- 1Division of Pediatric Dentistry, College of Dentistry, The Ohio State University, Columbus, OH, United States
- 2Division of Dentistry, Nationwide Children’s Hospital, Columbus, OH, United States
- 3Department of Pediatrics, College of Medicine, The Ohio State University, Columbus, OH, United States
Oral lesions associated with SARS-CoV-2 (COVID-19) include aphthous-like ulcers, herpetiform eruption of vesicles and erosions and other findings. Reactive infectious mucocutaneous eruption (RIME) has recently been used to describe non-Mycoplasma pneumoniae pathogens that can lead to rash and mucositis. RIME secondary to SARS-CoV-2 infection is consistent with reports in the literature. The patient in this case report is significant in that it involves only the oral mucosa, although there are cases reported where mucosal involvement is limited to one site. The degree of mucosal involvement in our case report was in the presence of an acute COVID-19 infection without ocular or genital involvement. Oral lesions associated with COVID-19 infection vary in presentation. This paper adds to the understanding of systemic manifestations of COVID-19 infection and provides a reference of clinical findings, management, and interdisciplinary collaboration for caring for this patient.
1 Introduction
Oral lesions associated with SARS-CoV-2 (COVID-19) vary in prevalence of presentation and association with systemic manifestations (1–6). A case series reports characteristics of oral lesions in 10 patients with COVID-19 that is consistent with reports of other patients published systematic reviews (3, 4, 6). One systematic review and practical guideline published in 2023 reports the oral manifestations of COVID-19, reviewing 24 studies involving over 2,000 pediatric patients with COVID-19 (5).
The literature reports varying prevalence of oral lesions associated with COVID-19 infections and Current research does not definitively conclude that oral lesions are directly related to COVID-19 (4, 7). Some examples of oral lesions associated with COVID-19 include aphthous-like ulcers, herpetiform eruption of vesicles and erosions, Candida-associated lesions, recurrent herpesvirus infection, blisters, dark pigmentations, erythema multiforme-like lesions, white plaques, yellowish ulcers, inflammatory and purulent areas on pharyngeal wall, sialadenitis, overgrowth of filiform papillae, tongue depapillation, necrotizing gingivitis, salivary gland infections, angular cheilitis, hyposalivation, xerostomia, burning mouth sensation, facial pain, and oral submucous fibrosis (1–6).
Reactive infectious mucocutaneous eruption (RIME) has recently been used to describe non-Mycoplasma pneumoniae pathogens that can lead to rash and mucositis (8). RIME typically presents with mucositis in at least two or more oral, ocular, and/or genital mucosal tissues, with or without cutaneous involvement, and is associated with a prodrome that includes cough (8–11). Pathogens associated with RIME include Chlamydophila pneumoniae, metapneumovirus, parainfluenza virus 2, rhinovirus, influenza B virus, and enterovirus (8, 12, 13). RIME may present similar to, but is distinct from, M. pneumoniae–induced rash and mucositis (MIRM), erythema multiforme (EM), and Stevens-Johnson syndrome (SJS)/toxic epidermolytic necrosis (TEN) (8, 12, 13). The mechanism of RIME may involve polyclonal B-cell proliferation antibody production, which leads to immune complex deposition, complement activation, and molecular mimicry resulting in skin damage (12, 14).
2 Patient information
A 15-year-old-male presented with symptoms of fever, cough, and congestion, and was diagnosed with COVID-19 and acute bronchitis three days after initial symptoms. The diagnosis of COVID-19 was made via reverse transcription polymerase chain reaction (RT-PCR). The patient was otherwise healthy with a non-contributory medical history. Surgical, family, and social histories were unremarkable with no current medications. The patient was not vaccinated against SARS-CoV-2. There was a reported allergy to bee venom and no known drug allergies. The patient was undergoing comprehensive orthodontic treatment. The patient was prescribed prednisone (20 mg tablet, four times daily for 7 days) and azithromycin (500 mg once for 1 day, then 250 mg once daily for 4 days) by a local emergency department for management of COVID-19.
3 Case description
Eight days after diagnosis, the patient reported painful mouth sores that initially presented on the soft palate. Lesions progressed to generalized ulcerations to the upper lip and lower lip. At 10 days post-diagnosis, the patient returned to their primary care physician's office and was prescribed nystatin tablets and Magic Mouthwash.
