
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Earth Sci. , 16 September 2020
Sec. Paleontology
Volume 8 - 2020 | https://doi.org/10.3389/feart.2020.00367
This article is part of the Research Topic Early Avian Evolution View all 14 articles
 Xiaoting Zheng1,2*
Xiaoting Zheng1,2* Jingmai O’Connor3,4*
Jingmai O’Connor3,4* Yan Wang1,2
Yan Wang1,2 Xiaoli Wang1,2
Xiaoli Wang1,2 Yin Xuwei2
Yin Xuwei2 Xiaomei Zhang2
Xiaomei Zhang2 Zhonghe Zhou3,4
Zhonghe Zhou3,4The keratinous beak is inferred to have evolved multiple times in the Archosauria and in Aves. Unfortunately, this feature rarely preserves in the fossil record. Here we examine a collection of 603 specimens belonging to the Confuciusornithiformes, a clade of edentulous basal avians, only two of which preserve visible traces of the rhamphotheca. Preservation is very different between the two specimens, offering no clues as to the taphonomic conditions that are conducive to preservation of this feature. These differences suggest that preservation of the rhamphotheca is not limited to a very narrow set of specific chemical conditions. We suggest the more common preservation of feathers over rhamphotheca is due to the higher melanin content in the former. The well-preserved traces in one specimen described here suggests that the rhamphotheca covering the upper and lower jaws each may consist of a pair of right and left elements, thus differing from the condition in neornithines in which the premaxillary nail and mandibular nail covering the rostral half of the upper and lower jaws respectively each form a single unit.
The beaked rostrum is one of the most distinctive features of birds, present in all living species and exhibiting an enormous diversity of form and size relative to the body (Lovette and Fitzpatrick, 2004; Gill, 2007). Although this morphological diversity is commonly associated with various feeding strategies, the actual relationship between shape and function is far more complex (Bright et al., 2016; Navalón et al., 2019). In some extant birds the rhamphotheca, the keratinous sheath that covers the edentulous bony rostrum and together form the beak, forms specialized structures such as filters (e.g., flamingos) and “teeth” (e.g., mergansers) that facilitate certain feeding behaviors (Storer, 1960). In addition to its primary role in feeding, the beak has evolved a myriad of other functions including sound production (e.g., bill-clattering/culmen knocking in Storks) (Han et al., 1999), warfare (e.g., Toucans, Long-billed hermit hummingbird) (Rico-Guevara and Araya-Salas, 2015), thermoregulation (e.g., Toucans) (Tattersall et al., 2016), and inter and intraspecific signaling (e.g., Toucans, American goldfinches, King penguins) (Murphy et al., 2009; Nolan et al., 2010; Guaraldo et al., 2019). The keratinous beak is a dynamic feature – it grows continuously as it is also constantly worn away through use and can change in size, shape, and color in response to seasonal differences in functional requirements (Stettenheim, 1972; Matthysen, 1989; Bonser and Witter, 1993; Tattersall et al., 2016).
A keratinous beak was absent in the earliest bird, Archaeopteryx, which had a fully toothed rostrum (Elzanowski, 2002; Mayr et al., 2005; Rauhut et al., 2018). Tooth reduction and an edentulous rostrum evolved numerous times during the Mesozoic evolution of Aves given the phylogenetic distribution of edentulous clades and clades containing edentulous taxa (O’Connor et al., 2011, 2016; O’Connor and Zhou, 2013; Wang M. et al., 2018). The oldest and most basal occurrence of a toothless rostrum in Aves is in the Early Cretaceous basal pygostylian clade, the Confuciusornithiformes, in the oldest and most primitive member of this clade – Eoconfuciusornis from the 131 Ma Huajiying Formation (Hou et al., 1995b; Zhang et al., 2008). Tooth loss also evolved independently at least once in the diverse ornithothoracine clade, the Enantiornithes, as evidenced by the edentulous Gobipteryx minuta (Elzanowski, 1974; Chiappe et al., 2001). Tooth loss is far more common in the enantiornithine sister-clade the Ornithuromorpha, the clade that includes modern birds nested within (O’Connor et al., 2011). Several Early Cretaceous ornithuromorphs preserve edentulous rostra: Archaeorhynchus (Zhou and Zhang, 2006; Zhou et al., 2013), Eogranivora (Zheng et al., 2018), Xinghaiornis (Wang et al., 2013), Schizooura (Zhou et al., 2012), and Dingavis (O’Connor et al., 2016). The phylogenetic distribution of these taxa strongly suggests that tooth loss most likely evolved independently in each lineage and thus numerous times in this clade alone (O’Connor et al., 2016; Wang M. et al., 2018). Complete tooth loss in Mesozoic birds has been linked to herbivory (Louchart and Viriot, 2011; O’Connor, 2019), which is also suggested in some data from non-avian dinosaurs (Zanno and Makovicky, 2011; Wang et al., 2017).
In addition, some ornithuromorphs (e.g., Yanornis, Yixianornis) possess a rostrum that is only edentulous at the tip of the premaxillae, caudally followed by teeth (Zhou and Zhang, 2001). This edentulous tip, which ventrally articulates with the edentulous avian predentary, is inferred to have been covered by a small rhamphotheca (Bailleul et al., 2019). The entire premaxilla is edentulous in the Early Cretaceous Iteravis (Zhou et al., 2014) and in the Late Cretaceous ornithurines Ichthyornis (Field et al., 2018) and the Hesperornithiformes (Gingerich, 1973). However, the pattern of tooth loss in the Ornithuromorpha was not a straight-forward rostro-caudal reduction as previously hypothesized (Louchart and Viriot, 2011). Mengciusornis, a schizoourid ornithuromorph closely related to the edentulous Schizooura, possesses teeth only in the premaxilla, indicating that tooth loss in ornithuromorphs proceeded both rostro-caudally and caudo-rostrally (Wang et al., 2019a).
Like an edentulous rostrum, presumably rhamphothecae also evolved multiple times during the Cretaceous evolution of birds associated with each new occurrence of tooth loss or reduction (Hieronymus and Witmer, 2010). Although the absence of teeth is readily identifiable, actual traces of the rhamphothecae are rarely preserved in the fossil record (O’Connor, 2019). So far among Mesozoic Aves traces of the rhamphotheca have only been reported in four specimens referable to the Early Cretaceous basal pygostylian clade, the Confuciusornithiformes: the holotype of Eoconfuciusornis zhengi, the holotype of Confuciusornis dui, and two referred specimens of Confuciusornis sanctus (Hou et al., 1999; Zhang et al., 2008; Chiappe and Meng, 2016; Falk et al., 2019). More avian specimens from the Jehol Group are referable to the Confuciusornithiformes than to any other clade (Hou et al., 1995a, b, 1996, 1999, 2002; Chiappe et al., 1999, 2008; de Ricqlès et al., 2003; Dalsätt et al., 2006; Zhang et al., 2008, 2009; Li L. et al., 2010; Li et al., 2018; Marugán-Lobón et al., 2011; Chinsamy et al., 2013, 2019; Zheng et al., 2013, 2017; Falk et al., 2016; Jiang et al., 2017; Elzanowski et al., 2018; Wang and Zhou, 2018). A large number of described specimens boast well-preserved soft tissues most commonly in the form of feathers and keratinous ungual sheaths (e.g., Chiappe et al., 1999 – Figure 8, Falk et al., 2016 – Figure 1, Li et al., 2018 – Figure 1, Zheng et al., 2017 – Figure 1). Most specimens are recovered from the Yixian Formation and referred to C. sanctus (Wang et al., 2019b). Confuciusornis sanctus is without a doubt the fossil bird known from the greatest number of specimens in the world, with thousands reportedly known and scattered throughout collections primarily in China but also elsewhere (Elzanowski et al., 2018; Wang et al., 2019b). Most other Jehol birds with fully edentulous rostra are known from a single specimen (e.g., Xinghaiornis, Eogranivora) with the definite exception of Archaeorhynchus, which is known from five specimens, none of which preserve traces of the rhamphotheca (Zhou and Zhang, 2006; Zhou et al., 2013; Wang and Zhou, 2016; Wang X. et al., 2018).
The largest single collection of Confuciusornithiformes is that of the Shandong Tianyu Museum of Nature (STM) in Pingyi, China. The collection boasts 603 specimens, all of which consist of partial to nearly complete mostly articulated skeletons. It has been reported that 273 of these specimens preserve soft tissue in the form of feathers (Zheng et al., 2013). A survey of this collection identified only two specimens preserving traces of the soft tissue of the beak (Figures 1, 2). These specimens are described here and compared to other specimens preserving traces of the rhamphotheca with regards to shape and mode of preservation (Falk et al., 2019).
