
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Archaeol. , 09 May 2024
Sec. Zooarchaeology
Volume 3 - 2024 | https://doi.org/10.3389/fearc.2024.1405535
This article is part of the Research Topic Neanderthal Complex Behaviour Through the Lens of Faunal Resources View all 5 articles
 Eboni Westbury1*
Eboni Westbury1* Sofía Samper Carro1,2
Sofía Samper Carro1,2 Susana Vega Bolivar2
Susana Vega Bolivar2 Jezabel Pizarro2
Jezabel Pizarro2 Jorge Martínez-Moreno2
Jorge Martínez-Moreno2 Rafael Mora2
Rafael Mora2The examination of faunal assemblages through zooarchaeological analyses constitutes a fundamental approach for gaining insight into the intricate behaviours of Neanderthals. Previous investigations have primarily focused on periods of relative environmental stability, and this has provided a wealth of relevant archaeological data. However, our understanding of Neanderthal resilience during the MIS 4, a period presumably characterised by harsh environmental conditions, remains limited. This study presents the first comprehensive analysis of the faunal assemblages from Levels M and P at Abric Pizarro. The geographic location of Abric Pizarro in the southeast Pre-Pyrenees, combined with chronometric dating, offers a unique opportunity to explore Neanderthal behaviours during a poorly known chronological period. The detailed zooarchaeological analysis comprised taxonomic identification, taphonomic analysis and age-at-death profiling to explore the adaptability and flexibility in the Neanderthal diet. The findings indicate that Neanderthal groups incorporated a diverse range of protein resources from small herbivores (e.g., caprids) to very large herbivores (e.g., Bos/Bison). These results not only demonstrate an adaptability to changing environments in an area traditionally deemed unsuitable for long-term occupation, but also contributes significantly to our understanding of the complex behaviours exhibited by Neanderthals.
Since their discovery in the 19th century, Neanderthals have been historically characterised as “primitive,” “brutish,” and “ape-like,” denoting an inherent inferiority compared to anatomically modern humans (AMH) (e.g., Busk, 1865; Trinkaus and Shipman, 1993). This assumption was perpetuated by prevailing religious ideologies that influenced scientific research, resulting in a biassed exclusion of complex behaviours traditionally attributed solely to AMH (Trinkaus and Shipman, 1993). However, in recent decades, multidisciplinary investigations, encompassing archaeology and genetics among others, have challenged these prevailing perceptions, prompting a profound reassessment of Neanderthals' cognitive capacities and their position within the broader framework of human evolutionary history (see an updated review in Romagnoli et al., 2022).
Archaeological and biomolecular research conducted since the mid-20th century has generated compelling evidence that has reshaped our understanding of Neanderthals. This accumulating corpus of data reveals that Neanderthals exhibited behaviours previously regarded as exclusive to AMH (Sankararaman et al., 2014; Hajdinjak et al., 2021; Romagnoli et al., 2022). One such behaviour is the spatial organisation of Neanderthal living sites, implying cognitive complexity and social structure (Vaquero, 2022). Additionally, Neanderthals demonstrated a remarkable ability to adapt and thrive in diverse environments, displaying resilience amidst fluctuating conditions, thus attesting to their considerable adaptability (Hardy, 2022; Sánchez Goñi, 2022; Fernández-García et al., 2023; Nabais et al., 2023).
The field of zooarchaeology has emerged as a pivotal avenue for elucidating the complex behaviours of Neanderthals. Zooarchaeological inquiry provides critical insights into the dietary patterns, subsistence strategies, and hunting practises of ancient human populations. Recent advancements in zooarchaeology have significantly challenged conventional notions regarding Neanderthals' dietary preferences and hunting capabilities (Blasco et al., 2022; Rendu, 2022). Contrary to earlier assumptions that Neanderthals subsisted primarily on large game, emerging evidence suggests their consumption of small prey such as rabbits (Lloveras et al., 2009, 2011; Fa et al., 2013; Martínez-Polanco et al., 2017; Carvalho et al., 2018; Pelletier et al., 2019), tortoises/turtles (Blasco, 2008; Nabais and Zilhão, 2019), birds (Blasco and Fernández Peris, 2009; Blanco et al., 2021), and other marine resources (Zilhão et al., 2020; Nabais et al., 2023).
The present study seeks to contribute further to the burgeoning field of zooarchaeological research by investigating the adaptability and flexibility of the Neanderthal diet during the Marine Isotope Stage 4 (MIS 4; ca. 71–58 ka), a period traditionally characterised by climatic instability (d'Errico and Sánchez Goñi, 2003; Fernández-García et al., 2023). This temporal window offers a unique opportunity to explore how Neanderthals adjusted their subsistence strategies in response to fluctuating environmental conditions.
By employing a comprehensive analysis of animal remains derived from a well-preserved Neanderthal site within MIS 4, this study aims to address key research inquiries. Firstly, it seeks to delineate the spectrum of animal species exploited by Neanderthals during this interval, examining any discernible shifts in prey preferences. Moreover, through the examination of animal bone assemblages for evidence of processing and butchery techniques, this research endeavours to shed light on the level of the standardisation of butchery practises within Neanderthal subsistence practises.
The Abric Pizarro site is situated in the Barranc de les Coves valley, 697 m above sea level at the foothills of the southeastern Pre-Pyrenees (Figure 1; Pizarro et al., 2013; Vega Bolivar et al., 2015; Samper Carro et al., under review1). The rock shelter is 35 m in length, 4.3 m in height, and 6.4 m in depth, with a sedimentary sequence exceeding 1.5 m in depth (Vega Bolivar et al., 2015).
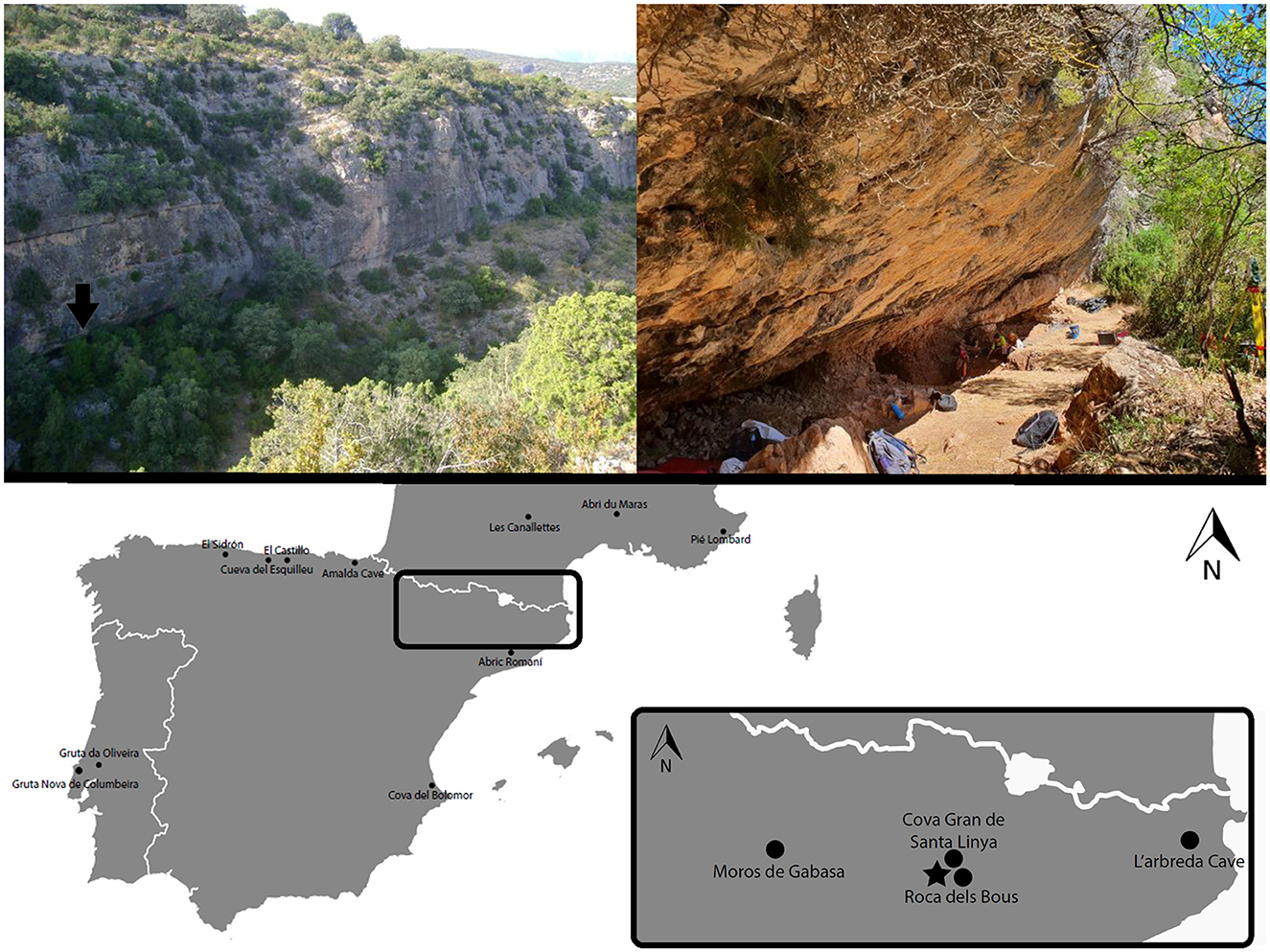
Figure 1. Location of Abric Pizarro rockshelter and other key temporally synonymous Middle Palaeolithic Neanderthal sites in the immediate Pre-Pyrenean region and broader Iberian Peninsula and southern France. The arrow in the top left photograph and ⋆ in the bottom map indicates the location of Abric Pizarro.
Discovered in 2007, the site underwent its first test excavation in 2009, followed by extended excavation seasons between 2010–2013, 2016, 2017, and 2022. The excavations revealed four distinct archaeological units, labelled as M, P, Q, and S, which were separated by culturally sterile layers. The identification of these discrete Neanderthal occupations was made possible through the application of three-dimensional recording techniques for artefacts, features (e.g., hearths), and sedimentary elements (e.g., large rocks; Figure 2).
