- 1National High Magnetic Field Laboratory Geochemistry Group and Department of Earth, Ocean, and Atmospheric Science, Florida State University, Tallahassee, FL, United States
- 2Water Mission Area, United States Geological Survey, Boulder, CO, United States
Increased permafrost thaw due to climate change in northern high-latitudes has prompted concern over impacts on soil and stream biogeochemistry that affect the fate of dissolved organic carbon (DOC). Few studies to-date have examined the link between molecular composition and biolability of dissolved organic matter (DOM) mobilized from different soil horizons despite its importance in understanding carbon turnover in aquatic systems. Additionally, the effect of mixed DOM sources on microbial metabolism (e.g., priming) is not well understood. No studies to-date have addressed potential priming effects in northern high-latitude or permafrost-influenced aquatic ecosystems, yet these ecosystems may be hot spots of priming where biolabile, ancient permafrost DOC mixes with relatively stable, modern stream DOC. To assess biodegradability and priming of DOC in permafrost-influenced streams, we conducted 28 day bioincubation experiments utilizing a suite of stream samples and leachates of fresh vegetation and different soil horizons, including permafrost, from Interior Alaska. The molecular composition of unamended DOM samples at initial and final time points was determined by ultrahigh resolution mass spectrometry. Initial molecular composition was correlated to DOC biodegradability, particularly the contribution of energy-rich aliphatic compounds, and stream microbial communities utilized 50–56% of aliphatics in permafrost-derived DOM within 28 days. Biodegradability of DOC followed a continuum from relatively stable stream DOC to relatively biolabile DOC derived from permafrost, active layer organic soil, and vegetation leachates. Microbial utilization of DOC was ∼3–11% for stream bioincubations and ranged from 9% (active layer mineral soil-derived) to 66% (vegetation-derived) for leachate bioincubations. To investigate the presence or absence of a priming effect, bioincubation experiments included treatments amended with 1% relative carbon concentrations of simple, biolabile organic carbon substrates (i.e., primers). The amount of DOC consumed in primed treatments was not significantly different from the control in any of the bioincubation experiments after 28 days, making it apparent that the addition of biolabile permafrost-derived DOC to aquatic ecosystems will likely not enhance the biodegradation of relatively modern, stable DOC sources. Thus, future projections of carbon turnover in northern high-latitude region streams may not have to account for a priming effect.
KEY POINTS
• Biodegradability of DOC followed a continuum from relatively stable stream DOC to relatively biolabile DOC from permafrost, active layer organic soil, and vegetation leachates.
• DOM composition, especially the relative contribution of aliphatic compounds, largely controlled biodegradability and we observed evidence for selective utilization/preservation of certain compounds with depth in soil horizons.
• Nutrient availability played a role in DOC biodegradability, while priming did not appear to be a relevant mechanism for enhancing DOC biodegradation.
Introduction
Climate change in northern high-latitudes (Acia, 2005; Settele et al., 2014) has prompted concern over changes in soil and stream biogeochemistry that affect the fate of dissolved organic carbon (DOC). Increasing permafrost thaw associated with warming in these regions is of particular concern as permafrost soils currently store approximately twice as much carbon as in the Earth’s atmosphere (Zimov et al., 2006; Schuur et al., 2008; Tarnocai et al., 2009). As near-surface permafrost thaws and maximum thaw depth in the summer increases (Zhang, 2013), more permafrost-derived DOC will be mobilized (Wickland et al., 2018) and exported to streams, where it has been shown to be highly susceptible to microbial degradation (Mann et al., 2014; Spencer et al., 2015; Vonk et al., 2015; Drake et al., 2017; Stubbins et al., 2017). Therefore, permafrost thaw represents a potential positive feedback to the atmosphere as aged permafrost carbon may enter the contemporary carbon cycle as DOC is mobilized (Schuur et al., 2008; Schaefer et al., 2011; Spencer et al., 2015) and remineralized as CO2 in the aquatic pathway due to microbial respiration.
Warming may also affect the molecular composition of dissolved organic matter (DOM) exported to streams in discontinuous permafrost regions, which is inherently linked to the biodegradability of DOC (Dittmar and Kattner, 2003; Mann et al., 2012; Ward and Cory, 2015; O’Donnell et al., 2016). Microbial turnover of DOC previously stored in frozen soils is critical to our knowledge of how carbon export in high-latitude streams will respond to climate change (Striegl et al., 2005; Zhang et al., 2017). However, limited studies have examined the link between molecular composition and biolability of DOM mobilized from different soil horizons despite its importance in understanding carbon turnover in aquatic systems. While biodegradability of DOC is an overriding control on ecosystem respiration, regulating how much organic carbon (OC) is remineralized as CO2 or exported downstream (Holmes et al., 2008, 2012; Mann et al., 2012; Wickland et al., 2012; Abbott et al., 2014), the processes controlling DOC degradation are not well understood (Mann et al., 2012; Wickland et al., 2012; Vonk et al., 2015), particularly the effect of mixed DOM sources on microbial metabolism (e.g., priming).
Boreal and arctic discontinuous permafrost regions are hot spots for DOC degradation, especially at the soil-stream interface (Hutchins et al., 2017). This provides an opportunity to study microbial responses to the presence of mixed DOC sources, which can lead to increased respiration compared to the sum of the sources alone (Attermeyer et al., 2014). Soil studies have demonstrated enhanced carbon turnover due to a “priming effect” (Bingeman et al., 1953) in which moderate inputs of biolabile substrates enhance microbial degradation and mineralization of more stable OC (Bingeman et al., 1953; Kuzyakov, 2010; Guenet et al., 2012). Priming is well documented in the soil sciences, however, it has only recently been suggested in the aquatic sciences, and studies in a variety of aquatic ecosystems have yielded both evidence for and against a priming effect (Guenet et al., 2010, 2014; Bianchi, 2011; Bengtsson et al., 2014, 2018; Hotchkiss et al., 2014; Koch et al., 2014; Bianchi et al., 2015; Catalán et al., 2015; Ward et al., 2017; Textor et al., 2018).
No studies to-date have addressed priming effects in northern high-latitude, permafrost-influenced aquatic ecosystems, yet they may be hot spots of priming as highly biolabile permafrost DOC mixes with modern, stable stream DOC (Abbott et al., 2014). Additionally, hot moments of priming have the potential to enhance microbial respiration in northern high-latitude streams and rivers; during spring freshet, a large proportion of biolabile DOC is exported in a short time period (Striegl et al., 2005; Raymond et al., 2007; Spencer et al., 2008, 2009; Holmes et al., 2012; Wickland et al., 2012) and in summer and fall, DOC from newly thawed permafrost soils may be released (Drake et al., 2015; Wickland et al., 2018). As riverine processing of allochthonous DOC is a critical component of the global carbon cycle, the importance of priming is underscored by its potential as a missing link in the study of mechanisms that may be contributing to removal of terrigenous DOM from the ocean (Bianchi, 2011).
This study was conducted to investigate: (1) how the molecular composition of DOM leached from various terrestrial sources affects DOC bioavailability in streams; and (2) if the mixing of fresh inputs of terrestrial DOM, specifically permafrost DOC, can “prime” relatively modern, stable stream DOC in northern high-latitudes, resulting in enhanced DOC respiration. Samples were collected in the Yukon River Basin in interior Alaska, a region underlain by discontinuous permafrost that has recently been subject to rapidly warming temperatures (Osterkamp, 2007; Chapin et al., 2010; IPCC, 2013), permafrost thaw and degradation (Osterkamp, 2007; Lu and Zhuang, 2011; Belshe et al., 2013) and associated changes in hydrology and vegetation (Neff and Hooper, 2002; Schuur et al., 2007; Wickland et al., 2007; Walvoord et al., 2012; Jorgenson et al., 2013). We quantified the biodegradability of DOC in streams as well as vegetation and soil leachates during 28 day bioincubation experiments. The molecular composition of the various DOM sources at initial and final timepoints was determined via ultrahigh resolution mass spectrometry to investigate which DOM compound classes are particularly susceptible to microbial utilization. The role of priming was determined by statistical differences between DOC turnover in controls and bioincubation treatments amended with a variety of biolabile OC substrates such as acetate, a low molecular weight acid that is highly bioavailable and abundant in ancient permafrost DOM (Drake et al., 2015). By assessing possible controls on DOC biodegradability in a variety of sources, we can address the urgent need to understand the fate of allochthonous DOM released into streams by thawing and leaching processes and the mechanisms controlling DOC turnover in permafrost landscapes.
Materials and Methods
Site Descriptions and Sample Collection
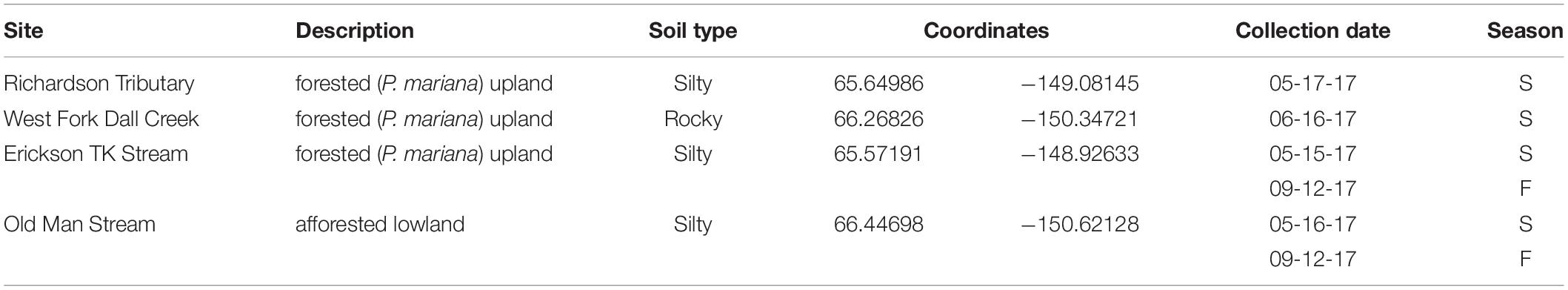
Sampling occurred at four different watersheds underlain by discontinuous permafrost in the Yukon River Basin of Interior Alaska (Figure 1). Seasonal discharge patterns in the region are commonly used to divide seasons into spring flush (May through June), summer to fall (July through October), and winter (November through April) (Striegl et al., 2005). Stream samples were collected at all four sites during spring flush and at two of the sites during the fall (Table 1). Old Man Stream is an afforested, silty lowland site while the remaining sites are uplands forested with black spruce (Picea mariana) (Table 1). Richardson Tributary and Erickson Thermokarst (TK) watersheds are classified as silty, while West Fork Dall Creek watershed is classified as rocky (Table 1; Jorgenson et al., 2013). On each sampling occasion, a 4 L bulk stream sample was filtered through a pre-rinsed high capacity 0.45 μm capsule filter and the filtrate was collected in an acid-washed, high-density polyethylene (HDPE) bottle. In addition 1 L of stream water was filtered through a GF/A (1.6 μm) glass microfiber filter (precombusted at 450°C, >5 h) and collected in acid-washed, polycarbonate bottles for use as an inoculum in bioincubations (see below). Samples were stored in the dark at 4°C until bioincubations were setup.
