- 1Department of Earth Sciences, University of Oxford, Oxford, UK
- 2Department of Earth Sciences, Natural History Museum, London, UK
- 3Image and Analysis Centre, Natural History Museum, London, UK
As the closest living relatives of tetrapods, lungfishes are frequently used as extant models for exploring the fin-to-limb transition. These studies have generally given little consideration to fossil taxa. This is because even though lungfish fins are relatively common in the fossil record, the internal structure of these fins is virtually unknown. Information on pectoral-fin endoskeletons in fossil representatives of Dipnomorpha (the lungfish total group) is limited to effectively poorly preserved remains in the lungfishes Dipterus and Conchopoma and more complete material in the porolepiform Glyptolepis. Here we describe a well-preserved pectoral-fin endoskeleton in the Middle Devonian (Givetian) lungfish Pentlandia macroptera from the John o'Groats fish bed, Caithness, northeastern Scotland. The skeleton is in association with a cleithrum and clavicle, and consists of a series of at least eight mesomeres. Extensive series of preaxial and postaxial radials are present. Some of the radials are jointed, but none branch. No mesomere articulates with multiple radials on either its pre- or post-axial face. The first two mesomeres, corresponding to the humerus and ulna, bear well-developed axial processes. Uniquely among dipnomorphs, a distinct ossification center corresponding to the radius is present in Pentlandia. A review of anatomy and development of the pectoral-fin endoskeleton in the living Neoceratodus is presented based on cleared and stained material representing different size stages. These developmental data, in conjunction with new details of primitive lungfish conditions based on Pentlandia, highlight many of the derived features of the pectoral-fin skeleton of Neoceratodus, and clarify patterns of appendage evolution within dipnomorphs more generally.
Introduction
Lungfishes occupy a key phylogenetic position as the living sister group of terrestrial vertebrates (Cloutier and Ahlberg, 1996; Broughton et al., 2013; Liang et al., 2013), and provide important comparative data bearing on our understanding of the fish-tetrapod transition (Coates et al., 2008; Clack, 2012). The paired fins of modern lungfishes have attracted particular attention as models for understanding the origin of tetrapod limbs from both an evolutionary and developmental perspective (Holmgren, 1933; Shubin and Alberch, 1986; Joss and Longhurst, 2001; Johanson et al., 2004, 2007; Cole et al., 2011; Boisvert et al., 2013; Pierce et al., 2013). These comparisons rest almost exclusively upon Neoceratodus forsteri (the Queensland lungfish), although the reduced, whip-like paired fins of Protopterus (African lungfishes, comparable to the South American lungfish Lepidosiren paradoxa) have recently been studied to comment on the evolution of the tetrapod gait (King et al., 2011).
However, comparative analyses suggest that the paired fins of all modern lungfishes, including Neoceratodus, are highly specialized (Ahlberg, 1989; Coates et al., 2002; Friedman et al., 2007). Lungfishes diverged from tetrapods no later than the Early Devonian (Lochkovian, ca. 415 Ma) (Jessen, 1980; Chang and Yu, 1984), but crown-group lungfishes are relatively modern, only emerging around the Permo-Triassic boundary (ca. 252 Ma) (Cavin et al., 2007). This considerable offset between the age of crown and total groups means that information on the first third of lungfish paired fin evolutionary history can only be sought in the fossil record. Patterns of change apparent in median fin geometries of Paleozoic lungfishes represent an often reproduced paleontological “transformation series” (Dollo, 1895; Ahlberg and Trewin, 1995; Friedman, 2010), but lungfish paired fins from the same interval have not received the same level of attention.
Where preserved, pectoral and pelvic fins of Paleozoic lungfishes and their closest relatives are represented by scales and fin-rays (Figure 1) and provide only circumstantial evidence for the geometry of the underlying endoskeleton (e.g., Cloutier, 1996; Clement, 2004; Long and Clement, 2009). Paired-fin endoskeletons are known in only a handful of stem lungfishes, with the most detailed accounts based on incomplete remains in the Middle Devonian (Eifelian-Givetian; Dineley and Metcalf, 1999; Blom et al., 2007) porolepiform Glyptolepis (G. groenlandica: Jarvik, 1980: Figure 200c; Ahlberg, 1989: Figures 4, 6–7, 10; G. leptopterus: Ahlberg, 1989: Figure 8; G. ?leptopterus: Ahlberg, 1989: Figures 5, 9, 11; Figure 2). The Middle Devonian Dipterus and Permo-Carboniferous Conchopoma are the only fossil lungfishes (in the apomorphy-based sense) in which the pectoral-fin endoskeleton has been described in any detail (Schultze, 1975; this study also includes an account of the pelvic fin of Conchopoma). Ahlberg and Trewin (1995: Figures 1,2) figured and described a poorly-preserved fin skeleton in a specimen of Dipterus from the Middle Devonian (Eifelian) Achanarras horizon of Caithness, Scotland (Figure (3A). Although radials and mesomeres are apparent in this specimen, overlapping relationships between many of the poorly mineralized cartilages preclude a clear structural account. Conchopoma fins preserve only pre- and post-axial radials, with no trace of associated mesomeres (Schultze, 1975: Figures 8, 9, 11, pl. 1.2, Figures 3F,G). In his review of paired-fin endoskeletons in sarcopterygians, Ahlberg (1989) described—but did not figure—remains in two Devonian taxa: Chirodipterus (pectoral and pelvic) and Fleurantia (pelvic only; noted as Scaumenacia, but subsequently identified as Fleurantia by Cloutier, 1996). Excluding the supporting girdles, the pectoral and pelvic fin endoskeletons in Chirodipterus are represented only by a few mesomeres. The pelvic fin endoskeleton of Fleurantia is more intact, with a chain of at least seven mesomeres and faint traces of spindle-shaped pre- and post-axial radials. We include photographs of these specimens of Chirodipterus and Fleurantia for completeness (Figures 3C–E).

Figure 1. Articulated specimens of Dipterus valenciennesi (Middle Devonian, Scotland), showing external details of pectoral fins. (A) NHMUK PV P22189; (B) NHMUK PV P17638; (C) NHMUK PV P22187. All lateral view, white arrows indicate pectoral fins.
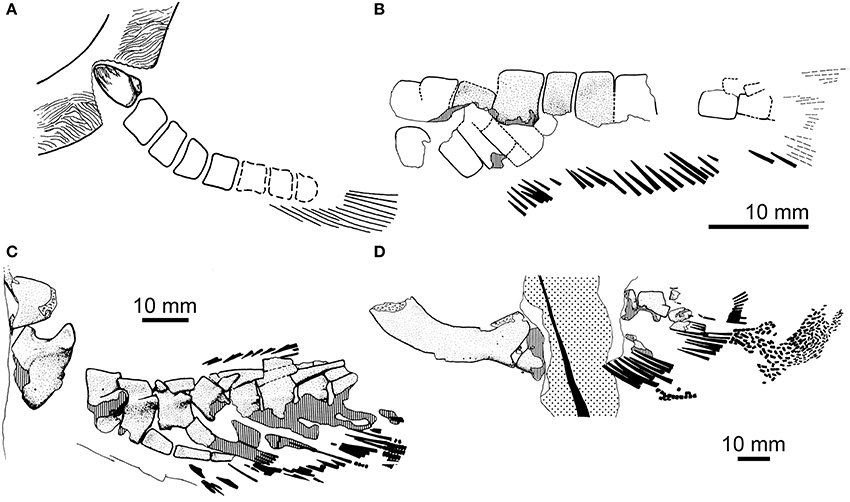
Figure 2. Paired-fin endoskeletons of fossil dipnomorphs: porolepiforms. (A) Shoulder girdle and pectoral-fin endoskeleton of Glyptolepis groenlandica from the Middle Devonian (Eifelian) of Greenland (modified from Jarvik, 1980; no scale indicated. Reproduced from Basic structure and evolution of vertebrates volume 1 by permission of Elsevier Press); (B) Pelvic-fin endoskeleton of Glyptolepis groenlandica (modified from Ahlberg, 1989). (C) Pectoral-fin endoskeleton with associated endoskeletal girdle of Glyptolepis ?leptopterus from the Middle Devonian (Eifelian) of Scotland (modified from Ahlberg, 1989); (D) Pelvic-fin endoskeleton with associated endoskeletal girdle of Glyptolepis ?leptopterus (same individual as in C; modified from Ahlberg, 1989. Reproduced by permission of John Wiley and Sons). Anterior is to the left in all panels.