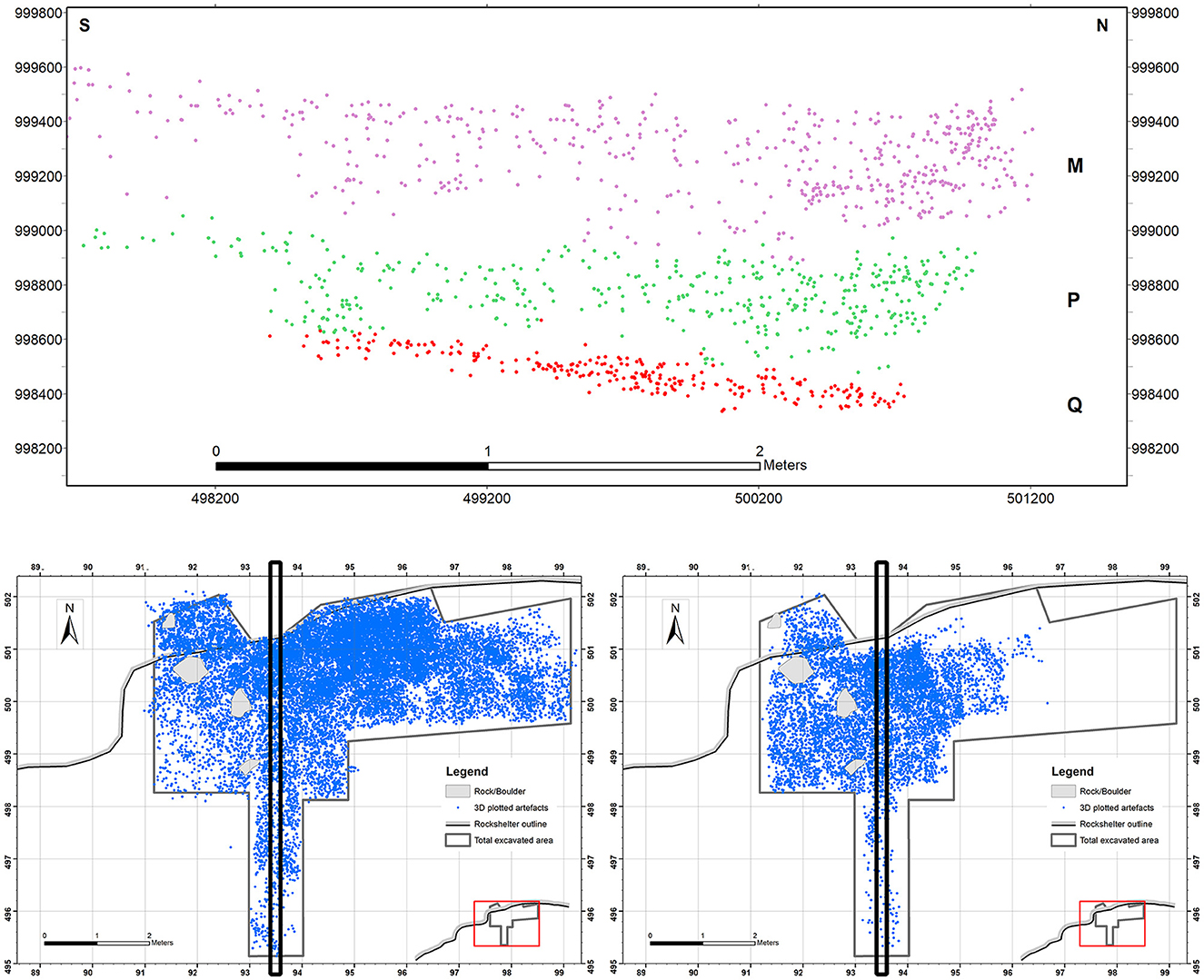
Figure 2. Vertical (top) and horizontal (bottom) distribution of 3D plotted cultural material recovered in units M (bottom left) and P (bottom right) from Abric Pizarro.
To establish the chronological framework for the analysed levels, thermoluminescence (TL) dating was initially performed on thermally altered chert artefacts. The obtained dates position level M at 58,971 ± 5,359 BP and level P at 62,602 ± 6,110 BP, placing them within MIS 4 even when considering the statistical error margins associated with this dating method (Vega Bolivar et al., 2015). Optically stimulated luminescence (OSL) samples were subsequently collected from units P and Q to enhance the chronological resolution of the deposit. These samples returned an age of 67.7 ± 4.9 ka for level P and an age of 74.4 ± 5.1 ka for level Q, in broad agreement with the previously obtained TL dates, and supporting a MIS 4 chronology for the deposit (see text footnote 1).
A general overview of the complete faunal assemblage recovered in Abric Pizarro has been previously published (Vega Bolivar et al., 2015) (see text footnote 1). This study focuses on the comprehensive analysis of the large vertebrates recovered from levels M and P within the Abric Pizarro site. The analysis included quantitative assessment, taxonomic identification, and taphonomic analysis to gain insights into subsistence practises, taphonomic processes, and potential cultural activities during the period of occupation at Abric Pizarro.
It is important to note that due to bone sampling and recovery strategies, many bones of small vertebrates were collected in “non-coordinate” bags and omitted from detailed recording. Resultingly, the small vertebrates analysed during the zooarchaeological investigation do not constitute the entire assemblage of small vertebrate remains and are therefore not discussed in this study. Current research is focusing on assessing the collection of small vertebrate remains from Abric Pizarro to provide a more comprehensive and holistic interpretation of Neanderthal behaviour at the site.
Bone preservation was evaluated based on the degree of weathering, adapting the criteria established by Behrensmeyer (1978) to match the fossilisation state of the assemblage (Samper Carro et al., 2020). The weathering stages included stage 1 (minimal calcareous coating and cracking), stage 2 (less than half of cortical surface affected by calcareous coatings and some cracking), and stage 3 (more than half of cortical surface covered with calcareous coatings or extreme cracking and flaking). Abrasion was classified into stage 0 (no abrasion), stage 1 (light abrasion), and stage 2 (polishing of the cortical surface).
Quantitative units were calculated to assess the faunal composition. This involved, where appropriate, distinguishing between diaphyses and other skeletal components following standardised guidelines (e.g., Marean and Kim, 1998). The total number of fragments (NRt) was determined, encompassing both identified and unidentified specimens. Additionally, the number of remains (NR) was recorded, including fragments identified to taxa or size/weight-class. The number of identifiable specimens (NISP) represented the identified fragments at the lowest taxonomic level. Furthermore, the minimum number of elements (MNE) was calculated, considering aspects such as age-at-death, side, and anatomical position, to assess the minimum representation of each skeletal element within taxa (i.e., skeletal representation). MNE was calculated in tandem with the percentage of minimum animal units (%MAU) to assess the relative representation of the different species and size classes within the assemblage. The minimum number of individuals (MNI) was calculated to estimate the relative abundance of each taxonomic group present in the assemblage. Where appropriate, age-at-death determinations contributed to the calculation of MNI.
Taxonomic identification was conducted with assistance from the osteological collection housed at the Centre d'Estudis del Patrimoni Arqueologic (UAB) and osteological reference manuals (Pales and Garcia, 1981; Brown and Chapman, 1991; Hillson, 1991, 2005). For non-diagnostic bone fragments that could not be identified to specific taxa, classification by size classes was employed, following criteria established in previous studies (Bunn, 1986; Saladié et al., 2011; Samper Carro et al., 2020). The size classes included size 1 (up to 100 kg; Sus scrofa, Capra pyrenaica, infantile Cervus elaphus), size 2 (100–300 kg; sub-adult and adult C. elaphus, infantile Equus ferus and Equus hydruntinus), size 3 (300–500 kg; sub-adult and adult E. ferus and E. hydruntinus), size 4 (500–800 kg; sub-adult and adult Bos/Bison), and size 5 (over 800 kg; Rhinocerotidae). It is important to note that the size classes excluded remains attributable to small prey, such as turtles and rabbits.
Age-at-death profiling was conducted to gain insights into the age composition of the faunal assemblages. This process involved the examination of tooth eruption and wear patterns, and epiphyseal fusion. This age-at-death profiling analysis was carried out following established methodologies and reference standards for age determination in extant mammalian species (Grant, 1982; Brown and Chapman, 1991; Hillson, 2005; Greenfield and Arnold, 2008; Lemoine et al., 2014). Age categories include infantile (no epiphyseal fusion, deciduous teeth with no wear, incomplete tooth eruption sequence), juvenile (some epiphyseal fusion, deciduous teeth with minimal wear, eruption of first permanent teeth), immature/sub-adult (nearly complete epiphyseal fusion, loss of deciduous teeth, eruption of most permanent teeth with minimal wear), adult (complete epiphyseal fusion, eruption of all permanent teeth with some wear), and senile (presence of all permanent teeth with extreme wear). Skeletal indicators were compared with age categories defined based on the developmental stages of modern comparative species and known growth patterns of related taxa.
Additionally, bone colour was documented to assess potential diagenetic alterations, including natural burning and manganese staining (López-González et al., 2006; Marín Arroyo et al., 2008). The colour stages included stage 0 (no change to bone colour), stage 1 (black spots; manganese staining), stage 2 (brown; manganese staining), stage 3 (black; burning), stage 4 (grey and/or white; burning), and stage 5 (bluish white; burning). The identification of bone surface modifications, both anthropogenic and non-anthropogenic, was conducted through preliminary observations at 30 × magnification, followed by more detailed examination at up to 80 × magnification using a DinoLite microscope (AM4815-FJT Dino-Lite Edge). Anthropogenic modifications included butchery-related marks, such as incisions, scrapes, and chop marks, as well as intentional breakage through impact points and indirect percussion, following identification criteria previously published (e.g., Fisher, 1995; Fernández-Jalvo and Andrews, 2016). Furthermore, bone fracture angles, morphology, diaphysis length, and circumference were recorded following the methodology established by Villa and Mahieu (1991). The identification of burning considered the presence of colour changes, cracking, and shrinking (Nicholson, 1993; Stiner et al., 1995).
The complete faunal assemblage from levels M and P of the Abric Pizarro rockshelter comprises a total of 246,913 fragments (NRt; the “complete assemblage”). Notably, a large proportion of these fragments (95.90%), were smaller than 2 cm, indicating a high fragmentation rate and the preservation integrity of the site. For the purpose of this study, only fragments larger than 2 cm were considered for taphonomic and taxonomic analysis (the “identifiable assemblage”; n = 10,116).
Bone preservation within the identifiable assemblage was generally characterised by the good integrity of cortical surfaces. Approximately 99.95% of the identifiable assemblage was found with sediment concretion, indicating favourable preservation conditions through the protection and stabilisation of cortical bone surfaces. Only a small proportion of the identifiable assemblage (0.06%) exhibited signs of abrasion.
