- 1Department of Orthopaedic Surgery, Keck School of Medicine of USC, University of Southern California (USC), Los Angeles, CA, United States
- 2Center for Craniofacial Molecular Biology, Ostrow School of Dentistry of University of Southern California (USC), Los Angeles, CA, United States
- 3Department of Oral and Maxillofacial Surgery, Peking University School and Hospital of Stomatology, National Center of Stomatology, National Clinical Research Center for Oral Diseases, National Engineering Research Center of Oral Biomaterial and Digital Medical Devices, Beijing, China
- 4Department of Orthopaedic Surgery, Sir Run Run Hospital, Nanjing Medical University, Nanjing, China
- 5Center for Temporomandibular Disorders and Orofacial Pain, Peking University School and Hospital of Stomatology, National Center of Stomatology, National Clinical Research Center for Oral Diseases, National Engineering Research Center of Oral Biomaterial and Digital Medical Devices, Beijing, China
- 6State Key Laboratory of Oral Diseases, Department of Oral and Maxillofacial Surgery, West China Hospital of Stomatology, Sichuan University, Chengdu, China
- 7Department of Orthopaedics, The Affiliated Jiangning Hospital With Nanjing Medical University, Nanjing, China
- 8Department of Orthopaedic Surgery, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China
- 9Broad Center for Stem Cell Research and Regenerative Medicine Keck School of Medicine of USC, University of Southern California (USC), Los Angeles, CA, United States
Temporomandibular joint disorders (TMJs) are a multifaceted group of chronic disorders characterized by stiffness in the jaw, limited jaw mobility and pain when opening or closing the mouth. TMJs are relatively common, with incidence rates in the range of 5–12%, with nearly twice as many women as men being affected. One of the primary causes of TMJs is a degenerative disease of joints, such as osteoarthritis (OA), characterized by progressive loss of cartilage, which causes stiffness, swelling, and pain. Currently, there are no disease-modifying agents on the market for OA. We have recently discovered a small molecule, R805, acting as a modulator of glycoprotein 130 (gp130) receptor for the IL-6 family of cytokines. R805 enables regenerative outputs of endogenous joint stem and progenitor cells through immunomodulation in the joint microenvironment by reducing the levels of destructive cytokines and supporting chondrocyte survival and anabolism. Extensive testing has shown R805 to be safe at doses far above the therapeutic level. Here, we have conducted a pivotal efficacy study in our newly established pig model of TMJ post-traumatic OA. IA injection of R805 has shown a highly significant reduction of articular cartilage degeneration, reduced synovitis and reduced degenerative changes in subchondral bone in the mandibular condyle compared to the vehicle-treated group. These data will support additional preclinical development of R805 as a first-in-class injectable therapeutic for TMJ osteoarthritis.
Introduction
Temporomandibular joint disorders (TMDs) are a heterogeneous group of chronic pain disorders characterized by pain and/or stiffness in the jaw, limited jaw mobility and pain when opening or closing the mouth (1). TMDs are relatively common, with incidence rates in the range of 5–12%, with nearly twice as many women as men being affected (2, 3). One of the primary causes of TMDs is arthritis, with both rheumatoid arthritis (RA) and OA associated with the condition in 40–75% of the cases (4, 5). Arthritis is a degenerative disease of joints, characterized by progressive loss of cartilage, which causes stiffness, swelling, and pain (6). RA is considered an autoimmune disease, with acute inflammation leading to the destruction of cartilage (4). In contrast, inflammation plays more of an enabling role in cartilage degeneration in OA, with chronic, lower levels of pro-inflammatory cytokines supporting a feed-forward process of chondrocyte loss (7). As of 2015, over 22% (~20% with OA and ~2% with RA) of Americans have been diagnosed with arthritis, and this number is projected to climb steeply due to a rapidly aging population (8).
Proposed risk factors for TMJ OA are in line with those suggested for other joints: age, sex, genetics, infection/inflammation, congenital, and developmental abnormalities (1). In the vast majority of patients, TMJ OA develops with aging without obvious predisposing conditions such as trauma and affects more than one joint (9). TMJ OA is common in hand OA patients (10), suggesting that TMJ OA may be part of the generalized OA phenotype.