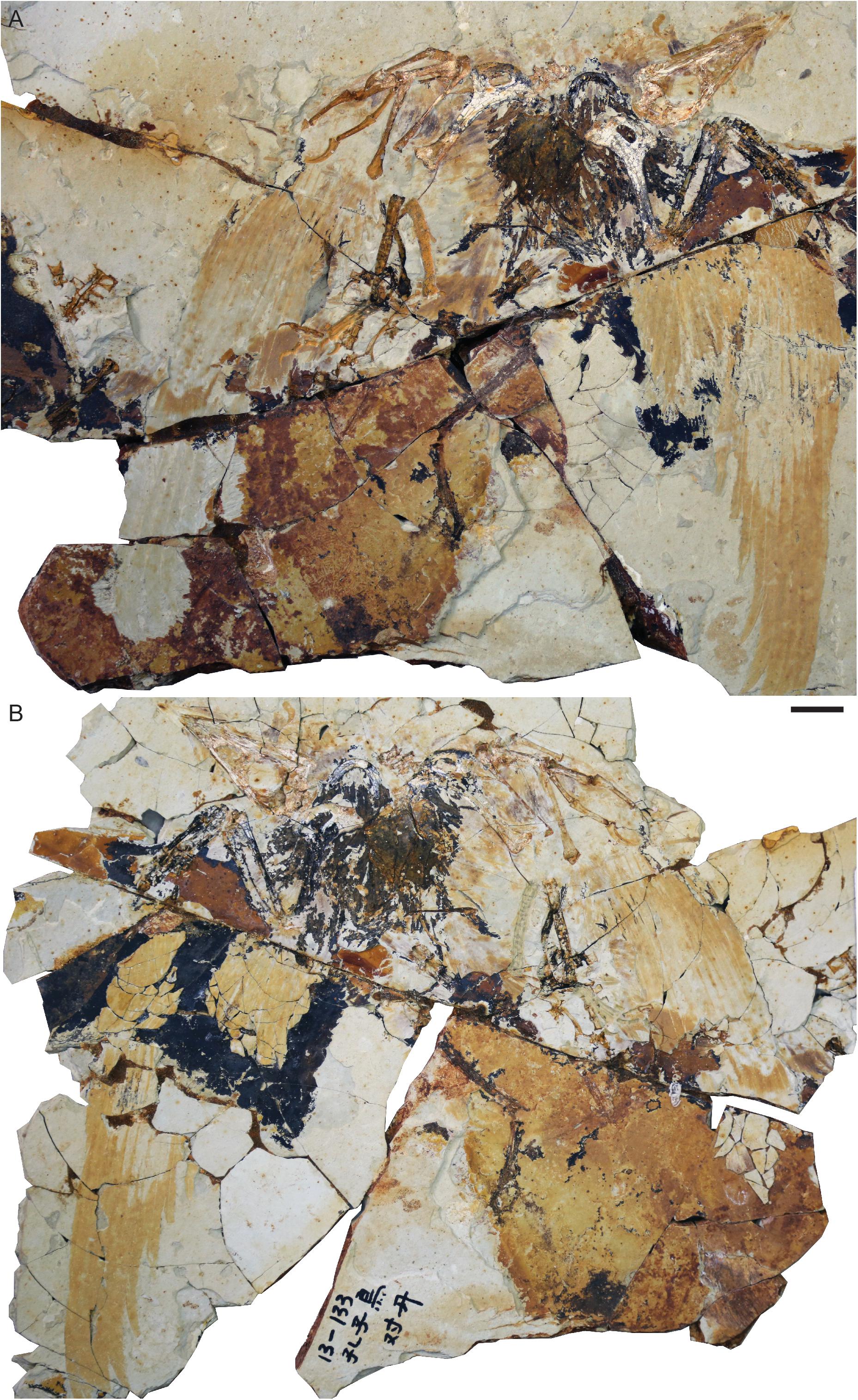
Figure 1. Confuciusornis sp. STM13-133: (A) main slab; (B) counter-slab. Scale bar equals two centimeters.
603 specimens belonging to the Confuciusornithiformes in the collection of the STM were surveyed for preserved rhamphothecae. This feature was identified in two previously undescribed specimens, STM13-133 and STM13-162. As in all specimens preserving this feature, the traces are visible under normal light. These two specimens were studied using a Leica binocular microscope and photographed using a Canon EOS 5DS. Measurements were taken using Fiji (ImageJ) 2.0. Figures and illustrations were generated using Adobe CC 2018.
Osteological terminology primarily follows Baumel and Witmer (1993) using the English equivalents for the Latin terms (Baumel and Witmer, 1993). For terminology concerning the rhamphothecae we follow Hieronymus and Witmer (2010). Institutional abbreviations for other specimens preserving rhamphothecae: BMNHC, Beijing Museum of Natural History Collection, Beijing, China; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China.
This specimen consists of a nearly complete and articulated individual preserved with feather impressions in a slab and counter-slab (Figure 1). STM13-133 can be readily identified as Confuciusornis based on the robust edentulous rostrum, stout boomerang shaped furcula lacking a hypocleidium, massive perforated deltopectoral crest on the humerus, and characteristic manual morphology with reduced major digit ungual (Chiappe et al., 1999). Where unbroken, the bones are preserved white with cracks distinctly visible in black. Where the bones are broken they are reveal a reddish coloration indicative of iron oxidative taphonomic processes. The feathers are preserved primarily as reddish impressions with the rachis clearly visible as a gap in some primaries. In the distal half of the left wing the barbs are preserved a pale white-pink color. In addition to the wings, in which impressions of the primaries, secondaries, and some coverts are fairly well-preserved, body feathers are preserved on the dorsal and ventral margins of the skull, ventral surface of the neck, left lateral surface of the body, and near the right knee. The keratinous sheaths covering the manual and pedal claws are mostly not preserved although the sheath covering the left minor digit ungual is partially preserved as a reddish stain.
The skull is preserved in left lateral view (Figures 1, 3). Although the general shape of the skull is well-preserved, the cranial bones themselves are very poorly preserved, with most elements preserved as reddish voids or elements torn in half between the slab and counter-slab. The traces of the rhamphotheca appear to consist of four distinct parts, suggesting that the rhamphotheca covering the upper jaw and the rhamphotheca covering the lower jaw each consisted of two elements. This is evident from the morphology of the preserved traces themselves and further suggested by the fact that short feathers appear to extend nearly to the rostral tip of the rostrum (approximately, only the rostral 4 mm of the premaxillary corpus lack feathers) and toward the tip of the mandibular symphysis (Figure 3). However, it cannot be ruled out that these “separate parts” of the rhampthothecae are not the result of post-mortem breakage (Foth and Rauhut, 2017).
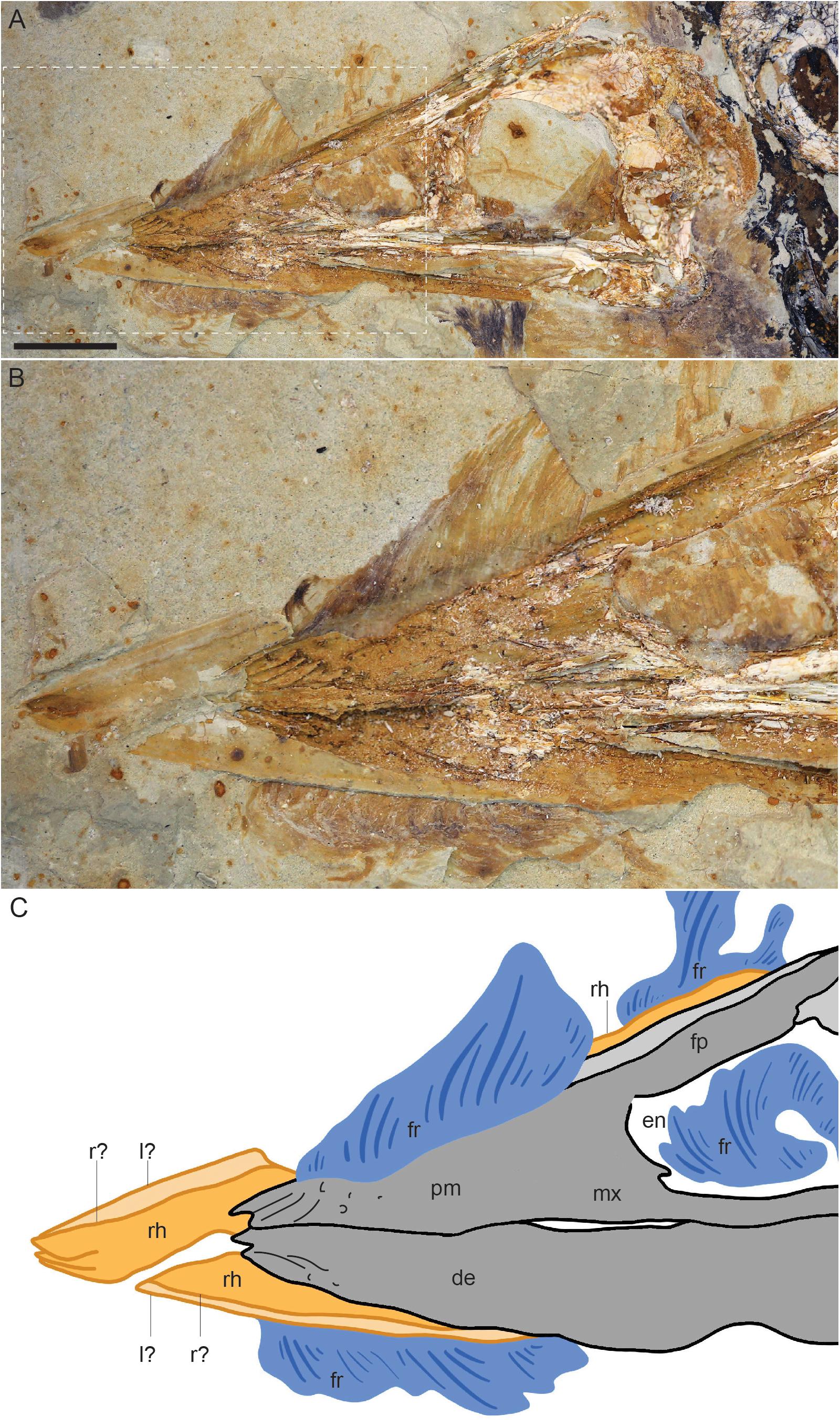
Figure 3. Close up of the skull of Confuciusornis STM13-133: (A) photograph; (B) close up of the rhamphothecal traces (C), interpretative drawing of (B). Dark gray indicates poorly preserved bone, light gray indicates bone preserved as voids, orange indicates the traces of the rhamphothecae, and blue indicates feather traces. Anatomical abbreviations: de, dentary; en, external nares; fp, frontal process of the premaxilla; fr, feathers; l?, possible left side of the rhamphotheca; mx, maxilla; pm, premaxilla; r?, possible right side of the rhamphotheca; rh, rhamphotheca.