Most fragments (86.72%) from the level M identifiable assemblage (n of level M identifiable fragments = 8,373) displayed a brown colouration. A dominance (71.37%) of bones from the level M identifiable assemblage also featured black spots, including on burned fragments. Within the level M identifiable assemblage, burning was observed on 11.44% of fragments. Similar patterns were observed in the level P identifiable assemblage (n of level P identifiable fragments = 1,743), with a dominance of bones (84.22%) exhibiting brown colouration, a significant portion (76.94%) of bones displaying black spots and burned remains contributing 10.90% to the assemblage.
In Level M, the burned faunal assemblage (n = 958 fragments) consists of 37.58% indeterminate bones and 62.42% identifiable bones. Fragments predominantly exhibit black colouration (70.67%), with lesser occurrences of grey (22.65%) and white (6.68%) hues. Among the burned assemblage, caprines and size 1 herbivores account for 4.59%, primarily represented by unidentifiable long bones alongside few humeri, isolated instances a radius, femur, metacarpal, and metatarsal, and small, irregular bones like patellae. Cervids and size 2 herbivores are the most commonly occurring identifiable species, constituting 20.04% of the burned assemblage. These fragments predominantly feature long bones (primarily unidentifiable, though also including humeri, ulnae, radii, femora, tibiae, metapodials, and phalanges), as well as a few teeth and small bones such as sesamoids. Equids and size 3 herbivores contribute 3.77% to the burned assemblage, primarily represented by unidentifiable long bones with minimal instances of humeri, radii, and tibiae, with few teeth and an unidentifiable vertebra. Size 4 herbivores are exclusively represented by unidentifiable long bones, accounting for 0.42% of the burned assemblage. Size classes that defy definitive classification, such as size 1/2, 2/3, and 3/4 herbivores, account for 14.41%, 2.51%, and 0.84% of the burned assemblage, respectively, predominantly comprising unidentifiable long bones. Undiagnostic ungulates or mammals constitute 14.61% of the burned assemblage, primarily characterised by unidentifiable epiphyses and flat bones, with occasional cranial and mandibular elements, ribs, scapulae, and vertebrae represented.
In Level P, the burned faunal assemblage (n = 190 fragments) consists of 44.21% indeterminate bones and 55.79% identifiable bones. Over half of the fragments display a black coloration (55.27%), with lesser occurrences of grey (35.26%) and white hues (9.47%). Among the identifiable remains, caprines and size 1 herbivores account for 14.74% of the burned assemblage, primarily represented by unidentifiable long bones, along with isolated instances of a humerus, femur, and metapodial, and a few radii and teeth. Cervids and size 2 herbivores are again the most commonly occurring identifiable species, constituting 24.74% of the burned assemblage, predominantly represented by unidentifiable long bones, with some humeri, single instances of an ulna and tibia, and a few metapodials and isolated teeth. Equids and size 3 herbivores contribute 6.84% to the burned assemblage, primarily through isolated teeth, as well as a few femora, and single instances of a humerus, radius, tibia, and unidentified long bone. No size 4 herbivores are identified. Size 1/2 herbivores (4.74%), size 2/3 herbivores (0.53%), and size 3/4 herbivores (0.53%) are exclusively represented by unidentifiable long bones. Undiagnostic mammals account for 14.21% of the burned assemblage, primarily characterised by unidentifiable epiphyses, alongside a few ribs, unidentifiable flat bones, and teeth, and single instances of a mandible and dorsal vertebra.
A relatively small number of specimens from both levels M and P (n = 6,090; 2.47% of the complete faunal assemblage), were confidently identified to taxa or size class (NR; i.e., excluding undefined and unidentified specimens). Despite the limited identification, the taxonomic composition of the faunal assemblage revealed a diverse array of fauna, with variations between the two archaeological units (Table 1).
In the level M identifiable assemblage, the faunal composition comprises 5.21% caprines and small-sized (size 1) herbivores (including Capra pyrenaica), 29.20% cervids and medium-sized (size 2) herbivores, 8.94% equids and large-sized (size 3) herbivores, and 0.74% large bovids and very large-sized (size 4) herbivores. However, certain fragments could not be confidently assigned to a single size class, resulting in 8.56% being categorised as size 1/2 herbivores, 3.10% as size 2/3 herbivores, and 0.84% as size 3/4 herbivores. Other species including moles, rodents, lynx, wild cats, hyenas, foxes, canids, bears, undefined carnivores, wild boars, rhinoceros, very large (size 5) herbivores and birds constituted only 0.37% of the level M identifiable assemblage.
Similarly, in the level P identifiable assemblage, the faunal composition includes 12.28% caprines and small-sized (size 1) herbivores, 28.11% cervids and medium-sized (size 2) herbivores, 7.23% equids and large-sized (size 3) herbivores, and 1.09% large bovids and very large-sized (size 4) herbivores. As in level M, some fragments in level P could not be confidently assigned to a single size class, with 4.07% being designated as size 1/2 herbivores, 1.43% as size 2/3 herbivores, and 0.52% as size 3/4 herbivores. Other species, including frogs, wild boar, and rhinoceros, constituted only 0.29% of the level P identifiable assemblage. No carnivores were identified in this unit.
The prevailing composition of the assemblage is characterised by highly fragmented long and flat bone fragments, which impeded precise determinations of age-at-death for most specimens. To mitigate this challenge and gain insights into the age structure of the assemblage, comparisons were undertaken with a subset of material from several taxa (caprines and size 1 herbivores, cervids and size 2 herbivores, and equids and size 3 herbivores) that permitted reliable age-at-death identifications (n = 377; Table 2). This subset comprised isolated teeth exhibiting preserved dentine, complete and nearly complete mandibles demonstrating tooth eruption sequences, as well as long bones and epiphyses displaying discernible fusion rates. Within this subset, a notable proportion (83.82%) comprised juveniles, constituting 84.78% of the Level M subset and 76.19% of the Level P subset.
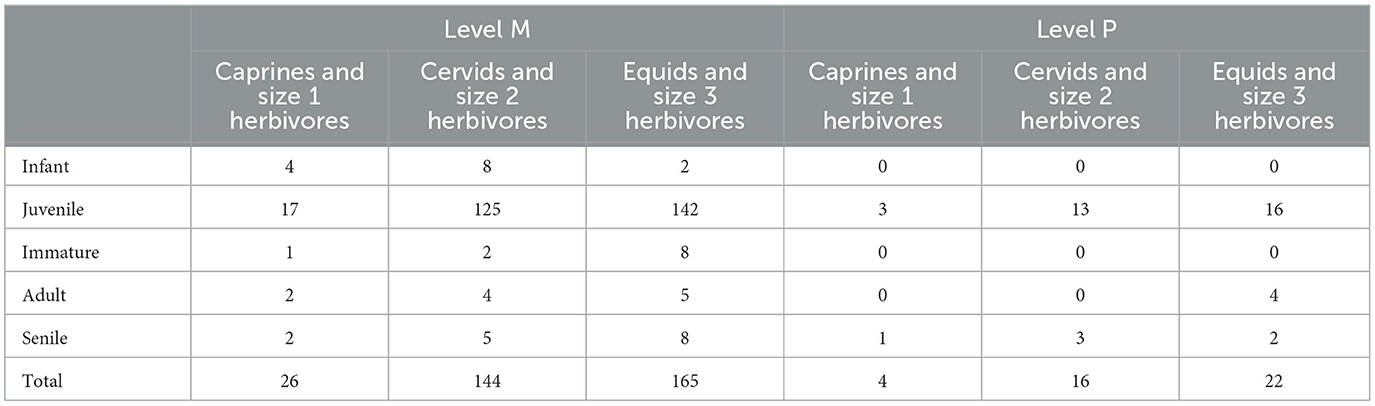
Table 2. Age-at-death determinations for caprines/size 1 herbivores, cervids/size 2 herbivores, and equids/size 3 herbivores from Abric Pizarro levels M and P.
When considering the subset of material permitting age-at-death determinations to the larger identifiable assemblage of Level M, the mortality profiles indicate minimal representation of infants (0.17%), a modest presence of juveniles (3.39%), a marginal presence of immature individuals (0.13%) and adults (0.13%), and a nominal presence of senile individuals (0.18%). In contrast, consideration with the identifiable assemblage of Level P exhibited a distinct composition with 1.84% juveniles, 0.23% adults, and 0.34% senile individuals.
The cumulative age-at-death determinations derived from the subset of material allowing for demographic analysis reveal a notable predominance of juveniles compared to other age categories, particularly evident within Level M. However, the extensive fragmentation observed in the complete faunal assemblage results in the majority of specimens being classified as 'unknown' in terms of age-at-death. Consequently, this poses a significant challenge in comprehensively assessing mortality profiles, necessitating a cautious interpretation of these findings.
Skeletal profiles of the most abundant prey species (i.e., caprines; C. elaphus, Cervidae; E. ferus, E. hydruntinus, Equus sp.), were documented for each archaeological level using MNE and %MAU calculations for each skeletal element within taxa (Table 3; Figure 3). In level M, size 1 herbivores exhibit representation by teeth, mandibles, vertebrae, scapulae, humeri, radii, ulnae, pelvises, femora, patellae, tibiae, metapodials, and phalanges, with humeri and femora being the most well-represented elements. Similarly, size 2 herbivores are represented by teeth, mandibles, scapulae, humeri, radii, ulnae, femora, tibiae, metapodials, and sesamoids, with metatarsals and tibiae among the most well-represented elements. Size 3 herbivores in Level M are represented by teeth, mandibles, vertebrae, ribs, scapulae, humeri, radii, ulnae, pelvises, femora, tibiae, metapodials, and phalanges, with humeri and femora being most abundant.
