- Department of Health Sciences, Anna Meyer Children’s University Hospital, University of Florence, Florence, Italy
Paediatricians are often called on to weigh up potential side effects and interferences associated with drug treatments. Ethical concerns often prevent clinical trials in children, meaning that specific data for the paediatric population can be lacking. This is true for pharmacological therapies and also natural remedies used as add-on therapy. Among natural health products are “medical devices made of substances” (MDMS) which have become increasingly important in the treatment of many disorders; the substances contained in MDMSs frequently consist of molecular structures present in a standardized preparation derived from a natural source which act as a “system.” The benefits of using MDMSs to treat paediatric conditions such as gastrointestinal disorders and obesity have been proven, although there remains a degree of uncertainty about the precise mechanism of action underlying their therapeutic effectiveness. This paper argues in favour of using MDSMs when there is scientific grounds to prove their efficacy.
Introduction
Paediatricians are often called on to address questions regarding potential side effects and interference associated with drug treatments (Nakama et al., 2019).
Since the early 1960s, when it became increasingly clear that children were often “therapeutic or pharmaceutical orphans” (Wilson, 1999), there has been a growing global awareness of the need to improve the health of children by reducing the potential risks of pharmacological treatments. Medical science no longer considers children as “little adults”, but as a special and very heterogeneous group of individuals (infants, for example, have very different needs and characteristics from adolescents) (Joseph et al., 2015). The diseases of childhood are different from adult diseases, and the same diseases can present themselves differently in children and adults; unlike adults, the physiological characteristics of paediatric patients depend very much on their age and stage of development (Joseph et al., 2015).
The availability of specific paediatric medicinal products is limited (Ernest et al., 2010), and data regarding dosage and efficacy in children for drugs developed for the adult population are often lacking (Klassen et al., 2009).
Given the difficulties of tailoring drugs to the needs of children, there is a high risk that therapies will be sub-optimal, unexpected responses will occur and adverse reactions and toxicity could be problems (Joseph et al., 2015). This is particularly true for children suffering from chronic diseases or with special health care needs, who are typically exposed to multiple concurrent medications in inpatient and ambulatory settings (Feinstein et al., 2014).
The discovery and use of natural remedies as add-on therapies should, therefore, be considered on the one hand with greater open-mindedness, seeking, through clinical studies as accurate as possible, to obtain proven clinical data and benefits in paediatrics; on the other hand as a potential means of reducing problems associated with traditional treatments, which are often connected to a clear key-lock mechanism (Beer et al., 2016). There is growing interest among parents, clinicians and researchers in using “natural products” for the treatment or alleviation of diseases or in association with traditional drugs to limit adverse effects. Complementary and alternative medicines (CAM) have most often been used to treat musculoskeletal problems (particularly back and neck pain), head and chest colds, anxiety and stress, and attention deficit disorder (ADHD) (Godwin et al., 2013). Complementary compounds are used together with chemical drugs in two thirds of children pharmacologically treated, in particular for upper respiratory tract infections, infant colic and other gastrointestinal disorders, and sleep disturbances, making it important to understand potential interactions with chemical drugs (Beer et al., 2016).
In the past, studies of Natural Health Products (NHPs), have led to the discovery of new drugs. Having been structurally “optimized” by evolution to serve particular biological functions (Atanasov et al., 2015; Atanasov et al., 2021), the molecules NHPs contain have a much greater scaffold diversity and structural complexity than synthetic molecules (Atanasov et al., 2015). Natural substances allow active principles to be isolated, while keeping the complexity of the starting material (Bilia et al., 2021). At present, in many European countries, numerous botanical products are present on the market as Medical devices (MDs).
Medical Devices Made of Substances
A medical device is defined as any device intended to be used for medical purposes. There are many items which fall within this definition and are used for disease management (Popov et al., 2020). In the European Union, botanical products sold as MDs are subjected to regulation by Directive 93/42/EEC and the more recent Regulation 2017/745/EC, which introduces the official term “medical devices made of substances” (MDMS) (Bilia et al., 2021), referring to natural substances composed of a large number of molecules, which act in synchrony, through a non-pharmacological mechanism of action, in a way, that is, best represented by the concept of a “system” (Bilia et al., 2021). MDMSs and medicinal products, therefore, both have therapeutic effects, although their mechanisms of action are different (Bilia et al., 2021). Medicinal products are mostly composed of a single Active Pharmaceutical Ingredient (API) and generally have one main target, whereas MDMSs, as mentioned, act in a “systemic” way (Bilia et al., 2021).