The dorsal feathers are short, extend caudodorsally, and increase in height caudally until they disappear due to poor preservation (the layer of matrix preserving the soft tissue is chipped away in this region) just rostral to the level of the caudal margin of the external nares. From their rostral-most point until the cranial margin of the external nares the feathers appear to overlap the right frontal process of the premaxilla, however, it is likely that the basal portions of the feathers are not complete. Caudal to this point portions of the feather apices are preserved but not their basal portions, exposing a caudal portion of the rhamphotheca extending along the dorsal margin of the frontal process of the right premaxilla (Figure 3). This trace extends for approximately the length of the dorsal margin of the external nares with a fairly even thickness of 2.8 mm and ends rather abruptly just cranial to the caudal margin of the external nares suggesting it may be caudally incomplete.
The rhamphothecae extend rostrally from the premaxillae and dentaries just over 9 mm (Table 1). From the rostral position of the feathers and the pre-rostral length of the rhamphothecal traces it may be that both the rhamphothecae and the feathers are displaced rostrally. The traces of the upper and lower rhamphothecae overlap obscuring the dorsoventral thickness of these traces although the tips of each can be distinguished, preserved very close to each other consistent with the closed morphology of the jaws. The upper rhamphotheca appears to consist of two separate parts. The dorsal margin of each part can be distinctly observed due to the fact these margins are preserved a darker color than the remaining rhamphothecal traces (Figure 3). The dorsal margins of the right and left portions depart from a single point, approximately demarcating an angle of 8°. For most of their preserved length cranial to the premaxilla the dorsal margins extend caudally nearly parallel to each other, separated by a distance of 0.86 mm. Both traces are interrupted by the feathers on the dorsal (or dorsolateral) margin of the skull.
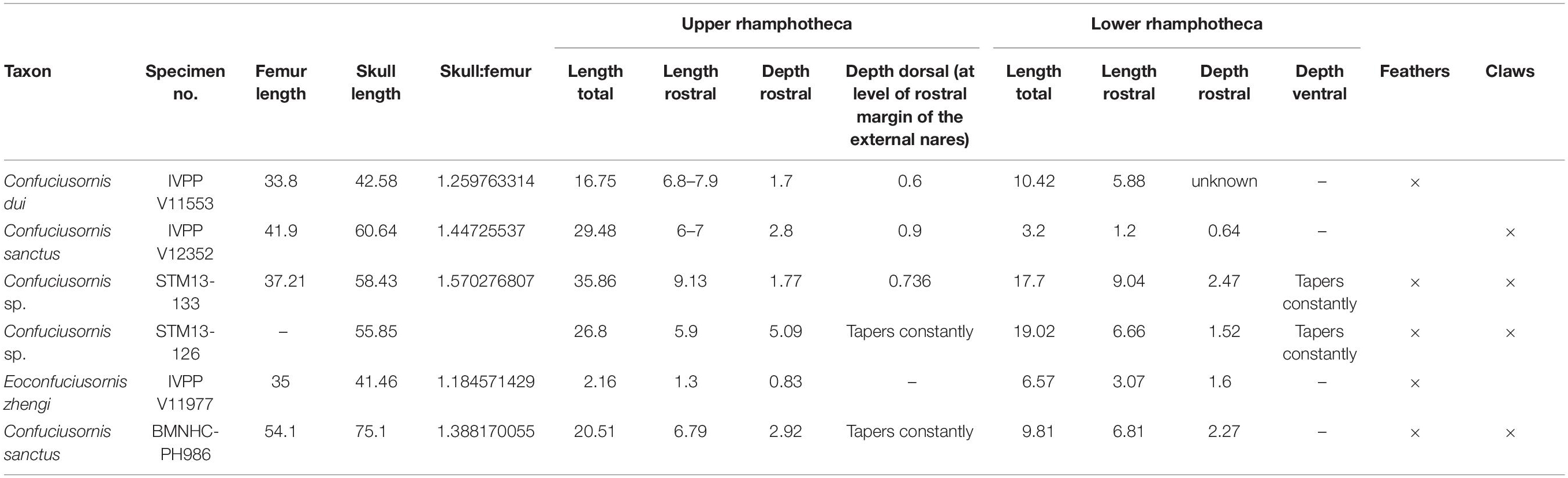
Table 1. Comparative measurements of the rhamphothecae traces and body size in the six published confuciusornithiform specimens preserving this trace.
The rhamphothecae covering the mandible also appears to be formed by left and right parts, the tips of which are clearly preserved slightly offset from each other (Figure 3). This could be due to the notched morphology of the tip of the mandible itself (Chiappe et al., 1999). However, a faint line of darker reddish color extends through the lower rhamphothecal traces parallel to the ventral margin, continuous with the offset tip of the presumably right half of the mandibular rhamphotheca. The mandibular rhamphothecae extend rostrally to approximately the same level as the premaxillary rhamphothecae. As preserved the traces have a maximum thickness of 2.47 mm just rostral to the mandibular symphysis. Caudally it steadily decreases in thickness disappearing just rostral to the level of the cranial margin of the external nares. Similar to the upper jaw, the presence of left and right elements forming the mandibular rhamphothecae is supported by submalar feathers that extend far rostrally, presumably extending between the two halves of the mandibular rhamphothecae. These ventral feathers begin just caudal to the rostral tip of the mandibular bones and are separated from the dentary by the preserved trace of the mandibular rhamphothecae. The feather traces are interrupted by poor preservation (the layer preserving the soft tissue is clearly not preserved, exposing a lower level of sediment) at the level of the external nares, but appear again at the level of the antorbital fenestra and continue caudally, continuous with feathers along the ventral surface of the neck.
STM13-162 represents a complete specimen preserved in lateral view in a slab and counter-slab (Figure 2). The specimen is preserved in an unusual position with all the limbs folded close to the body. Faint traces of feathers are visible around the skeleton including traces of the wings. The keratinous sheaths covering the manual and pedal unguals are preserved. Dark material in the orbit may be soft tissue traces of the eye. Like STM13-133, STM13-162 is referable to Confuciusornis based on the robust edentulous rostrum, stout boomerang shaped furcula lacking a hypocleidium, massive perforated deltopectoral crest on the humerus, and characteristic manual morphology with hypertrophied alular ungual and reduced major digit ungual (Chiappe et al., 1999).
The skull is preserved in lateral view (Figures 2, 4). Although the skull is complete and it retains its general shape the bones are crushed with little to no clearly preserved anatomical details. Notably, the tip of the fused premaxillae are slightly but distinctly downturned (Figure 4). Traces of the rhamphothecae from both the upper and lower jaws are visible in both slabs but more clearly observed in the main slab. These traces appear to be slightly displaced from their natural positions. The upper rhamphotheca trace is displaced dorsally so that the ventral margin is not level with the ventral margin of the premaxilla (Figure 4). The traces extend 5.9 mm from the premaxillae with a total length of 26.8 mm, dorsally ending near the caudal margin of the external nares. The pre-rostral portion of the traces form a triangular impression, divided dorsally and ventrally by the fact the dorsal portion that is continuous with the trace extending caudally over the frontal processes of the premaxillae is much darker. The margin dividing the darker (dorsal) and lighter (ventral) traces is interpreted as the ventral margin of the trace of either the right or left upper rhamphotheca, with the opposing trace somewhat displaced ventrally, forming the lighter portion of the pre-rostral impression of the rhamphothecae (Figure 4).