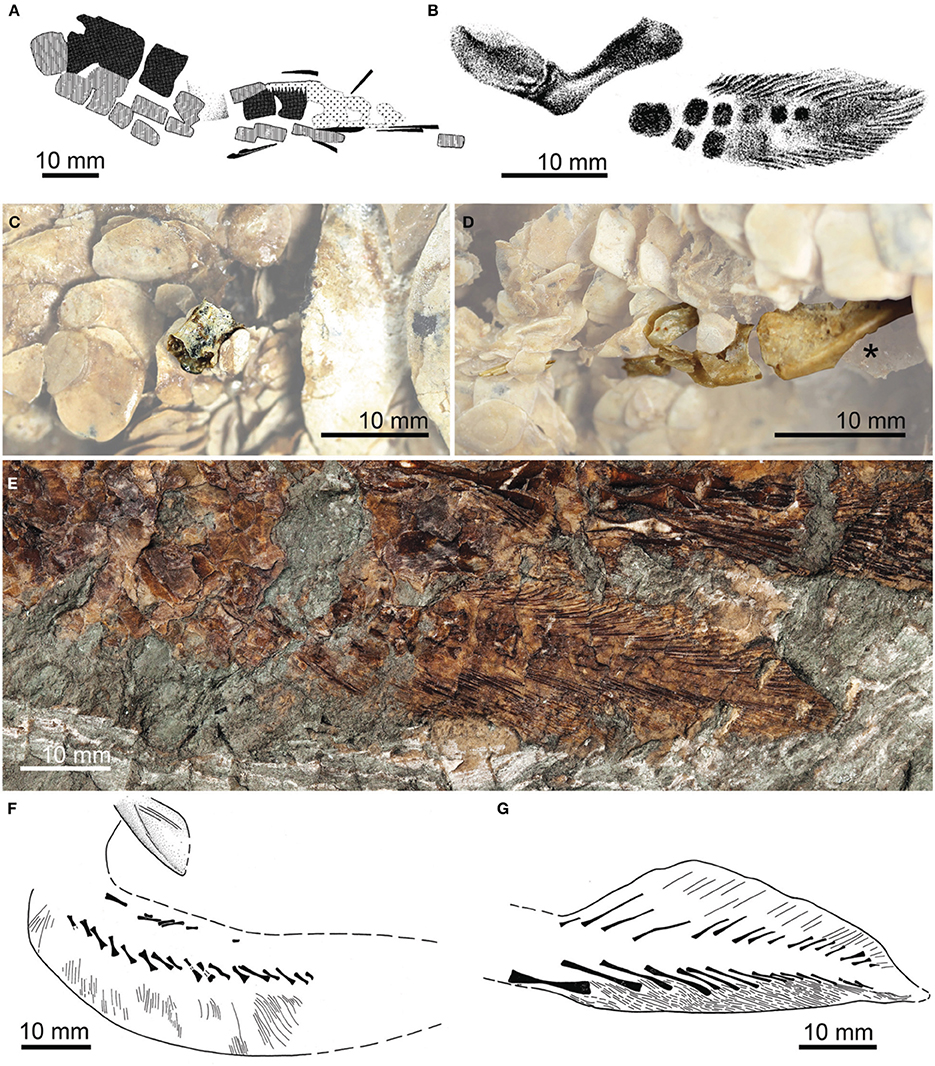
Figure 3. Paired-fin endoskeletons of fossil dipnomorphs: lungfishes. (A) Pectoral-fin endoskeleton of Dipterus sp. from the Middle Devonian (Eifelian) of Scotland [modified from Ahlberg and Trewin, 1995. Reproduced by permission of The Royal Society of Edinburgh and the authors from Transactions of the Royal Society of Edinburgh: Earth Sciences volume 85 (1995, for 1994), pp. 159–175]. (B) Pentlandia macroptera from the Middle Devonian (Givetian) of Scotland (modified from Traquair, 1889. Reproduced by permission of Cambridge University Press). (C) Pectoral-fin mesomere of Chirodipterus australis NHMUK PV P.52586 from the Late Devonian of Gogo, Western Australia. (D) Endoskeletal pelvic girdle (marked with asterisk; “*”), associated mesomeres, and lepidotrichia of Chirodipterus ?australis NHMUK PV P.62102 from the Late Devonian of Gogo, Western Australia. (E) Pelvic-fin endoskeleton of Fleurantia denticulata NHMUK PV P.60487 from the Late Devonian (Frasnian) of Miguasha, Québec, Canada, showing mesomeres and pre- and post-axial radials. (F) Cleithrum and pectoral-fin endoskeleton of Conchopoma gadiforme from the early Permian of Germany (modified from Schultze, 1975; reversed relative to original). (G) Pelvic-fin endoskeleton of Conchopoma gadiforme from the early Permian of Germany [modified from Schultze, 1975. Reproduced by permission of Senkenberg Gesellschaft für Naturforschung from: schultze, H.-P., Die Lungenfisch-Gattung Conchopoma (Pisces, Dipnoi), Senckenbergiana lethaea volume 56 (1975), pp. 191–231].
The uniquely complete pectoral-fin endoskeletons of Dipterus and Conchopoma are joined in the literature by another example that is arguably better preserved but has not been subject to further study since its original description over a century ago. In his review of fossils then attributed to Dipterus, Traquair (1888) noted that material from the John o'Groats fish bed was distinct from the type species Dipterus valenciennesi found at other Scottish sites like Lethen Bar, Cromarty, and Tynet Burn. Traquair (1888) placed specimens from John o'Groats in the new species Dipterus macroptera in an initial note that was followed by a more complete description the following year (Traquair, 1889). This account included anatomical illustrations based on several referred specimens. Traquair (1889) considered all of these materials conspecific, but did not identify a holotype. Woodward (1891: 240), in his capacity as first reviser, designated the articulated individual depicted by Traquair (1889: Figure 1) as the holotype of D. macroptera. Watson and Day (1916) subsequently recognized this taxon as being generically distinct from Dipterus, and erected the new genus Pentlandia to accommodate it.
In addition to figuring an essentially complete specimen of Pentlandia, Traquair (1889: Figure 4) also illustrated an isolated dermal shoulder girdle associated with a pectoral fin (National Museums of Scotland [NMS] specimen 1875.29.45). Traquair did not elaborate on the anatomy of the pectoral fin beyond noting that it was “‘archipterygian’ in configuration” (1889, p. 99), but his figure clearly shows components of the internal skeleton (Figure 3B). Although preservation of this specimen is clearly exceptional, Traquair's sketch is too small and diagrammatic to permit reliable interpretations of structure. In light of the rarity of fossil lungfish pectoral-fin endoskeletons, we have re-examined this important specimen of Pentlandia with a variety of imaging tools. By providing a detailed account of structure in Pentlandia, we look to establish primitive conditions for lungfish pectoral fin endoskeletons and identify the specializations of modern species.
Geological Context
Pentlandia macroptera is known exclusively from the John o'Groats fish bed of the Last House Formation of the upper part of the John o'Groats Sandstone Group of the Orcadian Basin of northeast Scotland. The John o'Groats Sandstone consists mainly of channel-containing, cross-bedded yellow and buff sandstones filling the Orcadian Basin. Sequences in the John o'Groats Sandstone represent an alluvial fan periodically transgressed—as in the case of the fish bed—by lacustrine facies (Dineley and Metcalf, 1999). The fish bed is the lowest of three thin (0.25 m) varved carbonate laminites within the formation and the only one containing fish.
Evidence for the age of the John o'Groats fish bed is biostratigraphic. On the basis of fishes, Donovan et al. (1974) placed this fossiliferous horizon within their faunal zone 7, which has been correlated with the Gauja regional stage of the Baltics (Dineley and Metcalf, 1999). Spores in the Eday Flagstone Formation, a lateral equivalent to the John o'Groats fish bed, permit correlation with standard conodont zonation. Marshall et al. (2011) interpret the John o'Groats fish bed as lying within the middle of the Polygnathus varcus Conodont Zone. This zone has an interpolated age of 387.30–384.99 Ma (Becker et al., 2012), providing a narrow age constraint for Pentlandia.
The fauna of the John o'Groats fish bed is species-poor in comparison to older horizons in the Old Red Sandstone like Achanarras (Trewin, 1986), and yields only five fish taxa in addition to Pentlandia: the antiarch placoderm Microbrachius dicki, the arthrodire placoderm Watsonosteus fletti, the tetrapodomorph sarcopterygian Tristichopterus alatus, and specifically indeterminate material assigned to Dipterus sp.
Materials and Methods
Imaging of Fossils and Modern Comparative Material
NMS 1875.29.45 is preserved as a flattened specimen in a brown-gray varved limestone. The specimen was photographed under polarized light (Figure 4A) and dusted with a sublimate of ammonium chloride (Figure 4B). Interpretive drawings of the specimen were made using these photographs in combination with a camera lucida (Figure 4C). NMS 1875.29.45 was also examined using the JEOL 5900LV variable pressure s.e.m. (Image and Analysis Centre, Natural History Museum) in energy dispersive X-ray (EDX) mapping and backscattered electron imaging (BEI) modes (Figure 5). EDX images were made for carbon, aluminum, silicon, phosphorus, and calcium. Of these, phosphorus and calcium abundance images showed the clearest differences between fossilized material (including some scales not seen with the naked eye) and the matrix (Figures 5D–F). BEI mapping provided monochrome images illustrating relative mineral mean atomic number (Figure 5G).