Currently, there are no disease-modifying agents (DMAs) available on the market for the treatment of OA. Treatment modalities for OA focus on pain management and reducing inflammation, both of which can be achieved with NSAIDs and local (IA) or systemic corticosteroids (6, 11). In OA, additional care can include opioid-based pain medications and IA injections to increase joint viscosity (6).
The pathogenesis of OA often involves chronic, low-grade inflammation mediated by interleukin-6/glycoprotein 130 (IL-6/gp130) and other factors that promote matrix degradation over time and eventual destruction of cartilage (12). Gp130 and its downstream pathways have not only been previously implicated in cartilage degeneration and chronic joint inflammation but have also been shown to play a major role in chronic inflammatory pain (13). Previous attempts to completely inhibit gp130 by drugs have shown significant side effects due to the highly pleiotropic role of this receptor. In agreement with that, we have demonstrated that IL-6 cytokines drive both regenerative and destructive outcomes through selective and context-specific activation of signaling residues (modules) within the intracellular domain of gp130 receptor. Most recently, we have identified a signaling residue (Y814) within gp130 that represents an initiating factor responsible for the activation of a highly destructive c-SRC/MAPK circuit in response to IL-6 cytokines (14). To translate these findings into clinically relevant technology, we have created a new class of chemicals capable of selective inactivation of the gp130Y814/c-SRC/MAPK module and showed their efficacy in small and large animal models of knee post-traumatic osteoarthritis (PTOA) (14). The current study was designed to explore the therapeutic potential of a novel gp130 modulator R805 in a porcine model of TMJ OA.
Materials and Methods
Large Animal Model of TMJ Post-traumatic Osteoarthritis (PTOA)
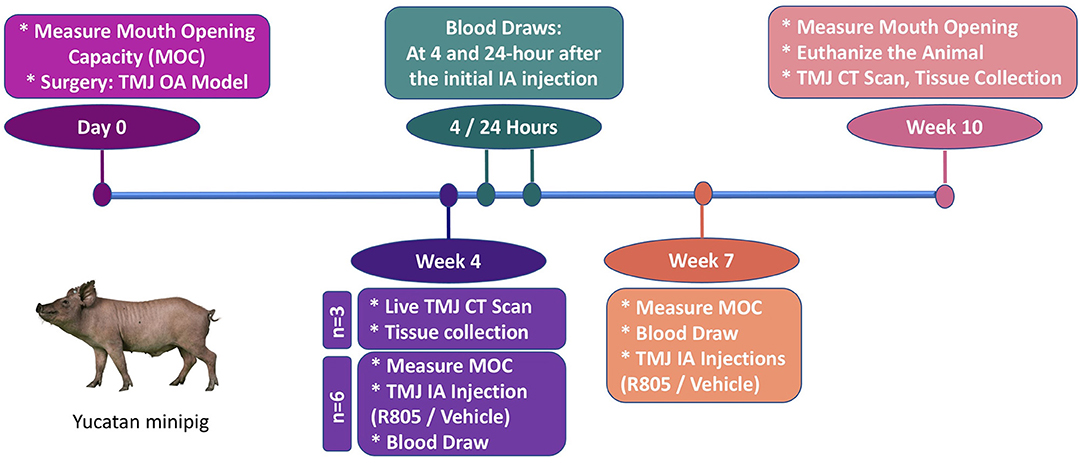
Yucatan minipigs were purchased from Premier BioSource—Swine for Biomedical Research (Formerly S & S Farms) at 5 months of age and housed under the supervision of the University of Southern California (USC) Department of Animal Resources (DAR). All preoperative, surgical and postoperative procedures were conducted following USC DAR guidelines and were overseen by the USC Institutional Animal Care and Use Committee (IACUC). Six animals per group (main study) with unilateral partial TMJ discectomy were included based on power calculations to yield the minimum number of animals projected to acquire statistically significant results. Briefly, we used the formula based on a 30–40% difference and 20% standard deviation between experimental groups, with these parameters based on our previous study of articular cartilage repair in large animal models (15). Animals were anesthetized for surgery using Telazol/Xylazine 2.2–4.4 mg/ kg administered intramuscularly. A 6–8 cm incision was made along the outer edge of the mandibular ramus (around the middle section between tragus and the outer canthus). The TMJ joint is about 1–2 cm below the line that connects tragus and the outer canthus. The condyle of the TMJ joint can be palpated while moving the jaw of the pig. The superior chamber of the joint was opened by horizontal incisions through the joint capsule. The lateral portion of the disc that was not covered by the zygomatic process was elevated from the condyle by using periosteal elevator to avoid damaging the underlying mandibular condyle surface. This exposed portion of the disc (~16 × 7 mm) was then excised by scissors. The surgical incision was closed with both internal and external sutures. Pigs were fed with a regular diet. No changes occurred in dietary habits or total mass. Three of the pigs were euthanized at 4 weeks following the surgery to confirm the development of OA in TMJ. The rest of the pigs were randomly divided into drug- or vehicle-treated groups and euthanized 10 weeks after the surgery.