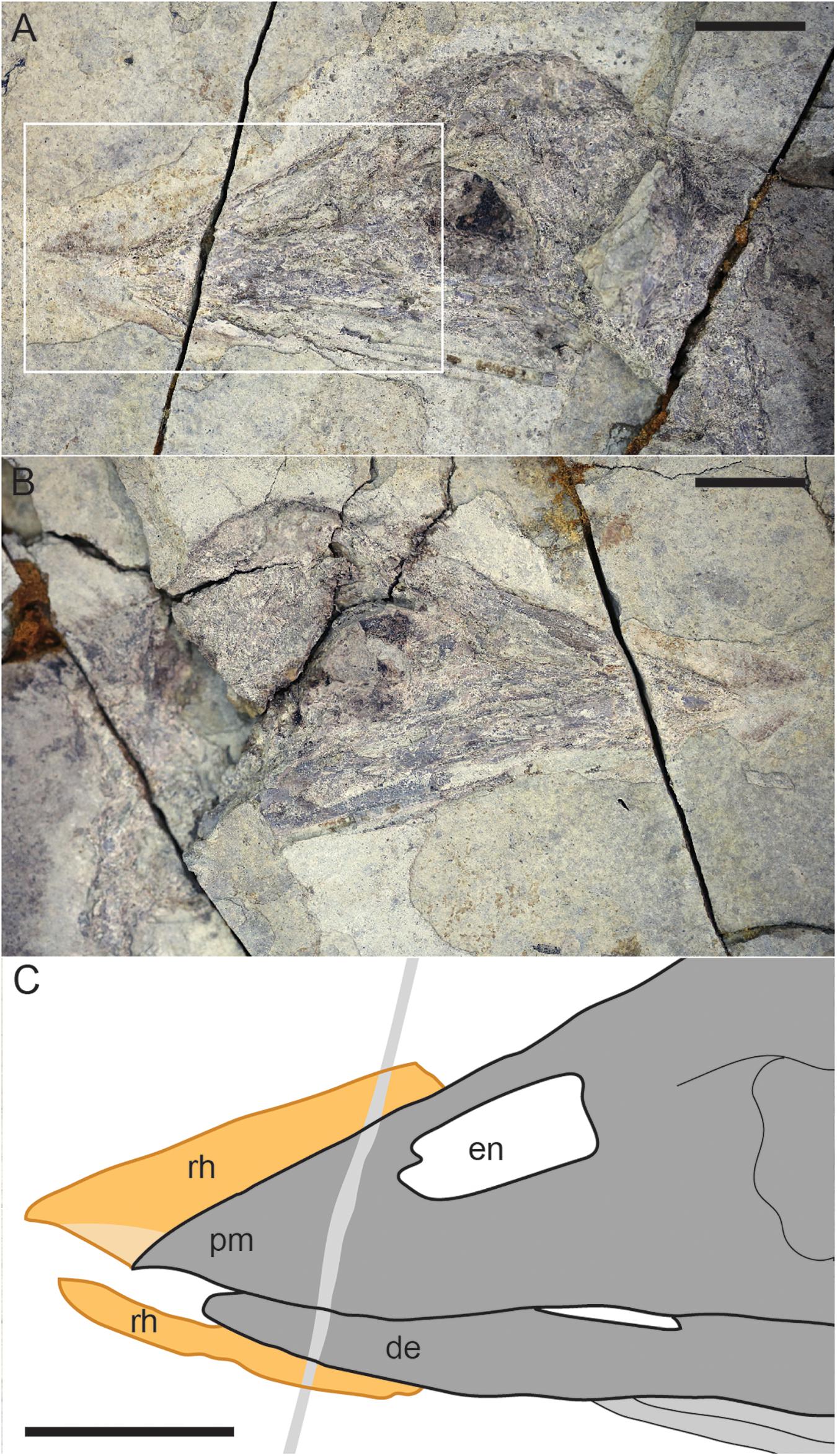
Figure 4. Close up of the skull of Confuciusornis STM13-162: (A) photograph of slab; (B) photograph of counter slab; (C) interpretative drawing. Scale bars equal one centimeter. For anatomical abbreviations see Figure 3 caption.
The trace of the mandibular rhampotheca extends 6.66 mm from the dentaries and has a total length of 19.02 mm. The traces have a fairly even thickness of 1.52 mm except where they taper away caudally.
Due to the rare preservation of the rhamphotheca in the fossil record, very little is known about this feature in extinct organisms. As such, each new specimen has the potential to provide new information. So far among Mesozoic birds, data has only been recovered for the Cretaceous Confuciusornithiformes (Hou et al., 1999; Zhang et al., 2008; Chiappe and Meng, 2016; Falk et al., 2019). Most birds collected in deposits corresponding to the Lower Cretaceous Yixian Formation are referable to this clade. Counter intuitively, the horny sheath or rhamphotheca that covers the rostrum in edentulous birds is less likely to preserve than the more pliable feather integument (Falk et al., 2019). In a collection of 603 specimens of confuciusornithiforms nearly half preserve feathers on some part of the body (273 specimens, 45%) (Zheng et al., 2013). In contrast, only two specimens preserve some trace of the beak – that is 0.33% of the collection. This percentage is likely exaggerated by at least two factors. First, most fossils in the STM collection are not fully prepared and what preparation has been done was conducted prior to being placed in the collections of the STM and was not done by professionals. This may make it more difficult to identify the subtle traces of the preserved rhamphothecae, which are proportionately small compared to traces of the plumage. Furthermore, these subtle traces may also have been lost during cursory non-professional preparation attempts. The rhamphothecal traces preserved in Confuciusornis IVPP V12352 are surrounded by clear marks of preparation (Falk et al., 2019 – Figure 1A) – unfortunately, there is not enough data currently available to assess to what extent the morphology of these and other soft tissue traces are affected by the work of preparators. Second, as the collection was aggregated an emphasis may have been placed on acquiring feathered specimens, which are visually striking, but not on specimens preserving the rhamphotheca, a soft tissue feature that is less obvious and easily overlooked. Unsurprisingly, the two specimens preserving beak traces described here also preserve feathers. Of the two new specimens, STM13-133 is the better preserved and offers the most significant new morphological information.
The appearance of the two new specimens STM13-133 and STM13-162 suggests very different modes of preservation, presumably contributing to the diversity of taphonomic processes that apparently lead to the preservation of rhampthothecae in Jehol specimens. Preservation is very different in in all six known specimens that record these traces, among these differences varying with respect to the quality of bone preservation (excellent in E. zhengi IVPP V11977 and C. sanctus IVPP V12352) and preservation of feathers (preserved in all but C. sanctus IVPP V12352). Notably, several specimens appear to preserve some non-keratinous soft tissue (e.g., probable eye tissue in STM13-162; abdominal tissues in E. zhengi IVPP V11977) indicative of exceptional preservation.
In STM13-133 the mandibular rhamphothecae extend rostrally to approximately the same level as the premaxillary rhamphothecae as in C. dui IVPP V11553 and C. sanctus BMNHC-PH986, whereas the premaxillary traces extend farther in C. sanctus IVPP V12352 and STM13-162 and the mandibular traces extend farther in E. zhengi IVPP V11977. With the limited information available it is impossible to determine to what degree this is due to taphonomy vs. actual interspecific differences. The traces in the two STM specimens are much more comparable to those in C. sanctus IVPP V12352 – so far C. dui IVPP V11553 is unique in having a rhamphotheca that is rostrally upturned and this may be a diagnostic feature of this taxon. However, the holotype and only known specimen has been lost making C. dui a nomen dubium.
Notably, in the five specimens in which both skull and femur length can be measured the skull is proportionately much larger in specimens of greater body size (Table 1). Interpretations are obscured by the taxonomic diversity of these confuciusornithiforms. However, at least E. zhengi IVPP V11977 is regarded as a subadult. This may suggest that the skull becomes proportionately longer in mature individuals. A proportionately short rostrum is a common indicator of ontogenetic immaturity (Bhullar et al., 2016). This also suggests that ontogeny is not a factor that plays into preservation of the rhamphotheca since this feature is preserved in both subadult and adult specimens.
Ichthyornis and Hesperornis were described as having osteological correlates that indicate the presence of compound rhamphothecae with the upper jaws covered by a premaxillary nail, culminicorn and paired latericorns and the lower jaw rhamphothecae consisting of a mandibular nail and paired ramicorns (Hieronymus and Witmer, 2010). This in turn was used to infer that the presence of compound rhamphothecae covering the rostrum is plesiomorphic to the crown clade (Hieronymus and Witmer, 2010). However, a recent comprehensive description of the skull of Ichthyornis based on multiple new specimens indicates that osteological features of compound rhamphothecae, i.e., a nasolabial groove on the upper jaw and mentolabial grooves on the mandibles (Hieronymus and Witmer, 2010), are in fact absent (Field et al., 2018). Only a premaxillary nail is inferred to be present in this taxon based on the presence of numerous neurovascular foramina on the rostral portion of the edentulous premaxilla, presumably accentuating the hooked morphology of this element (Field et al., 2018). A mandibular nail was absent but a small keratinous sheath likely covered the outer surface of the predentary bone present in these non-neornithine ornithurines (Zhou and Martin, 2011; Bailleul et al., 2019), thus the condition in Hesperornis and Ichthyornis represents a primitive rhamphothecal morphology not present in neornithines (the predentary being a feature restricted to non-neornithine ornithuromorphs).