At 11 days post-diagnosis, the patient returned to a local emergency department due to worsening mouth pain and was prescribed oral acyclovir (400 mg four times daily for 7 days) and a compounded “Magic Mouthwash” (active ingredients are 15 ml of a 1:1:1:1 mixture of 100,000 U/ml Nystatin, diphenhydramine 12.5 mg/ml, alum-magnesium hydroxide, and 2% viscous lidocaine every 6 h).
At 13 days post-diagnosis, the patient returned to another emergency department due to severe oral pain (eight out of 10 using the visual analogue pain rating scale), limited opening, and inability to tolerate oral intake. The patient had eight kilograms of weight loss 13 days since the initial COVID-19 diagnosis. Extraoral examination revealed erosive, yellowish ulcerations with crusted surfaces, and sloughing of mucosa on the upper and lower lip vermilion border. Patient had limited opening due to pain from sores. Intraoral examination revealed generalized, wide and shallow, erythematous ulcerations with overlying sloughing mucosa with a moderately coated dorsum of tongue (Figure 1) and numerous, localized, raised fluid-filled vesicular lesions on the posterior soft palate (Figure 2). Slight ulcerations were noted in the floor of the mouth. The patient denied eye pain or genital pain. No additional mucosal membrane involvement was noted. Further testing with a respiratory infection PCR array at 13 days post-diagnosis did not detect rhinovirus, enterovirus, influenza virus, parainfluenza, respiratory syncytial virus, Bordetella pertussis, Chlamydophila pneumoniae, or M. pneumoniae.
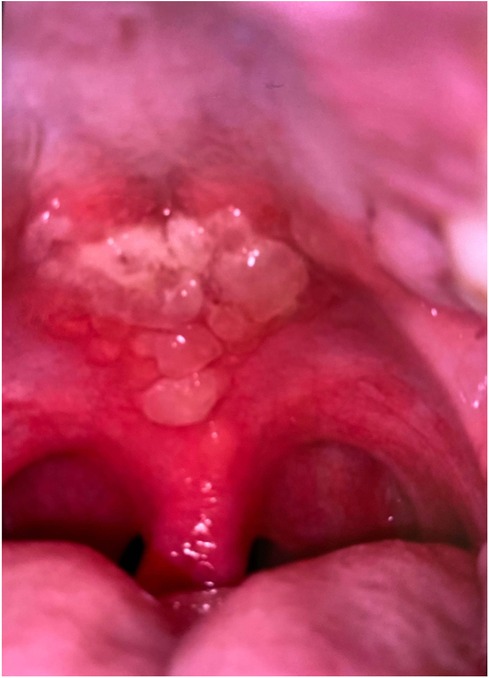
Figure 1. Intraoral photograph, initial presentation for admission, 13 days post-diagnosis, demonstrating mucosal lesions on hard and soft palate.

Figure 2. Photograph during admission, 14 days post-diagnosis, frontal view showing extent of mucosal lesions from intraoral to extraoral.
This patient was admitted to a children's hospital for 4 days. He had no improvement with three doses of acyclovir (400 mg tablets) and a topical oral rinse comprised of lidocaine:diphenhydramine:alum-magnesium hydroxide:water in a 1:1:1:2 ratio (one dose tolerated only). He improved after intravenous methylprednisone (60 mg) for 3 days and supportive treatment; pain control with ketorolac, ibuprofen, acetaminophen; fluconazole suspension; and nutritional support including intravenous dextrose and ad lib feeding with Pediasure®, Pedialyte®, and Ensure® (Abbott Laboratories, Abbott, IL, USA). Dental wax was placed over the orthodontic brackets as the patient was experiencing severe pain from the appliance abrading against the oral mucosa. By day 18, the patient had resolution of oral lesions except for remaining ulcers on mucosa of the lower lip (Figures 3, 4).