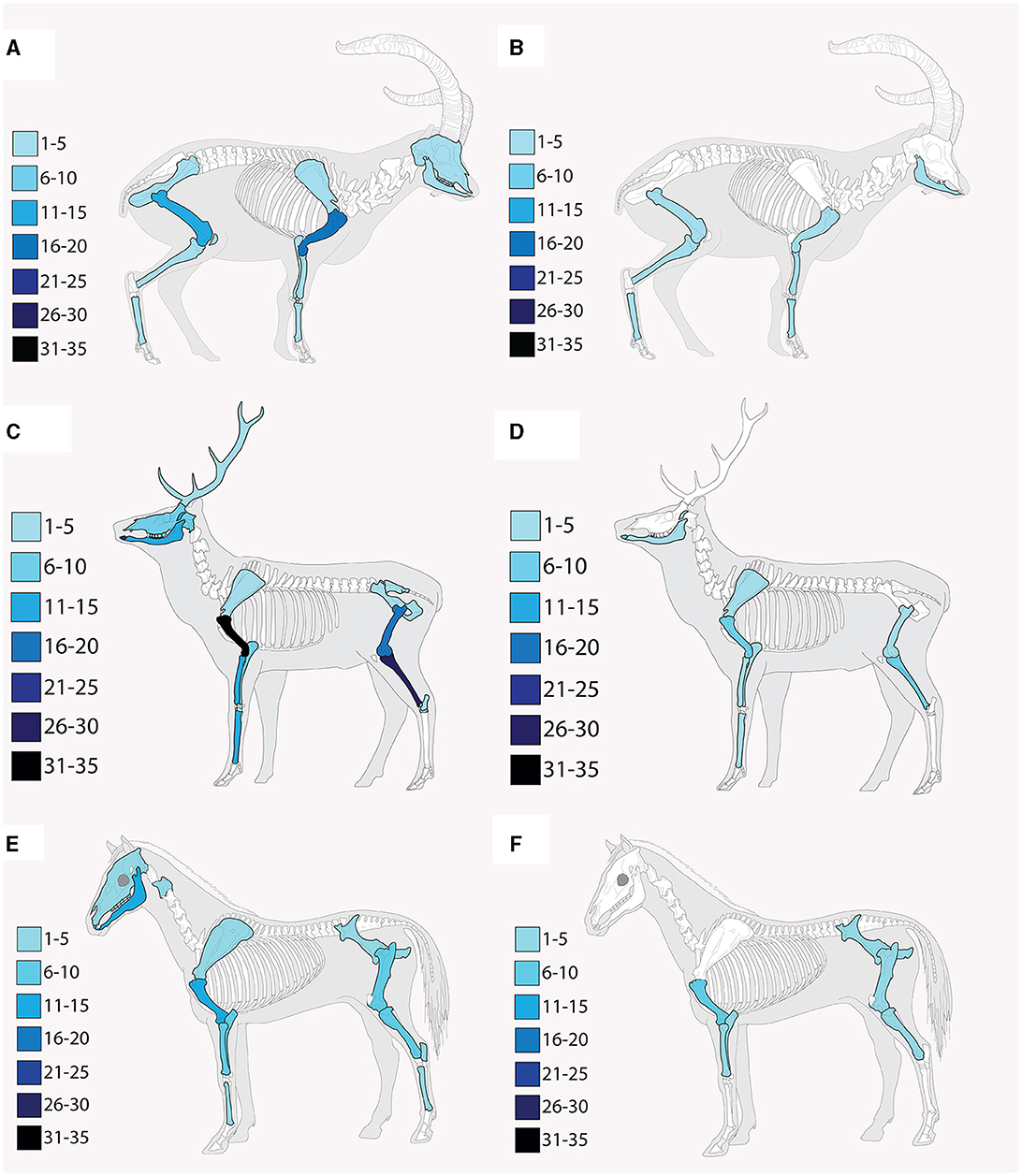
Figure 3. Skeletal profiles based on the MNE of the dominant hunted prey species at Abric Pizarro. (A) Level M caprines, (B) Level P caprines, (C) Level M cervids, (D) Level P cervids, (E) Level M equids, (F) Level P equids. Skeletal representations are based exclusively on identifiable remains, excluding indeterminate elements that cannot be confidently attributed to specific anatomical features (such as indeterminate teeth, cranial fragments, and metapodials, unnumbered vertebrae and ribs, and phalanges and sesamoids that cannot be assigned to the front or rear of the skeleton).
In Level P, size 1 herbivores are represented by a narrower range of bones, including mandibles, vertebrae, humeri, radii, femora, tibiae, and metapodials, with a relatively even representation across all bones. Size 2 herbivores are represented by the same skeletal elements as in Level M, with femora and metatarsals being most well-represented. Size 3 herbivores in Level P exhibit a reduced representation compared to Level M, including humeri, radii, ulnae, pelvises, femora, tibiae, metapodials, and phalanges, with humeri being the most abundant element as observed in Level M.
Long bones from each archaeological unit demonstrated varying shaft lengths and circumferences. In level M (n of level M long bones = 3,748), only 3.33% of shafts were complete in length. Rather, there was a dominance (53.69%) of shafts less than half of their original length, while 42.98% of shafts were more than half of their original length. Similarly, there was a marginal portion of shafts with complete circumference (1.28%) and of shafts with more than half of their original circumference (1.14%). Instead, most (97.58%) long bone shaft circumferences were less than half of their original circumference.
A similar pattern of long bone completeness was observed in the level P long bone assemblage (n of level P lone bones = 799). A marginal percentage (4.95%) of shafts were complete in length, with most (62.83%) shaft lengths less than half of their original length, and some (32.22%) shafts more than half of their original length. Echoing this, complete shaft circumferences accounted for 3.07% of the long bone assemblage and shafts with more than half of their original circumference accounted for 1.07% of the long bone assemblage. The majority (95.86%) of level P long bones had shafts with less than half of their original circumference.
Distinct trends of bone fracture morphology were observed on long bones and epiphyses from both archaeological units. In level M (n of level M long bones and epiphyses = 4,261), spiral fracturing affected 97.75% of long bones and epiphyses. Irregular breakage was observed on 8.11% of bones. Transversal (3.44%) and V-shaped (1.30%) bone morphology contributed marginally to breakage patterns. A similar trend was observed in level P (n of level P long bones and epiphyses = 893), with a dominance (99.73%) of spiral fractures, marginal transversal (0.27%) and irregular (0.27%) breakage, and no V-shaped morphology.
Orthogonal bone morphology, as defined by the presence of right angles or sharp breaks on the cortical surface, was observed in 21.91% of the long bones and epiphyses contributing to the identifiable assemblage (n of level M and level P long bones and epiphyses = 5,154). Orthogonal bone morphology was slightly more pronounced in level P, accounting for 35.72% of the level P long bones and epiphyses, while affecting 26.50% of the level M long bones and epiphyses.
Very few remains demonstrated non-anthropogenic bone surface modifications. Amongst the level M faunal assemblage, tooth pits from medium-sized carnivores were observed on 0.05% (n = 4) of the identifiable assemblage, affecting one C. elaphus second phalanx, a single Cervidae metacarpal and tibia, and two mammal epiphyses. Evidence of digestion, including crenulated edges, was observed on two size 2 herbivore long bone fragments, impacting 0.02% of the identifiable assemblage. Marks from rodent scavenging were additionally identified an Equidae humerus and a mammal epiphysis (0.02% of the level M identifiable assemblage).
Tooth pitting from medium-sized carnivores was the only non-anthropogenic modification observed within the level P faunal assemblage, affecting a C. elaphus tibia, two size 2 herbivore long bone fragments, and a mammal epiphysis (0.23% of the level P identifiable assemblage).
Anthropic bone surface modifications were documented in both levels M and P of the identifiable assemblage, encompassing a variety of features such as incisions, scrapes, chop marks, and direct (impact points and notches) and indirect percussion (Table 4; Figure 4). These modifications are primarily concentrated to undiagnostic fragments and unidentifiable herbivore long bones. However, other prime meat- and marrow-bearing portions (i.e., long bones, epiphyses, phalanges, and mandibles) from key prey species (i.e., caprids, cervids, and equids) are also well represented.
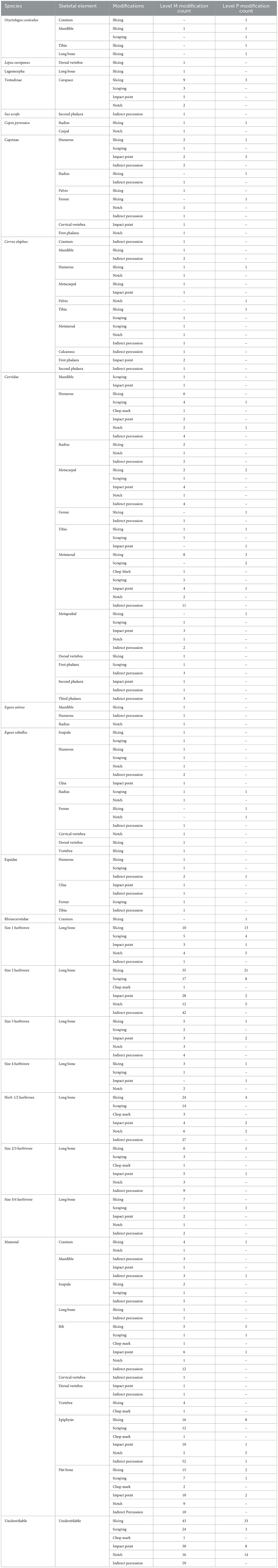
Table 4. Anthropogenic modifications identified on the Abric Pizarro levels M and P faunal material.

Figure 4. Examples of anthropogenic bone surface modifications identified within the Abric Pizarro faunal assemblage. (A) Slicing marks on Cervus elaphus radius, (B) scraping marks on size 2 herbivore long bone, (C) indirect percussion on Caprinae humerus, (D), orthogonal bone breakage of Cervidae radius, (E) direct percussion on Cervus elaphus metacarpal.
In the level M identifiable assemblage, 2.70% of bones displayed incisions, while 1.35% exhibited scrapes. Incisions were predominantly observed on unidentifiable long bone fragments (1.04%) from all sized herbivores and undiagnostic bone fragments (0.51%). Identifiable long bones with slicing marks include humeri (0.19%), radii (0.04%), a single tibia (0.01%), and metapodials (0.14%). Epiphyses (0.19%) were also affected by slicing marks. Several flat bones exhibited incisions, including crania (0.05%), mandibles (0.04%), scapulae (0.04%), ribs (0.06%), a single pelvis (0.01%), and unidentifiable flat bones (0.18%). Several vertebrae additionally demonstrated incisions (0.10%). Scrapes were similarly observed primarily on unidentifiable long bone fragments (0.47%) and non-diagnostic bone fragments (0.27%). Within the identifiable assemblage, scrapes impacted humeri (0.12%), a single radius (0.01%), a single femur (0.01%), tibiae (0.02%), metapodials (0.11%), a single mandible (0.01%), scapulae (0.02%), a single rib (0.01%), a single phalange (0.01%), epiphyses (0.14%), and unidentifiable flat bones (0.08%).
Chop marks were rare, affecting only 0.16% of bones in level M. Chop marks were identified on humeri (0.02%), a single metatarsal (0.01%), a single rib (0.01%), a single vertebra (0.01%), a single epiphysis (0.01%), unidentifiable long bones (0.06%), and unidentifiable flat bones (0.02%).