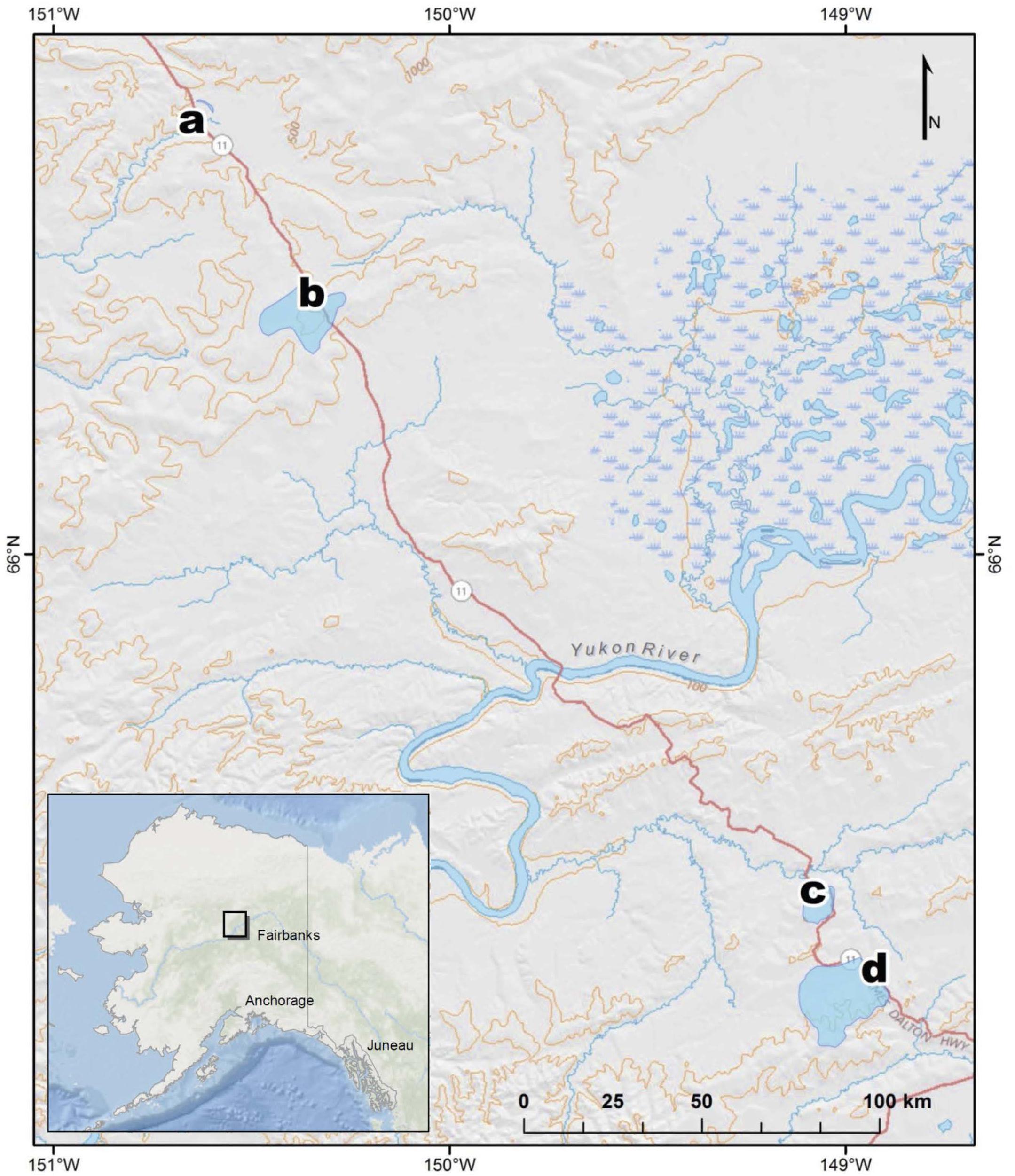
Figure 1. Map of stream sampling site locations and watersheds in Interior Alaska, United States, at (a) Old Man, (b) West Fork Dall, (c) Richardson, and (d) Erickson TK.
Groundcover vegetation and soil samples from the active layer and near-surface permafrost were collected during spring sampling at the two sites where seasonal stream sampling occurred at an active thermokarst slump (Erickson TK watershed), and a lowland underlain by discontinuous permafrost (Old Man watershed). Representative groundcover vegetation, mostly feather mosses and vascular plants, was collected with a soil knife directly above the location of soil collection. At the Old Man site, a SIPRE (Snow, Ice, and Permafrost Research Establishment) corer (7.5 cm diameter, 1 m length) was used to collect soils after the groundcover vegetation was removed. Three replicate cores that included seasonally frozen active layer soils and near-surface permafrost soils were taken within the same area (∼25 m2), which had an approximate active layer depth of ∼40–65 cm based on measurements at the same site the previous fall. Cores were extruded, wrapped in aluminum foil at the field site, and kept frozen on dry ice during transport to laboratory facilities. The site at Erickson TK is an active incised thermokarst slump where samples were collected at multiple depths along the face of the exposed soil profile using a soil knife and hand trowel. Exposed soil layers were first removed from the face of the slump and underlying, unexposed soils were collected. Vegetation and soil samples were stored in the dark on dry ice immediately following sample collection.
Vegetation and Soil Leachates
Leachates were made from the groundcover vegetation and soil samples collected from Erickson TK and Old Man watersheds. Soils were classified as organic (active layer), mineral (active layer), or permafrost based on depth of horizon (Table 2). Soils collected at three discrete depths from Erickson TK were leached individually. The frozen soil cores from Old Man were cut into discrete depth increments using a band saw. Replicate corresponding soil layers (organic active layer, mineral active layer, and permafrost) from the three cores were combined prior to leaching. Materials were leached in a 0.001 N sodium bicarbonate (NaHCO3) solution of ultrapure water, as NaHCO3 acts as a pH buffer that simulates the ionic strength of the sampled systems (Wickland et al., 2007; Spencer et al., 2008). Dried vegetation (50°C for 24 h) was added to the buffer solution in a 1:4 ratio by weight, while field-moist frozen soils were homogenized with a mallet and added in a 1:2 ratio by weight. Vegetation and soils were leached at room temperature (20°C) in the dark on a stir plate for 24 h before filtering. Leachates were filtered through high capacity capsule filters (0.45 μm) using a peristaltic pump (Cole and Parmer, Masterflex L/S) and immediately used for bioincubation experiments.
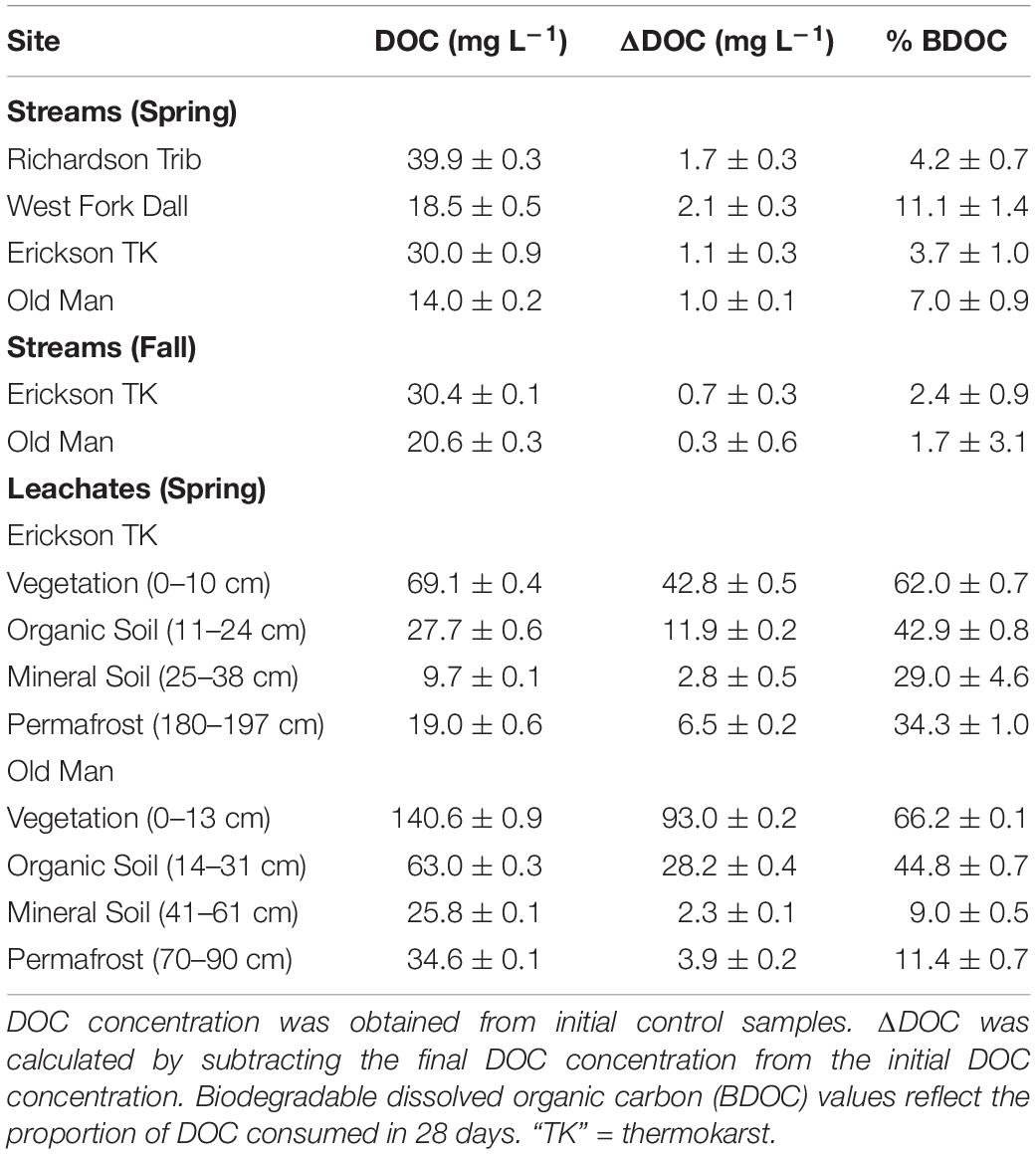
Table 2. DOC concentration, ΔDOC over 28 days, and percent of biodegradable dissolved organic carbon (BDOC), all reflecting the average and standard deviation of triplicate samples.
Bioincubation Experiments
Filtered (0.45 μm) stream samples and leachates were amended with a 1% microbial inoculum (Wickland et al., 2012; Vonk et al., 2015) obtained from stream samples from each site filtered with precombusted (450°C, 5 h) GF/A (1.6 μm) glass microfiber filters. An unamended control treatment quantified background DOC loss, while all other treatments were nutrient-amended to observe DOC loss under nutrient replete conditions. Nutrients were added for each sample based on doubled Redfield ratios of inorganic nitrate and phosphate in the form of KNO3 and Na3PO4 (i.e., 106C: 32N: 2P; Mann et al., 2015; Vonk et al., 2015). Therefore, an equal proportion of nutrients was added to each sample, accounting for differences in initial DOC concentrations between samples. To test for a priming effect with carbon as the limiting factor, there were three separate treatments of biolabile OC substrates (e.g., primers) plus the same proportion of KNO3 and Na3PO4. Primer treatment groups were amended with 1% relative carbon concentrations of acetate (C2H9NaO5), glucose (C6H12O6), or cellobiose (C12H22O11) based on the initial concentration of filtered, unamended samples. The 1% primer amendment was chosen based on the upper range of previously tested primer amendments (Catalán et al., 2015). Stream bioincubation experiments consisted of a five-point time series (0, 2, 7, 14, and 28 days), while leachate bioincubations only had two time points (0 and 28 days) to assess DOC loss.
Triplicate 30 mL samples from each treatment and time point were partitioned into 40-mL acid-washed, precombusted (550°C, 5 h) amber glass vials capped with plastic lids sealed by Teflon-coated septa. Bioincubations were conducted in the dark at room temperature (20°C). At each time point, samples were filtered with 0.45 μm glass microfiber syringe filters to remove any microbial biomass (Vonk et al., 2015) and acidified to pH 2 using 12 M HCl and stored in the dark at 4°C until analysis. Every 7 days remaining samples in the time series were opened and aerated to keep the samples oxygenated.
Dissolved Organic Carbon Analyses
Dissolved organic carbon concentrations of the bioincubation sample sets were analyzed on a Shimadzu TOC-L CPH total organic carbon analyzer using the high temperature combustion catalytic oxidation method. All sample sets were tested against a calibration curve consisting of five different standard concentrations from zero to 50 mg L–1. DOC concentration was measured as non-purgeable organic carbon (NPOC), with a maximum coefficient of variance of 2%.
DOC loss was calculated as the difference between the final (t = 28) and average initial (t = 0) concentrations (ΔDOC) for each sample type (i.e., each site and treatment).
Biodegradable dissolved organic carbon (BDOC) was calculated as the proportion of DOC loss,
during the 28 day bioincubation experiments (Vonk et al., 2015).
Molecular-Level Characterization of Dissolved Organic Matter
Concentrations of DOC in initial bioincubation samples were used to calculate the required aliquot volume for solid phase extraction (100 mg Bond Elut PPL, Agilent Technologies) following the method described by Dittmar et al. (2008). We aimed for a target concentration of 40 μg C mL–1 for DOM extracts eluted with 1 mL of methanol. Extracts were stored at −20°C before analysis. A 21 tesla Fourier transform ion cyclotron resonance mass spectrometer (FT-ICR MS; National High Magnetic Field Laboratory, Tallahassee, FL, United States) was used to analyze the molecular composition of DOM extracts of initial and final control bioincubation samples for each site. The instrument was set to negative ion mode and produced ions via electrospray ionization (ESI). Due to its high resolving power and extreme mass accuracy, ESI-FT-ICR MS is currently unparalleled in its ability to characterize DOM as it can detect thousands of individual molecular peaks separated out in a spectrum by their mass to charge ratio (m/z) (Sleighter and Hatcher, 2007; D’Andrilli et al., 2015; Hendrickson et al., 2015; Smith et al., 2018).