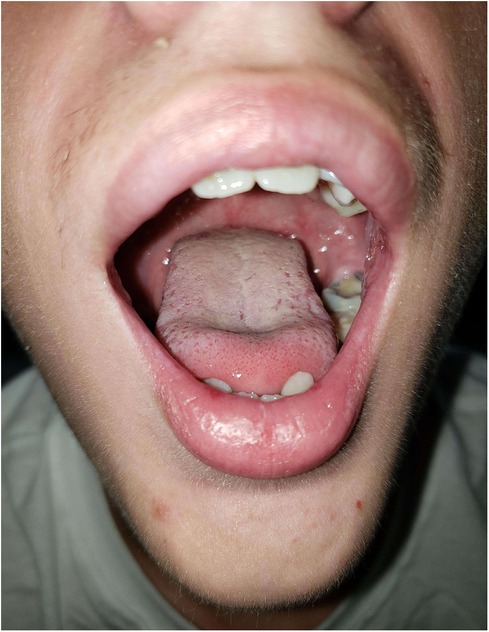
Figure 3. Photographs prior to discharge, 18 days post-diagnosis, extraoral photograph demonstrating healing lesions.
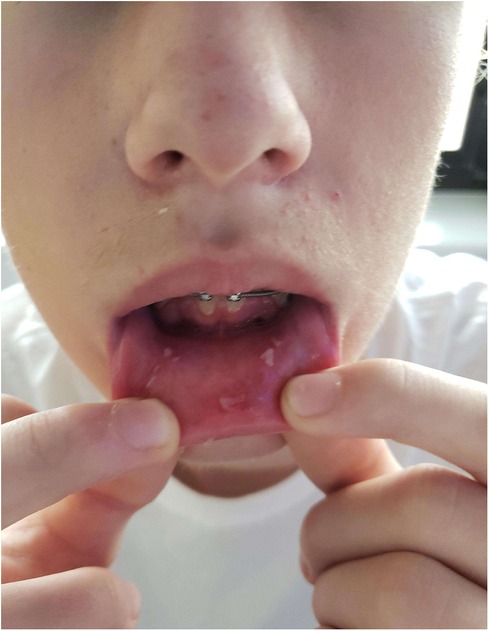
Figure 4. Photographs prior to discharge, 18 days post-diagnosis, intraoral photograph demonstrating healing lesions.
4 Discussion
The differential diagnosis for this patient includes: Stevens-Johnson syndrome (SJS)/toxic epidermolytic necrosis (TEN), erythema multiforme, herpetic gingivostomatitis, or reactive infectious mucocutaneous eruption. Reactive infectious mucocutaneous eruption (RIME) secondary to SARS-CoV-2 infection is consistent with reports in the literature (8–11, 14).
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN) is a continuum of a severe mucocutaneous reaction preceded by fever and caused by an exposure to drugs (15). Lesions were limited to the oral mucosa for the current patient and he did not have lesions present on his trunk which progressed to skin detachment which makes SJS/TEN less likely compared to RIME.
Herpetic gingivostomatitis (HG) is a common clinical manifestation of herpes simplex virus type 1 (HSV-1) (16, 17). The patient in our case presented with similar ulcerative and erosive lesions but they did not appear until after his prodromal symptoms had resolved and most importantly, HSV DNA was not detected in the oral mucosal swab by PCR. Nonetheless, a primary HG infection could not be completely ruled out as a clinical diagnosis due to similarity in symptoms with RIME.
Erythema multiforme (EM) appears to be an immune-mediated entity presenting with an acute onset of classic target-like blistering ulcerative mucocutaneous lesions (18–21). The characteristic target lesions differentiate EM from other conditions and EM differs in cause from SJS/TEN (20). The lack of characteristic target lesion morphology and the clinical appearance inconsistent with oral EM clinically distinguishes EM for our patient compared to RIME. This patient is similar to other cases of RIME associated with COVID-19 in the literature due to the presence of oral lesions in the absence of other mucocutaneous involvement. Some oral findings reported with RIME include erosions, ulcers, vesiculobullous lesions, or denudation of the buccal mucosa (22). This case report is significant in that it involves only the oral mucosa (14, 23). The degree of mucosal involvement in our case report was in the presence of an acute COVID-19 infection without ocular or genital involvement. Given the patient's degree of discomfort and inability to tolerate oral intake, the medical team attempted a trial of IV steroid treatment as initial treatment did not improve symptoms. This is similar in management to other cases of RIME in children, where systemic corticosteroids may benefit the management in combination with pain management, hydration, nutrition, and supportive care (8, 9, 24). However, without an evidence-based guidelines for treatment, a systematic review describes the different approaches in treatment including supportive care, systemic immunosuppression, and/or antibiotics (22, 25). Dental professionals who may encounter similar cases in practice should consider COVID-19 infections in association with oral lesions. Dental professionals can collaborate with the medical team to weigh the risks and benefits of treatment such as systemic immunosuppression, especially if supportive care failed to improve the patient's status.