Direct percussion, represented by impact points and notches affected 2.53% of bones, and indirect percussion was observed on 3.12% of bones. Again, direct percussion is predominantly focused on unidentifiable long bones (0.90%) and undiagnostic bone fragments (0.55%). Identifiable long bones exhibiting direct percussion include humeri (0.11%), ulnae (0.02%), radii (0.04%), a single femur (0.01%), and metapodials (0.20%). Directly percussed epiphyses were also noted (0.18%). Flat bones exhibiting direct percussion are mostly unidentifiable (0.23%), though also include a single cranial fragment (0.01%), mandibles (0.02%), and ribs (0.08%). Other bones contributing to directly percussed elements include vertebrae (0.04%), phalanges (0.05%), and a single carpal (0.01%). Following the observed trend, indirect percussion is concentrated to unidentifiable long bones (1.03%) and non-diagnostic fragments (0.70%). Identifiable long bones with evidence of indirect percussion include humeri (0.14%), ulnae (0.02%), radii (0.04%), femora (0.04%), tibiae (0.02%), and metapodials (0.24%). A similar proportion (0.19%) of indirectly percussed epiphyses was noted to that of directly percussed epiphyses. Indirectly percussed flat bones include cranial fragments (0.05%), mandibles (0.06%), scapulae (0.08%), ribs (0.14%), and unidentifiable long bones (0.12%). Other indirectly percussed skeletal elements include vertebrae (0.02%), phalanges (0.10%), and a single calcaneus (0.01%).
In the level P identifiable assemblage, the incidence of anthropic bone surface modifications was notably varied from that of level M. Incisions were found on 6.43% of bones, and scrapes affected 1.32% of bones. Similar to level M, incisions primarily impacted unidentifiable long bones from all herbivore sizes (2.41%) and non-diagnostic fragments (1.89%). Identifiable long bones demonstrating slicing marks include a single humerus (0.01%), radii (0.11%), femora (0.17%), tibiae (0.17%), and metapodials (0.34%). Incised epiphyses account for 0.46% of the identifiable assemblage. Flat bones affected by incisions include cranial fragments (0.17%), a single mandible (0.01%), ribs (0.29%), and unidentifiable long bones (0.15%). Scraping was primarily noted on unidentifiable long bones (0.75%), though was also observed on a single humerus (0.01%), a single radius (0.01%), metatarsals (0.11%), a single mandible (0.01%), a single rib (0.01%), a single unidentifiable flat bone (0.01%), and undiagnostic fragments (0.17%). No chop marks were identified in this level.
Direct percussion within level P was documented on 3.50% of bones, while indirect percussion was observed on a smaller proportion, affecting only 0.17% of bones. Mimicking the pattern observed throughout both archaeological strata, direct percussion primarily impacts unidentifiable long bones from size 1 through size 3 herbivores (1.38%) and non-diagnostic fragments (1.09%). Epiphyses and undiagnostic flat bones contribute 0.34 and 0.11%, respectively, to the modified identifiable assemblage. Directly percussed identifiable elements include humeri (0.29%), a single femur (0.01%), a single tibia (0.01%), a single metatarsal (0.01%), a single rib (0.01%), and a single pelvis (0.01%). Indirectly percussed elements include an equid humerus (0.01%), a mammal mandible (0.01%), and a mammal epiphysis (0.01%).
The Abric Pizarro rockshelter, situated in the Pre-Pyrenean region, presents a unique opportunity to investigate Neanderthal resilience and adaptability during the climatically challenging period of MIS 4. Traditionally, this period, characterised by extreme aridity and changing landscapes, has been considered unsuitable for Neanderthal occupation (d'Errico and Sánchez Goñi, 2003; Fernández-García et al., 2023). However, the zooarchaeological analysis of faunal remains from levels M and P at Abric Pizarro provides compelling data that challenge these assumptions and sheds light on Neanderthal hunting and butchery behaviours, as well as their ability to exploit diverse dietary resources.
It is worth noting the disparity in the number of bones between levels M and P at Abric Pizarro. Level M represents a palimpsest with a depth of ~80 cm, contributing to a larger quantity of bones, whereas Level P constitutes a more discrete unit, with a depth of only 5–10 cm. Given the extensive deposition over thousands of years, it becomes challenging to formulate hypotheses regarding site use or seasonality. Observations from numerous other sites within the same spatiotemporal region, including Covalejos Cave (Sánchez-Hernández et al., 2019), Cova Gran de Santa Linya (Samper Carro et al., 2020), Abric Romaní (Fernández-Laso et al., 2010; Rosell et al., 2012), Cueva del Esquilleu (Yravedra Sáinz de los Terreros et al., 2014), El Castillo (Pike-Tay et al., 1999), Pié Lombard (Texier et al., 2011), and Abri du Maras (Daujeard et al., 2019; Moncel et al., 2021) indicate a general pattern of seasonal site use. However, the highly fragmented nature of the assemblage, along with potential biases in conducting age-at-death determinations, such as the greater ease in identifying very young individuals, presents challenges in confidently assessing occupation patterns at Abric Pizarro.
Levels M and P at Abric Pizarro exhibit a conspicuous abundance of Neanderthal-deposited prey species, coupled with a notably limited presence of carnivore remains. Moreover, these strata are delineated by culturally sterile layers. The conspicuous dearth of carnivore remains observed in comparison to other sites in Spain, such as Abric Romaní (Rosell et al., 2012, 2019), L'Arbreda Cave (Estévez, 1987; Lloveras et al., 2010), and Moros de Gabasa (Blasco, 1995, 1997; Blasco Sancho and Montes Ramírez, 1997), underscores the exceptional nature of the faunal assemblage at Abric Pizarro. In contrast to these sites, where the percentage of carnivore remains is considerably higher, Abric Pizarro and its neighbouring sites, including Cova Gran de Santa Linya and Roca dels Bous (Benito-Calvo et al., 2020; Samper Carro et al., 2020), exhibit a significantly lower prevalence of carnivore remains. While the prevalence of carnivore remains at Moros de Gabasa is attributed to its general function as a hyena den (Blasco Sancho and Montes Ramírez, 1997), the relatively high number of carnivores at Abric Romaní and L'Arbreda Cave are attributable to alternating periods of occupation between carnivores and hominins (Estévez, 1987; Lloveras et al., 2010; Rosell et al., 2012). As such, the carnivore remains are often found independent of the archaeological levels in which the hominin prey species are observed (Estévez, 1987; Rosell et al., 2012). Moreover, the occurrence of bone surface modifications generated by carnivores is generally low, indicating that human occupations are responsible for primary deposition of faunal assemblages (Rosell et al., 2012). This pattern lends support to the interpretation that the faunal assemblages at Abric Pizarro were primarily accumulated through hominin activities rather than carnivore interactions.
Despite the limited occurrence of carnivore remains at Abric Pizarro, they offer valuable insights into the potential mechanisms by which they were introduced to the site. Absence of taphonomic indicators indicative of natural processes, such as bone abrasion and polishing, suggests that phenomena like water movement or slope washes were not responsible for the deposition of carnivore remains. The predominance of isolated elements, such as teeth and phalanges, occasionally accompanied by larger post-cranial bones like ulnae, vertebrae, and pelvises, implies their introduction through secondary mechanisms, likely involving the activities of other fauna. Processes such as scavenging or predation events by other carnivores could have contributed to their presence. However, the culturally sterile layers and the absence of concentrated bone accumulations typical of carnivore dens suggest that carnivore-mediated deposition was not the primary mode of introduction. It is also plausible that the remains were transported to the site through the actions of other animals, such as burrowing species, capable of displacing carcasses. The absence of Neanderthal-mediated modifications on carnivore bones further supports the inference that Neanderthals did not bring the bones to the site, particularly as typically non-meat- and marrow-bearing bones are present. Nevertheless, the scarcity of carnivore remains complicates the determination of the mechanisms underlying their deposition, leaving open the possibility that they are the result of Neanderthal scavenging or the transportation of carnivore elements for alternate purposes, such as toolmaking. Further analyses, particularly of spatial distribution patterns, may yield additional insights into the intricate interactions between hominins and carnivores at Abric Pizarro.
The presence of black spots on the cortical surface of bones raises questions regarding site formation processes. This phenomenon may be attributed to post-depositional taphonomic processes impacting the bones, or alternatively, to the presence of manganese-rich sediments owing to the calcareous composition of the rockshelter (López-González et al., 2006; Marín Arroyo et al., 2008). Manganese deposition on bone surfaces can result from soil humification subsequent to anthropic occupation and the decomposition of organic matter, as observed in sites characterised by seasonal occupation, exemplified by the case of El Mirón Cave (Marín Arroyo et al., 2008). Yet, the evidence for seasonal occupation at Abric Pizarro remains limited, and therefore, it is conceivable that the black staining observed on bone surfaces predominantly stems from the intrinsic geological characteristics of the rockshelter.
The identification of burned bones at the Abric Pizarro site, in the absence of evident hearth-related features, poses intriguing questions. One plausible explanation for the lack of identifiable hearths may stem from preservation challenges. It is pertinent to note that despite the absence of conspicuous hearth features, the presence of concentrated black stains within the sediment matrix and the recovery of charcoal fragments from both levels M and P suggest past fire-related activities. If indeed hearths were integral to the Neanderthal occupation at Abric Pizarro, it would imply a level of spatial organisation consistent with other prehistoric sites of similar antiquity (e.g., Gesher Benot Ya'aqov in Israel, and Abric Romaní, Cova Gran de Santa Linya and Roca dels Bous in Spain; Alperson-Afil et al., 2009; Marín et al., 2019; Samper Carro et al., 2020). However, to substantiate assertions of spatial organisation, further investigations into the spatial distribution of bone fragments are imperative. Such inquiries represent a promising avenue for advancing our understanding of site use dynamics and Neanderthal behavioural patterns at Abric Pizarro.
The faunal assemblage from Abric Pizarro reveals a diverse spectrum of dietary resources exploited by Neanderthals, ranging from smaller herbivorous prey like caprids to very large herbivores such as Bos/Bison. This rich array of dietary choices lends support to the hypothesis that the site was surrounded by a resource-rich environment. Notably, the prevalence of cervids and medium-sized herbivores, coupled with a notable abundance of meat-bearing bones (i.e., long bones and mandibles), suggests the implementation of methodical hunting and butchery practices.