The initial treatment with R805 or vehicle via TMJ intra-articular (IA) injection began at 4 weeks postsurgery (n = 6). At this timepoint, significant chronic degenerative changes are present in the condylar cartilage of the operated joint. Two drug/vehicle (R805, 10 mg) TMJ IA injections were administrated for the entire study with 3 weeks apart (4 and 7 weeks postsurgery). The functional evaluation for the treatment was performed by measuring the passive maximal mouth opening capacity (MOC) at multiple time points: before the surgery (baseline, day 0), 4 weeks postsurgery prior to the TMJ IA injection, 7 weeks postsurgery prior to the second TMJ IA injection, and 10 weeks postsurgery prior to euthanizing the animal. Pharmacokinetic study of R805 was also performed. Serial blood samples were collected at the same time points including 24 h, 72 h, and 3 weeks following the initial drug/vehicle TMJ IA injection. The pigs were euthanized at 10 weeks postsurgery for further radiographic and histological evaluation. n = 6 per group.
Live Computer Tomography (CT) and ex vivo mCT Scan Analyses
In vivo computer tomography (CT) images were acquired at the Department of Radiology at Keck School of Medicine, USC, using a Toshiba scanner. The animal was transported in a cage from Vivarium to the PET Imaging Sciences Center at USC. For full skull scans, X-ray parameters were used at 429 micro pixel size resolution: 120 kV energy, 200 uA current slice thickness 1, exposure time 500. Dicom images were reconstructed using a soft and sharp kernel.
Two sets of data were acquired using ex vivo MicroCT using Nikon Metrology microfocus XT H225 CT scanner at the Molecular Imaging Center at the Department of Radiology at Keck School of Medicine, USC. First, we obtained imaging for the complete skull to demonstrate three-dimensional anatomical association of the TMJ as it is known for its bilateral synovial articulation between the temporal bone of the cranium and mandibular condyle. This data allows us to not only visualize the anatomical association but also quantify the depth of this synovial articulation and compare it from right to left side. X-ray parameters were used for full skull scans at 130 micro pixel size resolution: 80 kV energy and 180 uA current. The specimens were rotated 360° around the detector, and 1,440 projects at one frame per second acquisition time were collected. A 0.2-mm copper filter was used to filter low-energy X-rays. The obtained 1,440 frames were reconstructed using software of Nikon metrology CT Pro 3D images to 16-bit unsigned images and were saved as. dicom stack for data analysis.
The second dissected right and left mandibular condyles were scanned at a much higher pixel size resolution to assess the subchondral bone apparent density maps. The subchondral bone material properties including bone mineral density (BMD), % of relative bone volume formation (BV/TV), bone formation rate (BFR), and bone resorption rate (BRR) for each individual TMJ were calculated at a high resolution. The same X-ray parameters were used for full skull scans but were scanned at 20 micro pixel size resolution: 80 kV energy and 180 uA current. The specimens were rotated 360° around the detector, and 1,440 projects at one frame per second acquisition time were collected. A 0.2 mm copper filter was used to filter low-energy X-rays. Obtained 1,440 frames were reconstructed using software of Nikon metrology CT Pro 3D images to 16-bit unsigned images and were saved as. dicom stack for data analysis.
Histological Analysis
Tissues were fixed in 10% formalin, decalcified with 14% EDTA, and sectioned at 5 μm (15, 16). Paraffin sections were deparaffinized and rehydrated by passage through xylene and 100, 95, and 70% ethanol. Safranin O/ Fast Green staining was performed as previously described (17). Slides were viewed using a Zeiss Axio Imager. The images were taken using an Axiocam 105 color camera with Zen 2 program. Standard microscope camera settings were used. Auto-exposure was used to normalize background light levels across all images (16).