An edentulous rostrum covered by rhamphothecae in the Confuciusornithiformes evolved independently from that in ornithurines like Ichthyornis, enantiornithines like Gobipteryx, basal ornithuromorphs like Archaeorhynchus and Eogranivora, and other stem lineages that display some form of tooth reduction and thus may have had small beaked portions of the rostrum similar to Ichthyornis (e.g., Jeholornis, Sapeornis) (Hieronymus and Witmer, 2010). Therefore, it would not be unexpected to find morphological differences in this feature between Cretaceous avian clades. In most neornithines the caudal extent of the rhamphothecae of the upper jaw is approximated by the caudal extent of the maxillary process of the premaxilla (Hieronymus and Witmer, 2010). If confuciusornithiforms shared this condition with neornithines it would suggest that the ventral and lateral portion of the rhamphotheca below the external nares would be rostrally limited due to the fact that the maxilla makes up a majority of the facial margin (Figure 5A). It cannot be determined if the ventral margin of the rhamphotheca ends level with the premaxillomaxillary contact since the ventral margin below the premaxilla is not preserved in any specimen and similarly the lateral portions of the rhamphotheca are also not preserved (presumably lost during preparation). However, we suggest it is more likely that confuciusornithiforms differed from neornithines in this feature and that the rhamphotheca extended onto the maxilla in confuciusornithiforms (Figure 5B). This is supported by the fact that the maxilla, like the premaxilla and dentary, also bears pits and grooves indicative of neurovasculature (Figure 6). Compared to the premaxillary body and the rostral portion of the dentary, the maxilla is dominated by grooves and pits are less common. These pits and grooves are only present on the premaxillary process of the maxilla suggesting the caudal-most extension of the rhamphotheca on the upper jaw was the caudal margin of the external nares, which is partially enclosed caudally by the nasal process of the maxilla that divides this element into premaxillary and jugal processes. Notably there are no osteological correlates (pits, grooves) that support the caudal extension of the rhamphotheca along the dorsal surface of the skull although the presence of the rhamphotheca is demonstrated by direct evidence in several specimens (IVPP V12352, STM13-133).
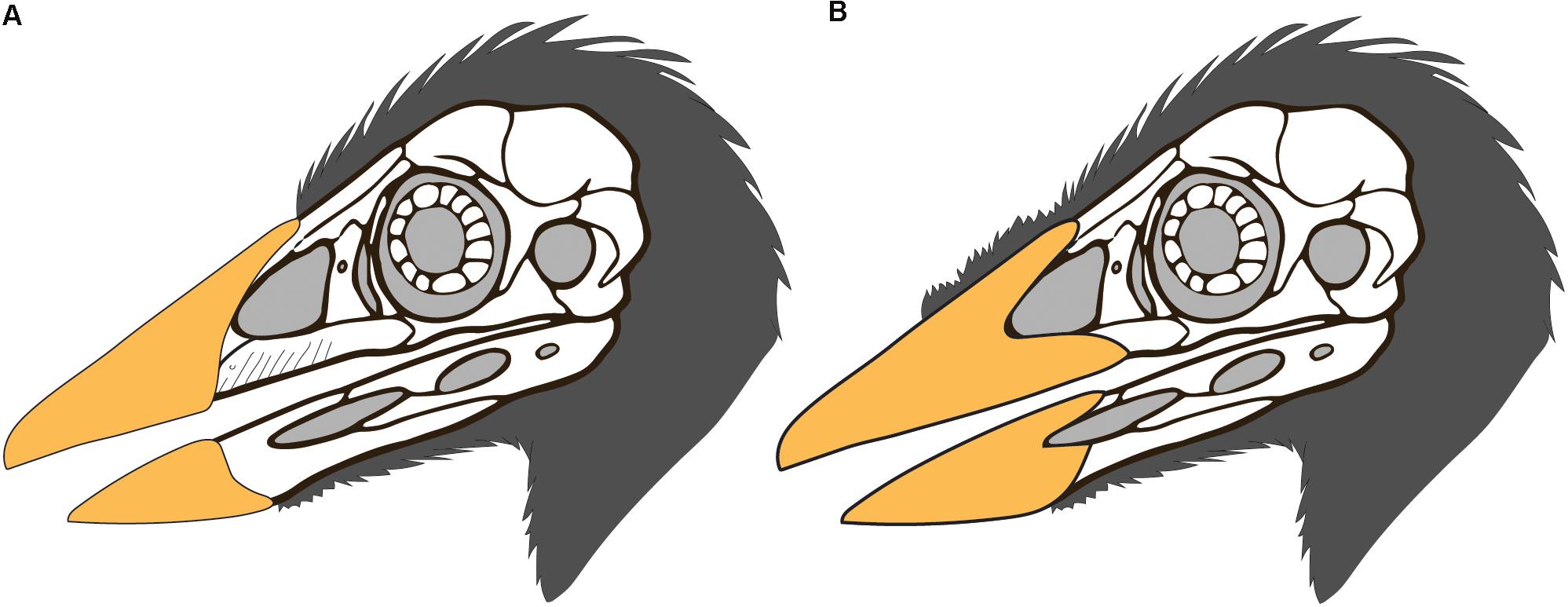
Figure 5. Possible reconstructions of the skull integument in Confuciusornis (skull drawing modified from that of S. Abramowicz): (A) the rhamphotheca is limited to the premaxilla as in neornithines, and the cranial feathers extend only as far rostrally as the rhamphotheca; (B) the upper rhamphotheca is reconstructed extending along the premaxillary process of the maxilla and the dorsal cranial feathers are reconstructed extending rostrally, possibly medially between the two halves of the rhamphotheca as suggested by STM13-133.
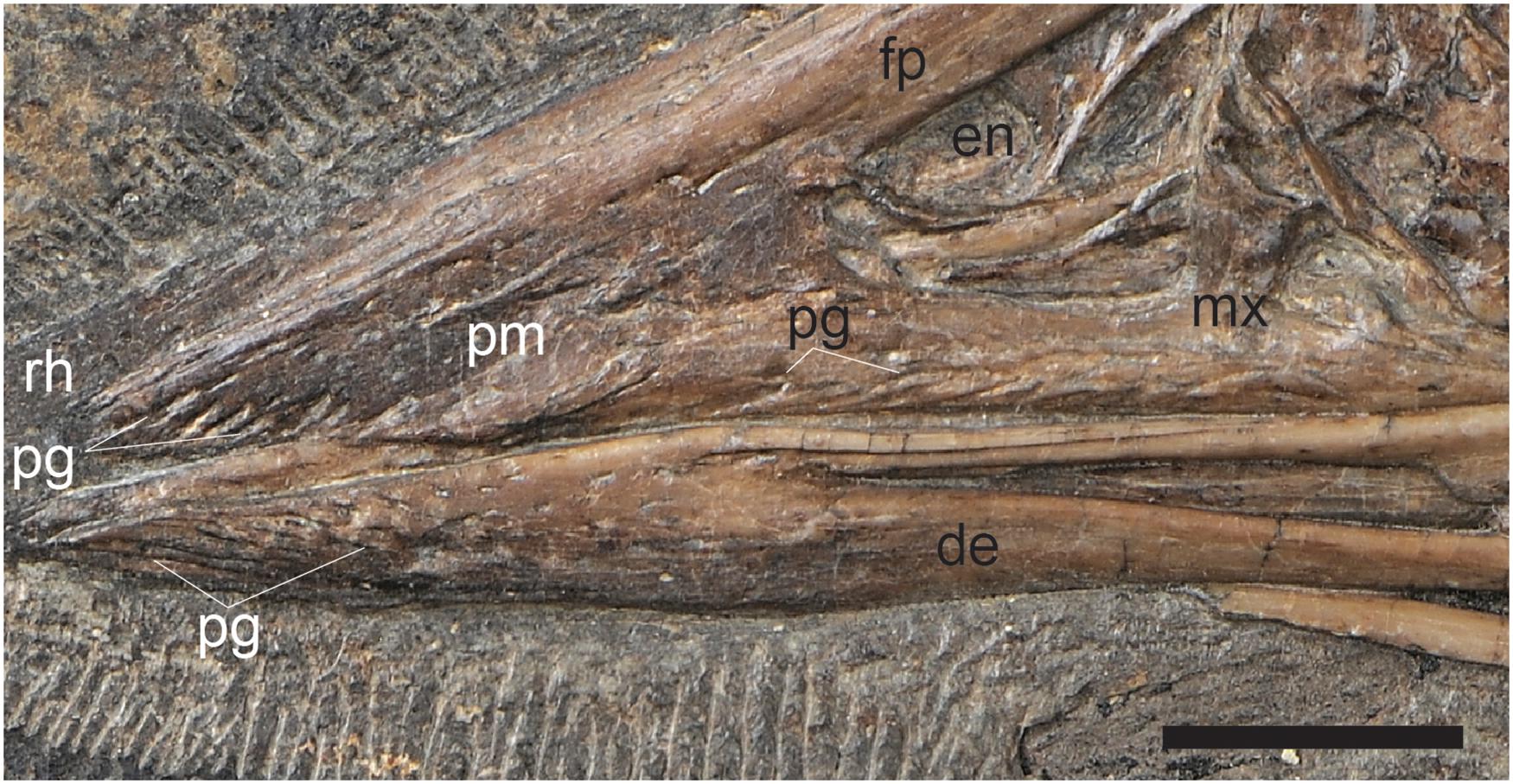
Figure 6. A close up of the cranial half of the rostrum in C. sanctus IVPP V12352 showing the pits and grooves that cover the premaxillary body, premaxillary process of the maxilla, and the rostral portion of the dentaries, inferred to reflect neurovasculature related to the presence of a keratinous beak. Scale bar equals five millimeters. Anatomical abbreviations not listed in Figure 3 caption: pg, pits and grooves.