Systems Biology and Systems Medicine
The concepts of “systems biology” and “systems medicine” have gained attention in recent years. Systems biology investigates biological organisms as integrated systems composed of dynamic and interrelated components (Kesić, 2016).
Living systems are immensely complicated, relying on constellations of constantly interacting networks, each of which is complex in its own right (Kesić, 2016). Systems biology has progressed rapidly in recent years due to advances in technology that enable the analysis of data from the fields of genomics, epigenomics, transcriptomics, proteomics, and metabolomics (collectively known as the “omics”). A systems approach to biology acknowledges that a molecular or biochemical factor does not act alone but is connected to many other factors (Kesić, 2016). There is a growing desire to shed light on the multiple interactive systems that are part of the complex physiological mechanisms of living organisms (Kesić, 2016). This approach is also gaining ground in paediatric care although the directions it could take are difficult to predict.
Systems medicine is an approach that uses the concepts and methods of systems biology to understand disease through an integration of data at multiple levels of biological organization (Saqi et al., 2016). An important feature of systems medicine is the interplay of biology, computation, and technology (Saqi et al., 2016). The primary goal is to improve and individualize patient care. The approach has led to the discovery that many diseases are heterogeneous and associated with several phenotypes and subtypes, each characterized by different aberrant pathways and processes (Saqi et al., 2016), which in turn has led to the development of more personalized and effective treatments, as well as subtype-specific medicines.
The past decades have seen the emergence of a new taxonomy of disease, based on molecular mechanistic features rather than the presentation of clinical symptoms (Saqi et al., 2016).
Paediatrics represents a new field in systems medicine. Novel approaches utilising cutting edge technologies are increasingly being used to identify new biomarkers which may be involved in the pathogenesis of paediatric conditions (Cheung, 2021). There have been many recent studies in paediatrics which have helped unveil more specific diagnostic markers in childhood conditions and develop more specific treatments which take into account the whole-body system (Cheung, 2021). As already specified, a fundamental concept of systems medicine is to view the human body as a network of networks. Each level of biological complexity, from genome to phenome, from cells to organ, and from molecules to individuals, can be conceptualised and modelled as networks with specific components and interactions with other networks (Cheung, 2021).
Paediatrics and its care subspecialties such as paediatric endocrinology may also be seen as component disciplines of a complex and holistic medical approach. Thinking in terms of systems can help paediatricians avoid a reductive view of disease, promote research into disease mechanisms and improve treatments. As Edgar Morin wrote in “Complexity and new science”: “.....in the time of globalization, specialization guides the progress of knowledge; however, it also pushes to break down the knowledge that should be kept as a whole.......” (Morin and Pieper, 1987).
It is essential to cultivate a transdisciplinary vision which places specialist knowledge in a systemic vision (Ehrich et al., 2021). Various conditions, such as, for example, altered foetal programming, can lead to disease. Maintaining a systemic vision which recognises the connections between childhood conditions and health in later life not only brings advantages to the individual but can also minimises healthcare costs for society (Ehrich et al., 2021).
Medical Device Made of Substances in Paediatrics
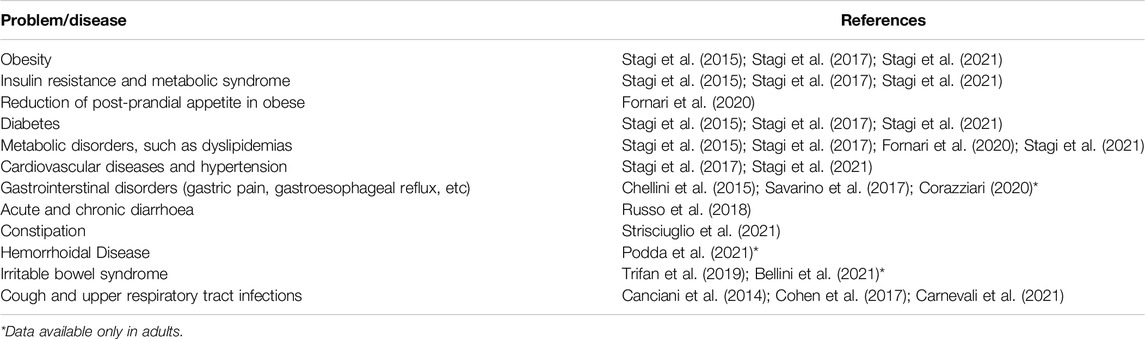
MDMSs can bring benefits in many fields of paediatrics (Table 1), including in the treatment of complex conditions such as metabolic syndrome or gastrointestinal disorders.