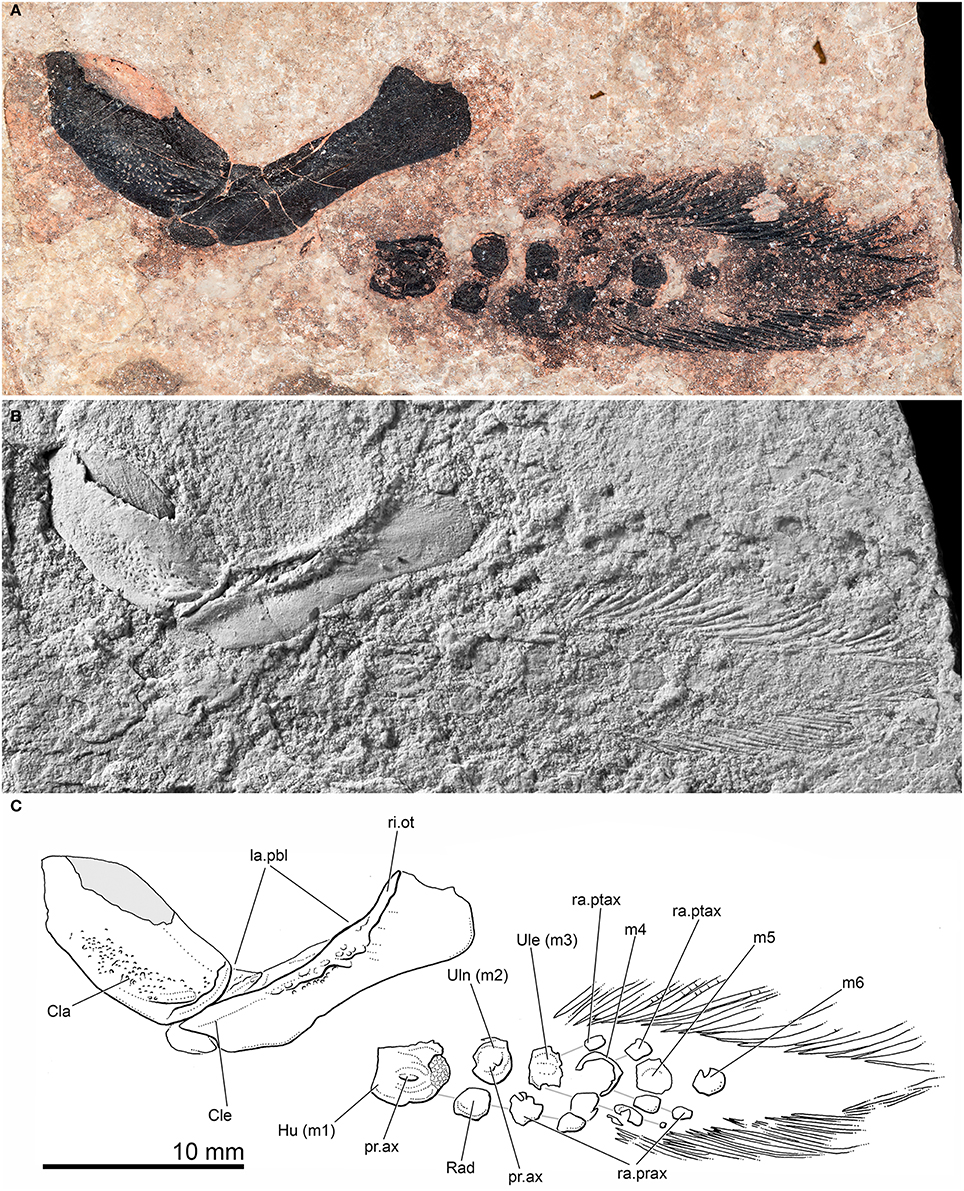
Figure 4. Pentlandia macroptera, NMS 1875.29.45, John o'Groats Sandstone, Middle Devonian (Givetian), Scotland, pectoral girdle and fin. Preaxial margin of fin directed toward bottom of figure. (A) Photograph of specimen taken in polarized light. (B) Photograph of specimen dusted with ammonium chloride. (C) Interpretive drawing of specimen. Solid gray infill represents area of clavicle preserved as impression. Individual radial ossifications belonging to the same series are shown linked by solid gray lines for clarity.
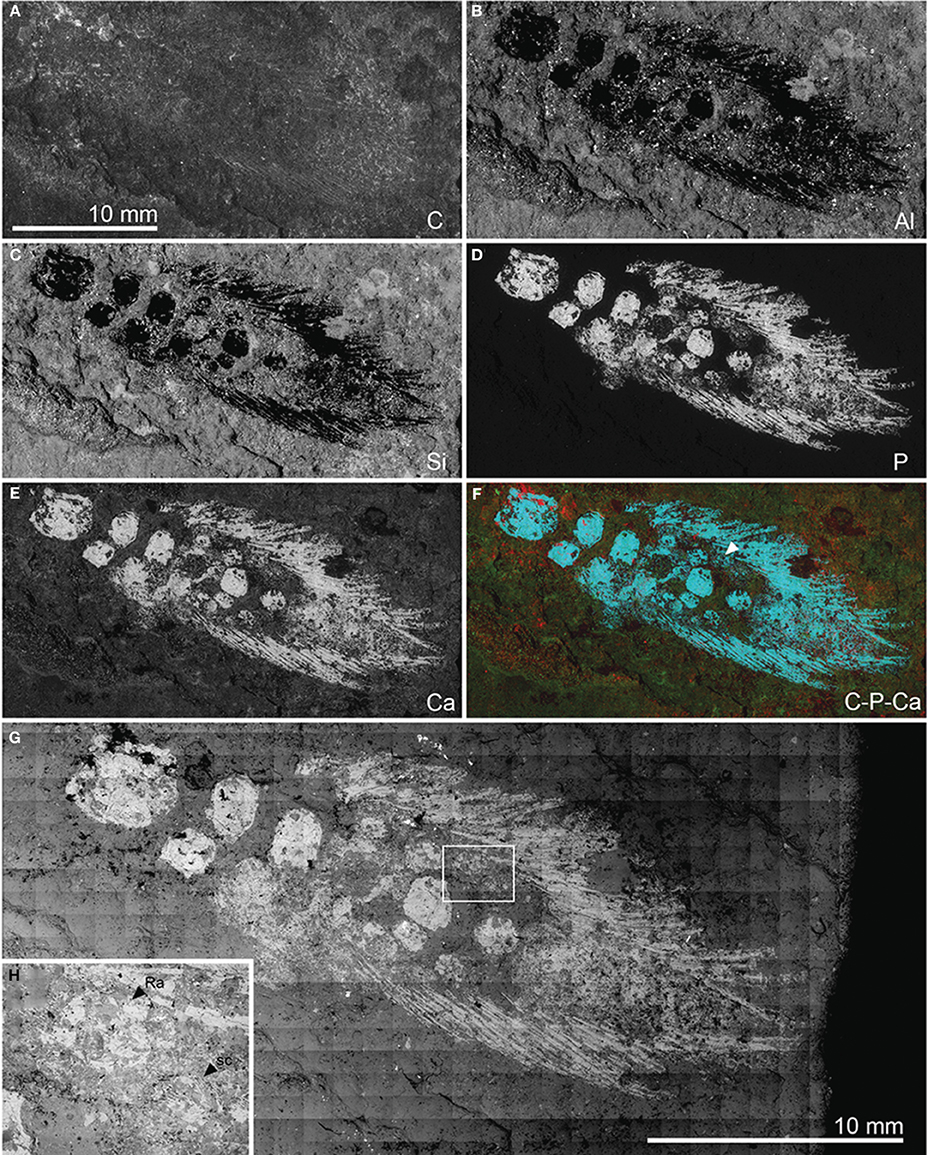
Figure 5. Pentlandia macroptera, NMS 1875.29.45, John o'Groats Sandstone, Middle Devonian (Givetian), Scotland, pectoral girdle and fin. Preaxial margin of fin directed toward bottom of figure. Energy dispersive X-ray (EDX) and backscattered electron (BEI) images. A-G, EDX mapping showing distribution of: (A) carbon; (B) aluminum; (C) silicon; (D) phosphorus; (E) calcium; (F) false-color image showing carbon (red), phosphorus (blue), and calcium (green). Arrowhead in F indicates traces of distal segment of the postaxial radial articulating with mesomere 4, and which is not visible in reflected light images (Figure 3). (G) BEI montage. (H) BEI close-up of region delimited by box in (G), and showing clear textural difference between radials (smooth perichondral bone) and scales (ornamented dermal bone). (B–F) Shown to same scale as (A).
Comparisons were made between fin structure in Pentlandia and that in the extant Neoceratodus forsteri. Pectoral fins and girdles were dissected from a cleared and stained juvenile specimen of Neoceratodus (Dingerkus and Uhler, 1977; Figure 6D, specimen unregistered) and photographed with a Leica MZ95 stereomicroscope. A fin skeleton from a larger individual of Neoceratodus (NHMUK IM499/E679) was also available for macrophotography (Figure 7).