Nasolabial, culminolabial, and mentolabial grooves, features that indicate the divisions between the compound rhamphothecal elements in neornithines, are not visible in the skull bones of Confuciusornis. The premaxillary nail, the only portion of the neornithine rhamphotheca considered present in Ichthyornis, is rostrally restricted (Hieronymus and Witmer, 2010). At least along the dorsocranial margin the rhamphotheca is caudally extensive in Confuciusornis (Falk et al., 2019). The absence of a culminolabial groove may suggest that the rhamphotheca covering the tip of the premaxilla and frontal processes of the premaxilla were not separated into a premaxillary nail and culminocorn as in neornithines with compound rhamphothecae. This is also supported by the morphology of the traces in IVPP V12352 in which the dorsal margin of the preserved rhampthothecal traces are smooth along their entirety (Falk et al., 2019). This suggests the condition in confuciusornithiforms was more reminiscent of the simple rhamphotheca in many living birds in which the compound elements are entirely fused and grooves are absent (Hieronymus and Witmer, 2010). However, unlike in neornithines, in which the premaxillary nail and mandibular nail are single elements (Kingsbury et al., 1953), evidence from STM13-133 may suggest that the rhamphotheca covering the upper and lower jaws including the rostral-most portions may have been compound elements medially divided into left and right components.
Although STM13-133 is the only specimen to preserve evidence suggesting the confuciusornithiform rhamphotheca was divided into right and left halves, it may be that only one side, presumably the side still buried in matrix (for example, the right in IVPP V12352), is preserved in most specimens due to the lateral exposure of the skull and or taphonomy, or that the two halves were tightly attached rostrally. The morphology in STM13-162 somewhat supports the interpretation at least that the upper rhamphotheca consisted of left and right halves but the weakly preserved traces provide no definitive information. However, without additional data it is impossible to rule out the alternative that the appearance of right and left components forming the rhamphotheca in STM13-133 is an artifact of preservation. Potentially the upper and lower rhamphothecae were crushed and broken giving the appearance of consisting of two parts. Crushed, two-dimensional fossils often preserve fractures than can be misinterpreted as true morphologies (Foth and Rauhut, 2017).
Falk et al. (2019) discussed the differential preservation of various keratinous integumentary structures in the numerous described specimens of confuciusornithiforms. Despite evidence that the rhamphotheca is the hardest of the beta-keratin integumentary structures (Bonser, 1996), it is the least commonly preserved and notably there appears no pattern in the preservation of specific keratin structures (Table 1). STM13-133 and STM-13-162 offer no information that elucidates this issue. Superficially, preservation appears very different between these two specimens, offering no clues as to the taphonomic conditions that are conducive to preservation of this feature. Differences in bone coloration hint at different mineralization processes (iron oxidative processes were clearly part of the taphonomic history of STM13-133 as indicated by the reddish color where the bones are broken and the reddish stains forming the feather traces, but not STM13-162). Differences in preservation of the plumage also point to different modes of preservation; a large portion of the plumage is preserved in STM13-133 and these are mostly preserved as reddish stains, whereas the feathers in STM13-162 are only faintly preserved. The stark contrasting modes of preservation (evident from comparing the quality of preservation of structures such as bone and feathers and differences in mineral composition as superficially determined by differences in color) between the two specimens confirms previous observations that preservation of the rhamphotheca is not limited to a very narrow set of specific chemical conditions (Falk et al., 2019). In the future, chemical analyses of specimens preserving rhamphothecal traces may shed light on the specific taphonomic processes and geochemical conditions conducive to the preservation of these traces.
Given the differential preservation of keratinous structures in various specimens, we hypothesize that the preservation of keratinous integumentary structures may not be related to properties of the keratin itself. The common preservation of feathers compared to the rhampthotheca may be due to the fact that the former is commonly melanized in confuciusornithiforms, as demonstrated in multiple specimens (Zhang et al., 2010; Zheng et al., 2017; Li et al., 2018). The addition of melanin to keratinous structures increases their relative hardness (Bonser and Witter, 1993). Melanin is extremely resistant to decay and most preserved feathers in Jehol specimens that have been studied using SEM have been described as remnants of the melanosomes rather than the keratinous matrix (Zhang et al., 2010; Li et al., 2012, 2018; Vinther, 2015; Peteya et al., 2017; Zheng et al., 2017). A survey of SEM studies conducted on feathers preserved in Jehol paravians reveals that most commonly melanosomes are preserved together with their impressions (Li Q. et al., 2010; Zhang et al., 2010; Peteya et al., 2017; Zheng et al., 2017; Hu et al., 2018). This may be related to the fact that most Jehol specimens are preserved split between a slab and counter-slab; the act of splitting may be responsible for the formation of impressions of melanosomes in the surrounding matrix (whether consisting of degraded or petrified remnants of the feather keratin or sediment) just as voids are left by bones that remain in the opposite slab. The few studies in which only imprints (Li et al., 2012) or melanosomes (O’Connor et al., 2020) were recovered may reflect limited sampling. However, even where only impressions of melanosomes were recovered, keratin was reportedly not preserved (Li et al., 2012). Although understanding of feather preservation in the Jehol and other deposits is currently incomplete, available studies strongly suggest that a majority of feather traces contain preserved melanosomes. The rare preservation of the rhamphothecae in confuciusornithiforms may suggest this structure was not melanized in most members of this clade (in neornithines both melanic and non-melanic beak morphologies are present). The only known specimen of C. dui preserves traces of the rhamphotheca (Hou et al., 1999) and thus hypothetically it is possible the bill may have been melanized in this species. Unfortunately, this hypothesis cannot be tested since the specimen is lost. Alternatively, the bill may be seasonally melanized, like that of some extant birds (Bonser and Witter, 1993), and only preserved in individuals that died during this particular season. Although the overall scarcity of such traces (now six specimens reported out of purportedly thousands) suggests that the rhamphotheca was simply not melanized, it is possible to test these two hypotheses by sampling bill traces preserved in specimens of Confuciusornis and studying them using scanning electron microscopy (SEM) in order to determine the presence or absence of preserved melanosomes. However, the rarity of these traces and their relatively limited surface area (compared to feathers) make it unlikely that this will be possible due to the destructive nature of SEM sampling. If confuciusornithiforms had non-melanic rhamphothecae this would indicate the bill was lightly colored, thus providing new data that will translate to more accurate reconstructions of this important clade of Cretaceous birds (Figure 5).
A total of six confuciusornithiforms preserving traces of the rhamphothecae have now been identified. Preservation is very different between all six specimens, thus providing no information pertaining to the specific taphonomic conditions that are conducive to the preservation of this feature. In the future, chemical analyses on all six specimens will hopefully elucidate the different diagenetic pathways that are conducive to preservation of the rhamphotheca, at least in confuciusornithiforms. The rare preservation of rhamphothecae may suggest that the beak in confuciusornithiforms was non-melanic and thus lightly colored. Although it was reasonable to assume that the rhamphothecae would have a different morphology in each independent evolutionary origin of this feature, this study provides the first discussion of the differences between the rhamphothecae in non-neornithine ornithurines, confuciusornithiforms, and neornithines. In addition to having rhamphotheca that extends onto the maxilla, one specimen suggests that the Confuciusornis rhamphotheca may have been a compound structure consisting of right and left halves on both the upper and lower jaws. Osteological and soft tissue evidence suggests that the rhamphothecae covering the upper jaw extended dorsally and laterally to the caudal margin of the external nares. Notably, evidence from several specimens indicates that although pits and grooves may indicate the presence of a rhamphotheca, they do not correlate strictly with the extent of this feature as the frontal processes of the premaxilla lack pits and grooves but were clearly covered in rhamphotheca in confuciusornithiforms.
All datasets generated for this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
XTZ, JO’C, ZZ, YW, and XW designed the project. JO’C wrote the manuscript. YW photographed the specimen. All authors examined the collection for rhamphothecae.
This research was supported by the National Natural Science Foundation (Grant Nos. 41688103, 41402017, and 41372014) and the Taishan Scholars Program of Shandong Province (Grant No. Ts20190954).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor is currently organizing a Research Topic with one of the authors JO’C, and confirms the absence of any other collaboration.
We thank Y. O’Connor for assistance with figures and L. Chiappe and S. Abramowicz for providing images of the BMNH specimen and allowing use of their reconstruction of the skull of Confuciusornis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2020.00367/full#supplementary-material
Bailleul, A. M., Li, Z.-H., O’Connor, J. K., and Zhou, Z.-H. (2019). Origin of the avian predentary and evidence of a unique form of cranial kinesis in Cretaceous ornithuromorphs. Proc. Natl. Acad. Sci. U.S.A. 116, 24696–24706. doi: 10.1073/pnas.1911820116
Baumel, J. J., and Witmer, L. M. (1993). “Osteologia,” in Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd Edn, eds J. J. Baumel, A. S. King, J. E. Breazile, H. E. Evans, and J. C. Vanden Berge (Cambridge, MA: Nuttall Ornithological Club), 45–132.
Bhullar, B.-A., Hanson, M., Fabbri, M., Pritchard, A., Bever, G. S., and Hoffman, E. (2016). How to make a bird skull: major transitions in the evolution of the avian cranium, paedomorphosis, and the beak as a surrogate hand. Integr. Comp. Biol. 56, 389–403. doi: 10.1093/icb/icw069
Bonser, R. H. (1996). Comparative mechanics of bill, claw and feather keratin in the Common Starling Sturnus vulgaris. J. Avian Biol. 27, 175–177.