Molecular formulae obtained from FT-ICR MS analysis were assessed in the mass range of 170–1500 m/z and reassigned in PetroOrg Software (Corilo, 2015). Here, we included elemental combinations of C1–45 H1–92N0–4O1–25S0–2 with mass errors less than 300 ppb and excluding noise signals (>6 σ root mean square baseline noise) (O’Donnell et al., 2016). Elemental stoichiometries and modified aromaticity indices (AImod; Koch and Dittmar, 2006, 2016) were used to assign molecular formulae into seven different compound classes as follows using a script developed by Hemingway (2017): unsaturated phenolic low O/C = AImod < 0.5, H/C < 1.5, O/C < 0.5, unsaturated phenolic high O/C = AImod < 0.5, H/C < 1.5, O/C ≥ 0.5, polyphenolic = AImod 0.50–0.67, condensed aromatic = AImod > 0.67, aliphatic = H/C ≥ 1.5, O/C < 0.9, N = 0, sugar-like = O/C > 0.9, and peptide-like = H/C ≥ 1.5, O/C < 0.9 N ≥ 1 (O’Donnell et al., 2016). Although molecular peaks detected during FT-ICR MS may represent multiple isomers, we interpret DOM composition based on the relative abundance of molecular formulae assigned to each compound class. Thus molecular formulae assigned to the same compound class may herein be collectively described as compounds. In this study, shifts in DOM composition due to changes in relative abundance of different compound classes are attributed to selective microbial utilization of certain compounds.
Statistical Analyses
Student’s t tests were performed to assess the presence or absence of nutrient and priming effects in the bioincubation experiments. To test for a priming effect, the amount of DOC consumed over 28 days (ΔDOC mg L–1) in the nutrient-amended control was compared to each nutrient-amended primer treatment group. A significant difference (p < 0.05) in DOC consumption would indicate that a priming effect occurred. Nutrient effects were indicated by a significant difference in DOC consumption between the unamended control and nutrient-amended control.
Additionally, we used Spearman rank correlations to assess the relationship between biodegradability and chemical composition of stream and leachate DOM. Spearman correlations were performed between percent BDOC and relative abundance of common compounds (present in ≥50% of samples) obtained from FT-ICR MS analysis. Only compounds that were significantly correlated (p < 0.05) with BDOC were assigned a Spearman rank coefficient and plotted in van Krevelen space (O:C vs. H:C).
Results
Biodegradability of Stream and Leachate Dissolved Organic Carbon
Initial background DOC concentrations of stream samples collected in spring ranged from 14.0 to 39.9 mg L–1 (Tables 1, 2). On average, 1.5 ± 0.5 mg L–1 DOC was lost over 28 days and the proportion of BDOC was 6.5 ± 3.2% (Figure 2 and Table 2). For comparison, our stream BDOC values fall on the lower range of those reported by Kawahigashi et al. (2004), who found 4–28% BDOC in permafrost-influenced streams, but this also included continuous permafrost regions which tend to have higher BDOC values (Vonk et al., 2015). Samples collected in the fall had background DOC concentrations that were 1.6 and 47.1% higher compared to spring at Erickson TK Stream and Old Man Stream, respectively (Table 2). In contrast, the proportions of BDOC at these sites were slightly lower in fall, averaging 2.0 ± 0.5% compared to 5.4 ± 2.4% in spring (Table 2).
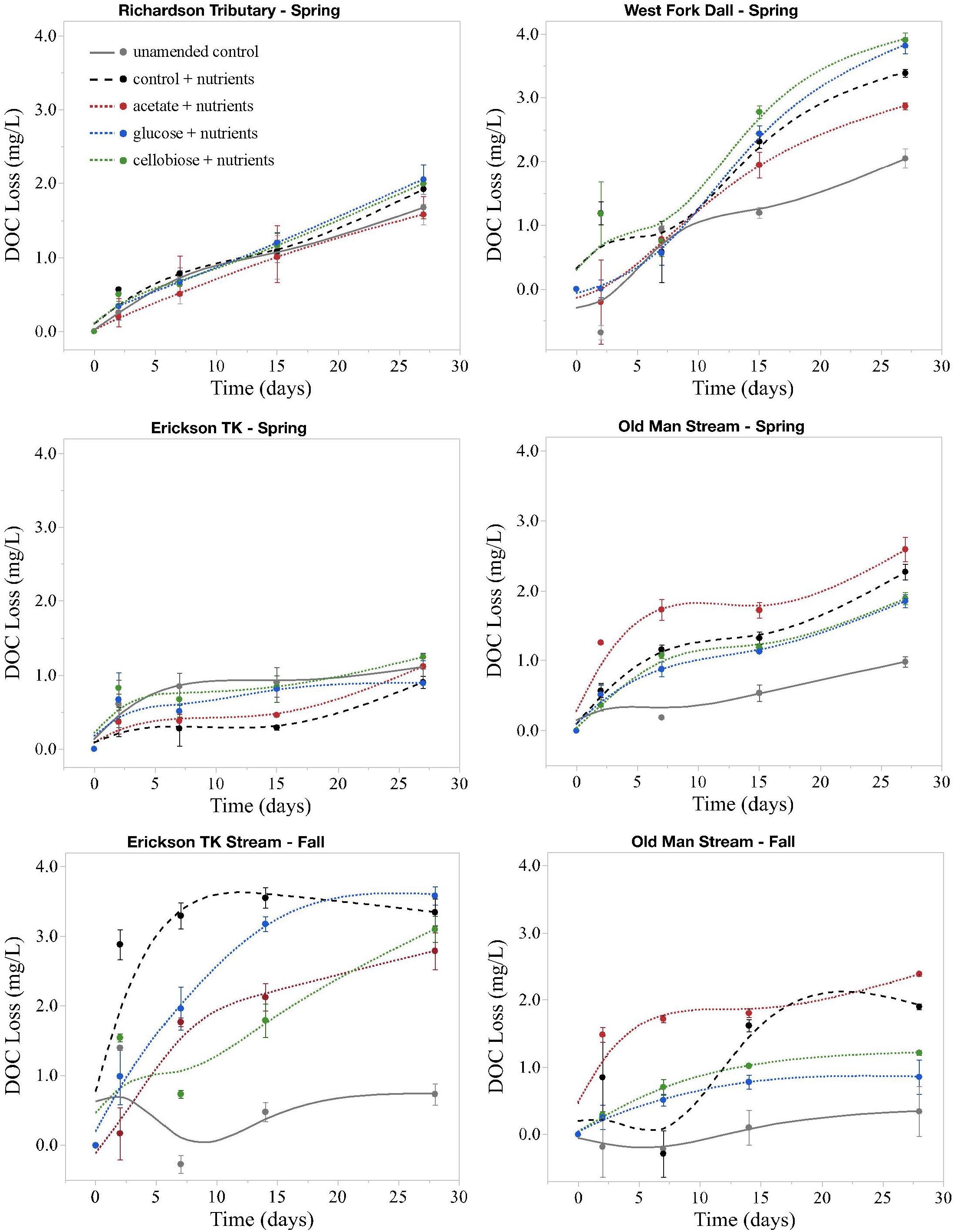
Figure 2. Loss of DOC in microbial incubation experiments. All changes are on the same scale, not exceeding 4 mg L– 1. Points are average changes in DOC concentration of the three replicates and bars represent one standard error from the mean. Spring = May; Fall = September.
Leachates of vegetation and active layer organic soils collected at the Old Man site had higher DOC concentrations compared to those at the Erickson TK site (Table 2). Leachate DOC exhibited a distinct pattern of biodegradability that was consistent across sites. Vegetation DOC was the most biodegradable, followed by active layer organic soil and permafrost soil DOC, with active layer mineral soil DOC being most stable (Table 2 and Figure 3). Average leachate BDOC was 64.1% for vegetation, 43.8% for active layer organic soil, 19.0% for active layer mineral soil, and 22.8% for permafrost soil across the sites (Table 2). Although BDOC values for vegetation and active layer organic soil leachates were comparable at the two sites, active layer mineral soil and permafrost soil leachates had much higher BDOC values at the Erickson TK site than the Old Man site (Table 2).
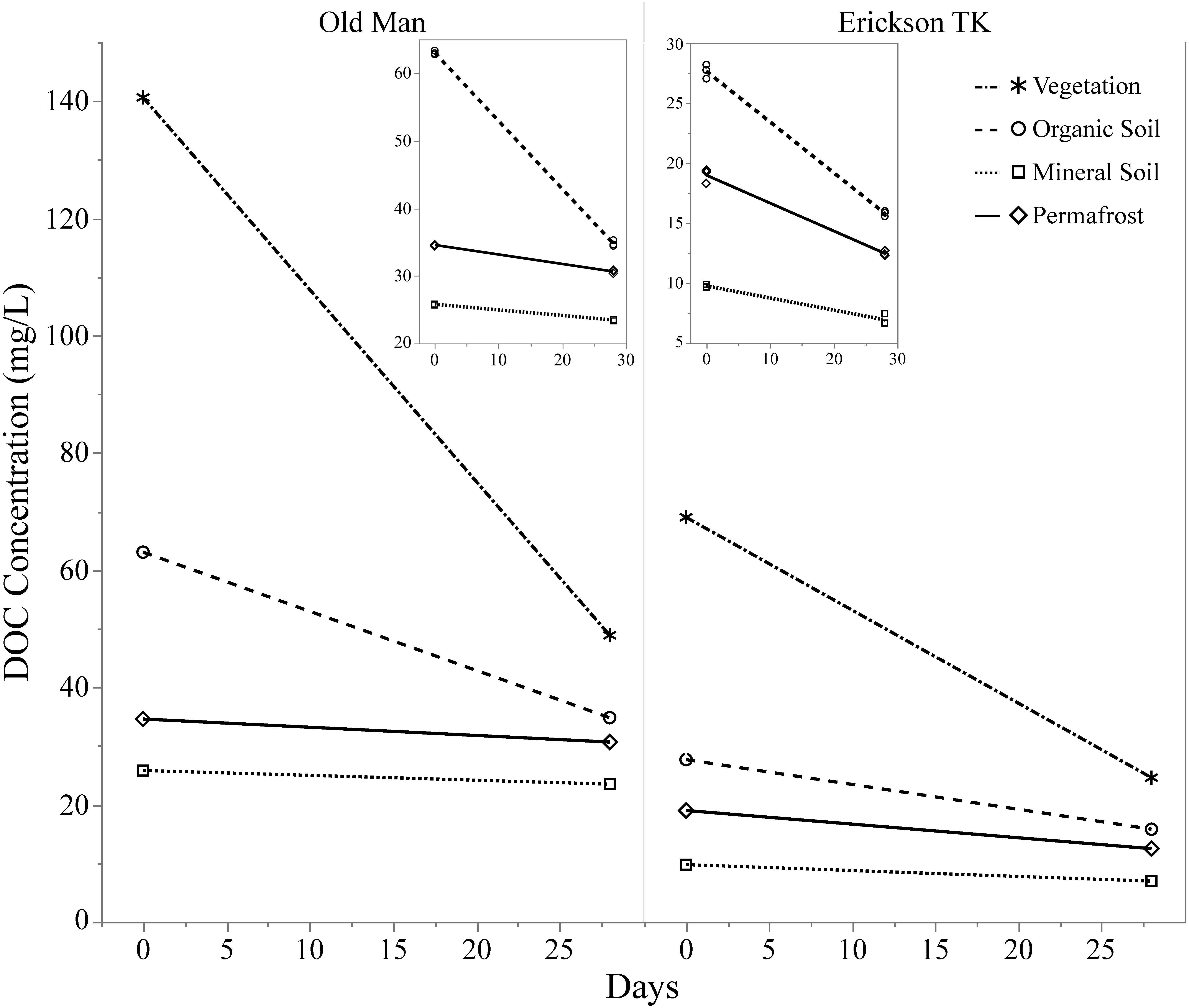
Figure 3. Continuum of dissolved organic carbon (DOC) biodegradability of leachates from different layer types. Compares background DOC loss (i.e., control samples) of layers and two different sites based on DOC concentration at t = 0 and t = 28. Insets show background DOC loss in the soil layers only.
Molecular Composition of Stream and Leachate Dissolved Organic Matter
Dissolved organic matter composition in streams, vegetation, and soil leachates was dominated by unsaturated phenolic compounds, mostly in the high O/C range, as measured by FT-ICR MS (Table 3 and Figures 4, 5). Although the unsaturated phenolic compound class was most prevalent in all DOM samples, these compounds come from diverse sources, and showed limited changes in relative abundance over the bioincubation period (Figures 4, 5). The next most abundant compound class was polyphenolics followed by condensed aromatics and aliphatics, respectively, while sugar- and peptide-like compounds contributed minimally to the DOM pools (Table 3 and Figures 4, 5).