For example, obesity, which will require huge economic resources in the future is best treated via a systemic approach because the pathophysiologic mechanisms underlying weight gain are much more complex and multifactorial than previously believed. It has recently been shown that polysaccharide macromolecule complexes are able to reduce plasma glucose and body weight in children and adolescents with obesity, diabetes and metabolic syndrome, opening the door to using this MDMS in association with or as an alternative to traditional drugs for suppressing appetite or contrasting insulin resistance (Guarino et al., 2013; Stagi et al., 2015; Stagi et al., 2017; Fornari et al., 2020). Indeed, treatment with this MDMS could reduce the need for treatments with drugs such as metformin which are potentially difficult to manage in children and associated with several adverse events (Stagi et al., 2017). Data for paediatric patients treated with these natural fiber complexes are similar to those for obese adult subjects (Grube et al., 2013), with a reduction in the incidence of metabolic syndrome and type 2 diabetes mellitus (Guarino et al., 2021). The MDMS was shown to significantly reduce Body Mass Index (BMI), body fat, and waist circumference, and to be non-inferior compared to metformin for glycaemic control and superior in terms of both serum lipid-lowering capacity and tolerability (Guarino et al., 2021). Ingested before meals, these macromolecular complexes can reduce the hormones that stimulate appetite as well as the post-meal triglyceride spike (Fornari et al., 2020). Importantly, they have also proved able to significantly decrease BMI and waist standard deviation score (SDS) and improve glucose control and variability in children with type 1 diabetes and metabolic syndrome (Stagi et al., 2021).
The benefits deriving from MDMS are also evident in paediatric gastrointestinal disorders, which are frequent in children and adolescents, functional constipation and diarrhoea (Russo et al., 2018; Strisciuglio et al., 2021). Moreover, although data for the paediatric population are currently lacking, in adults, the same MDMS ameliorates heartburn, gastroesophageal reflux, irritable bowel syndrome, and haemorrhoidal disease (Corazziari, 2020; Podda et al., 2021). In treating gastric acid (heartburn) or gastroesophageal reflux, MDMSs have been shown to be at least equal to drugs such as protonic pump inhibitors (Corazziari, 2020) due to their capacity to act upon pathophysiologic mechanisms that cannot be influenced by drug treatment (Corazziari, 2020). In the treatment of chronic constipation in children, MDMSs have proved as effective as oral PEG (Strisciuglio et al., 2021).
MDMSs are often used to treat coughs in children, and some data are present in the literature (Canciani et al., 2014; Cohen et al., 2017; Carnevali et al., 2021). Acute cough associated with upper respiratory tract infections is a frequent cause of distress and sleep disturbance, and the reason for many paediatric visits and drug prescriptions. MDMSs seem to be effective and safe in reducing acute and persistent cough in children, leading to a reduction in the use of specific drugs in this age group (Canciani et al., 2014; Cohen et al., 2017; Carnevali et al., 2021).
NHPs and MDMS must be subjected to the same ethical scrutiny as traditional medicines. It is fundamental that scientific data is gathered on efficacy and patient safety (Huijghebaert et al., 2020). As with drugs, treatment with any natural product must be individualized and tailored to each patient’s circumstances (Huijghebaert et al., 2020). It is vital to take into account the stage of the disease, the severity of symptoms, the patient’s quality of life and the existence/absence of valid therapeutic alternatives.
