmVhLnNwb2xhb3JAY25yLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.