5 Conclusion
Oral lesions associated with COVID-19 infection vary in presentation. This paper adds to the understanding of systemic manifestations of COVID-19 infection to help identify, manage, and coordinate care for patients with these lesions. This paper provides a reference of clinical findings, management, and interdisciplinary collaboration for caring for this patient. The dental team can contribute to the medical care team on appropriate diagnosis, management, and follow-up for patients with COVID-19 infections.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study or for the publication of any potentially identifiable images and data was not obtained from the minor(s)' legal guardian/next of kin as all patient data is de-identified and presented in accordance with local institutional policies.
Author contributions
DD: Conceptualization, Writing – original draft, Writing – review & editing. KL: Writing – original draft, Writing – review & editing. RW: Writing – original draft, Writing – review & editing. JT: Writing – original draft, Writing – review & editing. IM: Writing – original draft, Writing – review & editing. AK: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Acknowledgments to the care team at Nationwide Children's Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aragoneses J, Suárez A, Algar J, Rodríguez C, López-Valverde N, Aragoneses JM. Oral manifestations of COVID-19: updated systematic review with meta-analysis. Front Med (Lausanne). (2021) 8:726753. doi: 10.3389/fmed.2021.726753
2. Brandini DA, Takamiya AS, Thakkar P, Schaller S, Rahat R, Naqvi AR. COVID-19 and oral diseases: crosstalk, synergy or association? Rev Med Virol. (2021) 31(6):e2226. doi: 10.1002/rmv.2226
3. Prakash K, Bindu NR, Sanoj M. Prevalence of oral manifestations in COVID-19-diagnosed patients at a tertiary care hospital in Kerala. J Maxillofac Oral Surg. (2024) 23(2):296–300. doi: 10.1007/s12663-023-02049-5
4. Rueda CAC, Vinitzky Brener I. Oral lesions in patients with severe COVID-19 in the national institute of respiratory diseases in Mexico: case series. J Dent Res Dent Clin Dent Prospects. (2023) 17(1):54–60. doi: 10.34172/joddd.2023.37072
5. Nasiri K, Tehrani S, Mohammadikhah M, Banakar M, Alaeddini M, Etemad-Moghadam S, et al. Oral manifestations of COVID-19 and its management in pediatric patients: a systematic review and practical guideline. Clin Exp Dent Res. (2023) 9(5):922–34. doi: 10.1002/cre2.776
6. Gupta A, Shrivastav K, Agrawal A, Purohit A, Chanchlani R. Estimating the prevalence of oral manifestations in COVID-19 patients: a systematic review. Osong Public Health Res Perspect. (2023) 14(5):388–417. doi: 10.24171/j.phrp.2023.0033
7. Sarasati A, Agustina D, Surboyo MDC. The oral lesion in the COVID-19 patient: is it true oral manifestation or not? Infect Drug Resist. (2023) 16:4357–85. doi: 10.2147/IDR.S411615
8. Bowe S, O'Connor C, Gleeson C, Murphy M. Reactive infectious mucocutaneous eruption in children diagnosed with COVID-19. Pediatr Dermatol. (2021) 38(5):1385–6. doi: 10.1111/pde.14801
9. Holcomb ZE, Hussain S, Huang JT, Delano S. Reactive infectious mucocutaneous eruption associated with SARS-CoV-2 infection. JAMA Dermatol. (2021) 157(5):603–5. doi: 10.1001/jamadermatol.2021.0385
10. Binois R, Colin M, Rzepecki V, Prazuck T, Esteve E, Hocqueloux L. A case of erythema multiforme major with multiple mucosal involvements in COVID-19 infection. Int J Dermatol. (2021) 60(1):117–8. doi: 10.1111/ijd.15158