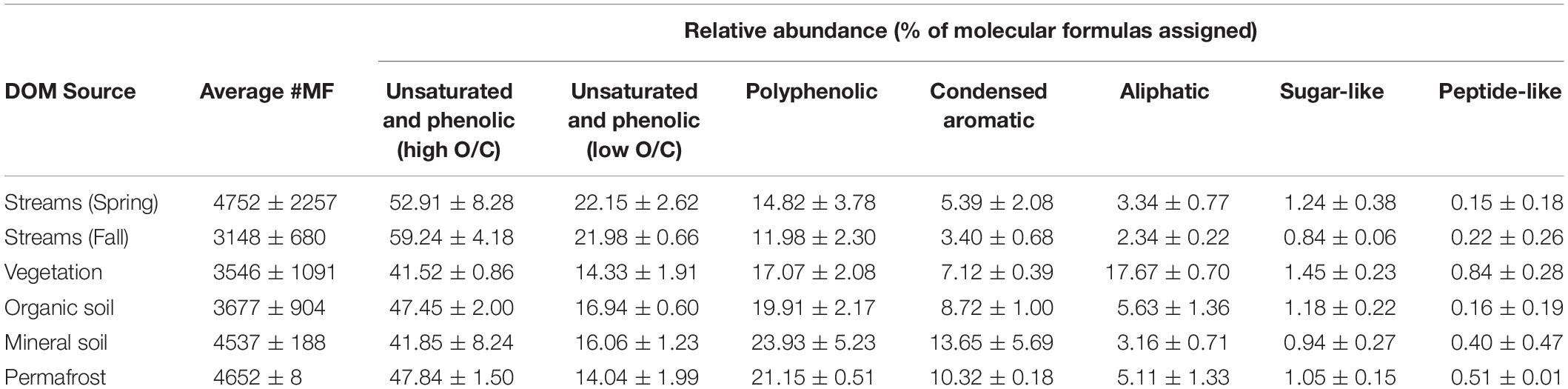
Table 3. Background chemical composition of each DOM source, reflecting the percent of molecular formulae assigned to each compound class (i.e., relative abundance).
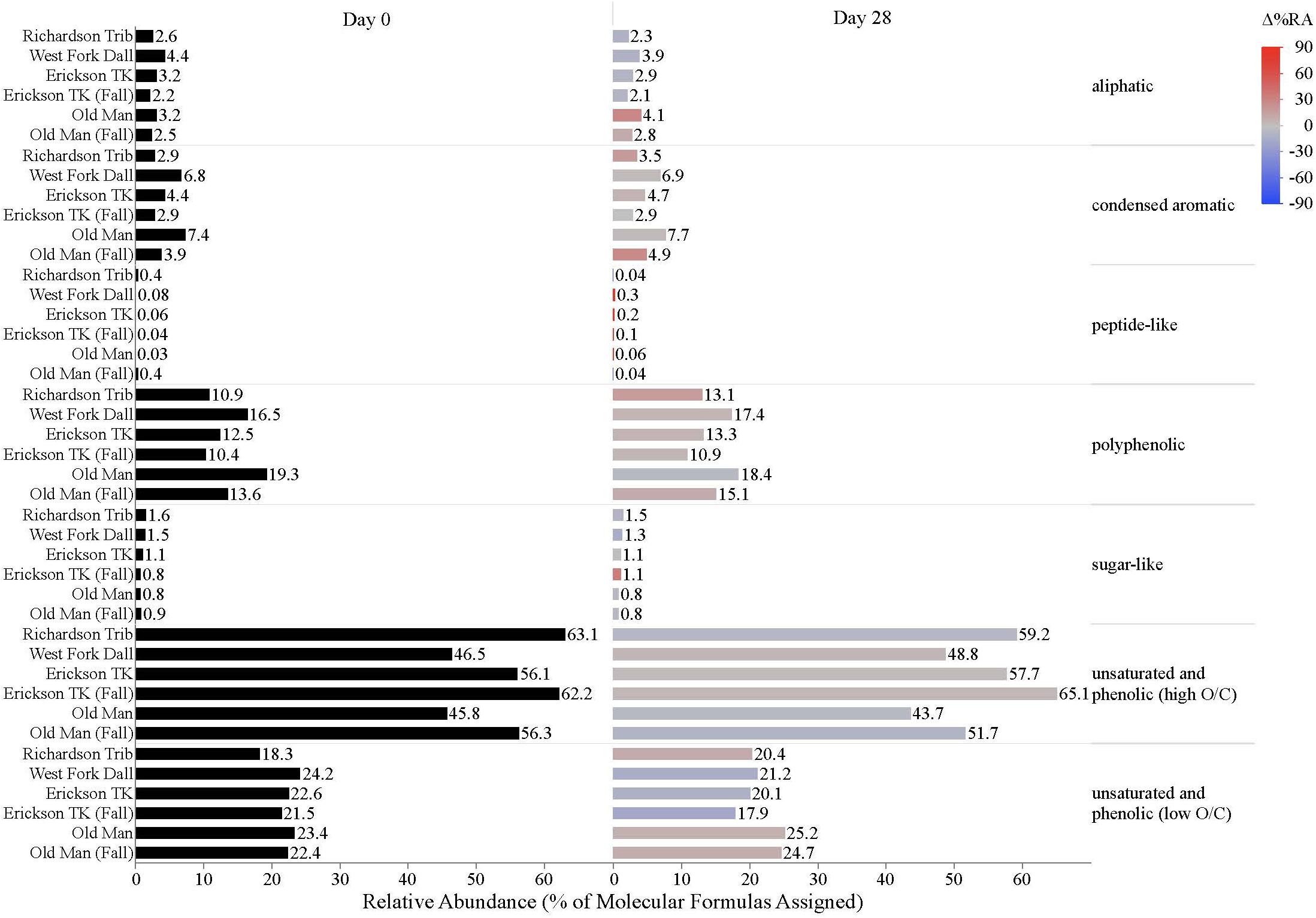
Figure 4. Stream DOM composition by compound class and site. Bars indicate the relative abundance of different compound classes, with black bars showing the relative abundance at t = 0 and colored bars representing the relative abundance by t = 28. The color scale reflects the percent change in relative abundance of each DOM compound class over the bioincubations, with red bars indicating a relative increase in abundance and blue bars indicating a relative decrease.
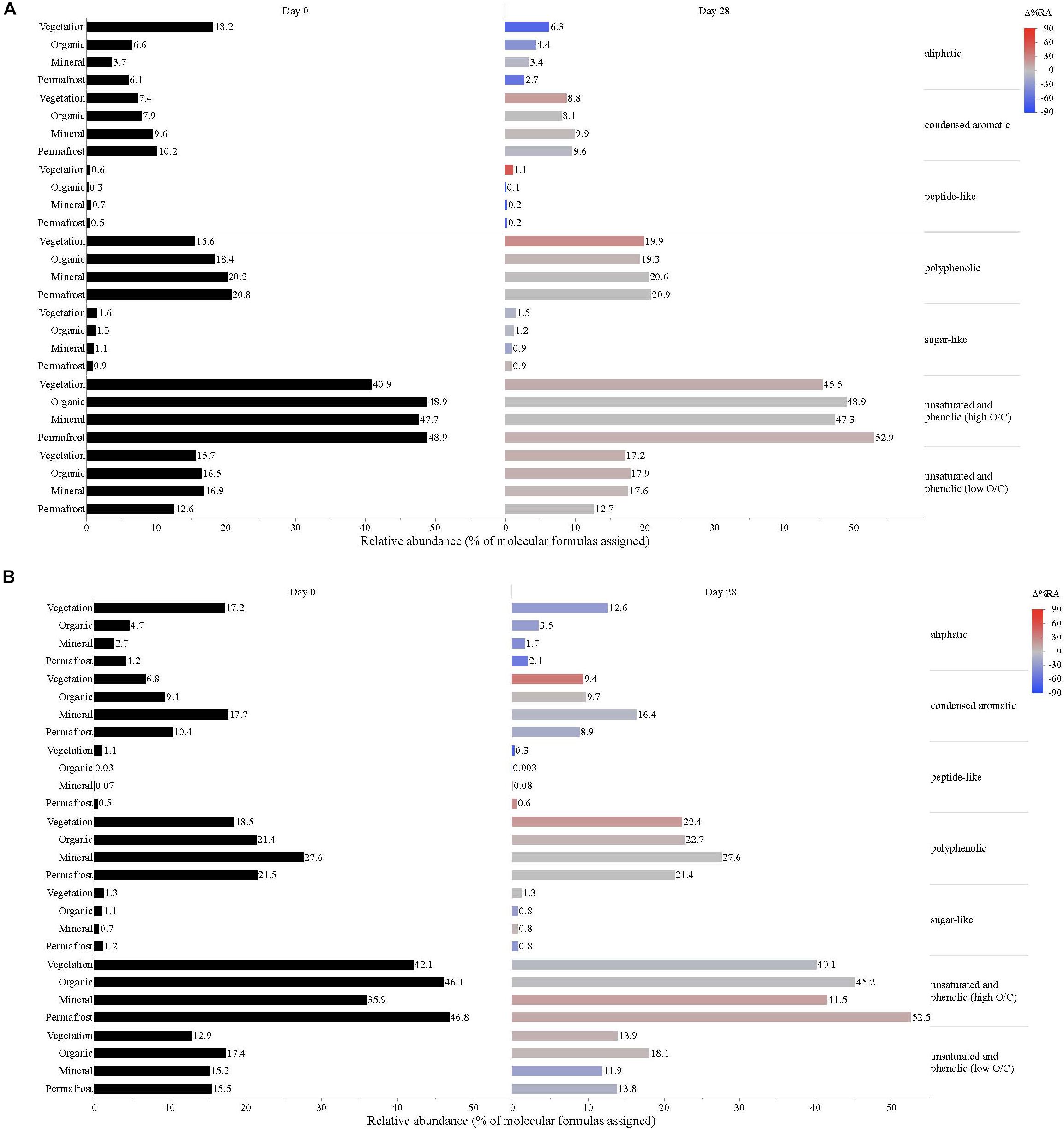
Figure 5. Leachate DOM composition at (A) Erickson TK and (B) Old Man site by compound class and layer. Bars indicate the relative abundance of different compound classes, with black bars showing the relative abundance at t = 0 and colored bars representing the relative abundance by t = 28. The color scale reflects the percent change in relative abundance of each DOM compound class over the bioincubations, with red bars indicating a relative increase in abundance and blue bars indicating a relative decrease.
Vegetation DOM leachates had the largest relative abundance of aliphatic, sugar-, and peptide-like compounds (Table 3 and Figure 5). The vegetation consisted of vascular plants and mosses, which have been shown to release highly biolabile DOM upon leaching (Wickland et al., 2007; Spencer et al., 2008). Out of all leachate DOM types, vegetation had the smallest contribution of polyphenolic compounds and condensed aromatics (Table 3 and Figure 5), consistent with other studies looking at the chemical composition of these types of mosses (Wickland et al., 2007; Spencer et al., 2008). DOM in the active layer organic soil and permafrost soil leachates had smaller relative abundances of aliphatic and sugar-like compounds in comparison to the vegetation leachates, while there was an increasing contribution of condensed aromatic and polyphenolic compounds in these soil horizons (Table 3 and Figure 5). However, compared to active layer mineral soils, DOM released from permafrost soil appears relatively enriched in aliphatic compounds that are highly susceptible to biodegradation (Vonk and Gustafsson, 2013; Mann et al., 2015; Spencer et al., 2015). The DOM leached from active layer mineral soils in this study had the smallest relative contribution of aliphatic compounds out of any of the leachate DOM samples, and the highest relative abundance of condensed aromatics and polyphenolics overall, including stream DOM samples (Table 3 and Figures 4, 5).
While stream DOM composition was relatively stable with respect to microbial degradation (Figure 4) there was an evident shift in leachate DOM composition by the end of the bioincubation period (Figure 5). The relative contribution of polyphenolics and condensed aromatics generally increased but, most notably, there was a large relative decrease in aliphatic compounds in leachate DOM. In permafrost DOM bioincubations, 50–56% of the energy-rich aliphatic compounds were lost over 28 days (Figure 5). The other leachate DOM types showed more variability between sites with respect to microbial utilization of aliphatics, with 27–66% loss in vegetation DOM, 25–34% loss in active layer organic soil DOM, and 6–37% loss in active layer mineral soil DOM (Figure 5).
Spearman rank correlations of common DOM compounds measured by FT-ICR MS and plotted in van Krevelen space indicated that there is a strong positive correlation between DOM composition and DOC biodegradability (p < 0.05; Figure 6C). When linear regressions were conducted separately for stream and leachate data, the positive correlation between percent BDOC and relative abundance of aliphatic compounds remained strong for both sample types (streams: r2 = 0.79; p = 0.008; leachates: r2 = 0.70; p = 0.026; Figures 6A,B).