Bonser, R. H., and Witter, M. S. (1993). Indentation hardness of the bill keratin of the European starling. Condor 95, 736–738. doi: 10.2307/1369622
Bright, J. A., Marugán-Lobón, J., Cobb, S. N., and Rayfield, E. J. (2016). The shapes of bird beaks are highly controlled by nondietary factors. Proc. Natl. Acad. Sci. U.S.A. 113, 5352–5357. doi: 10.1073/pnas.1602683113
Chiappe, L. M., Ji, S., Ji, Q., and Norell, M. A. (1999). Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the Late Mesozoic of northeastern China. Bull. Am. Mus. Nat. Hist. 242, 1–89.
Chiappe, L. M., Marugán-Lobón, J., Ji, S., and Zhou, Z. (2008). Life history of a basal bird: morphometrics of the early cretaceous Confuciusornis. Biol. Lett. 4, 719–723. doi: 10.1098/rsbl.2008.0409
Chiappe, L. M., Norell, M., and Clark, J. (2001). A new skull of Gobipteryx minuta (Aves: Enantiornithes) from the cretaceous of the Gobi desert. Am. Mus. Novit. 3346, 1–15. doi: 10.1206/0003-0082(2001)346<0001:ansogm>2.0.co;2
Chinsamy, A., Chiappe, L. M., Marugán-Lobón, J., Gao, C.-H., and Zhang, F.-J. (2013). Gender identification of the Mesozoic bird Confuciusornis sanctus. Nat. Commun. 4:1381. doi: 10.1038/ncomms2377
Chinsamy, A., Marugán-Lobón, J., Serrano, F. J., and Chiappe, L. M. (2019). Osteohistology and life history of the basal pygostylian, Confuciusornis sanctus. Anat. Rec. 303, 949–962. doi: 10.1002/ar.24282
Dalsätt, J., Zhou, Z., Zhang, F., and Ericson, P. G. P. (2006). Food remains in Confuciusornis sanctus suggest a fish diet. Naturwissenschaften 93, 444–446. doi: 10.1007/s00114-006-0125-y
de Ricqlès, A. J., Padian, K., Horner, J. R., Lamm, E.-T., and Myhrvold, N. (2003). Osteohistology of Confuciusornis sanctus (Theropoda: Aves). J. Vertebr. Paleontol. 23, 373–386. doi: 10.1671/0272-4634(2003)023[0373:oocsta]2.0.co;2
Elzanowski, A. (1974). Preliminary note on the palaeognathous bird from the Upper Cretaceous of Mongolia. Palaeontol. Pol. 30, 103–109.
Elzanowski, A. (2002). “Archaeopterygidae (Upper Jurassic of Germany),” in Mesozoic Birds: Above the Heads of Dinosaurs, eds L. M. Chiappe and L. M. Witmer (Berkeley, CA: University of California Press), 129–159.
Elzanowski, A., Peters, D. S., and Mayr, G. (2018). Cranial morphology of the early cretaceous bird Confuciusornis. J. Vertebr. Paleontol. 38:e1439832. doi: 10.1080/02724634.2018.1439832
Falk, A. R., Kaye, T. G., Zhou, Z.-H., and Burnham, D. A. (2016). Laser fluorescence illuminates the soft tissue and life habits of the early cretaceous bird Confuciusornis. PLoS One 11:e0167284. doi: 10.1371/journal.pone.0167284
Falk, A. R., O’Connor, J., Wang, M., and Zhou, Z.-H. (2019). On the preservation of the beak in Confuciusornis (Aves: Pygostylia). Diversity 11, 1–8.
Field, D. J., Hanson, M., Burnham, D. A., Wilson, L. E., Super, K., Ehret, D., et al. (2018). Complete Ichthyornis skull illuminates mosaic assembly of the avian head. Nature 557, 96–100. doi: 10.1038/s41586-018-0053-y
Foth, C., and Rauhut, O. W. M. (2017). Re-evaluation of the Haarlem Archaeopteryx and the radiation of maniraptoran theropod dinosaurs. BMC Evol. Biol. 17:236. doi: 10.1186/s12862-017-1076-y
Gingerich, P. D. (1973). Skull of Hesperornis and early evolution of birds. Nature 243, 70–73. doi: 10.1038/243070a0
Guaraldo, A. C., Antqueves, L. M. C., and Manica, L. T. (2019). Beyond a feeding and thermoregulatory structure: toucan’s bill as a sword and pincer. Rev. Bras. Ornitol. 27, 145–148. doi: 10.1007/bf03544462
Han, X.-D., Wu, Z.-G., Tian, F.-M., and Sun, F. (1999). Observation on behavior of adult oriental white stork in nesting period. J. For. Res. 10, 118–120. doi: 10.1007/bf02855541
Hieronymus, T. L., and Witmer, L. M. (2010). Homology and evolution of avian compound rhamphothecae. Auk 127, 590–604. doi: 10.1525/auk.2010.09122
Hou, L., Martin, L. D., Zhonghe, Z., Feduccia, A., and Zhang, F. (1999). A diapsid skull in a new species of the primitive bird Confuciusornis. Nature 399, 679–682. doi: 10.1038/21411
Hou, L., Martin, L. D., Zhou, Z., and Feduccia, A. (1996). Early adaptive radiation of birds: evidence from fossils from northeastern China. Science 274, 1164–1167. doi: 10.1126/science.274.5290.1164
Hou, L., Zhou, Z., Gu, Y., and Zhang, H. (1995a). Confuciusornis sanctus, a new late Jurassic sauriurine bird from China. Chin. Sci. Bull. 40, 1545–1551.
Hou, L., Zhou, Z.-H., Martin, L. D., and Feduccia, A. (1995b). A beaked bird from the Jurassic of China. Nature 377, 616–618. doi: 10.1038/377616a0
Hou, L., Zhou, Z., Zhang, F., and Gu, Y. (2002). Mesozoic Birds from Western Liaoning in China. Shenyang: Liaoning Science and Technology Publishing House.
Hu, D.-Y., Clarke, J. A., Eliason, C. M., Qiu, R., Li, Q.-G., Shawkey, M. D., et al. (2018). A bony-crested Jurassic dinosaur with evidence of iridescent plumage highlights complexity in early paravian evolution. Nat. Commun. 9:217.
Jiang, B.-Y., Zhao, T., Regnault, S., Edwards, N. P., Kohn, S. C., Li, Z.-H., et al. (2017). Cellular preservation of musculoskeletal specializations in the Cretaceous bird Confuciusornis. Nat. Commun. 8:14779.
Kingsbury, J. W., Allen, V. G., and Rotheram, B. A. (1953). The histological structure of the beak in the chick. Anat. Rec. 116, 95–115. doi: 10.1002/ar.1091160109
Li, L., Wang, J.-Q., and Hou, S.-L. (2010). A new species of Confuciusornis from lower cretaceous of Jianchang, Liaoning, China. Glob. Geol. 29, 183–187.
Li, Q., Gao, K.-Q., Vinther, J., Shawkey, M. D., Clarke, J. A., D’Alba, L., et al. (2010). Plumage color patterns of an extinct dinosaur. Science 327, 1369–1372. doi: 10.1126/science.1186290
Li, Q.-G., Clarke, J. A., Peteya, J. A., and Shawkey, M. D. (2018). Elaborate plumage patterning in a Cretaceous bird. PeerJ 6:e5831. doi: 10.7717/peerj.5831
Li, Q.-G., Gao, K.-Q., Meng, Q.-J., Clarke, J. A., Shawkey, M. D., D’Alba, L., et al. (2012). Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335, 1215–1219. doi: 10.1126/science.1213780
Louchart, A., and Viriot, L. (2011). From snout to beak: the loss of teeth in birds. Trends Ecol. Evol. 26, 663–673. doi: 10.1016/j.tree.2011.09.004
Lovette, I. J., and Fitzpatrick, J. W. (2004). The Handbook of Bird Biology. Princeton, NJ: Princeton University Press.
Marugán-Lobón, J., Chiappe, L. M., Ji, S.-A., Zhou, Z.-H., Gao, C.-H., Hu, D.-Y., et al. (2011). Quantitative patterns of morphological variation in the appendicular skeleton of the early cretaceous bird Confuciusornis. J. Syst. Palaeontol. 9, 91–101. doi: 10.1080/14772019.2010.517786
Matthysen, E. (1989). Seasonal variation in bill morphology of Nuthatches Sitta europaea: dietary adaptations or consequences? Ardea 77, 117–125.
Mayr, G., Pohl, B., and Peters, D. S. (2005). A well-preserved Archaeopteryx specimen with theropod features. Science 310, 1483–1486. doi: 10.1126/science.1120331
Murphy, T. G., Rosenthal, M. F., Montgomerie, R., and Tarvin, K. A. (2009). Female American goldfinches use carotenoid-based bill coloration to signal status. Behav. Ecol. 20, 1348–1355. doi: 10.1093/beheco/arp140
Navalón, G., Bright, J. A., Marugán-Lobón, J., and Rayfield, E. J. (2019). The evolutionary relationship among beak shape, mechanical advantage, and feeding ecology in modern birds. Evolution 73, 422–435. doi: 10.1111/evo.13655
Nolan, P. M., Dobson, F. S., Nicolaus, M., Karels, T. J., McGraw, K. J., and Jouventin, P. (2010). Mutal mate choice for colorful traits in King Penguins. Ethology 116, 635–644.