11. Fathi Y, Hoseini EG, Mottaghi R. Erythema multiform-like lesions in a patient infected with SARS-CoV-2: a case report. Future Virol. (2021) 16(3):157–60. doi: 10.2217/fvl-2020-0333
12. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, Del Rosal-Rabes T, González-Sainz FJ, Sánchez-Orta A, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol. (2017) 34(4):465–72. doi: 10.1111/pde.13140
13. Ramien ML, Bruckner AL. Mucocutaneous eruptions in acutely ill pediatric patients—think of mycoplasma pneumoniae (and other infections) first. JAMA Dermatol. (2020) 156(2):124–5. doi: 10.1001/jamadermatol.2019.3589
14. Miller MM, Kamath S, Hughes M, Harter N, Luu M. Evaluation of etanercept for treatment of reactive infectious mucocutaneous eruption. JAMA Dermatol. (2021) 157(2):230–2. doi: 10.1001/jamadermatol.2020.5166
15. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. (2013) 69(2):173.e1–13. doi: 10.1016/j.jaad.2013.05.003
16. Brice SL, Stockert SS, Bunker JD, Bloomfield D, Huff JC, Norris DA, et al. The herpes-specific immune response of individuals with herpes-associated erythema multiforme compared with that of individuals with recurrent herpes labialis. Arch Dermatol Res. (1993) 285(4):193–6. doi: 10.1007/BF00372008
17. Weston WL, Brice SL, Jester JD, Stockert S, Huff JC, Lane AT. Herpes simplex virus in childhood erythema multiforme. Pediatrics. (1992) 89(1):32–4. doi: 10.1542/peds.89.1.32
18. Huff JC. Erythema multiforme. Dermatol Clin. (1985) 3(1):141–52. doi: 10.1016/S0733-8635(18)30925-2
19. Huff JC, Weston WL, Tonnesen MG. Erythema multiforme: a critical review of characteristics, diagnostic criteria, and causes. J Am Acad Dermatol. (1983) 8(6):763–75. doi: 10.1016/S0190-9622(83)80003-6
20. Weston JA, Weston WL. The overdiagnosis of erythema multiforme. Pediatrics. (1992) 89(4 Pt 2):802. doi: 10.1542/peds.89.4.802b
21. Labé P, Ly A, Sin C, Nasser M, Chapelon-Fromont E, Ben Saïd P, Mahé E. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Eur Acad Dermatol Venereol. (2020) 34(10):e539–41. doi: 10.1111/jdv.16666
22. Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae–induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. (2015) 72(2):239–45.e4. doi: 10.1016/j.jaad.2014.06.026
23. Mazori DR, Nagarajan S, Glick SA. Recurrent reactive infectious mucocutaneous eruption (RIME): insights from a child with three episodes. Pediatr Dermatol. (2020) 37(3):545–7. doi: 10.1111/pde.14142
24. Ryder CY, Pedersen EA, Mancuso JB. Reactive infectious mucocutaneous eruption secondary to SARS-CoV-2. JAAD Case Rep. (2021) 18:103–5. doi: 10.1016/j.jdcr.2021.10.007
Keywords: COVID-19, oral pathology, pediatric dentistry, diagnostic challenge, oral ulcers
Citation: Danesh DO, Lee K, Wallihan RG, Townsend JA, Mulo I and Kumar A (2024) Painful ulcerations associated with COVID-19 in an adolescent patient: a case report. Front. Dent. Med 5:1412439. doi: 10.3389/fdmed.2024.1412439
Received: 4 April 2024; Accepted: 16 July 2024;
Published: 2 August 2024.
Edited by:
Rosalyn Sulyanto, School of Dental Medicine and Harvard University, United StatesReviewed by:
Pradeep Kumar Yadalam, Saveetha Dental College and Hospitals, IndiaHarleen Kumar, Sydney Dental Hospital, Australia
© 2024 Danesh, Lee, Wallihan, Townsend, Mulo and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David O. Danesh, ZGF2aWQuZGFuZXNoQG5hdGlvbndpZGVjaGlsZHJlbnMub3Jn
 David O. Danesh
David O. Danesh Kyulim Lee
Kyulim Lee Rebecca G. Wallihan
Rebecca G. Wallihan Janice A. Townsend1,2
Janice A. Townsend1,2 Ashok Kumar
Ashok Kumar