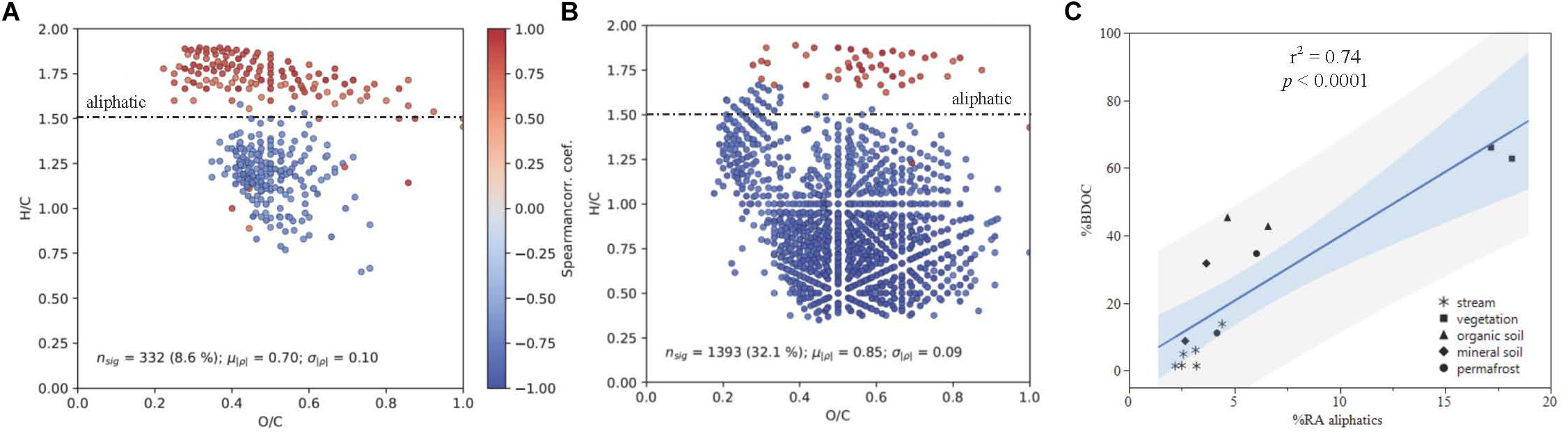
Figure 6. DOM composition as a function of % BDOC plotted in van Krevelen Space for (A) all samples and (B) leachates only, with the boundary for aliphatic formula assignment displayed (formulas with H/C > 1.50). Points represent molecular formulas measured by FT-ICR MS that were present in >50% of samples and were significantly correlated with % BDOC (p < 0.05). Colors indicate the Spearman rank correlation coefficient between the relative intensity of each molecular formula and % BDOC for its associated DOM sample. Red formulas have a higher relative abundance when the proportion of BDOC is high, while blue formulae have a higher relative abundance when the proportion of BDOC is low. (C) Shows % BDOC as a function of initial (t = 0) relative abundance of aliphatics (RAajiphatks) all DOM types, where the light blue and gray regions indicate the 95% confidence of fit and prediction, respectively.
Priming and Nutrient Effects
Priming effects were not evident in any of the stream or leachate bioincubations as there were no significant differences in DOC consumption between the nutrient-amended control and each nutrient- and primer-amended treatment group (p < 0.05, Student’s t test; Table 4 and Figure 2). Nutrient additions, however, significantly increased DOC consumption in the fall stream bioincubations and the active layer mineral soil leachate bioincubations (p = 0.003 and p = 0.001, respectively; Table 4 and Figure 2).
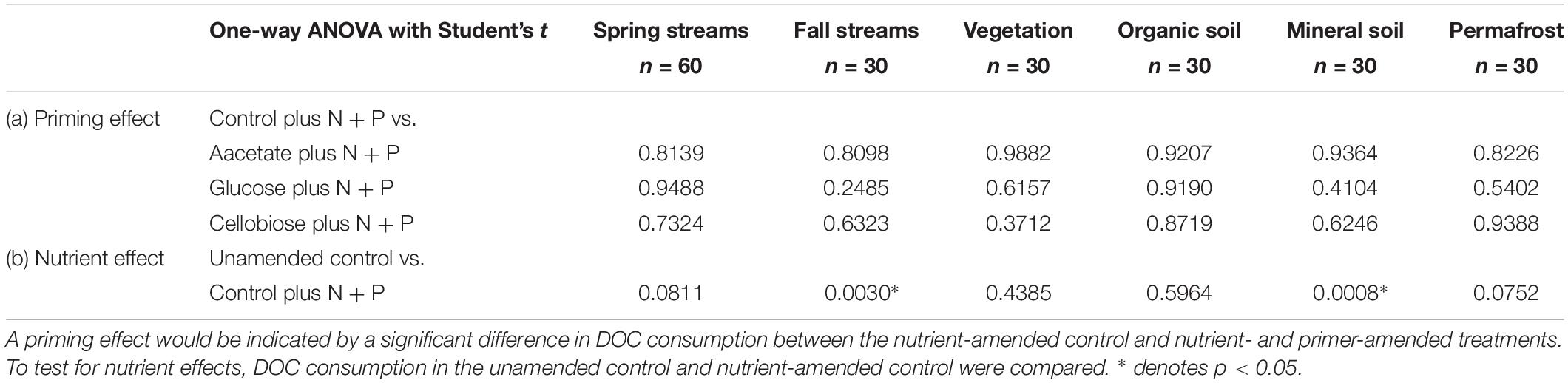
Table 4. Reported p-values from Student’s t tests to determine significant (a) priming and (b) nutrient effects in streams and leachates.
Discussion
Vulnerability of Different Dissolved Organic Matter Sources to Microbial Utilization
Dissolved organic matter followed a distinct continuum of biodegradability with respect to both bulk DOC utilization in bioincubation experiments and molecular composition. Stream DOM was relatively stable and resistant to microbial degradation, evidenced by low BDOC values compared to leachate DOM (Table 2) and minimal compositional changes during the bioincubation experiments (Figure 4). On the other end of the spectrum, vegetation leachate DOM had high BDOC values and higher contributions of energy-rich aliphatic, sugar-like, and peptide-like compounds, and lost up to 66% of aliphatic compounds over the bioincubation experiment (Table 2 and Figure 5). Recent studies have highlighted that elevated contributions of these compounds, particularly aliphatic compounds, are characteristic of highly biolabile DOM (Spencer et al., 2015; Textor et al., 2018).
Mosses dominate inputs to the vegetation leachate and despite the slow decomposition and poor substrate quality of mosses reported by many studies (Hobbie et al., 2000; Hodgkins et al., 2016) they have been shown to leach DOC with high hydrophilic content that is quickly consumed during bioincubations relative to DOC from other vegetation types like black spruce, for example, or fibric and amorphous soil horizons (Wickland et al., 2007; O’Donnell et al., 2016). Considering the high biodegradability of DOM leached from these mosses (Moore and Dalva, 2001; Wickland et al., 2007) and predicted short-term increases in primary productivity (Callaghan et al., 2004), they may represent an increasingly important supply of biolabile DOC to northern high-latitude aquatic ecosystems. Moving deeper into the soil column the biodegradability of active layer organic soil horizons has been shown to be inherently linked to decomposition processes and chemical characteristics of the overlying vegetation (Trumbore and Harden, 1997; Jonasson and Shaver, 1999; Jobbagy and Jackson, 2000; Wickland and Neff, 2008; O’Donnell et al., 2011, 2016). Compared to vegetation leachates, DOM from active layer organic soil had greater contributions of polyphenolic and condensed aromatic compounds (Table 3 and Figure 5). However, DOC leached from active layer organic soil was still highly biodegradable (Table 2 and Figure 3) and had high relative abundances of aliphatic and sugar-like DOM compounds that were readily utilized compared to DOM from streams and active layer mineral soils (Table 3 and Figures 4, 5).
Beneath the active layer organic soil, the DOM leached from the active layer mineral soil was the most resistant to microbial utilization, having the lowest BDOC values out of all the leachate types and exhibiting minimal compositional changes over the bioincubations (Table 2 and Figures 3, 5). The stability of active layer mineral soil-derived DOM to microbial utilization may be due to high relative contributions of polyphenolic and condensed aromatic compounds (Table 3 and Figure 5) that inhibit the enzyme synthesis or activity necessary for biodegradation (Mann et al., 2014), as well as sorption processes that are highly prevalent in mineral soils (Kalbitz et al., 2000). Mineral soils have been shown to preferentially adsorb higher molecular weight DOM fractions (Gu et al., 1995; Kalbitz et al., 2000) and hydrophobic acids (Qualls and Haines, 1992), which are less biologically available. It is also likely that the stability of DOM to microbial degradation increases as it is adsorbed to mineral surfaces (Kalbitz et al., 2000). In accordance with our finding that active layer mineral soil DOM was the least biodegradable of the soil layers analyzed, it had the lowest relative contribution of aliphatic compounds (Table 3 and Figure 5).
The type of permafrost under study has been shown to impact the biolability of exported organic matter (Kawahigashi et al., 2004; Schuur et al., 2008; Wickland et al., 2018). For example, Pleistocene-aged yedoma permafrost is widespread throughout Alaska and Siberia and releases extremely biolabile DOC upon thaw (Holmes et al., 2012; Vonk and Gustafsson, 2013; Abbott et al., 2014; Drake et al., 2015; Mann et al., 2015; Spencer et al., 2015; Vonk et al., 2015). As much as 65% of DOC from yedoma thaw is utilized within 30–40 day bioincubation experiments (Vonk et al., 2015) and a study by Spencer et al. (2015) revealed that up to >90% of aliphatic molecular formulae in DOM from Siberian yedoma thaw streams were utilized within 28 days. Past studies have also shown distinct differences in biolability between permafrost in continuous versus discontinuous permafrost regions (Kawahigashi et al., 2004; Vonk et al., 2015). In this study focused on discontinuous permafrost regions, we saw up to 34% loss of permafrost DOC and up to 56% loss of energy-rich aliphatic molecular formulae in permafrost DOM (Table 2 and Figure 5). While continuous permafrost regions contain preserved, unique DOC that is highly susceptible to microbial degradation (Kawahigashi et al., 2004), discontinuous permafrost regions are warmer and typical contain more aqueous environments allowing for microbial processing, and may have experienced previous leaching or microbial decomposition (Kawahigashi et al., 2004; Schuur et al., 2008; Vonk et al., 2015). This may explain the relatively lower loss of DOC and energy-rich aliphatic molecular formulae versus that observed in previous studies focused in continuous permafrost regions. However, it should be noted the permafrost in this study region still showed higher DOC loss than that of overlying active layer mineral soils and adjacent streams.
Role of Chemical Composition on the Fate of Dissolved Organic Matter
A warming climate will potentially change the chemical nature and fate of DOM draining from areas underlain by permafrost (Dittmar and Kattner, 2003; Mann et al., 2012; Ward and Cory, 2015; O’Donnell et al., 2016; Wickland et al., 2018), which has implications for aquatic carbon cycling in these regions. DOM is an extremely diverse mixture of substances (Thurman et al., 1985; Nebbioso and Piccolo, 2013) that, when characterized, can provide insight into DOM biodegradability (Sun et al., 1997; Spencer et al., 2008; Wickland et al., 2012; Zhang et al., 2017). By utilizing FT-ICR MS analysis, we were able to assess the link between underlying DOM composition and biodegradability at the molecular level. Further, by comparing DOM composition at the initial and final time points in bioincubation experiments, FT-ICR MS analysis allowed us to assess which compounds were susceptible to microbial utilization.
We observed that DOM composition from both streams and various terrestrial sources followed a distinct pattern of biodegradability. While stream DOM was relatively stable and experienced minor DOM compositional changes during the bioincubations, the DOM that would naturally be released into streams from vegetation and soils was typically more bioavailable and exhibited greater compositional changes. This pattern agrees strongly with previous findings that the biodegradability of DOM tends to decrease along a soil-stream-river continuum (Battin et al., 2008; Fellman et al., 2014; Mann et al., 2015; Spencer et al., 2015; Hutchins et al., 2017).
The positive correlation between DOM composition and DOC biodegradability was largely driven by the contribution of aliphatic compounds (H/C ≥ 1.5, O/C < 0.9) to DOM composition (Figure 6). Concurrent with these findings, consistent reductions in the relative abundance of aliphatic compounds over the bioincubations measured by FT-ICR MS indicate that these molecular formulae were preferentially utilized by stream microbes, which has been observed in other studies (Spencer et al., 2015; O’Donnell et al., 2016). Additionally, aliphatics had smaller relative contributions to the DOM pool sourced from deeper organic soil layers, which has been attributed to preferential microbial utilization of low molecular weight compounds (Drake et al., 2015; Spencer et al., 2015).
