- 1Department of Periodontics and Dental Hygiene, The University of Texas Health Science Center at Houston School of Dentistry, Houston, TX, United States
- 2The University of Texas Health Science Center at Houston School of Dentistry, Houston, TX, United States
Necrotizing periodontal disease (NPD) is a microbial infectious inflammatory disease of the gingiva and/or periodontium that is characterized by a rapid onset of inflammation, pain, and “punched out” cratered interdental papillae. Although NPD is not very common, diagnosis is mostly based on its unique clinical presentation. Some predisposing factors for developing NPD include poor oral hygiene, smoking, malnutrition, immunosuppression (such as in HIV infection, uncontrolled diabetes, and cancer), and stress. This report presents the case of a 22-year-old Caucasian woman, who was a heavy smoker but with otherwise showed no systemic disease and who suffered from severe inflammation of the gingiva, ulceration, and necrosis of the interdental papillae, and pseudomembrane formation. The patient had been hospitalized for a seizure episode and was diagnosed with malnutrition-induced seizure. NPD was diagnosed and the patient was treated successfully with nonsurgical scaling and root planing and behavioral modifications including smoking cessation and balanced nutrition intake. Disease remission was achieved after treatment. In addition, this case report reviews the effect of nutrients on the health of the periodontium.
Introduction
Necrotizing periodontal disease (NPD) is a noncommunicable microbial disease of the periodontium characterized by a rapid onset of inflammation, pain, and “punched out” cratered interdental papillae (1, 2). NPD has been described as far back as 401 BC and has been previously known as acute necrotizing ulcerative gingivitis, trench mouth, Vincent's disease, and Vincent's gingivostomatitis (3). In Western countries, it has been mostly reported among military personnel and HIV patients (3). The incidence of NPD in developed countries has decreased over the years. In developing countries, NPD is diagnosed mainly among malnourished children between the ages of 3 and 10 years hailing from the lower socioeconomic classes (3). In the previous 1999 American Academy of Periodontology (AAP) Classification System for Periodontal Diseases and Conditions, necrotizing ulcerative gingivitis (NUG) and necrotizing ulcerative periodontitis (NUP) were classified separately (4). The most recent 2017 AAP Classification of Periodontal and Peri-Implant Diseases and Conditions has now classified NUG and NUP together as NPD, believing that they characterize different stages of the same disease (5). Although NPD is a rare disease, affecting less than 1% of the population, it is of clinical importance because, without urgent management, it can rapidly progress into necrotizing stomatitis or cancrum oris (noma), a fatal disease that results in oral soft and hard tissue gangrene (1, 5, 6).
Some predisposing factors for a person developing NPD include poor oral hygiene, smoking, malnutrition, immunosuppression (such as in HIV infection, uncontrolled diabetes, and cancer), and stress (5, 7). A variety of nutrients can influence the health of the periodontium, such as the vitamin B complex, vitamin C, and calcium (8). Many studies have revealed that antioxidants, such as vitamin A, C, and E, glutathione, and melatonin, can help overcome the inflammation of periodontal tissues induced by reactive oxidative species during pathogenesis (8, 9). One study even indicated a sevenfold increase in the risk of NPD in patients with vitamin C deficiencies, when compared with patients who had sufficient levels of vitamin C (10). The vitamin B complex has also been shown to be important, as it can aid in wound healing in the periodontal disease onset, progression, and treatment process (8). It has also been found that calcium deficiency can lead to a more severe progression of periodontal disease.
This report aims to (1) present a unique case of NPD in a nutritionally deficient patient, who was treated nonsurgically, which resulted in disease remission, and (2) review the effect of nutrients on the health of the periodontium.
