An industry perspective on the use of machine learning in drug and vaccine safety
- 1GlaxoSmithKline, Global Safety, Durham, NC, United States
- 2GlaxoSmithKline, Global Safety, Upper Providence, PA, United States
- 3GlaxoSmithKline, Global Safety, Brentford, Middlesex, United Kingdom
- 4London School of Hygiene and Tropical Medicine, London, United Kingdom
A Corrigendum on
An industry perspective on the use of machine learning in drug and vaccine safety
by Painter JL, Kassekert R and Bate A (2023). Front. Drug. Saf. Regul. 3:1110498. doi: 10.3389/fdsfr.2023.1110498
In the published article, there was an error in Figure 1 as published. The figure failed to include the details on the individual node references. The corrected Figure 1 and its caption appear below.
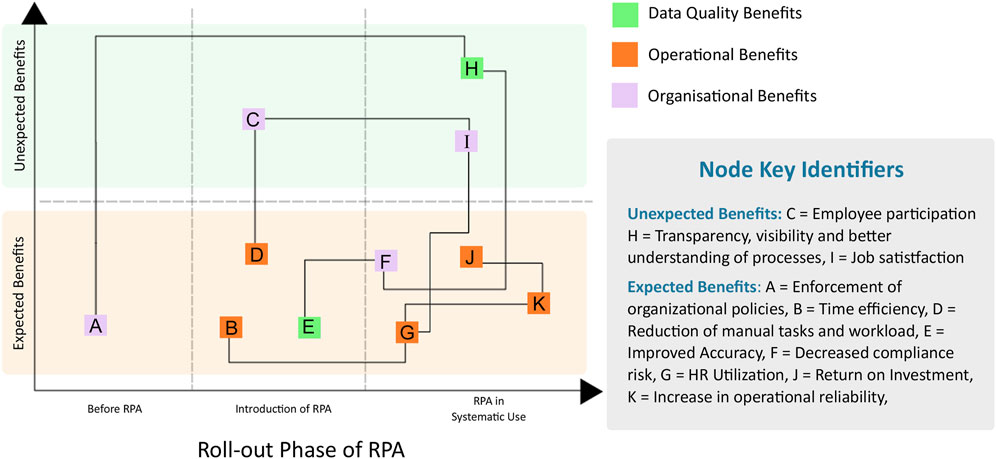
Figure 1. The benefits of robotic process automation (RPA). Additional details are found in Table 1.
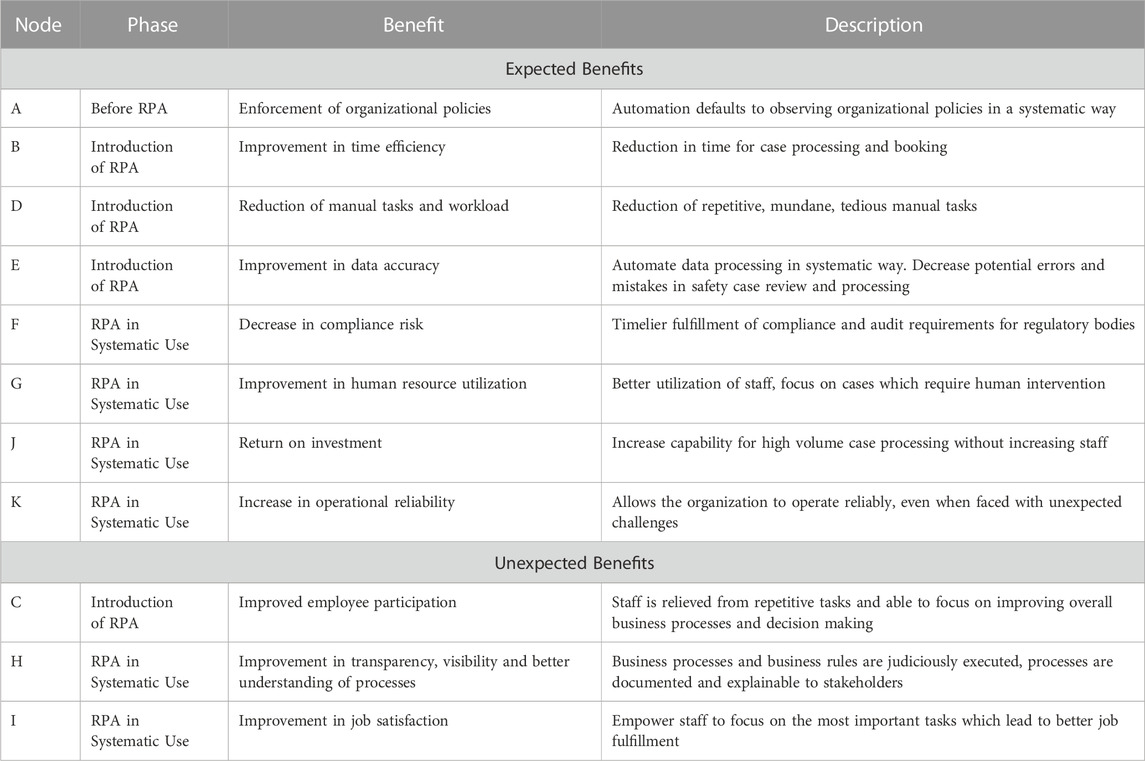
In the published article, Table 1 was mistakenly not included in the publication. The missing material appears below.
In the published article, Table 1 should now be updated as Table 2 and any associated references to Table 1 should refer to Table 2, with no changes to its legend or content.
The authors apologize for this errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: pharmacovigilance, machine learning-ML, drug safety, vaccines safety, artificial intelligence
Citation: Painter JL, Kassekert R and Bate A (2023) Corrigendum: An industry perspective on the use of machine learning in drug and vaccine safety. Front. Drug Saf. Regul. 3:1244115. doi: 10.3389/fdsfr.2023.1244115
Received: 21 June 2023; Accepted: 30 June 2023;
Published: 04 December 2023.
Edited by:
Taxiarchis Botsis, Johns Hopkins University, United StatesReviewed by:
Cristiano Matos, Escola Superior de Tecnologia da Saúde de Coimbra, ESTeSC-IPC, PortugalCopyright © 2023 Painter, Kassekert and Bate. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew Bate, YW5kcmV3LnguYmF0ZUBnc2suY29t
 Jeffery L. Painter1
Jeffery L. Painter1 Raymond Kassekert
Raymond Kassekert Andrew Bate
Andrew Bate