Contrarily, the relative abundance of polyphenolic and condensed aromatic compounds increased with soil depth, which has been previously reported for FT-ICR MS analysis of organic soils in boreal regions (O’Donnell et al., 2016) and suggests that these aromatic compounds are preferentially preserved with depth in organic soil horizons. Polyphenolic compounds are largely derived from vascular plant material, especially in boreal systems (Kellerman et al., 2015), while condensed aromatics are terrigenous combustion-derived compounds (Hockaday et al., 2006; Kellerman et al., 2018). The higher relative contribution of polyphenolic compounds with depth in DOM from organic soil horizons is likely due to the preferential preservation of vascular plant material from which polyphenols are derived (O’Donnell et al., 2016) and inhibitory effects of polyphenolic DOM content on biodegradation (Mann et al., 2014). Although the relative abundance of polyphenolics and condensed aromatics generally increased over the bioincubation period, consistent with their known stability to microbial degradation (Kim et al., 2006; Jaffé et al., 2013; O’Donnell et al., 2016; Kellerman et al., 2018), changes in relative abundance were generally small (Figure 5). We also observed an increase in the number of molecular formula assignments with depth (Table 3), indicating that deeper soil layers likely export DOM with greater chemodiversity (Zhang et al., 2017; Zark and Dittmar, 2018). Based on these findings, chemical composition of DOM, particularly the contribution of aliphatics, appears to be a major control on microbial respiration in boreal discontinuous permafrost regions.
No Priming Effect in Permafrost-Influenced Watersheds
This study is to the best of our knowledge the first to investigate the potential role of priming in northern high-latitude, permafrost-influenced watersheds. Out of the 18 different treatments of stream and leachate samples amended with OC substrates, none showed a significant priming effect (p < 0.05, Student’s t test, Table 4). The addition of simple OC substrates did not invoke a significant change in the amount of DOC consumed over 28 days (Table 4 and Figure 2), indicating that priming appears to play no role in altering DOC turnover in these regions.
The timescale over which priming is measured is important when considering the quantitative relevance of priming with respect to DOC mineralization and CO2 fluxes from aquatic ecosystems. Many aquatic studies on the priming effect that have reported evidence of priming describe the phenomenon as “apparent” or “transient” because, although they observed initial differences in DOC consumption between unamended and primed treatments, ultimately the amount of DOC consumed after about 1 month is not significantly different from background consumption (Steen et al., 2016; Textor et al., 2018).
An apparent priming effect occurs when microbial biomass turnover is affected, but not OM decomposition (Blagodatskaya and Kuzyakov, 2008; Kuzyakov, 2010). However, a real priming effect is evidenced by OM mineralization rates that are higher than ambient rates, resulting in the decomposition of more stable OM (Jenkinson et al., 1985). Studies that utilize isotopic labeling and modeled decay rates to study priming (Koehler et al., 2012; Bengtsson et al., 2014; Hotchkiss et al., 2014; Ward et al., 2016) can trace the degradation of biolable and biostable pools separately. While it is possible that priming may increase the rate of DOC mineralization initially in a triggering effect (Blagodatskaya and Kuzyakov, 2008), the quantitative importance of such priming effects in the long term may be limited (Steen et al., 2016; Ward et al., 2017; Textor et al., 2018). Thus, such studies that measure priming based on short-term modeled decay rates may overestimate the importance of priming.
We recognize that our pragmatic approach to assessing the priming effect through simple biodegradation experiments makes it difficult to distinguish between turnover of stable and biolabile DOC pools. However, throughout extensive replication, the amount of DOC consumed was statistically the same in the control and primed treatments after 28 day long bioincubations when the majority of BDOC has been respired (Wickland et al., 2007; Vonk et al., 2015). Therefore, we have no reason to believe that the stable DOC pools in the primed treatments were more susceptible to biodegradation than in the controls. Additionally, natural DOC in aquatic systems does not necessarily fall into “biolabile” or “stable” categories; rather, any given DOC pool is constantly evolving along the aquatic continuum and consists of multiple sub-pools of different ages and reactivities (Mostovaya et al., 2017; Vachon et al., 2017), presenting another challenge to assessing the priming effect.
A number of studies have shown that inorganic nutrient availability is strongly correlated to DOC biodegradability in arctic and boreal systems (Holmes et al., 2008; Wickland et al., 2012; Mann et al., 2014), while others have found that BDOC is unresponsive to inorganic nutrient additions (Abbott et al., 2014; Mann et al., 2015; Vonk et al., 2015). In this study, nutrient limitation in samples was evident for the fall stream and active layer mineral soil leachate DOC bioincubations, in which we found a significant difference between the percent of BDOC in the unamended and nutrient-amended samples (p < 0.05, Student’s t test; Table 4). Stream samples collected during fall had relatively high DOC concentrations and low proportions of BDOC (Table 2), as microbial metabolism was likely limited by naturally occurring stoichiometric constraints on inorganic nutrient availability in the Yukon River Basin during fall (Wickland et al., 2012). However, “primed” samples amended with nutrients and simple OC substrates, were not significantly different from the nutrient-amended control (i.e., no priming; p < 0.05, Student’s t test; Table 4). While many studies use algal or macrophyte exudates as primers (Danger et al., 2013; Attermeyer et al., 2014; Bengtsson et al., 2014; Hotchkiss et al., 2014; Ward et al., 2016), these sources of biolabile autochthonous OC also contain nutrients that may stimulate biodegradation, confounding the effect of added OC substrates on OM degradation with nutrients. The use of simple OC substrates and separate controls (i.e., with and without nutrients) ensures that priming can be measured separately from the confounding effect of nutrients (Textor et al., 2018). DOM turnover in response to biolabile OC inputs ultimately depends upon nutrient availability, which is also tightly coupled to microbial growth efficiencies (del Giorgio and Cole, 1998; Smith and Prairie, 2004; Franke et al., 2013). Thus it is critical to establish a clearer distinction between priming and the underlying mechanisms, such as nutrient stoichiometry and microbial activity, involved in DOM turnover in aquatic ecosystems.
The extent to which priming alters DOC cycling on a quantitatively relevant scale remains uncertain and, in permafrost-influenced watersheds, seems unlikely to play a major role. Bengtsson et al. (2018) recently conducted the most extensive meta-analysis of aquatic priming studies to date, including a variety of ecosystems along the aquatic continuum. By calculating log response ratios of mean OC degradation values for primed and control treatments to estimate the magnitude of priming effects, they found that a mean priming effect was not significantly different from zero across quantitative studies, with streams falling below the overall average (Bengtsson et al., 2018). Although DOM degradation rates are not likely altered by priming in various aquatic ecosystems, DOM molecular properties and underlying microbial processes hold promise in understanding some of the knowledge gaps in DOM turnover in the aquatic sciences. While this study is the first to investigate the potential of priming in northern high-latitude, permafrost-influenced watersheds, continued research will help develop a consensus on the role of priming in these dynamic systems.
Implications and Future Directions
Climate change and associated increases in permafrost thaw have implications for the quantity and biolability of DOC exported to and from streams and rivers in boreal and arctic regions (Vonk et al., 2015). Increasing thaw depth due to warming will release relatively preserved material from deeper soil layers that increases the amount of DOM available for microbial respiration, and has implications for stream DOM composition (Drake et al., 2015; O’Donnell et al., 2016; Wickland et al., 2018). This and other recent studies suggest that the chemical composition of DOM, especially aliphatic content, can be a good predictor of biodegradability (Figure 6) when examining permafrost draining watersheds and that permafrost-derived DOM is typically more susceptible to biodegradation than active layer mineral soil derived DOM in the same region.
While priming does not appear to be an important mechanism for increasing DOC turnover in boreal, discontinuous permafrost regions, the mobilization of inorganic nutrients from thawing permafrost may be important for alleviating nutrient constraints on microbial remineralization of DOC (Wrona et al., 2006; Holmes et al., 2012; Wickland et al., 2012). Permafrost thaw waters contain relatively high concentrations of inorganic nutrients (Tarnocai et al., 2009; Keuper et al., 2012; Abbott et al., 2014; Harms et al., 2014), and microbial metabolism in northern high-latitude aquatic ecosystems is often limited by inorganic nutrient availability (Dittmar and Kattner, 2003; McClelland et al., 2007; Holmes et al., 2008, 2012; Wickland et al., 2012). As seen in this study, inorganic nutrient additions can enhance DOC mineralization (Figure 2 and Table 4). Thus the concurrent release of biolabile permafrost DOC and inorganic nutrients with future warming in northern high-latitudes may enhance stream respiration by alleviating nutrient limitation (Striegl et al., 2005; Wrona et al., 2006; Wickland et al., 2012; Abbott et al., 2014; Harms et al., 2014; Mann et al., 2014) which may contribute to the predicted increases in greenhouse gas emissions from aquatic ecosystems in permafrost regions (Christensen et al., 2004; Schuur et al., 2009; Settele et al., 2014). Finally, results from this study suggest that priming of DOC derived from streams, vegetation, and soils across a variety of sites underlain by discontinuous permafrost seems unlikely due to the limited response of these DOC sources when amended with biolabile OC substrates. Therefore, it seems apparent that addition of biolabile permafrost DOM to aquatic ecosystems in northern high-latitude regions will not further prime turnover of relatively modern, more stable DOM sources, and thus future projections of carbon turnover in streams can be linked directly to permafrost thaw within the watersheds.
Author Contributions
RS, ST, and KW conceived the study and provided the materials. ST, KW, and SJ conducted the fieldwork. ST, DP, and SJ carried out the laboratory analyses in support of this study. ST drafted the initial manuscript. KW and RS edited extensively. All authors read and contributed to the final version of the manuscript.
Funding
This study was partly supported by the NSF Biological Oceanography award 1464392 to RS and the NASA-ABoVE Project 14-14TE-0012 award NNX15AU07A to RS and KW. A portion of this work was performed at the National High Magnetic Field Laboratory ICR User Facility, which is supported by the National Science Foundation Division of Chemistry through DMR-1644779 and the State of Florida. We thank the USGS LandCarbon Program for funding.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the people in the NHMFL ICR Program who work selflessly to facilitate data acquisition and processing for users of the facility. We also thank J. Koch, B. Minsley, and B. Ebel for field sample collection assistance.
References
Abbott, B. W., Larouche, J. R., Jones, J. B. Jr., Bowden, W. B., and Balser, A. W. (2014). Elevated dissolved organic carbon biodegradability from thawing and collapsing permafrost. Biogeosciences 119, 2049–2063. doi: 10.1002/2014JG002678
Acia. (2005). Impact of a Warming Arctic: Arctic Climate Impact Assessment. New York, NY: Cambridge University Press.
Attermeyer, K., Hornick, T., Kayler, Z. E., Bahr, A., Zwirnmann, E., Grossart, H. P., et al. (2014). Enhanced bacterial decomposition with increasing addition of autochthonous to allochthonous carbon without any effect on bacterial community composition. Biogeosciences 11, 1479–1489. doi: 10.5194/bg-11-1479-2014
Battin, T. J., Kaplan, L. A., Findlay, S., Hopkinson, C. S., Marti, E., Packman, A. I., et al. (2008). Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 1, 95–101.
Belshe, E. F., Schuur, E. A. G., and Grosse, G. (2013). Quantification of upland thermokarst features with high resolution remote sensing. Environ. Res. Lett. 8, 1–10. doi: 10.1088/1748-9326/8/3/035016
Bengtsson, M., Attermeyer, K., and Catalan, N. (2018). Interactive effects on organic matter processing from soils to the ocean: are priming effects relevant in aquatic ecosystems? Hydrobiologia 822, 1–17. doi: 10.1007/s10750-018-3672-2
Bengtsson, M. M., Wagner, K., Burns, N. R., Herberg, E. R., Wanek, W., Kaplan, L. A., et al. (2014). No evidence of aquatic priming effects in hyporheic zone microcosms. Sci. Rep. 4:5187. doi: 10.1038/srep05187
Bianchi, T. S. (2011). The role of terrestrially derived organic carbon in the coastal ocean: a changing paradigm and the priming effect. PNAS 108, 19473–19481. doi: 10.1073/pnas.1017982108
Bianchi, T. S., Thornton, D. C. O., Yvon-Lewis, S. A., King, G. M., Eglinton, T. I., Shields, M. R., et al. (2015). Positive priming of terrestrially derived dissolved organic matter in a freshwater microcosm system. Geophys. Res. Lett. 42, 5460–5467. doi: 10.1002/2015GL064765
Bingeman, C. W., Varner, J., and Martin, W. (1953). The effect of the addition of organic materials on the decomposition of an organic soil. Soil Sci. Soc. Am. J. 17, 34–38.