Conclusion
In conclusion, it is important to promote openminded discussion among professionals about MDMSs in paediatrics. In many ways, paediatrics can be viewed as “applied developmental biology,” and paediatric diseases as occurring in systems that are still growing and developing. It has become clear that many adult diseases contributing significantly to morbidity and mortality have their origins in childhood and early life. The challenge is to harness the potential of MDMSs in preventing and treating paediatric diseases, especially in the light of the shift towards systemic medicine. MDMSs represent a new philosophy in the treatment of diseases which employs complex substances as an alternative to or in association with traditional drugs.
It essential that we are not held back by fears or prejudices; in the words of Sir William Osler “The good physician treats the disease, the great physician treats the patient who has the disease.”
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
SS conceived, edited, and approved this manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Atanasov, A. G., Waltenberger, B., Pferschy-Wenzig, E.-M., Linder, T., Wawrosch, C., Uhrin, P., et al. (2015). Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 33 (8), 1582–1614. doi:10.1016/j.biotechadv.2015.08.001
Atanasov, A. G., Zotchev, S. B., Zotchev, S. B., Dirsch, V. M., and Supuran, C. T.International Natural Product Sciences Taskforce (2021). Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 20 (3), 200–216. doi:10.1038/s41573-020-00114-z
Beer, A.-M., Burlaka, I., Buskin, S., Kamenov, B., Pettenazzo, A., Popova, D., et al. (2016). Usage and Attitudes towards Natural Remedies and Homeopathy in General Pediatrics. Glob. Pediatr. Health 3, 2333794X1562540. doi:10.1177/2333794X15625409
Bellini, M., Berti, G., Bonfrate, L., Ciranni, F., Di Ciaula, A., Di Ruscio, M., et al. (2021). Use of GELSECTAN® in Patients with Irritable Bowel Syndrome (IBS): an Italian Experience. Patient Prefer Adherence 15, 1763–1774. doi:10.2147/PPA.S318859
Bilia, A. R., Corazziari, E. S., Govoni, S., Mugelli, A., and Racchi, M. (2021). Medical Devices Made of Substances: Possible Innovation and Opportunities for Complex Natural Products. Planta Med. 87 (12-13), 1110–1116. doi:10.1055/a-1511-8558
Canciani, M., Murgia, V., Caimmi, D., Anapurapu, S., Licari, A., and Marseglia, G. L. (2014). Efficacy of Grintuss® Pediatric Syrup in Treating Cough in Children: a Randomized, Multicenter, Double Blind, Placebo-Controlled Clinical Trial. Ital. J. Pediatr. 40, 56. doi:10.1186/1824-7288-40-56
Carnevali, I., La Paglia, R., Pauletto, L., Raso, F., Testa, M., Mannucci, C., et al. (2021). Efficacy and Safety of the Syrup "KalobaTUSS®" as a Treatment for Cough in Children: a Randomized, Double Blind, Placebo-Controlled Clinical Trial. BMC Pediatr. 21 (1), 29. doi:10.1186/s12887-020-02490-2
Chellini, E., Lavorini, F., Campi, G., Mannini, C., and Fontana, G. A. (2015). Effect of an Anti-reflux Medical Device in the Control of Deflation Cough: A Placebo-Controlled Comparative Study with an Antacid Drug in Chronic Coughers. Pulm. Pharmacol. Ther. 33, 11–14. doi:10.1016/j.pupt.2015.05.002
Cohen, H. A., Hoshen, M., Gur, S., Bahir, A., Laks, Y., and Blau, H. (2017). Efficacy and Tolerability of a Polysaccharide-Resin-Honey Based Cough Syrup as Compared to Carbocysteine Syrup for Children with Colds: a Randomized, Single-Blinded, Multicenter Study. World J. Pediatr. 13 (1), 27–33. doi:10.1007/s12519-016-0048-4
Corazziari, E. S. (2020). Medical Devices Made of Substances in the Management of Patients with Gastrointestinal Diseases. Pharm. Adv. 01, 27–29. doi:10.36118/pharmadvances.01.2020.08s
Ehrich, J., Manemann, J., Tasic, V., and DeSanto, N. G. (2021). The Implications of Complexity, Systems Thinking and Philosophy for Pediatricians. Ital. J. Pediatr. 47 (1), 76. doi:10.1186/s13052-021-01031-6
Ernest, T. B., Elder, D. P., Martini, L. G., Roberts, M., and Ford, J. L. (2010). Developing Paediatric Medicines: Identifying the Needs and Recognizing the Challenges. J. Pharm. Pharmacol. 59 (8), 1043–1055. doi:10.1211/jpp.59.8.0001
Feinstein, J. A., Feudtner, C., and Kempe, A. (2014). Adverse Drug Event-Related Emergency Department Visits Associated with Complex Chronic Conditions. Pediatrics 133 (6), e1575–e1585. doi:10.1542/peds.2013-3060
Fornari, E., Morandi, A., Piona, C., Tommasi, M., Corradi, M., and Maffeis, C. (2020). Policaptil Gel Retard Intake Reduces Postprandial Triglycerides, Ghrelin and Appetite in Obese Children: A Clinical Trial. Nutrients 12 (1), 214. doi:10.3390/nu12010214
Godwin, M., Crellin, J., Mathews, M., Chowdhury, N. L., Newhook, L. A., Pike, A., et al. (2013). Use of Natural Health Products in Children: Survey of Parents in Waiting Rooms. Can. Fam. Physician 59 (8), e364–71.