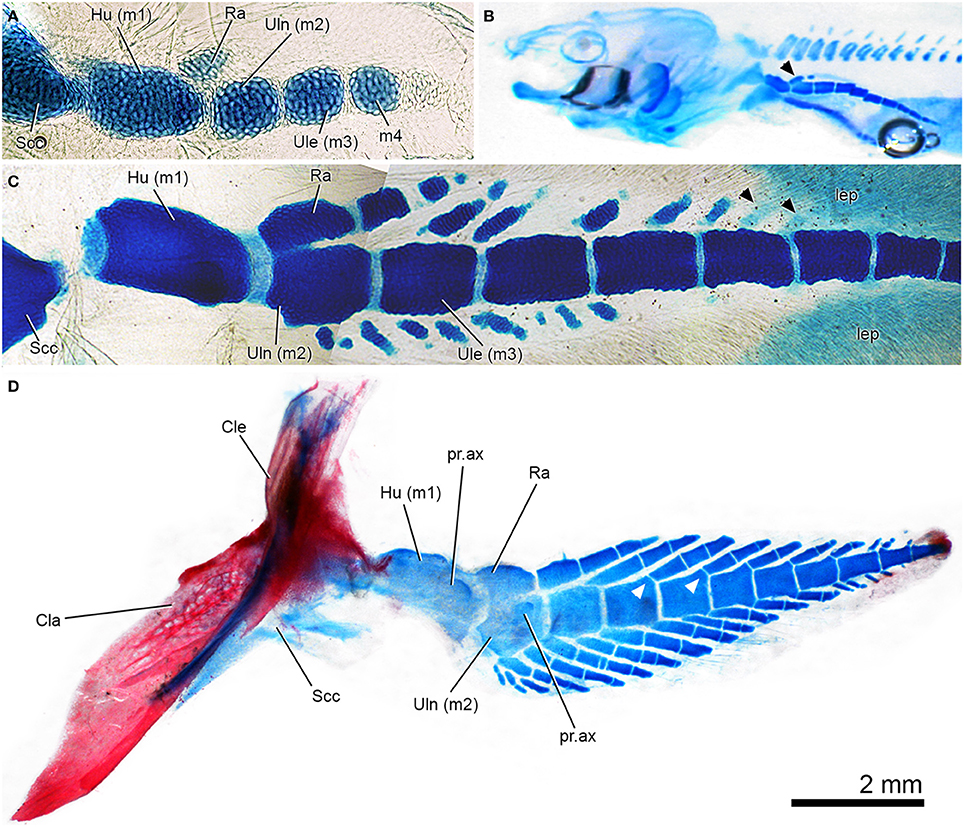
Figure 6. Patterns of pectoral-fin development in Neoceratodus forsteri, the extant Queensland lungfish. In all images, the preaxial margin of the fin faces the top of the figure. (A) Early ontogenetic stage of fin development showing mesomeres and radius anlage, which is separate from the ulna anlage. Specimen stained with Alcian blue (from Johanson et al., 2007: Figure 3A, used with permission from John Wiley and Sons). (B) Kemp stage 49 larval lungfish, Alcian blue staining of skeletal cartilages, including fin with series of mesomeres and radius now bearing a distal radial (black arrowhead). (C) Later stage larval lungfish, Alcian blue stained with axial mesomeres and developing fin radials preaxially and postaxially. Black arrowheads indicate radials developing distally, with more than one per mesomere (compare to Figures 6D, 7A). (D) Juvenile left pectoral fin, stained with Alcian and alizarin stained, shown in lateral view. Note fusion between ulna and radus, and one-to-one radial to mesomere relationship preaxially but variation in this pattern distally. White arrowheads indicate well-developed articular surfaces on mesomeres, which are not present in earlier stages.
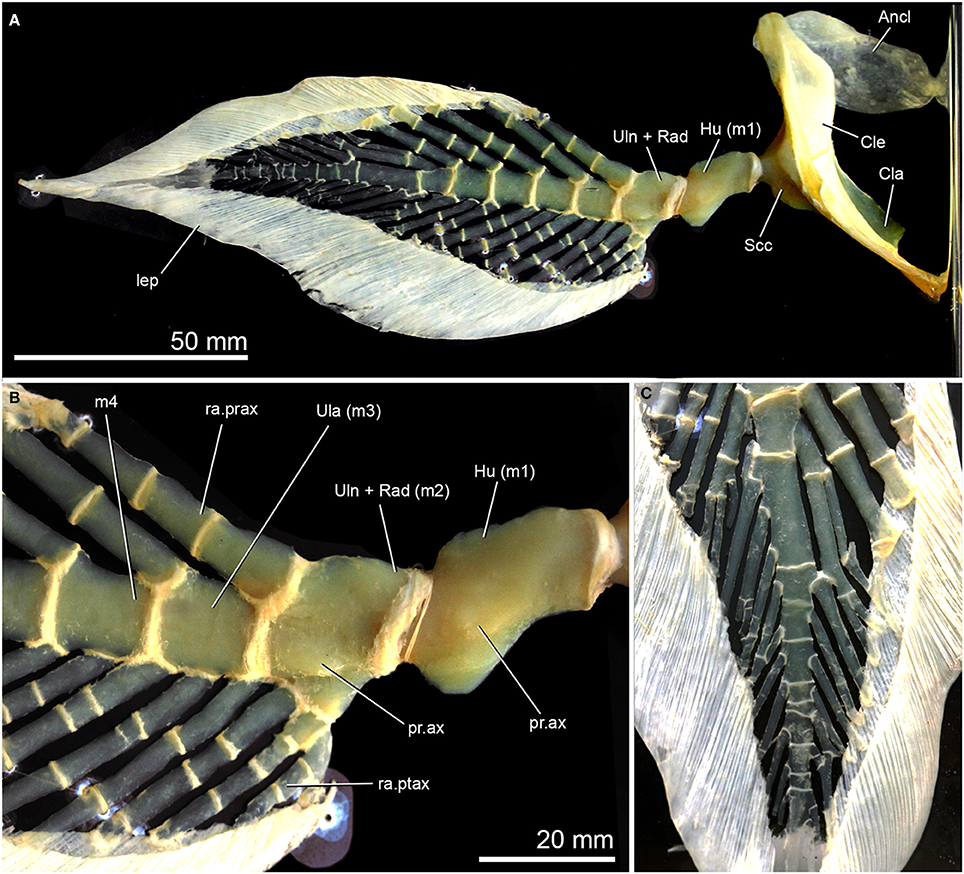
Figure 7. Pectoral-fin anatomy in adult Neoceratodus forsteri, NHMUK IM499/E679, skeletonised right pectoral fin and girdle shown in lateral view. (A) Complete fin; (B) Closeup of proximal region of pectoral-fin endoskeleton, highlighting axial processes on the humerus and fused ulna/radius; (C) Distal region of fin showing irregular branching and fusion of fin radials. Preaxial margin of the fin faces the top of the figure in (A,B), and left in (C).
Abbreviations
Institutional abbreviations: NHMUK, Zoological collections, Natural History Museum, London; NHMUK PV P, Palaeontological collections, Natural History Museum, London; NMS, National Museums of Scotland, Edinburgh. Anatomical abbreviations: Ancl, anocleithrum; Cla, clavicle; Cle, cleithrum; Hu, humerus; la.pbl, postbranchial lamina; lep, lepidotrichia; pr.ax, axial process of mesomere; ra.prax, preaxial radials; ra.ptax, postaxial radials; ri.ot, outturned ridge of the cleithrum; sc, scale; Scc, scapulocoracoid; Ule, ulnare; Uln, ulna; Uln + Rad, cartilage formed by fusion of radius and ulna. Mesomeres are indicated by “m” followed by a number indicating their position in a proximo-distal sequence.
Results
Description
Overview of NMS 1875.29.45
NMS 1875.29.45 consists of a left cleithrum and clavicle preserved in lateral (external) view and an associated pectoral fin with a series of endoskeletal components. The specimen is well preserved with most of the fin unobscured by matrix. EDX images indicate the presence of scale cover, particularly distally (Figure 5), obscuring internal fin structure, as is the case in most lungfish fossil specimens (Figure 1). The pectoral fin of NMS 1875.29.45 measures roughly 28 mm in length, and based on comparisons with more complete specimens we estimate of total length of the individual as approximately 150 mm. Pentlandia macroptera rarely exceeds sizes greater than approximately 170 mm in total length (pers. obs., EJ of material in NHM fossil fish collection), so we assume that NMS 1875.29.45 preserves the remains of an adult individual.
Shoulder girdle. We suggest that there has been only minor post-mortem disturbance to the pectoral girdle and fin, with loss of some scales from the latter (Figures 4, 5). The cleithrum is a splint-shaped bone with a broad, rounded dorsal margin. A thickened ridge extends along the anterior margin of the exposed portion of the bone, and represents the remains of the outturned ridge of the lateral lamina of the cleithrum that is characteristic of many Paleozoic lungfishes (e.g., Johanson, 2003; Campbell et al., 2006: Figure 6; Friedman, 2010: Figure 8). Small bony buttresses extend from the posterior margin of the outturned ridge. The remainder of the lateral lamina of the cleithrum is smooth, suggesting that it was deeply buried within soft tissue. The postbranchial lamina is largely exposed, and has a rough surface texture. The cleithrum of Pentlandia appears proportionally narrower than that in probable close relatives like Barwickia (Long and Clement, 2009: Figures 3a,b), Howidipterus (Long and Clement, 2009: Figures 3c,d), and Scaumenacia (Jarvik, 1980: Figure 335). The posterior margin of the cleithrum is gently concave. A shallow excavation along the anteroventral margin of the cleithrum accommodates the posterior margin of the clavicle. The clavicle is leaf-shaped. A broad, shallow trough extends along the ventral half of this bone. Its lateral surface bears a field of irregularly spaced pores. No scapulocoracoid is visible, suggesting it either was unmineralized or remains covered posteriorly by matrix. However, the wide gap between the dermal girdle and the articulated fin skeleton suggests that the scapulocoracoid might have been large and posteriorly extensive as in Dipterus (Campbell et al., 2006: Figure 3a).
Pectoral fin. The articulated pectoral fin in NMS 1875.29.45 is posterior to, and separated from, the pectoral girdle, indicating either a large scapulocoracoid, some taphonomic displacement, or a combination of the two. The outline of the pectoral fin itself is largely preserved, including lepidotrichia along the preaxial and postaxial margins, and is similar in shape to that of Dipterus (Figures 1, 3A) and Neoceratodus (Figures 6, 7). The preaxial margin is directed ventrally. The visible face of the pectoral-fin endoskeleton therefore corresponds to the surface designated as “dorsal” in many accounts of sarcopterygian appendicular anatomy (e.g., Ahlberg, 1989; Coates et al., 2008). Here we refer to this as the upper surface in order to avoid confusion with directional terminology applied to the positional arrangements of skeletal components as they are preserved.