Clinical case presentation
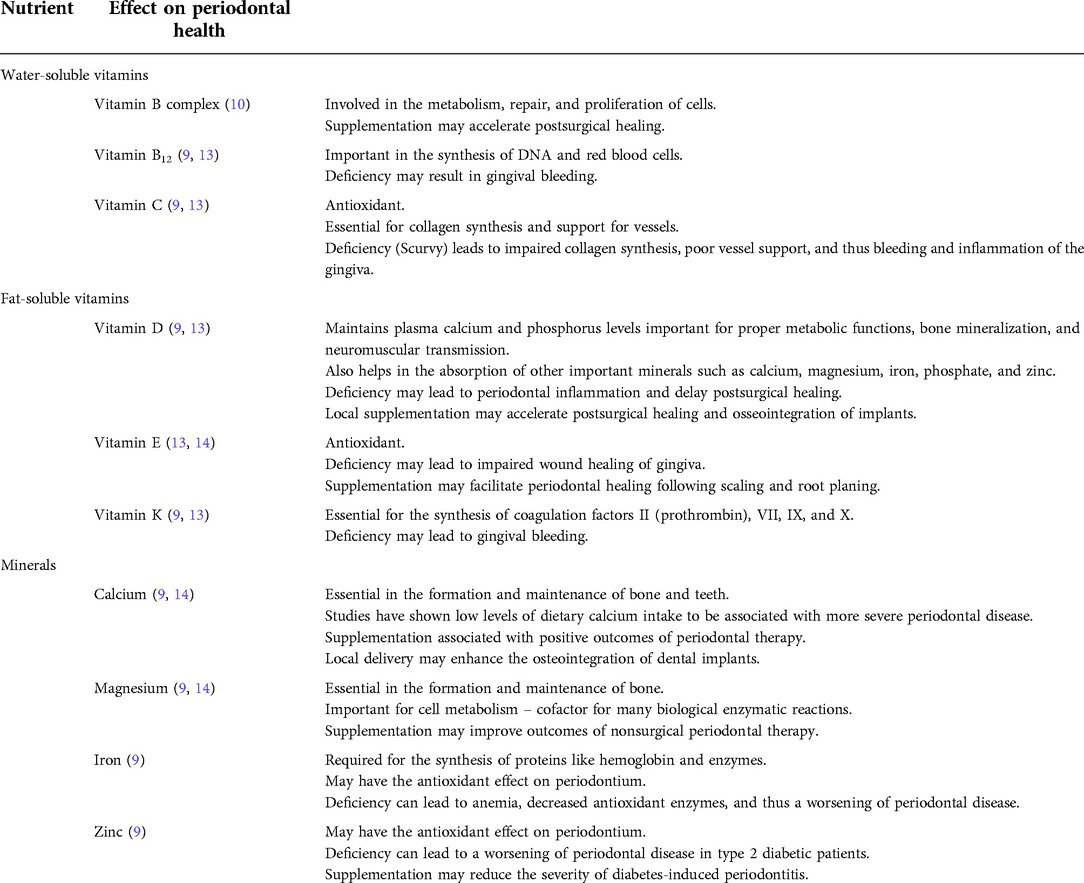
In September 2020, a 22-year-old Caucasian woman presented at The University of Texas Health Science Center at Houston, School of Dentistry, with a report of “painful and smelly bleeding gums”. Her medical history revealed that the patient had been hospitalized for a seizure episode and was diagnosed with malnutrition-induced seizure in July 2020. She was a heavy smoker (≥10 cigarettes/half a pack per day) with 3 pack-years of smoking history. The patient also reported that she was undergoing stressful life events to the extent that she often skipped meals. However, her vital signs were within normal limits. Overall, based on the American Society of Anesthesiologists (ASA) physical status classification, she was an ASA II patient. Upon an intraoral examination, it was revealed that the patient had severe inflammation of the gingiva and ulceration and necrosis of the interdental papillae, especially along the facial portions of the anterior teeth (Figure 1). A characteristic pseudomembrane formation was also observed, along with spontaneous hemorrhaging and halitosis. A periodontal examination revealed pocket probing depths of 1–6 mm, a plaque score of 100%, and a bleeding upon probing (BOP) of 86%. A slight alveolar bone loss (<15%) of the anterior dentition was observed upon a radiographic examination (Figure 2).
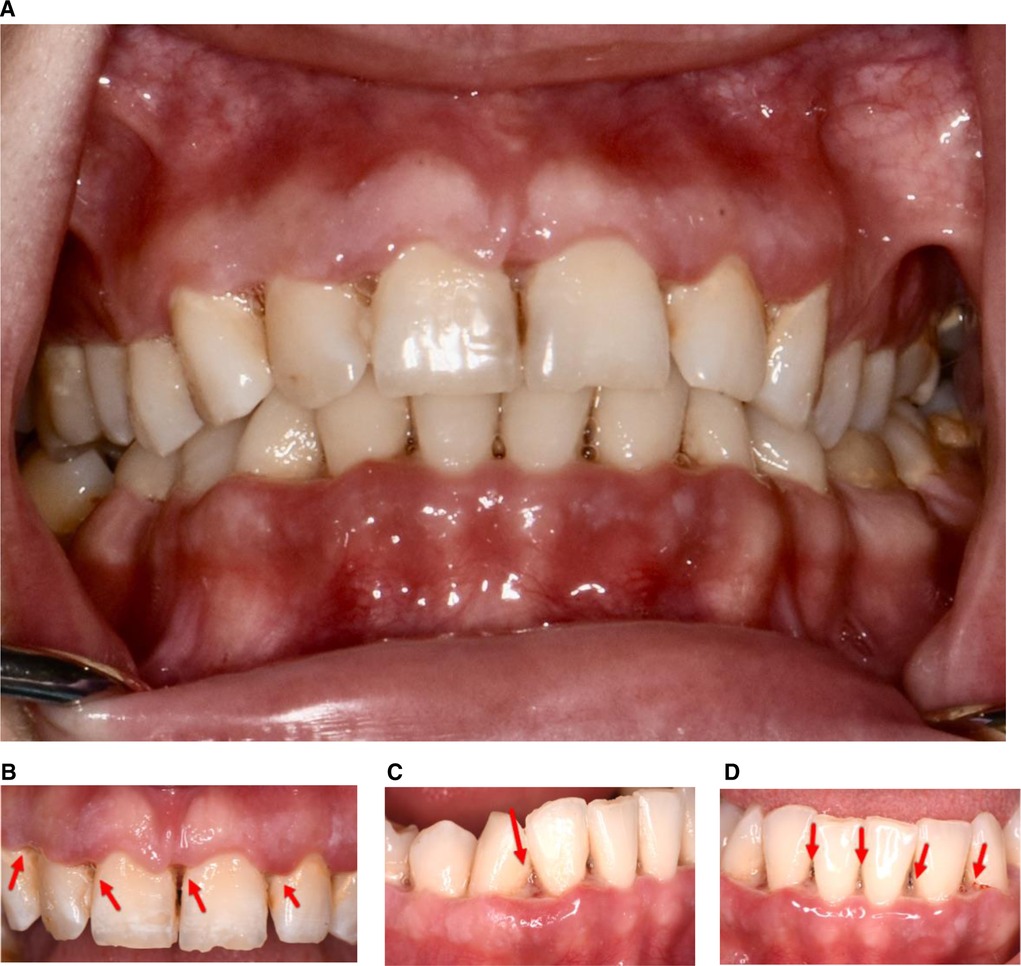
Figure 1. Clinical presentation prior to treatment. Notice the ulceration and necrosis of the interdental papillae and pseudomembrane formation denoted by the red arrows in 1B-1D.
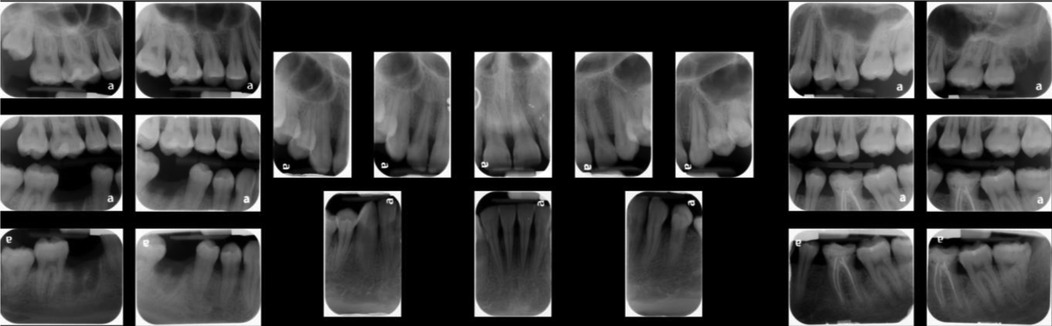
Figure 2. Full mouth radiographs. A slight (<15%) alveolar bone loss is noticed in anterior dentition upon a radiographic examination.
Diagnosis and treatment
Based on the oral and periodontal examination, the patient was diagnosed with NPD, exacerbated by nutrient deficiency and smoking. Treatment was focused on removal of the main etiological factor, bacteria, along with better management of her nutrition and smoking habits.
Treatment was broken down into three phases: acute phase, nonsurgical treatment of pre-existing conditions, and maintenance phase. During the acute phase, to halt the disease progression and manage the patient's pain, full mouth debridement with hand and ultrasonic instruments under local anesthesia was done. The patient was prescribed a 7-day course of ibuprofen 600 mg if necessary, amoxicillin 500 mg three times a day, and 0.12% chlorhexidine rinse two times a day for pain control and for reduction of the bacterial load. Proper oral hygiene instruction was given to the patient with a modified bass brushing technique and c-shaped flossing.
During the treatment of the pre-existing condition phase, the patient was treated with nonsurgical scaling and root planing under local anesthesia and oral hygiene re-enforcement. In addition, the patient's predisposing factors for NPD were addressed with patient education on behavior/diet modifications, including smoking cessation and balanced nutrition intake. Her primary care physician initially prescribed her multivitamins with minerals to be taken orally once a day and later instructed her patient to take the over-the-counter multivitamins upon 1-month follow-up. In addition, the patient was educated to maintain regular well-balanced meals. With regard to her efforts on smoking cessation, with a nicotine patch (21 mg patch/day for the first 6 weeks, 14 mg patch/day for weeks 7 and 8, and 7 mg patch/day for weeks 9 and 10), the patient significantly reduced her smoking amount to 1–2 cigarettes a day. The patient continued to follow up with her physician.
Finally, in the maintenance phase, when the patient was followed up 2 months after nonsurgical scaling and root planing, there were improvements in the patient's periodontal status. The gingiva tone was pink and firm with minimal signs of inflammation, and resolution of pain and halitosis was also noted. The patient's plaque score was 41%, and BOP was 14%, which were greatly reduced from the pretreatment values of 100% plaque score and 86% BOP. The patient gained a generalized clinical attachment of 1 mm, and her pretreatment deep pockets of 1–6 mm were reduced to a depth of 1–4 mm, which would be easier for her to maintain cleanliness with proper oral hygiene at home. Necrotized punched out interdental papillae healed. Interdental papillae were blunted in the anterior dentition as the patient had nonreversible bone loss (Figure 3). In areas without bone loss where the disease was limited to soft tissue, a complete fill of the interdental spacing with papillae was expected, emphasizing the importance of early detection and treatment of NPD.
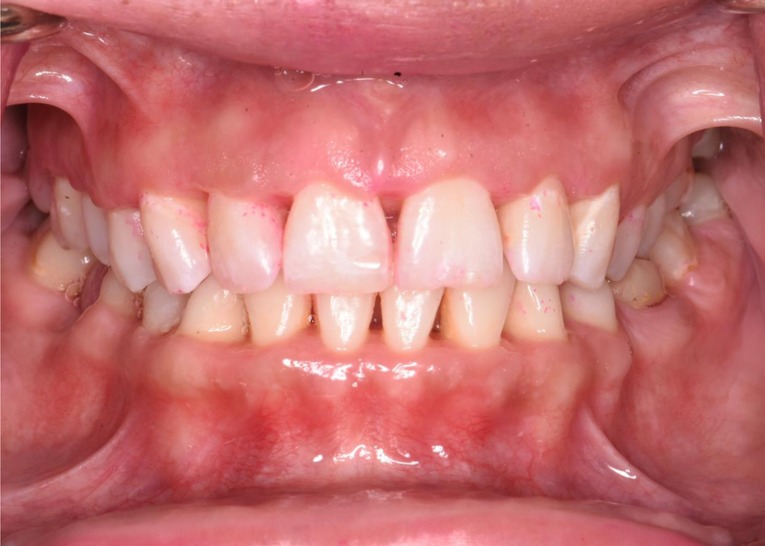
Figure 3. Clinical presentation after treatment. The pocket probing depth reduces to 1–4 mm after treatment. Necrotized punched out interdental papillae have healed. Interdental papillae are blunted in the anterior dentition as the patient had nonreversible bone loss. In the posterior area without bone loss, a complete fill of the interdental spacing with papillae is noticed.
Discussion
A diagnosis of NPD is primarily based on clinical findings and medical history (5). However, clinicians also need to rule out differential diagnosis, including periodontal conditions (such as recurrent gingivitis or generalized periodontitis), vesicular-bullous diseases (such as herpetic gingivostomatitis, gingival angiosarcoma, granulomatosis, or cicatricial pemphigoid), and systemic conditions or rare diseases with periodontal manifestations (such as leukemia or scurvy) (11).
The etiology of NPD is primarily caused by an opportunistic infection, mainly associated with fusiform and spirochete bacteria (1, 2, 12). More specifically, the microbiota associated with NPD include Treponema spp, Selenomonas spp, Fusobacterium spp, and Prevotella intermedia (3). Other etiological factors include poor oral hygiene, smoking, stress, immunosuppression, and malnutrition (2), especially deficiencies in micronutrients essential for periodontal health (Table 1), which will be further discussed below.
Vitamin B complex includes vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B5 (pantothenic acid), vitamin B6 (pyridoxine, pyridoxal, pyridoxamine), vitamin B7 (biotin), vitamin B9 (folic acid), and vitamin B12 (cobalamins). They are essential for the health of the periodontium because they are involved in the metabolism, repair, and proliferation of cells (9). A deficiency in vitamin B complex can manifest as a plethora of symptoms and diseases. However, the most significant B vitamin for periodontal health is vitamin B12, as its deficiency can lead to gingival bleeding (9). Vitamin B12 plays an important role in the synthesis of DNA (14). Thus, its deficiency causes inadequate DNA synthesis and subsequently results in defective maturation of red blood cells, which can lead to diseases such as anemia as well as result in periodontal symptoms such as gingival bleeding (9, 14).
Vitamin C is also vital for periodontal health as it has antioxidant properties and is essential for the synthesis of collagen. Collagen provides support for vessels, so a deficiency in vitamin C will result in impaired collagen synthesis, poor vessel support, and thus bleeding of the gingiva. Vitamin C deficiency also leads to a decrease in the ability to scavenge free radicals and results in inflammation of the gingiva (9, 14).
Vitamin D's main function is to maintain adequate plasma calcium and phosphorus levels in order to support proper metabolic functions, bone mineralization, and neuromuscular transmission (13). It also aids in the intestinal absorption of other important minerals such as calcium, magnesium, iron, phosphate, and zinc (9). Studies have suggested that deficiencies in vitamin D can lead to periodontal inflammation and delay postsurgical periodontal healing (9). It was also suggested that local use of vitamin D helped with the postsurgical healing and osseointegration of implants (9).
Vitamin E is a major antioxidant. It scavenges free radicals and helps reduce inflammation caused by reactive oxygen species (13). Deficiency in vitamin E can result in impaired wound healing of the gingiva. Conversely, supplementation of vitamin E has been shown to facilitate periodontal healing following scaling and root planing (15).