Grube, B., Chong, P. W., Lau, K. Z., and Orzechowski, H. D. (2013). A Natural Fiber Complex Reduces Body Weight in the Overweight and Obese: A Double-blind, Randomized, Placebo-controlled Study. Obesity 21 (1), 58–64. doi:10.1002/oby.20244
Guarino, G., Della Corte, T., Strollo, F., and Gentile, S.Nefrocenter Research Study Group (2021). Policaptil Gel Retard in Adult Subjects with the Metabolic Syndrome: Efficacy, Safety, and Tolerability Compared to Metformin. Diabetes & Metabolic Syndrome Clin. Res. Rev. 15 (3), 901–907. doi:10.1016/j.dsx.2021.03.032
Guarino, G., Strollo, F., Malfertheiner, P., Della Corte, T., Stagi, S., Masarone, M., et al. (2013). Efficacy and Safety of a Polysaccharide-Based Natural Substance Complex in the Treatment of Obesity and Other Metabolic Syndrome Components: a Systematic Review. Front. Drug Saf. Regul. 2. doi:10.3389/fdsfr.2022.844256
Huijghebaert, S., De Bruyne, P., Allegaert, K., Vande Velde, S., De Bruyne, R., Van Biervliet, S., et al. (2020). Medical Devices that Look like Medicines: Safety and Regulatory Concerns for Children in Europe. Arch. Dis. Child. 105 (2), 1. doi:10.1136/archdischild-2018-316391
Joseph, P. D., Craig, J. C., and Caldwell, P. H. Y. (2015). Clinical Trials in Children. Br. J. Clin. Pharmacol. 79 (3), 357–369. doi:10.1111/bcp.12305
Kesić, S. (2016). Systems Biology, Emergence and Antireductionism. Saudi J. Biol. Sci. 23 (5), 584–591. doi:10.1016/j.sjbs.2015.06.015
Klassen, T. P., Hartling, L., Hamm, M., van der Lee, J. H., Ursum, J., and Offringa, M. (2009). StaR Child Health: an Initiative for RCTs in Children. Lancet 374 (9698), 1310–1312. doi:10.1016/S0140-6736(09)61803-1
Morin, E. (1987). “Scienza Nuova,” in Lust Am Denken. Editor K. Pieper (München: Pieper Verlag), 119–124.