The fin endoskeleton comprises a series of distinct units, or mesomeres, that extend distally and form the central axis of the internal fin skeleton. Radial ossifications are visible in association with these mesomeres, and are described below and identified by comparison with the pectoral fin skeleton of Neoceratodus (Figures 6, 7). In some cases, bones of the fin endoskeleton are covered by, or in close proximity to, scales encasing the fin lobe. However, components of the fin endoskeleton are easily distinguished from the more weakly mineralized, ornamented scales in EDX images (e.g., Figure 5H). The fin skeleton appears largely undisturbed within the fin itself, permitting identification of fin mesomeres and radials when they are preserved in line with surrounding elements.
The proximal fin mesomeres are preserved as thin sheaths of perichondral bone, but more distal examples are not visible due to scale cover or what was likely weak mineralization in this region of the fin. More proximal radials are also preserved, but more distal radials cannot readily be observed.
The first mesomere, or humerus, is a squarish, flat bone. The proximal margin (articulating with the scapulocoracoid) is straight, while the distal margin of the bone is curved. The humeral mesomere is approximately twice as long as the second mesomere, or ulna. The exposed surface of the humerus carries a large axial process (“dorsal process” of Ahlberg, 1989), the long axis of which is aligned with that of the fin (Figure 4C). Endochondral bone is exposed at the distal end of the humerus, indicating either an unfinished articular facet that would have been capped in cartilage in life or, alternatively, damage to the overlying perichondral ossification. The second mesomere, or ulna, is wider anteroposteriorly with curved margins. It carries an irregularly-shaped axial process, though smaller and less distinct than that on the humerus. There is no evidence that the ulna bore either pre- (i.e., an intermedium) or post-axial radials.
As preserved, the ulna lies dorsal to a bone of slightly smaller size that we interpret as the radius. The radius does not carry an axial process like that found on the ulna, and its distal surface is aligned with a chain of two radial ossifications (Figure 3C, connected by gray lines). The radius designates the preaxial part of the pectoral fin, and is directed ventrally in NMS 1875.29.45 (i.e., toward the bottom of Figures 3B, 4, 5). This contrasts with the life orientation in Neoceratodus, where the dorsal edge of the pectoral fin corresponds to the preaxial margin. In this genus the radius and associated radials are dorsally directed in life position (Figures 6, 7). An equivalent “rotated” geometry has also been identified for Dipterus where the specimen is preserved in true lateral view (Ahlberg and Trewin, 1995); in other Dipterus shown in Figure 1, the skull and pectoral girdle and fin have been rotated slightly relative to the rest of the skeleton, so that the pectoral fin is held away from the body and rotated with the preaxial surface oriented ventrally. We cannot distinguish whether the arrangement apparent in Pentlandia represents post-mortem displacement comparable to specimens of Dipterus in Figure 1 (the skull and postcranial skeleton are not preserved) or whether that the fin was held with its preaxial margin directed ventrally in life.
Four more mesomeres are clearly visible distal to the ulna or second mesomere, with suggestions of at least two more toward the distal end of the fin that are largely obscured by overlying scales. These final preserved mesomeres are not clear under reflected light, but are apparent as higher abundances of phosphorus and calcium in EDX images (Figures 5D–F). Mesomeres three through six are all comparable in size to the ulna. Ossified pre- and post-axial radials are preserved in articulation with mesomeres three and four (Figure 4C, gray lines). The radial ossifications are roughly subquadrate and do not contact one another or their supporting mesomeres, suggesting the presence of extensive cartilaginous caps. The preaxial radial of mesomere three bears three ossified segments, while only a single ossification of the postaxial radial is preserved. Mesomere four bears a preaxial radial that consists of two ossifications in series. The postaxial radial of the mesomere is similarly represented by a chain of two ossifications. The most distal ossification is not apparent in specimen photographs, but is clear in false-color images derived from EDX for calcium and phosphorus (turquoise in combination, indicated by arrowhead in Figure 5F). A second associated region of high phosphorus and calcium concentration might be misinterpreted as another fin radial element, but targeted s.e.m. imaging of this ossification shows thin ridges of ornament suggesting that it is a scale. Radials are not preserved or visible in the more distal regions of the fin.
There is no definitive evidence for branching radials of the sort found in Neoceratodus (Figures 6, 7). Two bone fragments are preserved immediately distal to and in line with the proximal preaxial radial segment of the ulnare. These could either represent portions of a single damaged radial, or two separate radials representing a branching structure.
The bone of individual mesomeres is thin and appears to preserve a pattern of concentric circles on the surface (Figure 4). This suggests that ossification of the mesomeres progressed from the bone center both laterally and proximodistally, and that these cartilages had unmineralized proximal and distal surfaces. The bony mesomeres as preserved therefore do not accurately indicate the size of the fin cartilage. This conclusion is supported by two further observations: the absence of abutting relationships between successive mesomeres, and the lack of clear articular facets on the mesomeres to which the fin radials would have attached (e.g., compared to the distinct articular facets on the cartilaginous mesomeres of the fin of Neoceratodus; Figure 7) with the possible exception of a distal facet on the humerus.
Lepidotrichia are present along both sides of the fin. Their proximal extent is greatest along the postaxial margin as in Neoceratodus (Figure 7A), but unlike Glyptolepis where both the preaxial and postaxial series of lepidotrichia insert at a similar distance from the proximal end of the fin (Ahlberg, 1989: Figure 5B). All lepidotrichia have long unjointed bases. The lepidotrichia are segmented distally, but do not show any evidence of branching (Figure 4).
The pectoral fin of neoceratodus forsteri, with an emphasis on developmental patterns
The pectoral fin of Neoceratodus has been described several times previously, most notably by Günther (1871), Semon (1898), Braus (1906), Holmgren (1933: Figures 9–16), Goodrich (1958: Figure 152), Jarvik (1980: Figure 334), Rosen et al. (1981: Figures 30, 31), Shubin and Alberch (1986: Figure 18), Schultze (1987), Joss and Longhurst (2001) and Johanson et al. (2004, 2007). Holmgren (1933) was one of the first to describe the ontogeny of fin development in detail, including the mesomeres comprising the fin axis and pre- and postaxial radials, followed by Shubin and Alberch (1986), Joss and Longhurst (2001) and Johanson et al. (2004, 2007).
One of the earliest stages described in Neoceratodus shows the presence of the cartilaginous scapulocoracoid and humerus (first mesomere), as well as the second mesomere (Semon, 1898; Braus, 1906: Figure 206; Holmgren, 1933: Figures 10, 11; Joss and Longhurst, 2001: Figure 21.1b; Johanson et al., 2004: Figure 2). In the next stages, a small cartilaginous element begins to form in apparent association with the humerus (e.g., Holmgren, 1933; Ahlberg, 1989; Joss and Longhurst, 2001), representing the anlage of the radius (Figure 6A). The main axis of the fin continues to develop cartilaginous mesomeres distally, while more distal segments develop associated with the radius (Holmgren, 1933: Figures 11, 13; Joss and Longhurst, 2001: Figures 21.1d; Figure 6B, arrowhead). This is contrary to description of Shubin and Alberch (1986: Figure15), which suggests that the cartilaginous mesomeres continue to condense distally while branching to produce preaxial fin radials, with postaxial radials developing subsequently. In other words, Shubin and Alberch (1986: Figure15) suggest that the mesomeres of the axis and the preaxial radials developed concurrently from a sequence of branching events. As noted by Holmgren (1933: Figure 13), preaxial fin radials do develop in association with more distal mesomeres, as suggested by Shubin and Alberch (1986). However, postaxial fin radials begin to develop soon after, and from this point, fin radials condense and develop both pre- and postaxially (Joss and Longhurst, 2001: Figure 21.1e; Figure 6C). Fin radials also begin to develop in association with the ulna on the postaxial side of the fin, although this is a later ontogenetic event (Holmgren, 1933: Figures 14, 15; Figure 6C). Radials develop independently from the fin mesomeres (Holmgren, 1933; Joss and Longhurst, 2001; Johanson et al., 2007; Figure 6C), and only later do pronounced articular surfaces for radials develop on distal radials (Figure 6D, white arrowheads).
In larger specimens and adults (Figures 6D, 7A,B), the first two mesomeres develop small axial processes on the ventral surface, with the process on the humerus being the larger (Figures 6D, 7A,B). Processes are also developed on the ventral surface of these mesomeres. The preaxial radials have a one-to-one correspondence with the mesomeres (beginning with the ulnare), while postaxially, the numbers are more variable, with three radials associated with the ulna in and two with the ulnare (Figures 6D, 7A,B). The most proximal radials associated with the ulna form a complex structure, with the main radial and associated cartilages, which become partially fused in the adult to varying degrees (Günther, 1871; Holmgren, 1933; Rosen et al., 1981; Figures 6A,B). Interestingly, in Figure 6C (also Joss and Longhurst, 2001: Figure 21.1e), there are additional preaxial fin radials associated with the ulnare and the fourth mesomere, but these must be lost during growth (compare these mesomeres in Figure 6C with those in Figures 6D, 7A,B). In the adult pectoral fin, the mesomeres and radials are very irregular distally, with successive radials becoming fused (Figure 7C). This is likely due to individual variation, and suggests that patterning of radial development is not tightly constrained in distal parts of the fin. This extensive distal fusion is not readily apparent in existing figures of the pectoral-fin endoskeleton of Neoceratodus, many of which are highly diagrammatic (e.g., Günther, 1871; Rosen et al., 1981).