The marked preference for deer among the faunal remains attests to a pattern of high hunting efficiency, underscoring the Neanderthals' adeptness at effectively exploiting the surrounding landscape. Comparative analyses with sites across the broader geographical region, encompassing locales from Portugal, Spain and southern France, highlight striking similarities in hunting preferences (Steele, 2004; Baena Preysler et al., 2012; Blasco and Fernández-Peris, 2012; Uzquiano et al., 2012; Yravedra and Gómez Castanedo, 2014; Nabais, 2018; Bargalló et al., 2020; Samper Carro et al., 2020; Rendu, 2022). Yet not all Middle Palaeolithic sites in the Iberian Peninsula demonstrate a preference for deer. For example, caprines are preferred at Cueva del Esquilleu (Uzquiano et al., 2012; Yravedra and Gómez Castanedo, 2014), Abrigo de la Quebrada (Sanchis Serra et al., 2013; Real et al., 2018; Real Margalef et al., 2019), El Salt (Garralda et al., 2014; Real et al., 2018), and Valdegoba Cave (Rodríguez-Gómez et al., 2022). Considering the mountainous topography of these sites, it is likely that the exploitation of caprines is related to their abundance within the local environment. As such, the preference for deer within biomes abundant with available prey and between sites across the broader geographic expanse hints at a level of choice and preference exercised by Neanderthals. This implies a degree of cognitive complexity in their subsistence strategies, encompassing ecological awareness, deliberate decision-making, and knowledge transmission. Overall, the preference for deer within diverse ecological settings and across different sites implies that Neanderthals were not simply reacting to immediate environmental conditions. This aspect of their behaviour provides valuable insights into the complexity of Neanderthal cognitive abilities and their capacity to adapt to a variety of ecological contexts.
It is pertinent to underscore that the present analysis has primarily concentrated on the examination of large vertebrates within the faunal assemblage. However, small vertebrates, including lagomorphs and turtles, are presently undergoing detailed study. The outcomes derived from this analysis of small vertebrates will be amalgamated and compared with the findings obtained from the investigation of large vertebrates. Such a holistic approach contributes to a more nuanced understanding of Neanderthal resource exploitation practices.
The predominance of spiral fracturing observed in both level M and level P, affecting a significant majority of long bones and epiphyses, suggests a consistent pattern in Neanderthal butchery actions at the Abric Pizarro site. Spiral fractures, particularly in the relative absence of carnivore remains and taphonomic indicators of trampling, are typically associated with intentional, systematic bone breakage aimed at accessing the nutrient-rich marrow contained within the bones (Myers et al., 1980; Haynes, 1983; Villa and Mahieu, 1991; Bar-Oz et al., 2008; Rosell et al., 2012). This technique indicates a deliberate and skilful approach to carcass processing, wherein Neanderthals likely used stone tools such as hammerstones to create these distinctive fracture patterns. The marginal occurrence of other fresh or “green” breakages (i.e., V-shaped and irregular) further supports the interpretation of intentional butchery practices, likely focused on marrow extraction, with these variations possibly resulting from occasional differences in tool use or butchery techniques (Villa and Mahieu, 1991; Bar-Oz et al., 2008; Rosell et al., 2012). Minimal transversal morphology suggests a lack of accidental or incidental dry bone damage, reinforcing the notion of purposeful and controlled processing of faunal resources by Neanderthal populations at the site. It is imperative to note that while the fracture data elicits discernible patterns within the identifiable assemblage, the non-diagnostic assemblage (i.e., fragments < 2 cm) was not subject to detailed taphonomic analysis. As such, it is possible that incidences of dry bone damage are more common in the non-diagnostic assemblage.
The presence of distinctive orthogonal bone morphology on meat and marrow-bearing bones (i.e., long bones, phalanges, and epiphyses) further provides compelling evidence of intensive Neanderthal utilisation of edible animal portions for nutritional purposes. The relative scarcity of carnivore remains and carnivore-induced modifications within the assemblage further suggests that these breakages are likely the result of Neanderthal activities, possibly involving the percussion of short bones using stone tools like hammerstones. Analogous patterns of intense faunal processing in response to nutritional stress have been observed at Roc de Marsal and Pech IV in France during colder glacial periods (Hodgkins et al., 2016). Considering the relatively warm and stable environmental conditions indicated by preliminary paleoenvironmental analysis at the Abric Pizarro site (see text footnote 1) and the abundance of temperate taxa such as deer, it is plausible that Neanderthals favoured habitation in the region. The pronounced intensity of butchery activities at Abric Pizarro, aimed at maximising caloric extraction from prey, even during periods of relative climatic stability, suggests that Neanderthals regularly engaged in intensive faunal processing of carcasses. This possibly indicates that nutritional stress commonly affected Neanderthals throughout glacial and interglacial periods (see also Hodgkins et al., 2016). Through the examination of these zooarchaeological indicators and their contextual associations, valuable insights can be gained into the adaptive behaviours and dietary practises of Neanderthal groups during MIS 4.
Evidence of systematic butchery practises is additionally substantiated by the notably high proportion of meat-bearing bones, particularly long bones and mandibles, displaying characteristic anthropic surface modifications such as incisions, scrapes, chop marks, and percussion. The analysis of skeletal profiles suggests that the butchery activities took place away from the site, with a preference for prime, meat-bearing bones being selectively transported back to the rockshelter. This pattern is consistent across various prey species and is likely attributed to the challenging accessibility and topographical constraints of Abric Pizarro. These observations collectively underscore a level of technological sophistication in the Neanderthals' approach to processing animal remains, reflecting a deliberate and skilful methodology employed in the extraction of valuable resources from their prey.
To gain a more comprehensive understanding of the specific butchery practises and behaviours employed by Neanderthals, further investigation is currently underway. This involves the incorporation of spatial analysis of bone surface modifications, using the method proposed by Westbury and Samper Carro (under review)2. This ongoing research endeavour holds the potential to provide valuable comparative insights that will complement the findings of this study. It is anticipated to contribute to a more nuanced exploration of Neanderthal subsistence strategies and technological adaptations during the MIS 4 period at Abric Pizarro.
The zooarchaeological investigation at the Abric Pizarro rockshelter provides valuable insights into Neanderthal adaptability and resource exploitation capabilities. The diverse fauna, methodical hunting practices, and deliberate butchery behaviours offer a comprehensive understanding of Neanderthal subsistence and behaviours during the Middle Palaeolithic in the southeast Pre-Pyrenees region. These findings enrich the broader narrative of human evolution, reaffirming the intricate nature of Neanderthal behaviours and cognitive capabilities. The research at Abric Pizarro contributes significantly to the ongoing scholarly discourse on Neanderthals, highlighting their complexity and significance within the context of human evolution. This study provides important insights into the activities and strategies employed by Iberian Neanderthals at the Abric Pizarro site, shedding light on their adaptive behaviours during the critical MIS 4 epoch.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
EW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. SS: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. SV: Project administration, Resources, Software, Writing – review & editing. JP: Project administration, Resources, Software, Writing – review & editing. JM-M: Project administration, Resources, Software, Writing – review & editing. RM: Project administration, Resources, Software, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was financially supported in part by fieldwork funding awarded to EW by the School of Culture, History and Language (Australian National University). Fieldwork in 2022 and subsequent analysis was funded by a Research Scholarship granted by The Leakey Foundation and by an Australian Research Council (ARC) Discovery Early Career Researcher Award (DE240100530) awarded to SS. Fieldwork in Abric Pizarro was supported by the Generalitat de Catalunya, Culture Department. Research in Abric Pizarro was supported by the Spanish Ministry of Science and Innovation (PID2022-136363NB-I00).
The authors would like to acknowledge the Centro de Estudios del Patrimonio Arqueológico de la Prehistoria (Universitat Autònoma de Barcelona) for providing the lab and storage space to undertake this research. The authors would also like to acknowledge the Tartareu city council and Mr. Josep for their assistance (including the provision of water for wet sieving) and permission to work on their land. This is a contribution to research group 2021 SGR 00190 TEDA.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^Samper Carro, S. C., Vega Bolivar, S., Pizarro Barbera, J., Westbury, E., Connor, S., Martínez-Moreno, J., et al. (under review). Living on the edge: Abric Pizarro, a MIS 4 Neanderthal site in the lowermost foothills of the southeastern Prepyrenees (Lleida, Iberian Peninsula).
2. ^Westbury, E., and Samper Carro, S. (under review). A new open-access method applying GIS techniques to the study of bone surface modifications. Int. J. Osteoarchaeol.
Alperson-Afil, N., Sharon, G., Kislev, M., Melamed, Y., Zohar, I., Ashkenazi, S., et al. (2009). Spatial organization of hominin activities at Gesher Benot Ya'aqov, Israel. Science 326, 1677–1680. doi: 10.1126/science.1180695
Baena Preysler, J., Carrión, E., Cuartero, F., and Fluck, H. (2012). A chronicle of crisis: the Late Mousterian in north Iberia (Cueva del Esquilleu, Cantabria, Spain). Quat. Int. 247, 199–211. doi: 10.1016/j.quaint.2011.07.031
Bargalló, A., Gabucio, M. J., Gómez de Soler, B., Chacón, M. G., and Vaquero, M. (2020). “A snapshot of a short occupation in the Abric Romaní rock shelter: archaeo-level Oa,” in Short-Term Occupations in Paleolithic Archaeology: Definition and Interpretation, eds. J. Cascalheira and A. Picin (Cham: Springer), 217–235. doi: 10.1007/978-3-030-27403-0_9
Bar-Oz, G., Belfer-Cohen, A., Meshveliani, T., Djakeli, N., and Bar-Yosef, O. (2008). Taphonomy and zooarchaeology of the Upper Palaeolithic cave of Dzudzuana, Republic of Georgia. Int. J. Osteoarchaeol. 18, 131–151. doi: 10.1002/oa.926
Behrensmeyer, A. K. (1978). Taphonomic and ecologic information from bone weathering. Paleobiology 4, 150–162. doi: 10.1017/S0094837300005820
Benito-Calvo, A., Arnold, L. J., Mora, R., Martínez-Moreno, J., and Demuro, M. (2020). Reconstructing Mousterian landscapes in the southeastern Pyrenees (Roca dels Bous site, Pre-Pyrenees range, Spain). Quat. Res. 97, 167–186. doi: 10.1017/qua.2020.29
Blanco, G., Sánchez-Marco, A., and Negro, J. J. (2021). Night capture of roosting cave birds by Neanderthals: an actualistic approach. Front. Ecol. Evol. 9:733062. doi: 10.3389/fevo.2021.733062
Blasco Sancho, M. F., and Montes Ramírez, L. (1997). Los hiénidos del yacimiento musteriense de Gabasa 1 (Huesca, España). Bolskan 14, 9–27.