OARSI Histopathological Scoring
To further confirm the osteoarthritic changes in our model, we used OARSI recommendations for a macroscopic and histological scoring system (18, 19) to semiquantitate histological changes. The macroscopic analysis was done by evaluation of five aspects of the condyle surface: smooth / roughness, irregularity, fibrillation, erosion, and ulceration. The scales are 0–8, with 0 being normal and 8 indicating severe ulceration (18, 19). Histological evaluation included Safranin O (0–6), structure (0–11), chondrocyte Density (0–4), and cell cluster formation (0–3) (18, 19).
Synovitis Scoring System
The grading of the synovial membranes was carried out on routine hematoxylin and eosin (H&E)-stained slides, according to the three synovial membrane features (synovial lining cell layer, stroma cell density and inflammatory infiltrate), the ranking of alterations being on a scale from none (0), slight (1), and moderate (2) to strong (3). The values of the parameters were summarized and interpreted as follows: 0–1, no synovitis; 2–4, low-grade synovitis; and 5–9, high-grade synovitis (20).
TMJ Functional Analysis After Drug Treatment in TMJ PTOA Model
MOC was defined as the maximal interincisal distance on unassisted active mouth opening (21). In animal study, it is impossible to measure the active mouth opening. Therefore, passive mouth opening was measured under anesthesia. One examiner was responsible for passively opening the mouths of the pigs until the mouths cannot be further opened. The same examiner performed the mouth-opening procedure for all 12 pigs in both groups at all time points. The distance between the edges of the upper and lower central incisors was measured via a ruler with a millimeter scale by another examiner. The measurement was read and recorded to the nearest millimeter.
Results
Establishing a Large Animal Model of TMJ PTOA in a Yucatan Minipig
In human patients, pathological TMJ conditions entail involvement of multiple tissues (22). Due to such complexity, there is a significant paucity of established, preclinical large-animal models that can be employed to investigate the pathogenesis of TMJ OA and the appropriate therapeutic interventions. The majority of studies on degenerative joint diseases have been performed in small animal models, which do not always correlate to the clinical manifestations that occur in humans (18). Albeit groups have shown that TMJ OA in vivo can be induced through TMJ disc perforations, the surgeries were performed in rabbits (22). For the development of therapies for TMJ disorders, including TMJ OA, larger animal models are required to evaluate their safety and efficacy (18).
Our group has extensive surgical experience in successfully inducing OA in small and large animal models of degenerative joint disease (14, 23, 24). We have previously shown that partial meniscectomy in rats can induce OA formation in the knee joint (23). Both TMJ disc perforation and partial meniscectomy surgeries promote OA formation as the procedures involve destabilization of joints by partially damaging the disks. Studies have reported that pig disks have similar dimensions to human disks, and relative to other small and large animals, pig models have been regarded as the most suitable experimental model for TMJ studies (20, 25). Our group has established, for the first time, a preclinical, large animal model of TMJ post-traumatic OA (PTOA) by utilizing Yucatan minipigs. Minipigs are considered a superior model to other large animal model alternatives such as dogs and farm pigs since the size of minipig TMJ is comparable to humans (18).
In this model, we performed surgery to partially remove the TMJ disc of 5-month-old male Yucatan minipigs. The OA-like changes in TMJ were observed at 4 weeks following the surgery. The incision was made on the right side at the middle point of the line between the tragus and the outer canthus (Figure 1A) and continued down along the edge of the mandibular ramus (7–8 cm in length). After opening the upper chamber of the joint, the lateral portion of the disc that was not covered by the zygomatic process was elevated from the condyle by using periosteal elevator to avoid damaging the underlying mandibular condyle surface. This exposed portion of the disc (~16 × 7 mm) was then excised by scissors (Figures 1B–D). The TMJ disc of the minipig has dimensions of 25.6 and 13.1 mm in the anteroposterior and mediolateral directions, respectively, and is oval and biconcave (26). During the surgery, ~30% of the disc was removed. The surgical incision was closed with both internal and external sutures. Three of the pigs were euthanized at 4 weeks following the surgery to confirm the development of OA in the TMJ. The remaining pigs were randomly assigned to drug- or vehicle-treated groups and euthanized 10 weeks after the surgery.