Discussion
Additional interpretation of the pectoral fin skeleton of pentlandia in the light of development in neoceratodus
Further aspects of the anatomy of the pectoral fin of Pentlandia can be clarified by comparison to the pectoral fin of Neoceratodus, particularly in earlier ontogenetic stages. The most relevant comparison can be drawn between the structures immediately distal to the humerus. In Pentlandia, the fin skeleton bears two separate elements in this area. This pattern is not observed in the adult Neoceratodus, but matches the arrangement, position, and relative sizes of the ulna (larger) and radius (smaller element) present early in the ontogeny of the living genus (Figures 6A–C). As discussed above, NMS 1875.29.45 is considered to belong to an adult Pentlandia, so we consider two alternative interpretations of the radial and ulnar ossifications in this genus that are consistent with these observations. First, the radius and ulna might have been separate cartilages in adult Pentlandia, as is primitive for the sarcopterygian fin (Ahlberg, 1989; Friedman et al., 2007). Second, the constituent cartilages of the humerus and ulna might have fused in adult Pentlandia but maintained separate ossification centers. We prefer the former interpretation, as described in more detail below.
Two extra radial bones are associated distally with the radius in the fin of Pentlandia (Figures 3, 4), which compares well with the smaller specimens of Neoceratodus (Figures 6B–D). There is one preaxial and one postaxial radial associated with the ulnare (mesomere three) in Pentlandia, with one and three segments, respectively (Figures 4, 5). The next axial mesomere also has one pre- and postaxial radial, with two segments in each. Fin radials are not visible in the more distal parts of the fin. By comparison with Neoceratodus, these more distal radials develop later in ontogeny, after the main fin axis (e.g., Figure 6C). In Pentlandia, the cartilaginous fin radials in this region may have not yet condensed, or, more probably, not yet ossified.
Comparisons with other dipnomorphs and the evolution of lungfish pectoral appendages
Because of the rarity of pectoral-fin endoskeletons among fossil dipnomorphs, even the incomplete example in Pentlandia adds considerably to our understanding of structural diversity and evolution in this group (Figure 8). Our interpretations of trait evolution are informed by well-constrained hypotheses of deep divergences within sarcopterygians (Maisey, 1986; Ahlberg, 1991; Cloutier and Ahlberg, 1996; Friedman, 2007; Friedman et al., 2007; Zhu et al., 2009), along with placement of Glyptolepis, Dipterus, Pentlandia, and Conchopoma as successively more crownward members of the dipnoan stem lineage (Schultze, 2001; Lloyd et al., 2012). We assume these relationships to be fixed for the purposes of our character analysis. Following Friedman and Brazeau (2010), we regard the character state shown by the ingroup as derived when a contrasting arrangement is shared by two or more successive outgroups (the “first doublet” rule of Maddison et al., 1984).
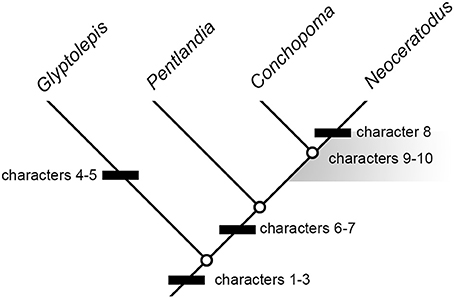
Figure 8. Summary cladogram indicating sequence of major character changes in dipnomorph pectoral-fin endoskeletons. Dipterus excluded on the account of highly incomplete information. Characters are as follows: (1) leaf-like fin geometry associated with very long metapterygial axis; (2) extensive series of postaxial radials; (3) loss of intermedium; (4) “naked” pectoral radials (i.e., radials not articulating with lepidotrichia); (5) ?loss of the radius (rather than ontogenetic fusion); (6) axial processes restricted to proximal mesomeres; (7) proximal insertion of preaxial finweb distal to that of postaxial finweb; (8) extensive branching of radials; (9) multiple postaxial radials in association with mesomeres; (10) postaxial radial(s) in association with humerus. Characters 8 and 9 arise crownward of Pentlandia, but cannot be mapped precisely due to unclear conditions in Conchopoma. The region where these changes could have occurred is indicated by a gray field. Fusion of the cartilages of the humerus and radius cannot be mapped, since the state of these cartilages (rather than their associated ossifications) cannot be assessed for Pentlandia. However, we hypothesize this fusion occurred on the lungfish stem crownward of Pentlandia.
As preserved, the pectoral-fin endoskeleton of Dipterus is represented only by a series of mesomeres (perhaps as many as eight; Ahlberg and Trewin, 1995: Figure 2), multiple unbranched but jointed preaxial radials, and a single postaxial radial. In Conchopoma, pre- and postaxial radials are unjointed, and the large number preserved suggest that a large number of mesomeres would have been present (Schultze, 1975: Figure 9; Figure 3F). There are no major structural disagreements between the arrangement found in Dipterus and that reported here for Pentlandia, although only the coarsest comparisons can be made due to the poor state of preservation in Dipterus. The most conspicuous difference between these two lungfishes is the apparently greater degree of mineralization of the pectoral-fin endoskeleton in Pentlandia relative to Dipterus, where the corresponding structure appears principally cartilaginous (Ahlberg and Trewin, 1995, p. 160).
More detailed comparisons can be drawn with the porolepiform Glyptolepis, which is represented by the most complete suite of pectoral-fin endoskeleton material of any fossil dipnomorph (Ahlberg, 1989; Figures 2A,C). This genus shares with Pentlandia a long chain of axial mesomeres and extensive series of both pre- and postaxial radials (also predicted for Conchopoma, Figure 8, characters 1–2). Outgroup comparison with tetrapodomorphs and coelacanths suggests that both represent derived dipnomorph features. In both Pentlandia and Glyptolepis, radials are unbranched but jointed (unjointed in Conchopoma), mesomeres articulate with only single pre- and post-axial radials, and there is no postaxial radial associated with the second mesomere (unknown in Conchopoma). This disagrees with conditions in Neoceratodus, where extensive branching of radials is present and some mesomeres, including the second, articulate with multiple postaxial radials (Figures 6, 7). We suggest that the arrangement common to Pentlandia and Glyptolepis is primitive for dipnomorphs, and that the features apparent in Neoceratodus are specialized within the group (cf. Friedman et al., 2007; Figure 8, characters 8–10).
Pentlandia shares with Glyptolepis the absence of a preaxial radial, or intermedium, on the second mesomere. By contrast, a distally segmented radial articulated with the second mesomere is present in adult specimens of Neoceratodus (Figure 7). However, developmental sequences for Neoceratodus clearly show that this chain of cartilages is associated with the radius, which fuses ontogenetically with the ulna to form the second mesomere in Neoceratodus. These same sequences also show that the ulna bears no preaxial radials at any developmental stage in Neoceratodus (Joss and Longhurst, 2001; Johanson et al., 2007; Figure 6). The condition shown by these dipnomorphs contrasts strongly with that seen in outgroups, which consistently bear an intermedium (Friedman et al., 2007; Coates et al., 2008). We regard loss of the intermedium as a potential dipnomorph synapomorphy (character 3). The condition in Conchopoma is unknown.
The most prominent differences between the pectoral-fin endoskeletons of Glyptolepis and Pentlandia relate to the structure of mesomeres, their relationships with associated radials, and symmetry of the fin itself. First, all well-preserved mesomeres in Glyptolepis bear prominent axial processes. By contrast, only the humerus and ulna bear well-developed axial processes in Pentlandia, a condition shared with Neoceratodus. Development of axial processes on distal mesomeres characterizes both fossil and living coelacanths (Millot and Anthony, 1958; Friedman et al., 2007; well-developed axial processes are not present in tetrapodomorphs), so we regard the condition in Glyptolepis as primitive relative to that common to Pentlandia and Neoceratodus (Figure 8, character 6). The state of the axial processes in Conchopoma is unknown, but we infer that they would be restricted to proximal mesomeres as in Pentlandia and Neoceratodus.
Second, there is no independent ossification representing the radius in Glyptolepis, but instead a single bone articulating with the distal surface of the humerus. By contrast, Pentlandia bears two separate ossification centers in articulation with the distal surface of the humerus and which correspond to the radius and ulna of other sarcopterygians. Neoceratodus has a single cartilaginous element formed by developmental fusion of separate radial and ulnar cartilages, but lacks any ossification. The arrangement in Conchopoma cannot be determined. These contrasting conditions make it difficult to infer possible sequences of character change. The presence of separate ossification centers for the radius and ulna in Pentlandia is clearly primitive based on comparison with other sarcopterygians (Ahlberg, 1989; Friedman et al., 2007). In Neoceratodus, the fused ulna and radius are noticeably broader than more distal mesomeres (Figures 6D, 7A,B), while in Glyptolepis, the second mesomere articulating with the humerus is the same width as more distal mesomeres (Ahlberg, 1989: Figures 4, 5; Figure 2C). This agreement in size suggests that the second mesomere of Glyptolepis does not represent a coalesced radius and ulna. Since it appears that the cartilaginous mesomere representing the ulna condenses before the radius (Joss and Longhurst, 2001: Figure 21.1a; Johanson et al., 2004: Figure 4; Figure 6A), it is possible that the mesomere in Glyptolepis represents the ulna and a radius did not form in this taxon, or was lost during ontogeny (Figure 8, character 5). In any event, this is a very different condition from that seen in Pentlandia and Neoceratodus. We therefore suggest that the condition of a single bone or cartilage articulating with the distal surface of the humerus in Glyptolepis and Neoceratodus is likely not homologous between these two taxa.