O’Connor, J. K. (2019). The trophic habits of early birds. Palaeogeogr. Palaeoclimatol. Palaeoecol. 513, 178–195. doi: 10.1016/j.palaeo.2018.03.006
O’Connor, J. K., Chiappe, L. M., and Bell, A. (2011). “Pre-modern birds: avian divergences in the Mesozoic,” in Living Dinosaurs: the Evolutionary History of Birds, eds G. D. Dyke and G. Kaiser (Hoboken, NJ: John Wiley & Sons), 39–114. doi: 10.1002/9781119990475.ch3
O’Connor, J. K., Wang, M., and Hu, H. (2016). A new ornithuromorph (Aves) with an elongate rostrum from the Jehol Biota, and the early evolution of rostralization in birds. J. Syst. Palaeontol. 14, 939–948. doi: 10.1080/14772019.2015.1129518
O’Connor, J. K., Zheng, X.-T., Pan, Y.-H., Wang, X.-L., Wang, Y., Zhang, X.-M., et al. (2020). New information on the plumage of Protopteryx (Aves: Enantiornithes) from a new specimen. Cretac. Res. 116:104577. doi: 10.1016/j.cretres.2020.104577
O’Connor, J. K., and Zhou, Z.-H. (2013). A redescription of Chaoyangia beishanensis (Aves) and a comprehensive phylogeny of Mesozoic birds. J. Syst. Palaeontol. 11, 889–906. doi: 10.1080/14772019.2012.690455
Peteya, J. A., Clarke, J. A., Li, Q.-G., Gao, K.-Q., and Shawkey, M. D. (2017). The plumage and colouration of an enantiornithine bird from the early cretaceous of China. Palaeontology 60, 55–71. doi: 10.1111/pala.12270
Rauhut, O. W. M., Foth, C., and Tischlinger, H. (2018). The oldest Archaeopteryx (Theropoda: Avialiae): a new specimen from the Kimmeridgian/Tithonian boundary of Schamhaupten, Bavaria. PeerJ 6:e4191. doi: 10.7717/peerj.4191
Rico-Guevara, A., and Araya-Salas, M. (2015). Bills as daggers? A test for sexually dimorphic weapons in a lekking hummingbird. Behav. Ecol. 26, 21–29. doi: 10.1093/beheco/aru182
Stettenheim, P. R. (1972). The integument of birds. Avian Biol. 2, 1–63. doi: 10.1016/b978-0-12-249402-4.50010-3
Storer, R. W. (1960). “Adaptive radiation in birds,” in Biology and Comparative Physiology of Birds, ed. A. J. Marshall (New York, NY: Academic Press), 15–55. doi: 10.1016/b978-1-4832-3142-6.50007-0
Tattersall, G. J., Arnaout, B., and Symonds, M. R. E. (2016). The evolution of the avian bill as a thermoregulatory organ. Biol. Rev. 11:e0154768.
Wang, M., O’Connor, J., Zhou, S., and Zhou, Z.-H. (2019a). New toothed early cretaceous ornithuromorph bird reveals intraclade diversity in pattern of tooth loss. J. Syst. Palaeontol. 18, 631–645. doi: 10.1080/14772019.2019.1682696
Wang, M., O’Connor, J., and Zhou, Z.-H. (2019b). A taxonomical revision of the Confuciusornithiformes (Aves: Pygostylia). Vertebrata Palasiatica 57, 1–37.
Wang, M., Stidham, T. A., and Zhou, Z.-H. (2018). A new clade of basal early cretaceous pygostylian birds and developmental plasticity of the avian shoulder girdle. Proc. Natl. Acad. Sci. U.S.A. 115, 10708–10713. doi: 10.1073/pnas.1812176115
Wang, X., O’Connor, J., Maina, J. N., Pan, Y.-H., Wang, M., Wang, Y., et al. (2018). Archaeorhynchus preserving significant soft tissue including probable fossilized lungs. Proc. Natl. Acad. Sci. U.S.A. 115, 11555–11560. doi: 10.1073/pnas.1805803115
Wang, M., and Zhou, Z.-H. (2016). A new adult specimen of the basalmost ornithuromorph bird Archaeorhynchus spathula (Aves: Ornithuromorpha) and its implications for early avian ontogeny. J. Syst. Palaeontol. 15, 1–18. doi: 10.1080/14772019.2015.1136968
Wang, M., and Zhou, Z.-H. (2018). A new confuciusornithid (Aves: Pygostylia) from the early cretaceous increases the morphological disparity of the Confuciusornithidae. Zool. J. Linn. Soc. 185, 417–430. doi: 10.1093/zoolinnean/zly045
Wang, S., Stiegler, J., Amiot, R., Wang, X., Du, G.-H., Clark, J. C., et al. (2017). Extreme ontogenetic changes in a ceratosaurian theropod. Curr. Biol. 27, 144–148. doi: 10.1016/j.cub.2016.10.043
Wang, X.-R., Chiappe, L. M., Teng, F.-F., and Ji, Q. (2013). Xinghaiornis lini (Aves: Ornithothoraces) from the early cretaceous of Liaoning: an example of evolutionary mosaic in early birds. Acta Geol. Sin. Engl. Ed. 87, 686–689. doi: 10.1111/1755-6724.12080
Zanno, L. E., and Makovicky, P. J. (2011). Herbivorous ecomorphology and specialization patterns in theropod dinosaur evolution. Proc. Natl. Acad. Sci. U.S.A. 108, 232–237. doi: 10.1073/pnas.1011924108
Zhang, F., Zhou, Z., and Benton, M. J. (2008). A primitive confuciusornithid bird from China and its implications for early avian flight. Sci. China Ser. D Earth Sci. 51, 625–639. doi: 10.1007/s11430-008-0050-3
Zhang, F.-C., Kearns, S. L., Orr, P. J., Benton, M. J., Zhou, Z.-H., Johnson, D., et al. (2010). Fossilized melanosomes and the colour of cretaceous dinosaurs and birds. Nature 463, 1075–1078. doi: 10.1038/nature08740
Zhang, Z.-H., Gao, C.-H., Meng, Q.-J., Liu, J.-Y., Hou, L.-H., and Zheng, G.-M. (2009). Diversification in an early cretaceous avian genus: evidence from a new species of Confuciusornis from China. J. Ornithol. 150, 783–790. doi: 10.1007/s10336-009-0399-x
Zheng, X.-T., O’Connor, J. K., Wang, X.-L., Pan, Y.-H., Wang, Y., Wang, M., et al. (2017). Exceptional preservation of soft tissue in a new specimen of Eoconfuciusornis and its biological implications. Natl. Sci. Rev. 4, 441–452. doi: 10.1093/nsr/nwx004
Zheng, X.-T., O’Connor, J. K., Wang, X.-L., Wang, Y., and Zhou, Z.-H. (2018). Reinterpretation of a previously described Jehol bird clarifies early trophic evolution in the Ornithuromorpha. Proc. R. Soc. B Biol. Sci. 285:20172494. doi: 10.1098/rspb.2017.2494
Zheng, X.-T., Zhou, Z.-H., Wang, X.-L., Zhang, F.-C., Zhang, X.-M., Wang, Y., et al. (2013). Hind wings in basal birds and the evolution of leg feathers. Science 339, 1309–1312. doi: 10.1126/science.1228753
Zhou, S., O’Connor, J. K., and Wang, M. (2014). A new species from an ornithuromorph dominated locality of the Jehol Group. Chin. Sci. Bull. 59, 5366–5378. doi: 10.1007/s11434-014-0669-8
Zhou, S., Zhou, Z.-H., and O’Connor, J. K. (2012). A new toothless ornithurine bird (Schizooura lii gen. et sp. nov.) from the lower cretaceous of China. Vertebrata Palasiatica 50, 9–24.
Zhou, S., Zhou, Z.-H., and O’Connor, J. K. (2013). Anatomy of the early cretaceous Archaeorhynchus spathula. J. Vertebr. Paleontol. 33, 141–152.
Zhou, Z., and Zhang, F. (2001). Two new ornithurine birds from the early cretaceous of western Liaoning, China. Kexue Tongbao 46, 371–377.
Zhou, Z.-H., and Martin, L. D. (2011). Distribution of the predentary bone in Mesozoic ornithurine birds. J. Syst. Palaeontol. 9, 25–31. doi: 10.1080/14772019.2010.504080
Keywords: rhamphotheca, keratin, beak, soft tissue, Jehol avifauna, Early Cretaceous, avian evolution, Confuciusornithiformes
Citation: Zheng X, O’Connor J, Wang Y, Wang X, Xuwei Y, Zhang X and Zhou Z (2020) New Information on the Keratinous Beak of Confuciusornis (Aves: Pygostylia) From Two New Specimens. Front. Earth Sci. 8:367. doi: 10.3389/feart.2020.00367
Received: 17 April 2020; Accepted: 07 August 2020;
Published: 16 September 2020.
Edited by:
Daniel J. Field, University of Cambridge, United KingdomReviewed by:
Valentina Rossi, University College Cork, IrelandCopyright © 2020 Zheng, O’Connor, Wang, Wang, Xuwei, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoting Zheng, dHk0MjkxNjY2QDE2My5jb20=; Jingmai O’Connor, amluZ21haUBpdnBwLmFjLmNu; amluZ21haS5vY29ubm9yQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.