Nakama, K. A., Dos Santos, R. B., Serpa, P., Maciel, T. R., and Haas, S. E. (2019). Organoleptic Excipients Used in Pediatric Antibiotics. Arch. Pédiatrie 26 (7), 431–436. doi:10.1016/j.arcped.2019.09.008
Podda, M., Laureti, S., Gentilini, L., Vittori, L., and Poggioli, G. (2021). Prospective Interventional Study to Evaluate the Effect of a Medical Device Made of Substances in Reducing Signs and Symptoms in Patients with Hemorrhoidal Disease. Ann. Clin. Laboratory Res. 9, 1–8. doi:10.36648/2386-5180.9.4.345
Popov, T. A., Passalacqua, G., González-Díaz, S. N., Plavec, D., Braido, F., García-Abujeta, J.-L., et al. (2020). Medical Devices in Allergy Practice. World Allergy Organ. J. 13 (10), 100466. doi:10.1016/j.waojou.2020.100466
Russo, M., Coppola, V., Giannetti, E., Buonavolontà, R., Piscitelli, A., and Staiano, A. (2018). Oral Administration of Tannins and Flavonoids in Children with Acute Diarrhea: a Pilot, Randomized, Control-Case Study. Ital. J. Pediatr. 44 (1), 64. doi:10.1186/s13052-018-0497-6
Saqi, M., Pellet, J., Roznovat, I., Mazein, A., Ballereau, S., De Meulder, B., et al. (2016). Systems Medicine: The Future of Medical Genomics, Healthcare, and Wellness. Methods Mol. Biol. 1386, 43–60. doi:10.1007/978-1-4939-3283-2_3
Savarino, V., Pace, F., and Scarpignato, C.Esoxx Study Group (2017). Randomised Clinical Trial: Mucosal Protection Combined with Acid Suppression in the Treatment of Non-erosive Reflux Disease - Efficacy of Esoxx, a Hyaluronic Acid-Chondroitin Sulphate Based Bioadhesive Formulation. Aliment. Pharmacol. Ther. 45 (5), 631–642. doi:10.1111/apt.13914
Stagi, S., Lapi, E., Seminara, S., Pelosi, P., Del Greco, P., Capirchio, L., et al. (2015). Policaptil Gel Retard Significantly Reduces Body Mass Index and Hyperinsulinism and May Decrease the Risk of Type 2 Diabetes Mellitus (T2DM) in Obese Children and Adolescents with Family History of Obesity and T2DM. Ital. J. Pediatr. 41, 10. doi:10.1186/s13052-015-0109-7
Stagi, S., Papacciuoli, V., Ciofi, D., Piccini, B., Farello, G., Toni, S., et al. (2021). Retrospective Evaluation on the Use of a New Polysaccharide Complex in Managing Paediatric Type 1 Diabetes with Metabolic Syndrome (MetS). Nutrients 13 (10), 3517. doi:10.3390/nu13103517
Stagi, S., Ricci, F., Bianconi, M., Sammarco, M., Municchi, G., Toni, S., et al. (2017). Retrospective Evaluation of Metformin And/or Metformin Plus a New Polysaccharide Complex in Treating Severe Hyperinsulinism and Insulin Resistance in Obese Children and Adolescents with Metabolic Syndrome. Nutrients 9 (5), 524. doi:10.3390/nu9050524
Strisciuglio, C., Coppola, V., Russo, M., Tolone, C., Marseglia, G. L., Verrotti, A., et al. (2021). Promelaxin Microenemas Are Non-inferior to Oral Polyethylene Glycol for the Treatment of Functional Constipation in Young Children: A Randomized Clinical Trial. Front. Pediatr. 9, 753938. doi:10.3389/fped.2021.753938
Trifan, A., Burta, O., Tiuca, N., Petrisor, D. C., Lenghel, A., and Santos, J. (2019). Efficacy and Safety of Gelsectan for Diarrhoea-predominant Irritable Bowel Syndrome: A Randomised, Crossover Clinical Trial. United Eur. Gastroenterol. J. 7 (8), 1093–1101. doi:10.1177/2050640619862721
Keywords: medical device, medical device made of substances, paediatrics, paediatrics (drugs and medicines), children
Citation: Stagi S (2022) Medical Devices Made of Substances: The Need for a Change in Approach in Paediatrics. Front. Drug Saf. Regul. 2:867143. doi: 10.3389/fdsfr.2022.867143
Received: 31 January 2022; Accepted: 26 May 2022;
Published: 28 June 2022.
Edited by:
Juan L. Tamargo, Complutense University of Madrid, SpainReviewed by:
Stefano Govoni, University of Pavia, ItalyCopyright © 2022 Stagi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Stagi, c3RlZmFuby5zdGFnaUB1bmlmaS5pdA==
 Stefano Stagi
Stefano Stagi