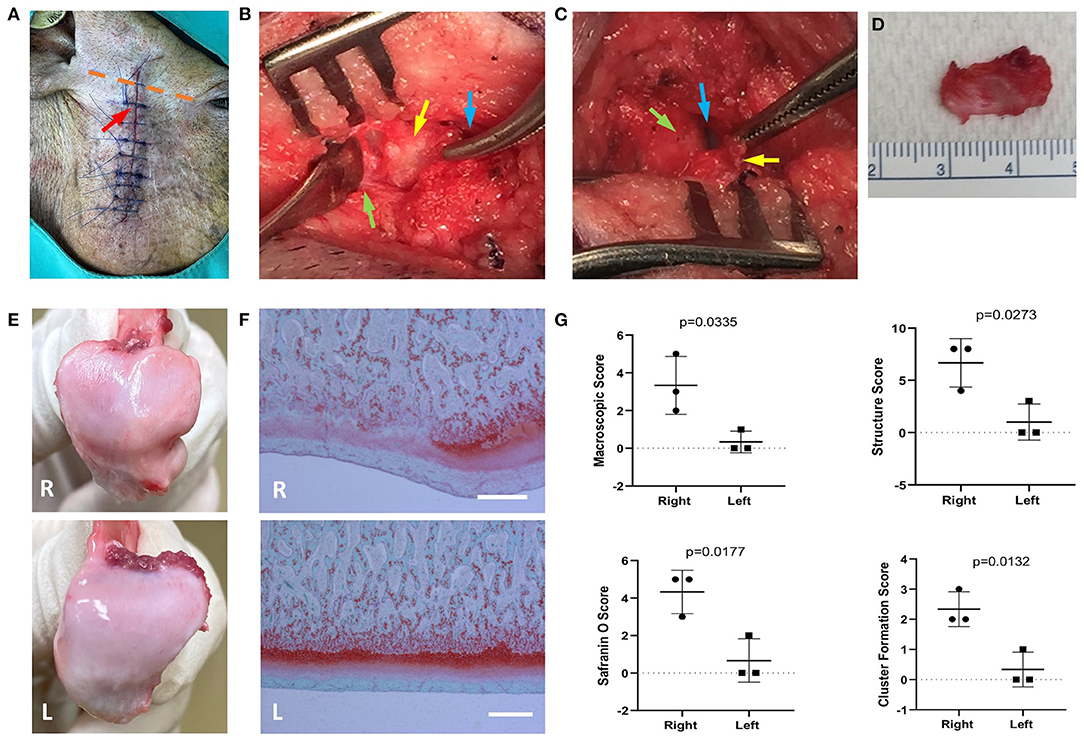
Figure 1. The preclinical pig temporomandibular joint (TMJ) post-traumatic osteoarthritis (PTOA) model was established by partial discectomy. Yucatan minipigs underwent unilateral partial discectomy on the right TMJ. (A) The incision was made at the middle of the indicated dashed line between tragus and the outer canthus (orange dotted line). The incision proceeded down along the edge of mandibular ramus. Red arrow indicates the location of the TMJ. (B) The superior synovial cavity of TMJ was opened (blue arrow), and the outer portion of the articular disc was exposed (yellow arrow), which is not covered by temporo-zygomatic arch. The periosteal elevator was used to separate the articular disc from the mandibular condyle (green arrow). (C) The articular disc was separated from the mandibular condyle (yellow arrow); blue arrow—inferior synovial cavity of TMJ; green arrow—surface of the mandibular condyle. (D) The exposed area of the articular disc was excised, which is about 30% of the total disc. (E) The macroscopic morphological changes on mandibular condylar surface 4 weeks postsurgery. (F) Safranin O/Fast green staining shows the condylar cartilage damaged. Scale bars = 400 μm (F). R, right (operated) side; L, left side. (G) Morphometric analysis of degenerative changes in articular cartilage. Unpaired t-test was used to determine the significant TMJ destruction following the surgery.
TMJ Condyle Degeneration and Synovial Inflammation in Pig TMJ PTOA Model
To further evaluate the changes in TMJ, three pigs were euthanized 4 weeks postsurgery. Macroscopic changes in TMJ mandibular condyle were observed (Figure 1E). In the TMJ disc partially excised condyle, gross morphological examination revealed a widespread rough and irregular surface (Figure 1E) compared to the smooth surface of the nonoperated left TMJ condyle (Figure 1E). Histological evaluation of TMJ condyle and synovium was also performed, and Safranin O staining (delineates proteoglycans) of the TMJ condyles revealed an irregular surface of the condyle (black arrows). In parallel to the structural changes, multiple areas of proteoglycan and chondrocyte loss (asterisks) in the condylar cartilage layer were also observed (Figure 1F). The macroscopic and histological scoring system (22, 27) were used to confirm the changes of the condyle (Figure 1G) in our model. The macroscopic changes and histologic alteration on structure, cell density, cellular cluster formation and Safranin O staining in the condyles were blindly scored.