Third, Pentlandia bears an ossification center in line with and distal to the radius. Distal segments of the radius remain independent in adult Neoceratodus, and articulate with, but are not incorporated into, the fused radial + ulnar cartilage. Conditions in Conchopoma are not known. In Glyptolepis, no radials articulate with the second mesomere, further supporting hypothesized loss of the radius rather than its fusion with the ulna as in Neoceratodus. The presence of additional bones or cartilages that articulate with the distal end of the radius is a distinctive feature of Neoceratodus and Pentlandia, but the polarity of this character is difficult to establish. The absence of a discrete radius in Glyptolepis means direct comparisons cannot be made with this taxon. Conditions in more distant outgroups are variable. Most non-digited tetrapodomorphs show no ossifications associated with the distal surface of the radius, but such additional segments are present in some rhizodonts (Coates et al., 2008: Figure 4). Modern coelacanths have a greatly reduced radius, but early fossil examples show long preaxial radials with no indication of segmentation (Friedman et al., 2007: Figure 1). At present, it is equally parsimonious to consider distal segmentation of the radius as: a feature of dipnomorphs and tetrapodomorphs that is secondarily lost in tetrapodomorphs crownward of rhizodonts, or a character derived independently in rhizodonts and dipnomorphs.
Fourth, the insertion of the proximal lepidotrichia of the preaxial finweb is distal to the equivalent insertion of the postaxial finweb in Pentlandia. This agrees with the condition in Neoceratodus (Figure 8, character 7), but differs from the arrangement in Glyptolepis. This porolepiform genus shows the primitive sarcopterygian arrangement (Ahlberg, 1989), with the insertion for the proximal insertion for the preaxial finweb lying proximal to that for the postaxial finweb (Friedman et al., 2007).
Among living lungfishes, it is clear that the pectoral-fin endoskeleton of Neoceratodus is more primitive in overall architecture than those of Lepidosiren and Protopterus, where this structure is reduced to a chain of mesomeres. However, it is also clear that the fin of Neoceratodus is modified relative to primitive dipnomorph conditions (cf. Friedman et al., 2007). When considered alongside that of other fossil dipnomorphs, the material of Pentlandia described here places additional constraints on the evolutionary timing of major anatomical changes that characterize Neoceratodus. The high number of axial mesomeres, extensive series of pre- and post-axial radials, and absence of the intermedium characteristic of porolepiforms and lungfishes must have arisen no later than the minimum time of divergence between those groups, which is indicated by the Lockhovian Powichthys, Youngolepis, and Diabolepis (Jessen, 1980; Chang and Yu, 1984). Pentlandia indicates that reduction of axial processes on distal mesomeres and expansion of the extent of the postaxial finweb relative to the preaxial finweb occurred prior to the Givetian. Given the paucity of available material for dipnomorphs in general and lungfish more specifically, it is not possible to provide a minimum date for the origin of four additional derived features of Neoceratodus: branching radials (Figure 8, character 8; also absent in Conchopoma); multiple postaxial radials in articulation with single mesomeres (Figure 8, character 9); the presence of postaxial radials in association with the ulna (Figure 8, character 10); and fusion of the radial and ulnar cartilages. However, it is clear that the first three characters, and likely the fourth, arose on the lungfish stem crownward of Pentlandia. Glyptolepis is characterized by its own specializations: the presence of radials that do not bear lepidotrichia (Friedman et al., 2007; convergent examples of “naked” radials are found in Latimeria and digit-bearing tetrapods), and possibly the loss of the radius (see above; Figure 8, characters 4–5).
Conclusion
The degree of preservation of the pectoral-fin endoskeleton in Pentlandia is unique among fossil lungfishes. With a long chain of mesomeres and extensive series of pre- and post-axial radials, the endoskeleton of Pentlandia agrees closely with those described for the porolepiform Glyptolepis, the fossil lungfishes Dipterus and Conchopoma, and the extant Neoceratodus. Pentlandia shares with Neoceratodus to the exclusion of Glyptolepis the restriction of well-developed axial processes to the first two pectoral mesomeres and an insertion of the postaxial finweb that lies proximal to that of the preaxial finweb. Pentlandia is unique among dipnomorphs in retaining separate ossification centers for the radius and ulna, and displays multiple characters that are primitive relative to Neoceratodus: absence of branching radials, absence of multiple postaxial radials in articulation with single mesomeres, and absence of postaxial radials associated with the ulna. Fossil taxa like Pentlandia indicate that while many gross aspects of lungfish fin architecture were established very early in the history of the group, many aspects of structure found in the modern Neoceratodus are clearly modified relative to conditions in Devonian dipnomorphs.
Author Contributions
All authors contributed to the conception and design of the work, data acquisition and interpretation. All authors contributed to various drafts of this paper including the final draft for submission. All authors agree to be accountable for all aspects of the work, including accuracy.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Jean Joss for allowing us to use images of Neoceratodus forsteri, from specimens raised in her lab at Macquarie University, Sydney. We thank Stig Walsh and Martha Richter for allowing us to access the NMS and NHM fossil fish collections, and allowing us to borrow NMS1875.29.45. Harry Taylor and Phil Hurst (Photographic unit, NHM) are thanked for high-quality photos of the fins of Pentlandia and Fleurantia. We also thank Oliver Crimmen and James MacLaine for allowing us access to the NHM fish collections, and to photograph NHMUK IM499/E679. We also thank the reviewers who helped to improve this paper.
References
Ahlberg, P. (1989). Paired fin skeletons and relationships of the fossil group Porolepiformes (Osteicthyes: Sarcopterygii). Zool. J. Linn. Soc. 96, 119–166. doi: 10.1111/j.1096-3642.1989.tb01824.x
Ahlberg, P. (1991). A re-examination of sarcopterygian interrelationships, with special reference to the Porolepiformes. Zool. J. Linn. Soc. 103, 241–287. doi: 10.1111/j.1096-3642.1991.tb00905.x
Ahlberg, P., and Trewin, N. (1995). The postcranial skeleton of the Middle Devonian lungfish Dipterus valenciennesi. Trans. Roy. Soc. Edinb. 85, 159–175. doi: 10.1017/S0263593300003588
Becker, R. T., Gradstein, F. M., and Hammer, O. (2012). “The devonian period,” in The Geological Time Scale 2012, eds F. M. Gradstein, J. G. Ogg, M. D. Schmitz, and G. M. Ogg, (Amsterdam: Elsevier), 559–601.
Blom, H., Clack, J. A., Ahlberg, P. E., and Friedman, M. (2007). Devonian vertebrates from East Greenland: a review of faunal composition and distribution. Geodiversitas 29, 119–141.
Boisvert, C. A., Joss, J. M. P., and Ahlberg, P. E. (2013). Comparative pelvic development of the axolotl (Ambystoma mexicanum) and the Australian lungfish (Neoceratodus forsteri): conservation and innovation across the fish-tetrapod transition. Evodevo 4:3. doi: 10.1186/2041-9139-4-3
Braus, H. (1906). “Die entwickelung der form der extremitaten und des extremitatenskeletts,” in Handbuch der Vergleichenden und Experimentellen Entwicklungslehre der Wirbeltiere. Dritter Band. Zweiter Teil, ed O. Hertwig (Jena: G. Fischer), 167–338.
Broughton, R. E., Betancur, -R. R., Li, C., Arratia, G., and Ortí, G. (2013). Multi-locus phylogenetic analysis reveals the pattern and tempo of bony fish evolution. PLoS Curr. doi: 10.1371/currents.tol.2ca8041495ffafd0c92756e75247483e
Campbell, K. S. W., Barwick, R. E., and den Blaauwen, J. (2006). Structure and function of the shoulder girdle in dipnoans: New material from Dipterus valenciennesi. Senckenb. Lethaea 86, 77–91. doi: 10.1007/BF03043636
Cavin, L., Suteethorn, V., Buffetaut, E., and Tong, H. (2007). A new Thai Mesozoic lungfish (Sarcopterygii, Dipnoi) with an insight into post-Palaezoic dipnoan evolution. Zool. J. Linn. Soc. 149, 141–177. doi: 10.1111/j.1096-3642.2007.00238.x
Chang, M.-M., and Yu, X. (1984). Structure and phylogenetic significance of Diabolichthys speratus gen. et sp. nov., a new dipnoan-like form from the Lower Devonian of eastern Yunnan, China. Proc. Linn. Soc. N.S.W. 107, 171–184.
Clement, G. (2004). Nouvelles donnés anatomiques et morphologie générale des «Porolepidae» (Dipnomorpha, Sarcopterygii). Rev. Paléobiol. Genève 9, 193–211.
Cloutier, R. (1996). “Dipnoi (Akinetia: Sarcopterygii),” in Devonian Fishes and Plants of Miguasha, Quebec, Canada, eds H.-P. Schultze and R. Cloutier (Munich: Dr Friedrich Pfeil), 198–226.
Cloutier, R., and Ahlberg, P. E. (1996). “Morphology, characters, and the interrelationships of basal sarcopterygians,” in Interrelationships of Fishes, eds M. L. J. Stiassny, L. R. Parenti, and G. D. Johnson (New York, NY: Academic Press), 445–479.