Blasco, M. F. (1995). Hombres, fieras y presas: studio arqueozoológico y tafonómico del yacimiento del paleolitico medio de la Cueva de Gabasa 1 (Huesca) [PhD Thesis]. Universidad de Zaragoza, Zaragoza.
Blasco, M. F. (1997). In the pursuit of game: the Mousterian cave site of Gabasa 1 in the Spanish Pyrenees. J. Anthropol. Res. 53, 177–217. doi: 10.1086/jar.53.2.3631276
Blasco, R. (2008). Human consumption of tortoises at level IV of Bolomor Cave (Valencia, Spain). J. Archaeol. Sci. 35, 2839–2848. doi: 10.1016/j.jas.2008.05.013
Blasco, R., Cochard, D., Colonese, A. C., Laroulandie, V., Meier, J., Morin, E., et al. (2022). “Small animal use by Neanderthals,” in Updating Neanderthals: Understanding Behavioural Complexity in the Late Middle Palaeolithic, eds. F. Romagnoli, F. Rivals, and S. Benazzi (Cambridge, MA: Academic Press), 123–206. doi: 10.1016/B978-0-12-821428-2.00010-X
Blasco, R., and Fernández Peris, J. (2009). Middle Pleistocene bird consumption at level XI of Bolomor cave (Valencia, Spain). J. Archaeol. Sci. 36, 2213–2223. doi: 10.1016/j.jas.2009.06.006
Blasco, R., and Fernández-Peris, J. (2012). Small and large game: human use of diverse faunal resources at level IV of Bolomor Cave (Valencia, Spain). Comptes Rendus Pale 1, 265–282. doi: 10.1016/j.crpv.2012.01.003
Brown, W. A. B., and Chapman, N. G. (1991). The dentition of red deer (Cervus elaphus): a scoring scheme to assess age from wear of the permanent molariform teeth. J. Zool. 224, 519–536. doi: 10.1111/j.1469-7998.1991.tb03783.x
Bunn, H. T. (1986). Patterns of skeletal representation and hominid subsistence activities at Olduvai Gorge, Tanzania, and Koobi Fora, Kenya. J. Hum. Evol. 15, 673–690. doi: 10.1016/S0047-2484(86)80004-5
Busk, G. (1865). “On a very ancient human cranium from Gibraltar,” in Report of the British Association for the Advancement of Science 1864 (London), 91–92.
Carvalho, M., Peireira, T., and Manso, C. (2018). Rabbit exploitation in the Middle Paleolithic at Gruta Nova da Columbeira, Portugal. J. Archaeol. Sci. Rep. 21, 821–832. doi: 10.1016/j.jasrep.2018.09.003
Daujeard, C., Vettese, D., Britton, K., Béarez, P., Boulbes, N., Crégut-Bonnoure, E., et al. (2019). Neanderthal selective hunting of reindeer? The case study of Abri du Maras (south-eastern France). Archaeol. Anthropol. Sci. 11, 985–1011. doi: 10.1007/s12520-017-0580-8
d'Errico, F., and Sánchez Goñi, M. F. (2003). Neandertal extinction and the millennial scale climatic variability of OIS 3. Quat. Sci. Rev. 22, 769–788. doi: 10.1016/S0277-3791(03)00009-X
Estévez, J. (1987). La fauna de l'Arbreda (sector Alfa) en el conjunt de faunes del Plistocè Superior. Cypsela 6, 73–87.
Fa, J. E., Stewart, J. R., Lloveras, L., and Vergas, J. M. (2013). Rabbits and hominin survival in Iberia. J. Hum. Evol. 64, 233–241. doi: 10.1016/j.jhevol.2013.01.002
Fernández-García, M., Vidal-Cordasco, M., Jones, J. R., and Marín-Arroyo, A. B. (2023). Reassessing palaeoenvironmental conditions during the Middle to Upper Palaeolithic transition in the Cantabrian region (Southwestern Europe). Quat. Sci. Rev. 301, 1–18. doi: 10.1016/j.quascirev.2022.107928
Fernández-Jalvo, Y., and Andrews, P. (2016). Atlas of Taphonomic Identifications: 1001+ Images of Fossil and Recent Mammal Bone Modification. Springer. doi: 10.1007/978-94-017-7432-1
Fernández-Laso, M. C., Rivals, F., and Rosell, J. (2010). Intra-site changes in seasonality and their consequences on the faunal assemblages from Abric Romaní (Middle Palaeolithic, Spain). Quaternaire. Rev. Assoc. Fr. l'Étude Quaternaire 21, 155–163. doi: 10.4000/quaternaire.5525
Fisher, J. W. (1995). Bone surface modifications in zooarchaeology. J. Archaeol. Method Theor. 2, 7–68.
Garralda, M. D., Galván, B., Hernández, C. M., Mallol, C., Gómez, J. A., Maureille, B., et al. (2014). Neanderthals from El Salt (Alcoy, Spain) in the context of the latest Middle Palaeolithic populations from the southeast of the Iberian Peninsula. J. Hum. Evol. 75, 1–15. doi: 10.1016/j.jhevol.2014.02.019
Grant, A. (1982). “The use of tooth wear as a guide to the age of domestic animals,” in Ageing and Sexing Animal Bones from Archaeological Sites, eds. B. Wilson, C. Grigson, and S. Payne (Oxford: B.A.R.), 91–108.
Greenfield, H. J., and Arnold, E. R. (2008). Absolute age and tooth eruption and wear sequences in sheep and goat: determining age-at-death in zooarchaeology using a modern control sample. J. Archaeol. Sci. 35, 836–849. doi: 10.1016/j.jas.2007.06.003
Hajdinjak, M., Mafessoni, F., Skov, L., Vernot, B., Hübner, A., Fu, Q., et al. (2021). Initial Upper Palaeolithic humans in Europe had recent Neanderthal ancestry. Nature 592, 253–257. doi: 10.1038/s41586-021-03335-3
Hardy, K. (2022). “The use of plant by Neanderthals as food, medicine, and raw materials,” in Updating Neanderthals: Understanding Behavioural Complexity in the Late Middle Palaeolithic, eds. F. Romagnoli, F. Rivals, and S. Benazzi (Cambridge, MA: Academic Press), 145–162. doi: 10.1016/B978-0-12-821428-2.00004-4
Haynes, G. (1983). Frequencies of spiral and green-bone fractures on ungulate limb bones in modern surface assemblages. Am. Antiq. 48, 102–114. doi: 10.2307/279822
Hillson, S. (1991). Mammal Bones and Teeth: An Introductory Guide to Methods of Identification. London: Routledge.
Hodgkins, J., Marean, C. W., Turq, A., Sandgathe, D., McPherron, S. J. P., Dibble, H., et al. (2016). Climate-mediated shifts in Neandertal subsistence behaviors at Pech de l'Azé IV and Roc de Marsal (Dordogne Valley, France). J. Hum. Evol. 96, 1–18. doi: 10.1016/j.jhevol.2016.03.009
Lemoine, X., Zeder, M. A., Bishop, K. J., and Rufolo, S. J. (2014). A new system for computing dentition-based age profiles in Sus scrofa. J. Archaeol. Sci. 47, 179–193. doi: 10.1016/j.jas.2014.04.002
Lloveras, L., Moreno García, M., and Nadal, J. (2009). Butchery, cooking and human consumption marks on rabbit (Oryctolagus cuniculus) bones: an experimental study. J. Taphonomy 7, 179–201.
Lloveras, L., Moreno-García, M., Nadal, J., Moroto, J., Soler, J., Soler, N., et al. (2010). The application of actualistic studies to assess the taphonomic origin of Musterian rabbit accumulations from Arbreda Cave (North-East Iberia). Archaeofauna 19, 99–119.
Lloveras, L., Moreno-García, M., Nadal, J., and Zilhão, J. (2011). Who brought in the rabbits? Taphonomical analysis of Mousterian and Solutrean leporid accumulations from Gruta do Caldeirão (Tomar, Portugal). J. Archaeol. Sci. 38, 2434–2449. doi: 10.1016/j.jas.2011.05.012
López-González, F., Grandal-d'Anglade, A., and Vidal-Romaní, J. R. (2006). Deciphering bone depositional sequences in caves through the study of manganese coatings. J. Archaeol. Sci. 33, 707–717. doi: 10.1016/j.jas.2005.10.006
Marean, C. W., and Kim, S. Y. (1998). Mousterian large-mammal remains from Kobeh Cave: behavioral implications for Neanderthals and Early Modern Humans. Curr. Anthropol. 39(S1), S79–S114. doi: 10.1086/204691
Marín Arroyo, A. B., Landete Ruiz, M. D., Vidal Bernabeu, G., Seva Román, R., González Morales, M. R., Straus, L. G., et al. (2008). Archaeological implications of human-derived manganese coatings: a study of blackened bones in El Mirón Cave, Cantabrian Spain. J. Archaeol. Sci. 35, 801–813. doi: 10.1016/j.jas.2007.06.007
Marín, J., Rodríguez-Hidalgo, A., Vallverdú, J., Gómez de Soler, B., Rivals, F., Rabuñal, J. R., et al. (2019). Neanderthal logistic mobility during MIS3: zooarchaeological perspective of Abric Romaní level P (Spain). Quat. Sci. Rev. 225:106033. doi: 10.1016/j.quascirev.2019.106033
Martínez-Polanco, M. F., Blasco, R., Rosell, J., Ibáñez, N., and Vaquero, M. (2017). Rabbits as Food at the end of the Upper Palaeolithic at Molí del Salt (Catalonia, Spain). Int. J. Osteoarchaeol. 27, 342–355. doi: 10.1002/oa.2541
Moncel, M.-H., Chacón, M. G., Vettese, D., Courty, M.-A., Daujeard, C., Eixea, A., et al. (2021). Late Neanderthal short-term and specialized occupations at the Abri du Maras (South-East France, level 4.1, MIS 3). Archaeol. Anthropol. Sci. 13, 1–28. doi: 10.1007/s12520-021-01285-5
Myers, T. P., Voorhies, M. R., and Corner, R. G. (1980). Spiral fractures and bone pseudotools at paleontological sites. Am. Antiq. 45, 483–490. doi: 10.2307/279863
Nabais, M. (2018). Neanderthal subsistence in Portugal: what evidence? Archaeol. Int. 21, 95–100. doi: 10.5334/ai-376
Nabais, M., Dupont, C., and Zilhão, J. (2023). The exploitation of crabs by Last Interglacial Iberian Neanderthals: the evidence from Gruta da Figueira Brava (Portugal). Front. Environ. Archaeol. 2:1097815. doi: 10.3389/fearc.2023.1097815
Nabais, M., and Zilhão, J. (2019). The consumption of tortoise among last interglacial Iberian Neanderthals. Quat. Sci. Rev. 217, 225–246. doi: 10.1016/j.quascirev.2019.03.024
Nicholson, R. A. (1993). A morphological investigation of burnt animal bone and an evaluation of its utility in archaeology. J. Archaeol. Sci. 20, 411–428. doi: 10.1006/jasc.1993.1025
Pales, L., and Garcia, M. A. (1981). Atlas Ostéologique pour Servir à l'Identification des Mammifères du Quaternaire. Paris: Editions du Centre National de la Recherce Scientifique.
Pelletier, M., Desclaux, E., Brugal, J.-P., and Texier, P.-J. (2019). The exploitation of rabbits for food and pelts by last interglacial Neandertals. Quat. Sci. Rev. 224, 1–20. doi: 10.1016/j.quascirev.2019.105972
Pike-Tay, A., Cabrera Valdés, V., and Bernaldo de Quirós, F. (1999). Seasonal variations of the Middle–Upper Paleolithic transition at El Castillo, Cueva Morín and El Pendo (Cantabria, Spain). J. Hum. Evol. 36, 283–317. doi: 10.1006/jhev.1998.0271
Pizarro, J., Roy, M., Roda, X., Vega, S., Samper, S., Plasencia, J., et al. (2013). Nous elements de reflexió al voltant del poblament del Prepirineu oriental: al llarg del plistocè superior i l'holocè. Colloqui d'Arqueologia d'Odèn 3, 17–26.
Real Margalef, C., Sanchís Serra, A., Morales Pérez, J. V., Bel Martínez, M. Á., Eixa Vilanova, A., Zilhão, J., et al. (2019). Estudio de la fauna del nivel IV del Abrigo de la Quebrada y su aportación al conocimiento de la economía y el comportamiento humano en el Paleolítico medio de la vertiente Mediterránea Ibérica. SPAL 28, 17–49. doi: 10.12795/spal.2019.i28.13
Real, C., Eixea, A., Sanchis, A., Morales, J. V., Klasen, N., Zilhão, J., et al. (2018). Abrigo de la Quebrada level IV (Valencia, Spain): interpreting a Middle Palaeolithic palimpsest from a zooarchaeological and lithic perspective. J. Paleolit. Archaeol. 3, 187–224. doi: 10.1007/s41982-018-0012-z
Rendu, W. (2022). “Selection versus opportunism: a view from Neanderthal subsistence strategies,” in updating Neanderthals: understanding behavioural complexity in the late Middle Palaeolithic, eds. F. Romagnoli, F. Rivals, and S. Benazzi (Cambridge, MA: Academic Press), 109–122. doi: 10.1016/B978-0-12-821428-2.00013-5
Rodríguez-Gómez, G., Pérez-Fernández, E., Fernandez, P., Arsuaga, J. L., Díez, C., Arceredillo, D., et al. (2022). Palaeoecology of the Southern chamois from Valdegoba Cave (Burgos, Spain) and its exploitation by the Neanderthals. Lethaia 55, 1–25. doi: 10.18261/let.55.4.3
Romagnoli, F., Rivals, F., and Benazzi, S. (2022). Updating Neanderthals: Understanding Behavioural Complexity in the Late Middle Palaeolithic. Cambridge, MA: Academic Press.
Rosell, J., Cáceres, I., Blasco, R., Bennàsar, M., Bravo, P., Campeny, G., et al. (2012). A zooarchaeological contribution to establish occupational patterns at Level J of Abric Romaní (Barcelona, Spain). Quat. Int. 247, 69–84. doi: 10.1016/j.quaint.2011.01.020
Rosell, J., Modesto-Mata, M., Fernández-Laso, M. C., Modolo, M., and Blasco, R. (2019). Refitting bones to reconstruct the diversity in Middle Palaeolithic human occupations: the case of the Abric Romaní site (Capellades, Barcelona, Spain). Archaeol. Anthropol. Sci. 11, 4601–4619. doi: 10.1007/s12520-019-00887-4
Saladié, P., Huguet, R., Díez, C., Rodríguez-Hidalgo, A., Cáceres, I., Vallverdú, J., et al. (2011). Carcass transport decisions in Homo antecessor subsistence strategies. J. Hum. Evol. 61, 425–446. doi: 10.1016/j.jhevol.2011.05.012
Samper Carro, S. C., Martínez-Moreno, J., and Mora Torcal, R. (2020). Winds of change: zooarchaeological approach to the Middle - Upper Palaeolithic transition in Cova Gran of Santa Linya (Lleida, south-eastern Pre-Pyrenees). J. Paleolit. Archaeol. 3, 989–1031. doi: 10.1007/s41982-020-00066-1
Sánchez Goñi, M. F. (2022). “The climatic and environment context of the Late Pleistocene,” in Updating Neanderthals: Understanding Behavioural Complexity in the Late Middle Palaeolithic, eds F. Romagnoli, F. Rivals, and S. Benazzi (Cambridge, MA: Academic Press), 17–38. doi: 10.1016/B978-0-12-821428-2.00017-2
Sánchez-Hernández, C., Gourichon, L., Pubert, E., Rendu, W., Montes, R., Rivals, F., et al. (2019). Combined dental wear and cementum analyses in ungulates reveal the seasonality of Neanderthal occupations in Covalejos Cave (Northern Iberia). Sci. Rep. 9:14335. doi: 10.1038/s41598-019-50719-7
Sanchis Serra, A., Morales Pérez, J. V., Real Margalef, C., Eixea Vilanova, A., Zilhão, J., Villaverde Bonilla, V., et al. (2013). “Los conjuntos faunísticos del Paleolítico medio del Abrigo de la Quebrada (Chelva, Valencia): problemática de estudio, metodología aplicada y síntesis de los primeros resultados,” in Animals i Arqueologia Hui: I Jornades d'Arqueozoologia del Museu de Prehistòria de València, eds. A. Sanchis and J. Ll. Pascual (Valencia: Museu de Prehistòria de València), 65–82.
Sankararaman, S., Mallick, S., Dannemann, M., Prüfer, K., Kelso, J., Pääbo, S., et al. (2014). The genomic landscape of Neanderthal ancestry in present-day humans. Nature 507, 354–357. doi: 10.1038/nature12961
Steele, T. E. (2004). Variation in mortality profiles of red deer (Cervus elaphus) in Middle Palaeoltihic assemblages from western Europe. Int. J. Osteoarchaeol. 14, 307–320. doi: 10.1002/oa.763
Stiner, M. C., Kuhn, S. L., Weiner, S., and Bar-Yosef, O. (1995). Differential burning, recrystallization, and fragmentation of archaeological bone. J. Archaeol. Sci. 22, 223–237. doi: 10.1006/jasc.1995.0024
Texier, P.-J., Renault-Miskovsky, J., Desclaux, E., de Lumley, M.-A., Porraz, G., and Tomasso, A. (2011). L'abri Pié Lombard à Tourrettes-sur-Loup (Alpes-Maritimes): anciennes fouilles, nouvelles données. Bull. Musée Anthropol. Préhistorique Monaco 51, 19–49.
Trinkaus, E., and Shipman, P. (1993). The Neanderthals: Changing the Image of Mankind. New York, NY: Knopf.
Uzquiano, P., Yravedra, J., Ruiz Zapata, B., José Gil Garcia, M., Sesé, C., Baena Preysler, J., et al. (2012). Human behaviour and adaptations to MIS 3 environmental trends (>53-30 ka BP) at Esquilleu cave (Cantabria, northern Spain). Quat. Int. 252, 82–89. doi: 10.1016/j.quaint.2011.07.023
Vaquero, M. (2022). “The organisation of living spaces in Neanderthal campsites,” in Updating Neanderthals: Understanding Behavioural Complexity in the Late Middle Palaeolithic, eds. F. Romagnoli, F. Rivals, and S. Benazzi (Cambridge, MA: Academic Press), 207–226. doi: 10.1016/B978-0-12-821428-2.00001-9
Vega Bolivar, S., Samper Carro, S. C., Pizarro Barberà, J., Mora, R., Martínez-Moreno, J., Benito-Calvo, A., et al. (2015). “Abric Pizarro (Àger, Lleida): un nou jaciment del Paleolític Mitjà al Prepireneu Oriental,” in Primeres Jornades d'Arqueologia i Paleontologia de Ponent (Lleida), 32−39.
Villa, P., and Mahieu, E. (1991). Breakage patterns of human long bones. J. Hum. Evol. 21, 27–48. doi: 10.1016/0047-2484(91)90034-S
Yravedra Sáinz de los Terreros, J., Gómez-Castanedo, A., Aramendi Picado, J., and Baena Preysler, J. (2014). Specialised hunting of Iberian ibex during Neanderthal occupation at El Esquilleu Cave, northern Spain. Antiquity 88, 1035–1049. doi: 10.1017/S0003598X00115303
Yravedra, J., and Gómez Castanedo, A. (2014). Taphonomic implications for the Late Mousterian of South-West Europe at Esquilleu Cave (Spain). Quat. Int. 337, 225–236. doi: 10.1016/j.quaint.2013.09.030
Keywords: Neanderthal behaviour, zooarchaeology, adaptability, resilience, Neanderthal diet, MIS 4, MIS 3
Citation: Westbury E, Samper Carro S, Vega Bolivar S, Pizarro J, Martínez-Moreno J and Mora R (2024) Neanderthal resilience and adaptability: insights from the Abric Pizarro faunal assemblage during the MIS 4. Front. Environ. Archaeol. 3:1405535. doi: 10.3389/fearc.2024.1405535
Received: 23 March 2024; Accepted: 22 April 2024;
Published: 09 May 2024.
Edited by:
Mariana Nabais, Institut Català de Paleoecologia Humana i Evolució Social (IPHES), SpainReviewed by:
Jose Yravedra, Complutense University of Madrid, SpainCopyright © 2024 Westbury, Samper Carro, Vega Bolivar, Pizarro, Martínez-Moreno and Mora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eboni Westbury, RWJvbmkuV2VzdGJ1cnlAYW51LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.