To demonstrate the inflammatory changes, grading of the synovial membranes was carried out on routine H&E-stained sections according to the three synovial membrane features (synovial lining cell layer, stroma cell density and inflammatory infiltrate) (28). The synovitis score analysis (28) has shown that synovial lining cell layer is enlarged, the cellular density of the synovial stroma is higher and the inflammatory cell infiltration is increased (Figure 2A).
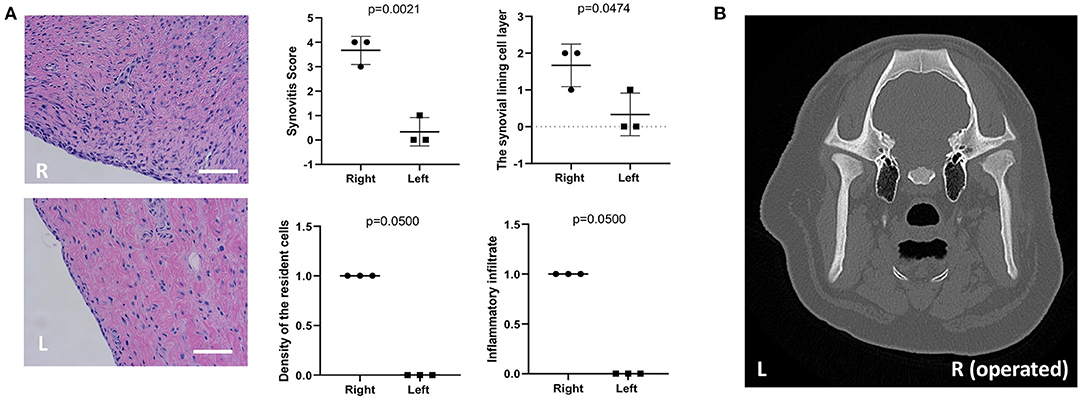
Figure 2. Histological assessment of the synovium and live CT scan at 4 weeks following partial TMJ discectomy. (A) Synovitis was assessed using previously-developed histological grading scale. For the scores of resident cell density and inflammatory infiltration of synovium, Mann-Whitney test (unpaired nonparametric test with single tail) was used. (B) To evaluate the development of TMJ OA, a live CT scan was performed on pig TMJs. Presented histological and CT images are representative image from three independent experiments. The scanning was done by using the Philips 64-Slice Brilliance CT scanner. From CT analysis, no obvious changes in bone structure was observed at this time point. R, right (operated) side; L, left side. Scale bar = 50 μm.
In pigs that underwent TMJ discectomy, the synovitis scores were 3–4. However, their collateral controls ranked the score 0–1 (n = 3). The macroscopic and histological changes in condyle and synovium delineate the pathological features of OA at 4 weeks following the partial TMJ discectomy in minipigs.
Live CT Scan Assessing Changes at 4 Weeks Following Partial TMJ Discectomy
To evaluate the development of TMJ OA, a live CT scan was performed on pig TMJs at 4 weeks postsurgery (Figure 2B). The animal was appropriately anesthetized and an i.v. line was maintained in the ear vein. From the CT scan analysis, no obvious changes or damage were observed at this time point in the subchondral bone of the mandibular condyle (Figure 2B).
R805 Prevents Mandibular Condyle Destruction and Condylar Cartilage Degeneration in vivo
Currently, there are no pharmacological agents available on the market that avert the progression of TMJ OA. Treatments, including NSAIDs, opioids, corticosteroids, anxiolytics, muscle relaxants, antidepressants, and anticonvulsants (29), only mask the symptoms and do not treat the underlying cause. We have previously reported that a small molecule agent, R805, synthesized by our group can not only promote tissue regeneration but also prevent articular cartilage degeneration in a rat model of PTOA (14). Strikingly, R805 inhibited inflammation in the joint, prevented articular cartilage degeneration, and mitigated pain and lameness in a post-traumatic joint injury dog model of OA (14). