- Department of Oral and Craniofacial Sciences, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, United States
Trigeminal neuralgia is highly debilitating, and its etiology is still undefined. The goal of this work was to define associations between well-characterized trigeminal neuralgia cases and common genetic variants in the population. Two hundred and fifty-seven individuals diagnosed with classical trigeminal neuralgia were compared to 865 individuals without classical trigeminal neuralgia and with an assessment for lower or higher pain threshold based on the amount of anesthetic required for routine dental treatment. Genotypes of 24 variants marking genes in the VGSC (voltage-gated sodium channels) or GABA (gamma-aminobutyric acid) pathways were obtained using TaqMan chemistry end end-point analysis. Chi-square was used for all comparisons with an alpha of 0.002. An association between classical trigeminal neuralgia and individuals requiring less or more anesthetic for routine dental treatments showed associations with SCN8A rs1601012 and GPHN rs723432 (p = 0.0009 and p = 0.0002, respectively). In conclusion, classical trigeminal neuralgia is associated with SCN8A and GPHN and markers rs1601012 rs723432 may be useful to determine individual risks for the condition.
Introduction
The term trigeminal neuralgia includes any “pain related to a lesion or disease of the trigeminal nerve” (1). Recognized variants of trigeminal neuralgia include those related to trauma, demyelination (e.g., multiple sclerosis), viruses (e.g., varicella zoster), tumors and neurovascular compression. Classical trigeminal neuralgia is characterized by severe, intermittent, and brief paroxysms of pain. Although approximately 80-90% of patients with classical trigeminal neuralgia have MRI- evidence of neurovascular compression of the trigeminal nerve (2, 3), the mechanism by which neurovascular compression may cause classical trigeminal neuralgia in these patients remains poorly understood (4). Demyelination of the trigeminal nerve in trigeminal neuralgia patients with and without neurovascular compression has been documented (5). Classical trigeminal neuralgia aggregates in families, affecting particularly women, suggesting there is a genetic basis for the condition (6). Based on prior observations concerning the role of voltage- gated sodium channel (VGSC) and gamma-aminobutyric acid (GABA) in various forms of trigeminal neuralgia (7–9), we hypothesized that genetic contributors to classical trigeminal neuralgia could be identified through existing techniques. Phenotypes that have been studied as a proxy for orofacial pain in the search of genetic contributors included opioid sensitivity, alveolar nerve changes after bilateral sagittal split ramus osteotomy, underlying temporomandibular disorders, mechanical, cold and heat pain threshold tests, and fear of pain scales (10–16). The framework of these analyses involves the comparison of affected and unaffected individuals. To bolster this framework, we created an additional comparison group, distinguishing individuals with higher from lower pain sensitivity, with the hypothesis that this distinction may facilitate the identification of genetic contributors to classical trigeminal neuralgia (i.e., the group with less sensitivity to pain may be a better comparison group). Therefore, the aim of this work was to test for over-representation of alleles of genes in the VGSC and GABA pathways in individuals with trigeminal neuralgia.
Materials and methods
Study sample
Participants were recruited through two registries. The University of Pittsburgh Orofacial Pain Registry and Sample Repository and the Dental Registry and DNA Repository project at the University of Pittsburgh School of Dental Medicine. Starting in September of 2006, all individuals who seek treatment at the University of Pittsburgh School of Dental Medicine have been invited to be part of the Dental Registry and DNA Repository project. Starting in January 2016, all individuals seeking treatment for various forms of trigeminal neuralgia at the University of Pittsburgh Presbyterian Hospital Department of Neurological Surgery have been invited to be part of the Orofacial Pain Registry project. All samples were categorized according to the International Headache Society's classification of “painful cranial neuropathies, other facial pains and other headaches” (1).
The provisions of the Declaration of Helsinki and US Federal Policy for the Protection of Human Subjects have been followed during the present study, which was approved by the University of Pittsburgh Institutional Review Board (IRB) under the protocols # 19050020 and 20050101. All subjects gave written informed consent to participate in this study after a full explanation of the procedures of the study and submitted to providing a saliva sample as a source of genomic DNA. The sample is typically collected at the first visit, before any necessary dental treatment is performed. From 5,025 records of participants in the Dental Registry and DNA Repository project, we selected 865 adults (513 women) who required more than one anesthetic tube to allow for a single posterior tooth restoration to be performed and compared to 365 adults (206 women) that did not need more than one tube to perform similar work (17). Although it can be argued that differences in anatomical structures, metabolism, and presence of infection may account for the need to give additional anesthetic solution to perform regular dental treatments, the assumption is that by matching subjects by the procedures done, the need for additional anesthetic solution may be related to higher levels of pain sensitivity. These two groups were compared to a group of 257 individuals (142 women) with purely paroxysmal classical trigeminal neuralgia out of the 660 individuals with various forms of trigeminal neuralgia participating in the Orofacial Pain Registry project. The reason for selecting only pure forms of trigeminal neuralgia (and not secondary or idiopathic cases) aimed to improve homogeneity. Trigeminal neuralgia is evoked by ectopic action potentials generated from the V root while compressed by a vessel. Idiopathic forms of trigeminal neuralgia have the same symptoms as classical trigeminal neuralgia but very mild or no compression by a vessel. Secondary forms of trigeminal neuralgia are reserved for cases that are associated with multiple sclerosis or resulting from trauma. Therefore, the molecular mechanisms underlying these subtypes of trigeminal neuralgia may be distinct. These three groups had similar ages (i.e., most individuals are above 45 years of age and reflect the demographic breakdown of the Pittsburgh area; 76% Whites, 18% Blacks, and the rest comprised by other groups).
Genotyping
Twenty-four single nucleotide polymorphisms (SNPs) were chosen (one for each gene) as markers for genes in the VGSC and GABA pathways (Table 1). These SNPs were selected based on their heterozygosity and the existence of an assay that has been optimized and were chosen based on their probability of having functional outcomes as tag-SNPs that were representative of the genes.
Saliva samples were obtained from volunteer participants, and no buffering reagent was added to the saliva samples and samples were stored at ice (2 h maximum) and then at −80 until extraction. Genomic DNA extraction from saliva samples was performed using the following protocol. Samples were brought to room temperature and centrifuged for 5 min at 10,000 × g. After the supernatant was removed, 1 ml of extraction buffer (10 mM Tris-HCL pH 7.8, 5 mM EDTA, 0.5% SDS) was added to the buccal cell pellet and thoroughly mixed. Five ul of proteinase K were added, and the samples were incubated in a 56°C water bath overnight. After removal, the samples were vortexed and 500 ul of 10 M ammonium acetate was added. Samples were inverted for 3 min and centrifuged at 21,000 × g for 15 min. The supernatant was then transferred to two eppendorfs with an equal volume of cold isopropanol and shaken vigorously. Samples were then incubated at −20°C for a minimum of 30 min, after which they were centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was poured off, and 1 ml of cold 70% EtOH was added and samples were inverted 3 times. Samples were then centrifuged at 10,000 × g for 5 min at 4°C. The supernatant was poured off and samples were allowed to air dry before 100 ul of TE buffer was added. Samples were kept in a 4°C refrigerator for 2–3 days before ensuring the DNA was completely dissolved before reading the concentration of the samples. The extracted DNA was preserved −20°C for further analysis.
The quality assessment and concentrations of the genomic DNA were conducted with a NanoDrop 2000 (Thermo Fisher Scientific, USA) spectrophotometer. A260/A280 curve was applied to determine the purity of DNA.
Taqman chemistry was used for the generation of the genotypes (18). Reactions were performed in 3 μl volumes in a QuantStudio 6 Flex automatic instrument and pre-designed assays (Applied Biosystems, Foster City, CA, USA), and reagents of the same system were used.
Statistical analysis
Overrepresentation of genotypes and alleles was tested using chi-square and alpha of 0.002 (0.05/23, SNP SCN3A rs34565727 was not informative and therefore not analyzed further). The distribution of genotypes (AA, AB, and BB) between the two groups was compared, as well as the number of alleles (A and B). Genotypic distributions were also tested for Hardy-Weinberg equilibrium using Pearson's chi-square test (two-tailed) based on the classical formula (p2 + 2pq + q2 = 1).
Results
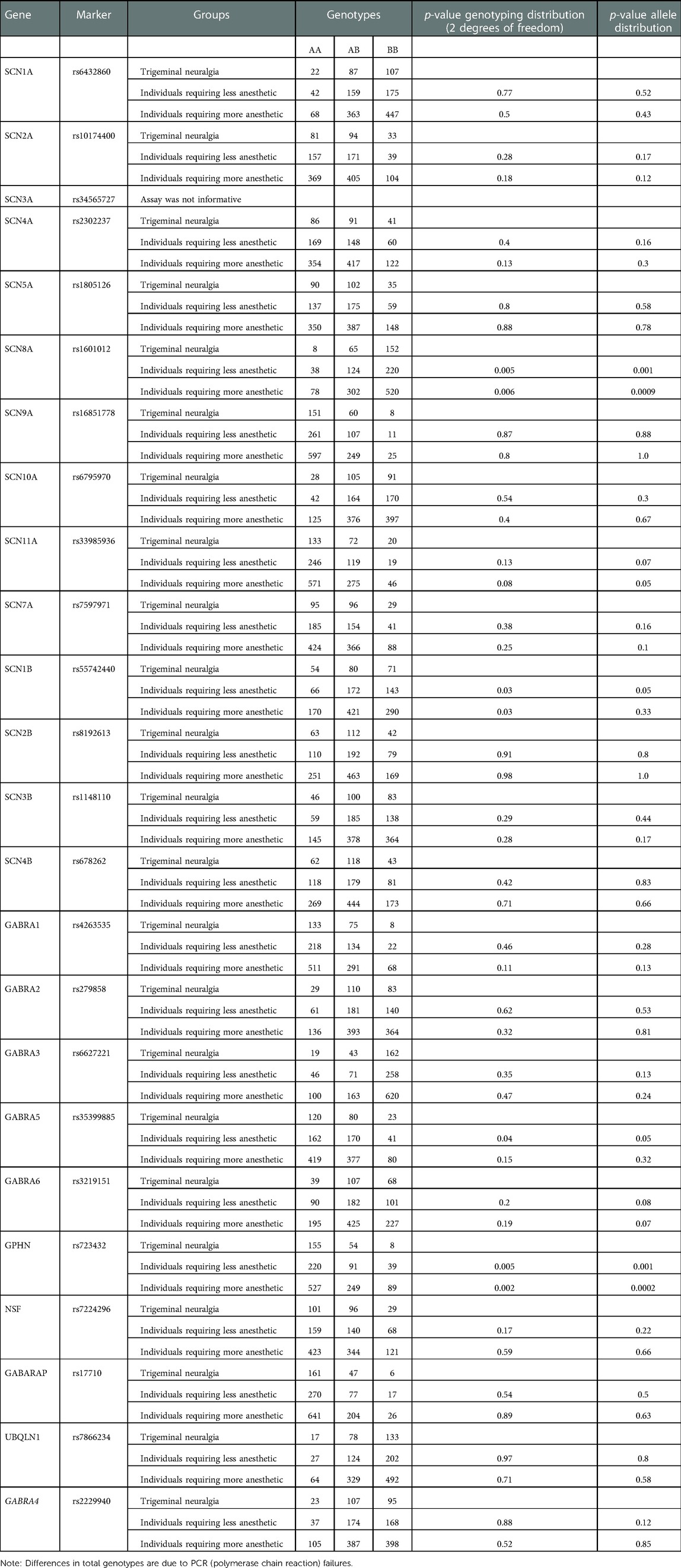
All genotypes were in Hardy-Weinberg equilibrium (p > 0.001) (data not shown). Table 1 presents all raw genotyping data. Comparisons between individuals with classical trigeminal neuralgia and individuals requiring less or more anesthetic for routine dental treatments showed associations with SCN8A rs1601012 and GPHN rs723432 (p = 0.0009 and p = 0.0002, respectively). There were no differences in the distributions of genotypes between individuals requiring less or more anesthetic for routine dental treatment.
Discussion
Classical trigeminal neuralgia is associated with genetic variations in the SCN8A and GPHN genes. Sodium channels are transmembrane proteins that can selectively conduct sodium. They determine the electrical excitability of sensory neurons modulating pain sensation. SCN8A (a sodium channel also called Nav1.6) is expressed in several painful and excitatory scenarios that have been tested in murine (19–35), zebrafish (36), Xenopus (37, 38), and human cell models (39, 40). In humans, SCN8A encephalopathy is a condition that presents in infancy with multiple seizure types and poor outcomes linked to mutations that for the most part arise de novo, although at least one case of a somatic mosaicism has been reported (41). Variants in SCN8A located in exons 13, 16, 21, and 26 have also been reported in cases diabetic and idiopathic neuropathy (42). A mutation in SCN8A was reported in a 64-year-old white woman who presented with classical trigeminal neuralgia. A Met136Val change produced a significant increase in peak transient and resurgent currents of Nav1.6, reduced the threshold for action potential in trigeminal ganglia neurons, and enhanced the neuronal evoked response and the fraction of neurons that fire at a higher rate than those expressing wild-type channels (43). We showed that this mutation is unlikely to be a common cause of classical trigeminal neuralgia, since we did not find it in 123 cases (9). Here, we expanded this work to additional cases (N = 660) and unveiled an association between trigeminal neuralgia and SCN8A. Individuals carrying the less common allele of rs1601012 were 36% less likely (odds ratio 0.64, 95% confidence interval 0.49–0.84) to show trigeminal neuralgia. Due to the evidence that coding mutation in SCN8A is associated with forms of encephalopathy, we suggest that hypomorphic alleles of SCN8A may underlie instances of trigeminal neuralgia and rs1601012 may be a risk marker for the condition.
Gephyrin is a cytoplasmatic protein that forms postsynaptic scaffolds to anchor GABA and glycine receptors, among other synaptic inhibitory functions, impacting in multiple ways pain sensation. Alterations in the human gephyrin (GPNH) gene are associated with leukemia (44), molybdenum cofactor deficiency (45), hyperekplexia (46), autism, schizophrenia, and seizures (47). The association we found between GPNH and trigeminal neuralgia is unlikely to be an indication that coding mutations in GPNH are common causes of the condition, since there is a range of neurological conditions that have been associated with the gene. Individuals carrying the less common allele of rs723432 were 41% less likely to have trigeminal neuralgia (odds ratio 0.59, 95% confidence interval 0.44–0.78). Hypomorphic GPNH alleles, however, could underlie some cases of trigeminal neuralgia, and rs723432 could serve as a genomic marker for the risk of the condition.
The three study groups, although very similar, were not precisely matched by sex, age, and ethnicity, but it is unlikely that population stratification explains the associations found. The phenotype we created related to the number of anesthetic tubetes used by the dentist for routine treatment is not easy to replicate since we are not aware of any other groups that keep comprehensive medical and dental records linked to biological samples. In conclusion, classical trigeminal neuralgia is associated with SCN8A and GPHN and markers rs1601012 rs723432 may be useful to determine individual risks for the condition.
Data availability statement
The authors acknowledge that the data presented in this study must be deposited and made publicly available in an acceptable repository, prior to publication. Frontiers cannot accept a manuscript that does not adhere to our open data policies.
Ethics statement
The studies involving human participants were reviewed and approved by the University of Pittsburgh Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors were involved in collecting and analyzing data and contributed to the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
Data supporting this work was obtained from the University of Pittsburgh School of Dental Medicine Dental Registry and DNA Repository project, which is supported by the University of Pittsburgh School of Dental Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Headache Classification Committee of the International Headache Society (HIS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. (2013) 33(9):629–808. doi: 10.1177/0333102413485658
2. Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L. Significance of neurovascular contact in classical trigeminal neuralgia. Brain. (2015) 138(Pt. 2):311–9. doi: 10.1093/brain/awu349
3. Traylor KS, Sekula RF, Eubanks K, Muthiah N, Chang Y-F, Hughes MA. Prevalence and severity of neurovascular compression in hemifacial spasm patients. Brain. (2021) 144(5):1482–7. doi: 10.1093/brain/awab030
4. Frederickson AM, Gold MS, Sekula RF Jr. Pathogenesis of trigeminal neuralgia. In: Li S-T, Zhong J, Sekula RF Jr, editors. Microvascular decompression surgery. Dordrecht, The Netherlands: Springer Science+Business Media B.V. (2016). 1st ed. with t(11;14)(q23;q24). Genes, Chromosomes & Cancer. (2016) 32(3):212–221. doi: 10.1002/gcc.1185
5. Rappaport ZH, Govrin-Lippmann R, Devor M. An electron-microscopic analysis of biopsy samples of the trigeminal root taken during microvascular decompressive surgery. Stereotact Funct Neurosurg. (1997) 68(1-4 Pt 1):182–6. doi: 10.1159/000099920
6. Rodríguez BF, Simonet C, Cerdán DM, Morollón N, Guerrero P, Tabernero C, et al. Familial classic trigeminal neuralgia. Neurología. (2019) 34(4):229–33. doi: 10.1016/j.nrl.2016.12.004
7. Dong W, Jin SC, Allocco A, Zeng X, Sheth AH, Panchagnula S, et al. Exome sequencing implicates impaired GABA signaling and neuronal ion transport in trigeminal neuralgia. iScience. (2020) 23(10):101552. doi: 10.1016/j.isci.2020.101552
8. Pineda-Farias JB, Loeza-Alcocer E, Nagarajan V, Gold MS, Sekula RF Jr. Mechanisms underlying the selective therapeutic efficacy of carbamazepine for attenuation of trigeminal nerve injury pain. J Neurosci. (2021) 41(43):8991–9007. doi: 10.1523/JNEUROSCI.0547-21.2021
9. Sekula RF, Deeley K, Denwood H, Vieira AR. Gain-of-function mutation Met136Val in SCN8A may not be a common cause of trigeminal neuralgia. Mol Genet Genomic Med. (2021) 9(2):e1587. doi: 10.1002/mgg3.1587
10. Fukuda K, Hayashida M, Ide S, Saita N, Kokita Y, Kasai S, et al. Association between OPRM1 gene polymorphisms and fentanyl sensitivity in patients undergoing painful cosmetic surgery. Pain. (2009) 147(1-3):194–201. doi: 10.1016/j.pain.2009.09.004
11. Kobayashi D, Nishizawa D, Takasaki Y, Kasai S, Kakizawa T, Ikeda K, et al. Genome-wide association study of sensory disturbances in the inferior alveolar nerve after bilateral sagittal split ramus osteotomy. Mol Pain. (2013) 9:34. doi: 10.1186/1744-8069-9-34
12. Nishizawa D, Fukuda K, Kasai S, Hasegawa J, Aoki Y, Nishi A, et al. Genome- wide association study identifies a potent locus associated with human opioid sensitivity. Mol Psychiatry. (2014) 19(1):55–62. doi: 10.1038/mp.2012.164
13. Parisien M, Khoury S, Chabot-Doré AJ, Sotocinal SG, Slade GD, Smith SB, et al. Effect of human genetic variability on gene expression in dorsal root ganglia and association with pain phenotypes. Cell Rep. (2017) 19(9):1940–52. doi: 10.1016/j.celrep.2017.05.018
14. Randall CL, Wright CD, Chernus JM, McNeil DW, Feingold E, Crout RJ, et al. A preliminary genome- wide association study of pain-related fear: implications for orofacial pain. Pain Res Manag. (2017) 2017:7375468. doi: 10.1155/2017/7375468
15. Takahashi K, Nishizawa D, Kasai S, Koukita Y, Fukuda KI, Ichinohe T, et al. Genome-wide association study identifies polymorphisms associated with the analgesic effect of fentanyl in the preoperative cold pressor-induced pain test. J Pharmacol Sci. (2018) 136(3):107–13. doi: 10.1016/j/jphs.2018.02.002
16. Smith SB, Parisien M, Bair E, Belfer I, Chabot-Doré AJ, Gris P, et al. Genome-wide association reveals contribution of MRAS to painful temporomandibular disorders in males. Pain. (2019) 160(3):579–91. doi: 10.1097/j.pain.0000000000001438
17. Vieira AR. Genetic basis of orofacial pain and temporomandibular joint dysfunction. In: Vieira AR, editors. Genetic basis of oral health conditions. 1ed. Cham: Springer (2019). p. 81–92.
18. Ranade K, Chang MS, Ting CT, Pei D, Hsiao CF, Olivier M, et al. High-throughput genotyping with single nucleotide polymorphisms. Genome Res. (2001) 11(7):1262–8. doi: 10.1101/gr.157801
19. Henry MA, Freking AR, Johnson LR, Levinson SR. Sodium channel Nav1.6 accumulates at the site of infraorbital nerve injury. BMC Neurosci. (2007) 8:56. doi: 10.1186/1471-2202-8-56
20. Byers MR, Rafie MM, Westenbroek RE. Dexamethasone effects on Na(v)1.6 in tooth pulp, dental nerves, and alveolar osteoclasts of adult rats. Cell Tissue Res. (2009) 338(2):217–26. doi: 10.1007/s00441-009-0842-6
21. Luo S, Perry GM, Levinson SR, Henry MA. Pulpitis increases the proportion of atypical nodes of Ranvier in human dental pulp axons without a change in Nav1.6 sodium channel expression. Neuroscience. (2010) 169(4):1881–7. doi: 10.1016/j.neuroscience.2010.06.044
22. Ren YS, Qian NS, Tang Y, Liao YH, Yang YL, Dou KF, et al. Sodium channel Nav1.6 is up-regulated in the dorsal root ganglia in a mouse model of type 2 diabetes. Brain Res Bull. (2012) 87(2-3):244–9. doi: 10.1016/j.brainresbull.2011.10.015
23. Sittl R, Lampert A, Huth T, Schuy ET, Link AS, Fleckenstein J, et al. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc Natl Acad Sci U S A. (2012) 109(17):6704–9. doi: 10.1073/pnas.1118058109
24. Deuis JR, Zimmermann K, Romanovsky AA, Possani LD, Cabot PJ, Lewis RJ, et al. An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain. (2013) 154(9):1749–57. doi: 10.1016/j.pain.2013.05.032
25. Xie W, Strong JA, Ye L, Mao J-X, Zhang J-M. Knockdown of sodium channel Nav1.6 blocks mechanical pain and abnormal bursting activity of afferent neurons in inflamed sensory ganglia. Pain. (2013) 154(8):1170–80. doi: 10.1016/j.pain.2013.02.027
26. Feng B, Zhu Y, La J-H, Wills ZP, Gebhart GF. Experimental and computational evidence for an essential role of NaV1.6 in spike initiation at stretch-sensitive colorectal afferent endings. J Neurophysiol. (2015) 113(7):2618–34. doi: 10.1152/jn.00717.2014
27. Xie W, Strong JA, Zhang JM. Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience. (2015) 291:317–30. doi: 10.1016/j.neuroscience.2015.02.010
28. Qin S, Jiang F, Zhou Y, Zhou G, Ye P, Ji Y. Local knockdown of Nav1.6 relieves pain behaviors induced by BmK I. Acta Biochim Biophys Sin. (2017) 49(8):713–21. doi: 10.1093/abbs/gmx064
29. Chen L, Huang J, Zhao P, Persson AK, Dib-Hajj FB, Cheng X, et al. Conditional knockout of Na(V)1.6 in adult mice ameliorates neuropathic pain. Sci Rep. (2018) 8(1):3845. doi: 10.1038/s41598-018-22216-w
30. Zhang X-L, Ding H-H, Xu T, Liu M, Ma C, Wu S-L, et al. Palmitoylation of δ-catenin promotes kinesin-mediated membrane trafficking of Nav1.6 in sensory neurons to promote neuropathic pain. Sci Signal. (2018) 11(523):eaar4394. doi: 10.1126/scisignal.aar4394
31. Ding HH, Zhang SB, Lv YY, Ma C, Liu M, Zhang KB, et al. TNF-alpha/STAT3 pathway epigenetically upregulates Nav1.6 expression in DRG and contributes to neuropathic pain induced by L5-VRT. J Neuroinflammation. (2019) 16(1):29. doi: 10.1186/s12974-019-1421-8
32. Israel MR, Tanaka BS, Castro J, Thongyoo P, Robinson SD, Zhao P, et al. Nav 1.6 regulates excitability of mechanosensitivity sensory neurons. J Physiol. (2019) 597(14):3751–68. doi: 10.1113/JP278148
33. Li L, Shao J, Wang J, Liu Y, Zhang Y, Zhang M, et al. MIR-30b-5p attenuates oxaliplatin-induced peripheral neuropathic pain through the voltage-gated sodium channel Na(v)1.6 in rats. Neuropharmacology. (2019) 153:111–20. doi: 10.1016/j.neuropharm.2019.04.024
34. Xie W, Zhang J, Strong JA, Zhang JM. Role of Na(V)1.6 and Na(V)beta4 sodium channel subunits in a rat model of low back pain induced by compression of the dorsal root ganglia. Neuroscience. (2019) 402:51–65. doi: 10.1016/j.neuroscience.2019.01.012
35. Chen L, Huang J, Benson C, Lankford KL, Zhao P, Carrara J, et al. Sodium channel Nav1.6 in sensory neurons contributes to vincristine-induced allodynia. Brain. (2020) 143(8):2421–36. doi: 10.1093/brain/awaa208
36. Weuring WJ, Singh S, Volkers L, Rook MB, van't Slot RH, Bosma M, et al. Nav1.1 and NaVq.6 selective compounds reduce the behavior phenotype and epileptiform activity in a novel zebrafish model for Dravet syndrome. PLoS One. (2020) 15(3):e0219106. doi: 10.1371/journal.pone.0219106
37. Okura D, Horishita T, Ueno S, Yanagihara N, Sudo Y, Uezono Y, et al. The endocannabinoid anandamide inhibits voltage-gated sodium channels Nav1.2, Nav1.6, Nav1.7, and Nav1.8 in Xenopus oocytes. Anesth Analg. (2014) 118(3):554–62. doi: 10.1213/ANE.0000000000000070
38. Horishita T, Yanagihara N, Ueno S, Okura D, Horishita R, Minami T, et al. Antidepressants inhibit Nav1.3, Nav1.7 and Nav1.8 neuronal voltage-gated sodium channels more potently than Nav1.2 and Nav1.6 channels expressed in Xenopus oocytes. Naunyn Schmiedeberg's Arch Pharmacol. (2017) 390(12):1255–70. doi: 10.1007/s00210-017-1424-x
39. Gonçalves TC, Boukaiba R, Molgó J, Amar M, Partiseti M, Servent D, et al. Direct evidence for high affinity blockade of Nav1.6 channel subtype by huwentoxin- IV spider peptide, using multiscale functional approaches. Neuropharmacology. (2018) 133:404–14. doi: 10.1016/j.neuropharm.2018.02.016
40. Israel MR, Thongyoo P, Deuis JR, Craik DJ, Vetter I, Durek T. The E15R point mutation in scorpion toxin Cn2 uncouples its depressant and excitatory activities of human Nav1.6. J Med Chem. (2018) 61(4):1730–6. doi: 10.101/acs.jmedchem.7b01609
41. Larsen J, Carvill GL, Gardella E, Kluger G, Schmiedel G, Barisic N, et al. The phenotypic spectrum of SCN8A encephalopathy. Neurology. (2015) 84(5):480–9. doi: 10.1212/WNL.0000000000001211
42. Almomani R, Marchi M, Sopacua M, Lindsey P, Salvi E, Koning B, et al. Evaluation of molecular inversion probe versus TruSeq® custom methods for targeted next-generation sequencing. PLoS One. (2020) 15(9):e0238467. doi: 10.1371/journal.pone.0238467
43. Tanaka BS, Zhao P, Dib-Hajj FB, Morisset V, Tate S, Waxman SG, et al. A gain-of-function mutation in Nav1.6 in a case of trigeminal neuralgia. Mol Med. (2016) 22:338–48. doi: 10.2119/molmed.2016.00131
44. Eguchi M, Eguchi-Ishimae M, Seto M, Morishita K, Suzuki K, Ueda R, et al. GPHN, a novel partner gene fused to MLL in a leukemia (2001).
45. Reiss J, Hahnewald R. Molybdenum cofactor deficiency: mutations in GPHN, MOCS1, and MOCS2. Hum Mutat. (2011) 32(1):10–8. doi: 10.1002/humu.21390
46. Rees MI, Harvey K, Ward H, White JH, Evans L, Duguid IC, et al. Isoform heterogeneity of the human gephyrin gene (GPHN), binding domains to the glycine receptor, and mutation analysis in hyperekplexia. J Biol Chem. (2003) 278(27):24688–96. doi: 10.1074/jbc.M301070200
Keywords: pain, orofacial pain, trigeminal nerve, trigeminal neuralgia, odontogenic pain, GABA, sodium channel
Citation: Vieira AR, Sekula Jr. RF and Deeley K (2023) Classical trigeminal neuralgia is associated with gephyrin and sodium voltage-gated channel alpha subunit 8. Front. Dent. Med 3:988094. doi: 10.3389/fdmed.2022.988094
Received: 6 July 2022; Accepted: 21 December 2022;
Published: 30 January 2023.
Edited by:
Cameron L Randall, University of Washington, United StatesReviewed by:
Henrique Ballassini Abdalla, São Leopoldo Mandic School, BrazilJun Zhong, Shanghai Jiao Tong University, China
Phillip R Kramer, Texas A&M University, United States
© 2023 Vieira, Sekula and Deeley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandre R. Vieira, YXJ2MTFAcGl0dC5lZHU=
†ORCID:
Alexandre R. Vieira
orcid.org/0000-0003-3392-6881
Specialty Section: This article was submitted to Systems Integration, a section of the journal Frontiers in Dental Medicine
 Alexandre R. Vieira
Alexandre R. Vieira Raymond F. Sekula Jr.
Raymond F. Sekula Jr. Kathleen Deeley
Kathleen Deeley