Vitamin K plays an important role in bleeding as it is required for the synthesis of coagulation factors II (prothrombin), VII, IX, and X (9, 14). Vitamin K deficiency can lead to gingival bleeding (9).
Calcium is important for the formation and maintenance of bone and teeth. Calcium deficiency can lead to low plasma calcium levels, which can stimulate the parathyroid gland to produce parathyroid hormone (PTH), causing osteoclastogenesis and bone resorption as a response to prevent hypocalcemia. Studies have shown that low dietary calcium intake is associated with more severe periodontal disease (15). Supplementation of calcium, along with vitamin D, is commonly used and is associated with positive outcomes with periodontal therapy (9, 15). Local calcium delivery can also enhance the osseointegration of dental implants (9).
Magnesium, such as calcium, is important for the formation and maintenance of bone (9). It is also important for cell metabolism as it is required as a cofactor for many biological enzymatic reactions (15). Magnesium supplementation may positively affect outcomes of nonsurgical periodontal therapy (9, 15).
Iron is required for the synthesis of proteins, such as hemoglobin and enzymes. Iron deficiency can lead to anemia, and a decrease in antioxidant enzymes, which can lead to increased oxidative stress and result in a worsening of periodontal disease (9). Similarly, zinc also plays an important role in antioxidant activity in the periodontium, and its deficiency has been shown to result in a worsening of periodontal disease in patients with type 2 diabetes mellitus (9). The effect of nutrients on the health of the periodontium is summarized in Table 1.
In addition to nutritional deficiency, stress and smoking are compounding factors for NPD in this case report. Stress can induce increased corticosteroid production (7). Chronically increased corticosteroid levels due to stress can lead to a plethora of downstream effects on the oral cavity, including, but not limited to, alveolar bone loss and an increased risk for periodontal attachment loss (16, 17). In addition, P. intermedia, one of the bacteria associated with NPD, requires a growth factor to proliferate, and it has been shown that corticosteroids can act as the growth factor for P. intermedia (3, 7). This can lead to the postulation that stress-induced corticosteroid increase could provide a nutritional advantage to the proliferation of NPD-specific bacterial species like P. intermedia over the other oral bacterial species (3, 7). Smoking has also been positively associated with a higher incidence and progression of periodontal disease (18). The mechanisms in which smoking affects the incidence and progression of periodontitis are not fully known. However, it has been hypothesized that smoking can affect the immune response and decrease the healing capacity of the periodontium (18). It has also been postulated that smoking can shift the composition of the oral microbiota to favor an increase in the number of pathogens that are associated with periodontitis, similar to how stress affects the microbiota (18).
Conclusion
Most risk factors for NPD, such as poor nutritional status, tobacco use, psychological stress, and poor oral hygiene, are modifiable, especially where malnutrition is concerned. In this article, the case of a patient with NPD exacerbated by malnutrition and smoking and provided with nonsurgical periodontal treatment was reviewed. The patient initially presented with painful and spontaneous gingival bleeding, malodor, and distinctive necrotized and punched out interdental papillae. After combined dental and medical treatments, along with behavior/diet modification efforts by the patient herself, disease remission was achieved. Early detection and treatment for NPD is important as the rapid progression of the disease can lead to irreversible periodontal damage, disfiguring cancrum oris, and can even prove potentially fatal.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
The authors contributed equally to the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to acknowledge the support from the Department of Periodontics and Dental Hygiene of the University of Texas Health Science Center at Houston, School of Dentistry.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aaron SL, DeBlois KW. Acute Necrotizing Ulcerative Gingivitis. Treasure Island (Fl): StatPearls Publishing (2021).