Blagodatskaya, E., and Kuzyakov, Y. (2008). Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol. Fertil. Soils 45, 115–131. doi: 10.1007/s00374-008-0334-y
Callaghan, T. V., Björn, L. O., Chernov, Y., Chapin, T., Christensen, T. R., Huntley, B., et al. (2004). Effects of changes in climate on landscape and regional processes, and feedbacks to the climate system. Ambio 33, 459–468. doi: 10.1579/0044-7447-33.7.459
Catalán, N., Kellerman, A. M., Peter, H., Carmona, F., and Tranvik, L. J. (2015). Absence of a priming effect on dissolved organic carbon degradation in lake water. Limnol. Oceanogr. 60, 159–168. doi: 10.1002/lno.10016
Chapin, F. S. III, McGuire, A. D., Ruess, R. W., Hollingsworth, T. N., Mack, M. C., Johnstone, J. F., et al. (2010). Resilience of Alaska’ s boreal forest to climatic change. Can. J. Forest Res. 40, 1360–1370. doi: 10.1139/X10-074
Christensen, T. R., Johansson, T., Akerman, J. H., Mastepanov, M., Mastepanov, M., Malmer, N., et al. (2004). Thawing sub-arctic permafrost: effects on vegetation and methane emissions. Geophys. Res. Lett. 31:L04501. doi: 10.1029/2003GL018680
D’Andrilli, J., Cooper, W. T., Foreman, C. M., and Marshall, A. G. (2015). An ultrahigh-resolution mass spectrometry index to estimate natural organic matter lability. Rapid Commun. Mass Spectrometr. 29, 2385–2401. doi: 10.1002/rcm.7400
Danger, M., Cornut, J., Chauvet, E., Chavez, P., Elger, A., and Lecerf, A. (2013). Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: a case of aquatic priming effect? Ecology 94, 1604–1613. doi: 10.1890/12-0606.1
del Giorgio, P. A., and Cole, J. J. (1998). Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 29, 503–541. doi: 10.1146/annurev.ecolsys.29.1.503
Dittmar, T., and Kattner, G. (2003). The biogeochemistry of the river and shelf ecosystem of the arctic ocean: a review. Mar. Chem. 83, 103–120. doi: 10.1016/S0304-4203(03)00105-1
Dittmar, T., Koch, B., Hertkorn, N., and Kattner, G. (2008). A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol. Oceanogr. 6, 230–235. doi: 10.4319/lom.2008.6.230
Drake, T. W., Raymond, P. A., and Spencer, R. G. M. (2017). Terrestrial carbon inputs to inland waters: a current synthesis of estimates and uncertainty. Limnol. Oceanogr. Lett. 3, 132–142. doi: 10.1002/lol2.10055
Drake, T. W., Wickland, K. M., Spencer, R. G. M., Mcknight, D. M., and Striegl, R. G. (2015). Ancient low-molecular-weight organic acids fuel rapid carbon dioxide production upon thaw. Proc. Natl. Acad. Sci. U.S.A. 112, 13946–13951. doi: 10.1073/pnas.1511705112
Fellman, J. B., Spencer, R. G. M., Raymond, P. A., Pettit, N. E., Skrzypek, G., Hernes, P. J., et al. (2014). Dissolved organic carbon biolability decreases along with its modernization in fluvial networks in an ancient landscape. Ecology 95, 2622–2632. doi: 10.1890/13-1360.1
Franke, D., Bonnell, J. E., and Ziegler, S. E. (2013). Mineralisation of dissolved organic matter by heterotrophic stream biofilm communities in a large boreal catchment. Freshwater Biol. 58, 2007–2026. doi: 10.1111/fwb.12187
Gu, B., Schmitt, J., Chen, Z., Liang, L., and McCarthy, J. F. (1995). Adsorption and desorption of different organic matter fractions on iron oxide. Geochim. Cosmochim. Acta 59, 219–229. doi: 10.1016/j.jhazmat.2013.10.074
Guenet, B., Danger, M., Abbadie, L., and Lacroix, G. (2010). Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 91, 2850–2861. doi: 10.1890/09-1968.1
Guenet, B., Danger, M., Harrault, L., Allard, B., Jauset-Alcala, M., Bardoux, G., et al. (2014). Fast mineralization of land-born C in inland waters: first experimental evidences of aquatic priming effect. Hydrobiologia 721, 35–44. doi: 10.1007/s10750-013-1635-1
Guenet, B., Juarez, S., Bardoux, G., Abbadie, L., and Chenu, C. (2012). Evidence that stable C is as vulnerable to priming effect as is more labile C in soil. Soil Biol. Biochem. 52, 43–48. doi: 10.1016/j.soilbio.2012.04.001
Harms, T. K., Abbott, B. W., and Jones, J. B. (2014). Thermo-erosion gullies increase nitrogen available for hydrologic export. Biogeochemistry 117, 299–311. doi: 10.1007/s10533-013-9862-0
Hemingway, J. D. (2017). Fourier Transform: Open-Source Tools for FT-ICR MS Data Analysis. Available at: http://github.com/FluvialSeds/fouriertransform (accessed January, 2018).
Hendrickson, C. L., Quinn, J. P., Kaiser, N. K., Smith, D. F., Blakney, G. T., Chen, T., et al. (2015). 21 tesla fourier transform ion cyclotron resonance mass spectrometer: a national resource for ultrahigh resolution mass analysis. J. Am. Soc. Mass Spectrometr. 26, 1626–1632. doi: 10.1007/s13361-015-1182-2
Hobbie, S. E., Schimel, J. P., Trumbore, S. E., and Randerson, J. R. (2000). Controls over carbon storage and turnover in high-latitudes. Glob. Change Biol. 6, 196–210. doi: 10.1016/j.cognition.2008.05.007
Hockaday, W. C., Grannas, A. M., Kim, S., and Hatcher, P. G. (2006). Direct molecular evidence for the degradation and mobility of black carbon in soils from ultrahigh-resolution mass spectral analysis of dissolved organic matter from a fire-impacted forest soil. Organ. Geochem. 37, 501–510. doi: 10.1016/j.orggeochem.2005.11.003
Hodgkins, S. B., Tfaily, M. M., Podgorski, D. C., McCalley, C. K., Saleska, S. R., Crill, P. M., et al. (2016). Elemental composition and optical properties reveal changes in dissolved organic matter along a permafrost thaw chronosequence in a subarctic peatland. Geochimica Cosmochimica Acta 187, 123–140. doi: 10.1016/j.gca.2016.05.015
Holmes, R. M., Mcclelland, J. W., Peterson, B. J., Tank, S. E., Bulygina, E., Eglinton, T. I., et al. (2012). Seasonal and annual fluxes of nutrients and organic matter from large rivers to the arctic ocean and surrounding seas. Estuaries Coasts 35, 369–382. doi: 10.1007/s12237-011-9386-6
Holmes, R. M., Mcclelland, J. W., Raymond, P. A., Frazer, B. B., Peterson, B. J., and Stieglitz, M. (2008). Lability of DOC transported by alaskan rivers to the arctic ocean. Geophys. Res. Lett. 35:L03402. doi: 10.1029/2007GL032837
Hotchkiss, E. R., Hall, R. O., Baker, M. A., Rosi-Marshall, E. J., and Tank, J. L. (2014). Modeling priming effects on microbial consumption of dissolved organic carbon in rivers. J. Geophys. Res. 119, 982–995. doi: 10.1002/2013JG002599
Hutchins, R. H. S., Aukes, P., Schiff, S. L., Dittmar, T., Prairie, Y. T., and del Giorgio, P. A. (2017). The optical, chemical, and molecular dissolved organic matter succession along a boreal soil-stream-river continuum. J. Geophys. Res. 122, 1–17. doi: 10.1002/2017JG004094
Jaffé, R., Ding, Y., Niggemann, J., Vähätalo, A. V., Stubbins, A., Spencer, R. G. M., et al. (2013). Global charcoal mobilization from soils via dissolution and riverine transport to the oceans. Science 340, 345–347. doi: 10.1126/science.1231476
Jenkinson, D. S., Fox, R. H., and Rayner, J. H. (1985). Interactions between fertilizer nitrogen and soil nitrogen—the so-called ‘priming’ effect. Soil Sci. 36, 425–444. doi: 10.1111/j.1365-2389.1985.tb00348.x
Jobbagy, E. G., and Jackson, R. B. (2000). The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. App. 10, 423–436. doi: 10.1890/1051-0761(2000)010
Jonasson, S., and Shaver, G. R. (1999). Within-stand nutrient cycling in arctic and boreal wetlands. Ecology 80, 2139–2150. doi: 10.1890/0012-9658(1999)080
Jorgenson, M. T., Harden, J., Kanevskiy, M., O’Donnell, J., Wickland, K., Ewing, S., et al. (2013). Reorganization of vegetation, hydrology and soil carbon after permafrost degradation across heterogeneous boreal landscapes. Environ. Res. Lett. 8:35017.
Kalbitz, K., Solinger, S., Park, J. H., Michalzik, B., and Matzner, E. (2000). Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci. 165, 277–304. doi: 10.1097/00010694-200004000-00001
Kawahigashi, M., Kaiser, K., Kalbitz, K., Rodionov, A., and Guggenberger, G. (2004). Dissolved organic matter in small streams along a gradient from discontinuous to continuous permafrost. Glob. Change Biol. 10, 1576–1586. doi: 10.1111/j.1365-2486.2004.00827.x
Kellerman, A. M., Guillemette, F., Podgorski, D. C., Aiken, G. R., Butler, K. D., and Spencer, R. G. M. (2018). Unifying concepts linking dissolved organic matter composition to persistence in aquatic ecosystems. Environ. Sci. Technol. 52, 2538–2548. doi: 10.1021/acs.est.7b05513
Kellerman, A. M., Kothawala, D. N., Dittmar, T., and Tranvik, L. J. (2015). Persistence of dissolved organic matter in lakes related to its molecular characteristics. Nat. Geosci. 8, 454–457. doi: 10.1038/ngeo2440
Keuper, F., van Bodegom, P. M., Dorrepaal, E., Weedon, J. T., van Hal, J., van Logtestijn, R. P., et al. (2012). A frozen feast: thawing permafrost increases plant-available nitrogen in subarctic peatlands. Glob. Change Biol. 18, 1998–2007. doi: 10.1111/j.1365-2486.2012.02663.x
Kim, S., Kaplan, L. A., and Hatcher, P. G. (2006). Biodegradable dissolved organic matter in a temperate and a tropical stream determined from ultra-high resolution mass spectrometry. Limnol. Oceanogr. 51, 1054–1063. doi: 10.4319/lo.2006.51.2.1054
Koch, B. P., and Dittmar, T. (2006). From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Comm. Mass Spectrom. 20, 926–932. doi: 10.1002/rcm.2386
Koch, B. P., and Dittmar, T. (2016). Erratum: From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 30:250. doi: 10.1002/rcm.7433
Koch, B. P., Kattner, G., Witt, M., and Passow, U. (2014). Molecular insights into the microbial formation of marine dissolved organic matter: recalcitrant or labile? Biogeosciences 11, 4173–4190. doi: 10.5194/bg-11-4173-2014
Koehler, B., von Wachenfeldt, E., Kothawala, D., and Tranvik, L. J. (2012). Reactivity continuum of dissolved organic carbon decomposition in lake water. J. Geophys. Res. 117:G01024. doi: 10.1029/2011JG001793
Kuzyakov, Y. (2010). Priming effects: interactions between living and dead organic matter. Soil Biol. Biochem. 42, 1363–1371. doi: 10.1016/j.soilbio.2010.04.003
Lu, X., and Zhuang, Q. (2011). Areal changes of land ecosystems in the alaskan areal changes of land ecosystems in the alaskan yukon river basin from 1984 to 2008. Environ. Res. Lett. 6:34012. doi: 10.1088/1748-9326/6/3/034012
Mann, P. J., Davydova, A., Zimov, N., Spencer, R. G. M., Davydov, S., Bulygina, E., et al. (2012). Controls on the composition and lability of dissolved organic matter in siberia’s kolyma river basin. J. Geophys. Res. 117, 1–15. doi: 10.1029/2011JG001798
Mann, P. J., Eglinton, T. I., Mcintyre, C. P., Zimov, N., Davydova, A., Vonk, J. E., et al. (2015). Utilization of ancient permafrost carbon in headwaters of arctic fluvial networks. Nat. Commun. 6, 1–7. doi: 10.1038/ncomms8856
Mann, P. J., Sobczak, W. V., and Larue, M. M. (2014). Evidence for key enzymatic controls on metabolism of arctic river organic matter. Glob. Change Biol. 20, 1089–1100. doi: 10.1111/gcb.12416
McClelland, J. W., Stieglitz, M., Pan, F., Holmes, R. M., and Peterson, B. J. (2007). Recent changes in nitrate and dissolved organic carbon export from the upper kuparuk river, north slope, alaska. J. Geophys. Res. 112, 1–13. doi: 10.1029/2006JG000371
Moore, T. R., and Dalva, M. (2001). Some controls on the release of dissolved organic carbon by plant tissues and soils. Soil Sci. 166, 38–47. doi: 10.1097/00010694-200101000-00007
Mostovaya, A., Hawkes, J. A., Koehler, B., Dittmar, T., and Tranvik, L. J. (2017). Emergence of the reactivity continuum of organic matter from kinetics of a multitude of individual molecular constituents. Environ. Sci. Technol. 51, 11571–11579. doi: 10.1021/acs.est.7b02876
Nebbioso, A., and Piccolo, A. (2013). Molecular characterization of dissolved organic matter (DOM): a critical review. Anal. Bioanal. Chem. 405, 109–124. doi: 10.1007/s00216-012-6363-2
Neff, J. C., and Hooper, D. U. (2002). Vegetation and climate controls on potential CO2, DOC and DON production in northern latitude soils. Glob. Change Biol. 8, 872–884. doi: 10.1046/j.1365-2486.2002.00517.x
O’Donnell, J. A., Aiken, G. A., Butler, K. D., Guillemette, F., Podgorski, D. C., and Spencer, R. G. M. (2016). DOM composition and transformation in boreal forest soils: the effects of temperature and organic-horizon decomposition state. J. Geophys. Res. 121, 2727–2744. doi: 10.1002/2016JG003431
O’Donnell, J. A., Harden, J. W., Mcguire, A. D., Kanevskiy, M. Z., Jorgenson, M. T., and Xu, X. (2011). The effect of fire and permafrost interactions on soil carbon accumulation in an upland black spruce ecosystem of interior alaska: implications for post-thaw carbon loss. Glob. Change Biol. 17, 1461–1474. doi: 10.1111/j.1365-2486.2010.02358.x
Osterkamp, T. E. (2007). Characteristics of the recent warming of permafrost in alaska. J. Geophys. Res. 112:F02S02. doi: 10.1029/2006JF000578
Qualls, R. G., and Haines, B. L. (1992). Biodegradability of dissolved organic matter in forest throughfall, soil solution, and stream water. Soil Sci. Soc. Am. 56, 578–586.
Raymond, P. A., Mcclelland, J. W., Holmes, R. M., Zhulidov, A. V., Mull, K., Peterson, B. J., et al. (2007). Flux and age of dissolved organic carbon exported to the arctic ocean: a carbon isotopic study of the five largest arctic rivers. Glob. Biogeochem. Cycles 21:GB4011. doi: 10.1029/2007GB002934
Schaefer, K., Zhang, T., Bruhwiler, L., and Barrett, A. P. (2011). Amount and timing of permafrost carbon release in response to climate warming. Tellus Ser. B Chem. Phys. Meteorol. 63B, 165–180. doi: 10.1111/j.1600-0889.2011.00527.x
Schuur, E. A. G., Bockheim, J., Canadell, J. G., Euskirchen, E., Field, C. B., Goryachkin, S. V., et al. (2008). Vulnerability of permafrost carbon to climate change implications for the global carbon cycle. Bioscience 58, 701–714.
Schuur, E. A. G., Crummer, K. G., Vogel, J. G., and Mack, M. C. (2007). Plant species composition and productivity following permafrost thaw and thermokarst in alaskan tundra. Ecosystems 10, 280–292. doi: 10.1007/s10021-007-9024-0
Schuur, E. A. G., Vogel, J. G., Crummer, K. G., Lee, H., Sickman, J. O., and Osterkamp, T. E. (2009). The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature 459, 556–559. doi: 10.1038/nature08031
Settele, J., Scholes, R., Betts, R., Bunn, S., Leadley, P., Nepstad, D., et al. (2014). “Terrestrial and inland water systems,” in Climate Change 2014: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, and T. E. Bilir (New York, NY: Cambridge University Press), 271–359.
Sleighter, R. L., and Hatcher, P. G. (2007). The application of electrospray ionization coupled to ultrahigh resolution mass spectrometry for the molecular characterization of natural organic matter. J. Mass Spectrometr. 42, 559–574. doi: 10.1002/jms.1221
Smith, D. F., Podgorski, D. C., Rodgers, R. P., Blakney, G. T., and Hendrickson, C. L. (2018). 21 Tesla FT-ICR mass spectrometer for ultrahigh-resolution analysis of complex organic mixtures. Anal. Chem. 90, 2041–2047. doi: 10.1021/acs.analchem.7b04159
Smith, E. M., and Prairie, Y. T. (2004). Bacterial metabolism and growth efficiency in lakes: the importance of phosphorus availability. Limnol. Oceanogr. 49, 137–147. doi: 10.4319/lo.2004.49.1.0137
Spencer, R. G. M., Aiken, G. R., Butler, K. D., Dornblaser, M. M., Striegl, R. G., and Hernes, P. J. (2009). Utilizing chromophoric dissolved organic matter measurements to derive export and reactivity of dissolved organic carbon exported to the arctic ocean: a case study of the yukon river. Alaska. Geophys. Res. Lett. 36:L06401. doi: 10.1029/2008GL036831
Spencer, R. G. M., Aiken, G. R., Wickland, K. P., Striegl, R. G., and Hernes, P. J. (2008). Seasonal and spatial variability in dissolved organic matter quantity and composition from the yukon river basin. alaska. Glob. Biogeochem. Cycles 22, 1–13. doi: 10.1029/2008GB003231
Spencer, R. G. M., Mann, P. J., Dittmar, T., Eglinton, T. I., Mcintyre, C., Holmes, R. M., et al. (2015). Detecting the signature of permafrost thaw in arctic rivers. Geophys. Res. Lett. 42, 2830–2835. doi: 10.1002/2015GL063498
Steen, A. D., Quigley, L. N. M., and Buchan, A. (2016). Evidence for the priming effect in a planktonic estuarine microbial community. Front. Mar. Sci. 3:1–9. doi: 10.3389/fmars.2016.00006
Striegl, R. G., Raymond, P. A., and Dornblaser, M. (2005). A decrease in discharge-normalized DOC export by the yukon river during summer through autumn. Geophys. Res. Lett. 32:L21413. doi: 10.1029/2005GL024413
Stubbins, A., Mann, P. J., Powers, L., Bittar, T. B., Dittmar, T., McIntry, C. P., et al. (2017). Low photolability of yedoma permafrost dissolved organic carbon. J. Geophys. Res. 122, 200–211. doi: 10.1002/2016JG003688
Sun, L., Perdue, E. M., Meyer, J. L., and Weis, J. (1997). Use of elemental composition to predict bioavailability of dissolved organic matter in a Georgia river. Limnol. Oceanogr. 42, 714–721. doi: 10.4319/lo.1997.42.4.0714
Tarnocai, C., Canadell, J. G., Schuur, E. A. G., Kuhry, P., Mazhitova, G., and Zimov, S. (2009). Soil organic carbon pools in the northern circumpolar permafrost region. Global 23:GB2023.
Textor, S. R., Guillemette, F., Zito, P. A., and Spencer, R. G. M. (2018). An assessment of dissolved organic carbon biodegradability and priming in blackwater systems. J. Geophys. Res. 123, 2998–3015. doi: 10.1029/2018JG004470
Thurman, E. M., Aiken, G. R., Ewald, M., Ficher, W. R., Förstener, U., Hack, A. H., et al. (1985). Isolation of Soil and Aquatic Humic Substances. Hoboken, NJ: John Wiley and Sons.
Trumbore, S. E., and Harden, J. W. (1997). Accumulation and turnover of carbon in organic and mineral soils of the BOREAS northern study area. J. Geophys. Res. 102, 817–830.
Vachon, D., Prairie, Y. T., Guillemette, F., and del Giorgio, P. A. (2017). Modeling allochthonous dissolved organic carbon mineralization under variable hydrologic regimes in boreal lakes. Ecosystems 20, 781–795. doi: 10.1007/s10021-016-0057-0
Vonk, J. E., and Gustafsson, O. (2013). Permafrost-carbon complexities. Nat. Geosci. 6, 675–676. doi: 10.1038/ngeo1937
Vonk, J. E., Tank, S. E., Mann, P. J., Spencer, R. G. M., Treat, C. C., Striegl, R. G., et al. (2015). Biodegradability of dissolved organic carbon in permafrost soils and aquatic systems: a meta-analysis. Biogeosciences 12, 6915–6930. doi: 10.5194/bg-12-6915-2015
Walvoord, M. A., Voss, C. I., and Wellman, T. P. (2012). Influence of permafrost distribution on groundwater flow in the context of climate-driven permafrost thaw: example from yukon flats basin, alaska, united states. Water Res. Res. 48:W07524. doi: 10.1029/2011WR011595
Ward, C. P., and Cory, R. M. (2015). Chemical composition of dissolved organic matter draining permafrost soils. Geochimica Cosmochimica Acta 167, 63–79. doi: 10.1016/j.gca.2015.07.001
Ward, N. D., Bianchi, T. S., Medeiros, P. M., Seidel, M., Richey, J. E., Keil, R. G., et al. (2017). Where carbon goes when water flows: carbon cycling across the aquatic continuum. Front. Mar. Sci. 4:7. doi: 10.3389/fmars.2017.00007
Ward, N. D., Bianchi, T. S., Sawakuchi, H. O., Gagne-Maynard, W., Cunha, A. C., Brito, D. C., et al. (2016). The reactivity of plant-derived organic matter and the potential importance of priming effects along the lower Amazon River. J. Geophys. Res. 121, 1522–1539. doi: 10.1002/2016JG003342
Wickland, K. P., Aiken, G. R., Butler, K., Dornblaser, M. M., Spencer, R. G. M., and Striegl, R. G. (2012). Biodegradability of dissolved organic carbon in the yukon river and its tributaries: seasonality and importance of inorganic nitrogen. Glob. Biogeochem. Cycles 26, 1–14. doi: 10.1029/2012GB004342
Wickland, K. P., and Neff, J. C. (2008). Decomposition of soil organic matter from boreal black spruce forest: environmental and chemical controls. Biogeochemistry 87, 29–47. doi: 10.1007/s10533-007-9166-3
Wickland, K. P., Neff, J. C., and Aiken, G. R. (2007). Dissolved organic carbon in alaskan boreal forest: sources, chemical characteristics, and biodegradability. Ecosystems 10, 1323–1340. doi: 10.1007/s10021-007-9101-4
Wickland, K. P., Waldrop, M. P., Aiken, G. R., Koch, J. C., Jorgenson, M. T., and Striegl, R. G. (2018). Dissolved organic carbon and nitrogen release from boreal holocene permafrost and seasonally frozen soils of Alaska. Environ. Res. Lett. 13:065011. doi: 10.1088/1748-9326/aac4ad
Wrona, F. J., Prowse, T. D., Reist, J. D., Hobbie, J. E., Lévesque, L. M. J., Vincent, W. F., et al. (2006). Climate change effects on aquatic biota, ecosystem structure and function. AMBIO 35, 359–369. doi: 10.1579/0044-7447(2006)35
Zark, M., and Dittmar, T. (2018). Universal molecular structures in natural dissolved organic matter. Nat. Commun. 9:3178. doi: 10.1038/s41467-018-05665-9
Zhang, X., Hutchings, J. A., Bianchi, T. S., Liu, Y., Arellano, A. R., and Schuur, E. A. G. (2017). Importance of lateral flux and its percolation depth on organic carbon export in arctic tundra soil: implications from a soil leaching experiment. J. Geophys. Res. 122, 796–810. doi: 10.1002/2016JG003754
Zhang, Y. (2013). Spatio-temporal features of permafrost thaw projected from long-term high-resolution modeling for a region in the hudson bay lowlands in canada. J. Geophys. Res. 118, 542–552. doi: 10.1002/jgrf.20045
Keywords: dissolved organic matter, dissolved organic carbon, biodegradation, permafrost, leachates, priming
Citation: Textor SR, Wickland KP, Podgorski DC, Johnston SE and Spencer RGM (2019) Dissolved Organic Carbon Turnover in Permafrost-Influenced Watersheds of Interior Alaska: Molecular Insights and the Priming Effect. Front. Earth Sci. 7:275. doi: 10.3389/feart.2019.00275
Received: 26 January 2019; Accepted: 07 October 2019;
Published: 24 October 2019.
Edited by:
Thomas S. Bianchi, University of Florida, United StatesReviewed by:
Jutta Niggemann, University of Oldenburg, GermanyBernhard Wehrli, ETH Zürich, Switzerland
Copyright © 2019 Textor, Wickland, Podgorski, Johnston and Spencer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sadie R. Textor, c3QxM2JAbXkuZnN1LmVkdQ==; Robert G. M. Spencer, cmdzcGVuY2VyQGZzdS5lZHU=
†Presentaddress: David C. Podgorski, Department of Chemistry, Pontchartrain Institute for Environmental Sciences, University of New Orleans, New Orleans, LA, United States
 Sadie R. Textor
Sadie R. Textor Kimberly P. Wickland
Kimberly P. Wickland David C. Podgorski
David C. Podgorski Sarah Ellen Johnston1
Sarah Ellen Johnston1 Robert G. M. Spencer
Robert G. M. Spencer