Coates, M. I., Jeffery, J. E., and Ruta, M. (2002). Fins to limbs: what the fossils say. Evol. Dev. 4, 390–401. doi: 10.1046/j.1525-142X.2002.02026.x
Coates, M. I., Ruta, M., and Friedman, M. (2008). Ever since Owen: changing perspectives on the early evolution of tetrapods. Annu. Rev. Ecol. Evol. Syst. 39, 571–592. doi: 10.1146/annurev.ecolsys.38.091206.095546
Cole, N. J., Hall, T. E., Don, E. K., Berger, S., Boisvert, C. A., Neyt, C., et al. (2011). Development and evolution of the muscles of the pelvic fin. PLoS Biol. 9:e1001168. doi: 10.1371/journal.pbio.1001168
Dineley, D., and Metcalf, S. (1999). Fossil Fishes of Great Britain. Peterborough: Joint Nature Conservancy Committee.
Dingerkus, G., and Uhler, L. (1977). Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Tech. 52, 229–232.
Dollo, L. (1895). Sur la phylogénie des dipneustes. Bull. Soc. Belge Geol. Paléont. Hydrol. 9, 79–128.
Donovan, R. N., Foster, R. J., and Westoll, T. S. (1974). A stratigraphical revision of the Old Red Sandstone of north-eastern Caithness. Trans. R. Soc. Edinb. Earth Sci. 69, 167–201. doi: 10.1017/S0080456800015118
Friedman, M. (2010). “Postcranial evolution in early lungfishes (Dipnoi: Sarcopterygii): new insights from Soederberghia groenlandica,” in Morphology, Phylogeny and Palaeobiogeography of Fossil Fishes, eds D. Elliot, J. Maisey, X.-B. Yu, and D. Miao, (Munich: Dr Friedrich Pfeil), 299–324.
Friedman, M. (2007). Styloichthys as the oldest coelacanth: implications for early osteichthyan interrelationships. J. Syst. Palaeontol. 5, 289–343. doi: 10.1017/S1477201907002052
Friedman, M., and Brazeau, M. D. (2010). A reappraisal of the origin and basal radiation of the Osteichthyes. J. Vert. Paleontol. 30, 36–56. doi: 10.1080/02724630903409071
Friedman, M., Coates, M. I., and Anderson, P. S. L. (2007). First discovery of a primitive coelacanth fin fills a major gap in the evolution of lobed fins and limbs. Evol. Dev. 9, 329–337. doi: 10.1111/j.1525-142X.2007.00169.x
Goodrich, E. S. (1958). Studies on the Structure and Development of the Vertebrates. Vol. I. New York, NY: Dover Publications.
Günther, A. (1871). Description of Ceratodus, a genus of ganoid fishes recently discovered in rivers of Queensland, Australia. Philos. Trans. R. Soc. Lond. B 161, 511–571.
Holmgren, N. (1933). On the origin of the tetrapod limb. Acta Zool. XIV, 185–295. doi: 10.1111/j.1463-6395.1933.tb00009.x
Jessen, H. L. (1980). Lower Devonian Porolepiformes from the Canadian Arctic with special reference to Powichthys thorsteinssoni Jessen. Palaeontogr. Abt. A 167, 180–214.
Johanson, Z. (2003). The clavobranchialis musculature in sarcopterygian fishes, and contribution to osteichthyans feeding and respiration. Contrib. Zool. 72, 17–37. Available online at: http://www.ctoz.nl/vol72/nr01/a02
Johanson, Z., Joss, J. M. P., Boisvert, C. A., Ericsson, R., Sutija, M., and Ahlberg, P. E. (2007). Fish fingers: digit homologues in sarcopterygian fish fins. J. Exp. Zool. B Mol. Dev. Evol. 308B, 757–768. doi: 10.1002/jez.b.21197
Johanson, Z., Joss, J. M. P., and Wood, D. (2004). Scapulocoracoid development in the Queensland lungfish Neoceratodus forsteri (Dipnoi; Sarcopterygii). Zoology 107, 93–110. doi: 10.1016/j.zool.2004.01.001
Joss, J. M. P., and Longhurst, T. (2001). “Lungfish paired fins,” in Major Events in Early Vertebrate Evolution, ed P. E. Ahlberg (London: Taylor and Francis), 370–376.
King, H. M., Shubin, N. H., Coates, M. I., and Hale, M. E. (2011). Behavioral evidence for the evolution of walking and bounding before terrestriality in sarcopterygian fishes. Proc. Natl. Acad. Sci. U.S.A 108, 21146–21151. doi: 10.1073/pnas.1118669109
Liang, D., Xing, X.-S., and Zhang, P. (2013). One thousand two hundred ninety nuclear genes from a genome-wide survey support lungfishes as the sister group of tetrapods. Mol. Biol. Evol. 30, 1803–1807. doi: 10.1093/molbev/mst072
Lloyd, G. T., Wang, S. C., and Brusatte, S. L. (2012). Identifying heterogeneity in rates of morphological evolution: discrete character change in the evolution of lungfish (Sarcopterygii; Dipnoi). Evolution 66, 330–348. doi: 10.1111/j.1558-5646.2011.01460.x
Long, J. A., and Clement, A. M. (2009). The postcranial anatomy of two Middle Devonian lungfishes (Osteichthyes, Dipnoi) from Mt. Howitt, Victoria, Australia. Mem. Mus. Vic. 66, 189–202.
Maddison, W. P., Donoghue, M. J., and Maddison, D. M. (1984). Outgroup analysis and parsimony. Syst. Zool. 33, 83–103. doi: 10.2307/2413134
Maisey, J. G. (1986). Heads and tails: a chordate phylogeny. Cladistics 2, 201–256. doi: 10.1111/j.1096-0031.1986.tb00462.x
Marshall, J. E. A., Brown, J. F., and Astin, T. R. (2011). Recognising the Taghanic Crisis in the Devonian terrestrial environment and its implications for understanding land-sea interactions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 304, 165–183. doi: 10.1016/j.palaeo.2010.10.016
Millot, J., and Anthony, J. (1958). Anatomie de Latimeria Chalumnae. Tome I. Squelette, Muscles, et Formation du Soitien. Paris: Éditions du Centre national de la Recherche scientifique.
Pierce, S. E., Hutchinson, J. R., and Clack, J. A. (2013). Historical perspectives on the evolution of tetrapodomorph movement. Integr. Comp Biol. 53, 209–223. doi: 10.1093/icb/ict022
Rosen, D. E., Forey, P. L., Gardiner, B. G., and Patterson, C. (1981). Lungfishes, tetrapods, paleontology and plesiomorphy. Bull. Am. Mus. Nat. Hist. 167, 159–276.
Schultze, H.-P. (1975). Die Lungenfisch-Gattung Conchopoma (Pisces, Dipnoi). Senckenb. Lethaea 56, 191–231.
Schultze, H.-P. (2001). Melanognathus, a primitive dipnoan from the Lower Devonian of the Canadian Arctic and the interrelationships of Devonian dipnoans. J. Vert. Paleontol. 21, 781–794. doi: 10.1671/0272-4634(2001)021[0781:MAPDFT]2.0.CO;2
Semon, R. (1898). Die Entwicklung der paarigen Flossen des Ceratodus forsteri. Jen. Denkschr. 4 Semon Zool. Forsch. 1, 61–111.
Shubin, N. S., and Alberch, P. (1986). A morphogenetic approach to the origin and basic organization of the tetrapod limb. Evol. Biol. 20, 319–387.
Traquair, R. H. (1888). Notes on the nomenclature of the fishes of the Old Red Sandstone of Great Britain. Geol. Mag. 5, 507–517.
Traquair, R. H. (1889). On a new species of Dipterus. Geol. Mag. 6, 97–99. doi: 10.1017/S0016756800176071
Trewin, N. H. (1986). Palaeoecology and sedimentology of the Achanarras fish bed of the Middle Old Red Sandstone, Scotland. Trans. R. Soc. Edinb. Earth Sci. 77, 21–46. doi: 10.1017/S0263593300010737
Watson, D. M. S., and Day, H. (1916). Notes on some Palaeozoic fishes. Mem. Proc. Manchest. Lit. Philos. Soc. 60, 1–47.
Woodward, A. S. (1891). A Catalogue of Fossil Fishes in the British Museum (Natural History). Part II. London: Trustees of the British Museum [Natural History].
Keywords: Dipnoi, appendage, archipterygium, development, limbs, Neoceratodus, Sarcopterygii, Tetrapoda
Citation: Jude E, Johanson Z, Kearsley A and Friedman M (2014) Early evolution of the lungfish pectoral-fin endoskeleton: evidence from the Middle Devonian (Givetian) Pentlandia macroptera. Front. Earth Sci. 2:18. doi: 10.3389/feart.2014.00018
Received: 13 May 2014; Paper pending published: 21 May 2014;
Accepted: 16 July 2014; Published online: 12 August 2014.
Edited by:
Hans-Dieter Sues, Smithsonian Institution, USAReviewed by:
Per Erik Ahlberg, Uppsala University, SwedenJennifer Alice Clack, University of Cambridge, UK
Copyright © 2014 Jude, Johanson, Kearsley and Friedman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zerina Johanson, Department of Earth Sciences, Natural History Museum, Cromwell Road, London SW75BD, UK e-mail:ei5qb2hhbnNvbkBuaG0uYWMudWs=
 Emma Jude
Emma Jude Zerina Johanson
Zerina Johanson Anton Kearsley3
Anton Kearsley3 Matt Friedman
Matt Friedman