2. Johnson BD, Engel D. Acute necrotizing ulcerative gingivitis. a review of diagnosis, etiology and treatment. J Periodontol. (1986) 57(3):141–50. doi: 10.1902/jop.1986.57.3.141
3. Loesche WJ, Syed SA, Laughon BE, Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. (1982) 53(4):223–30. doi: 10.1902/jop.1982.53.4.223
4. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodonto. (1999) 4(1):1–6. doi: 10.1902/annals.1999.4.1.1
5. Herrera D, Retamal-Valdes B, Alonso B, Feres M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J Periodontol. (2018) 89:S85–S102. doi: 10.1002/JPER.16-0642
6. Enwonwu CO, Falkler WA, Phillips RS. Noma (cancrum oris). Lancet. (2006) 368(9530):147–56. doi: 10.1016/S0140-6736(06)69004-1
7. Wade DN, Kerns DG. Acute necrotizing ulcerative gingivitis-periodontitis: a literature review. Mil Med. (1998) 163(5):337–42. doi: 10.1093/milmed/163.5.337
8. Neiva RF, Steigenga J, Al-Shammari KF, Wang HL. Effects of specific nutrients on periodontal disease onset, progression and treatment. J Clin Periodontol. (2003) 30(7):579–89. doi: 10.1034/j.1600-051x.2003.00354.x
9. Najeeb S, Zafar MS, Khurshid Z, Zohaib S, Almas K. The role of nutrition in periodontal health: an update. Nutrients. (2016) 8(9):530. doi: 10.3390/nu8090530
10. Melnick SL, Alvarez JO, Navia JM, Cogen RB, Roseman JM. A case-control study of plasma ascorbate and acute necrotizing ulcerative gingivitis. J Dent Res. (1988) 67(5):855–60. doi: 10.1177/00220345880670051201
11. Hanisch M, Hoffmann T, Bohner L, Hanisch L, Benz K, Kleinheinz J, et al. Rare diseases with periodontal manifestations. Int J Environ Res Public Health. (2019) 16(5):867. doi: 10.3390/ijerph16050867
12. Melnick SL, Roseman JM, Engel D, Cogen RB. Epidemiology of acute necrotizing ulcerative gingivitis. Epidemiol Rev. (1988) 10:191–211. doi: 10.1093/oxfordjournals.epirev.a036022
13. Malek R, Gharibi A, Khlil N, Kissa J. Necrotizing ulcerative gingivitis. Contemp Clin Dent. (2017) 8(3):496–500. doi: 10.4103/ccd.ccd_1181_16
15. Dommisch H, Kuzmanova D, Jönsson D, Grant M, Chapple I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontol 2000. (2018) 78(1):129–53. doi: 10.1111/prd.12233
16. Ng SKS, Keung Leung W. A community study on the relationship between stress, coping, affective dispositions and periodontal attachment loss. Community Dent Oral Epidemiol. (2006) 34(4):252–66. doi: 10.1111/j.1600-0528.2006.00282.x
17. Cirillo N. Role of tissue-specific steroid metabolism in oral disease: is there any clinical implication? Oral Dis. (2018) 24(1–2):224–7. doi: 10.1111/odi.12767
Keywords: periodontal diseases, oral pathology, nutrition disorder, state-of-the-art review, cigarette smoking
Citation: Sheng S, Kim HH, Meng H-W, Tribble GD and Chang J (2022) Necrotizing periodontal disease in a nutritionally deficient patient: A case report. Front. Dent. Med 3: 994442. doi: 10.3389/fdmed.2022.994442
Received: 14 July 2022; Accepted: 29 August 2022;
Published: 22 September 2022.
Edited by:
Gaetano Isola, University of Catania, ItalyReviewed by:
José Alcides Almeida de Arruda, Federal University of Minas Gerais, BrazilSiddhartha Varma, Krishna Institute of Medical Sciences, India
© 2022 Sheng, Kim, Meng, Tribble and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer Chang, SmVubmlmZXIuQ2hhbmdAdXRoLnRtYy5lZHU=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Periodontics, a section of the journal Frontiers in Dental Medicine
 Sally Sheng
Sally Sheng Gena D. Tribble
Gena D. Tribble Jennifer Chang
Jennifer Chang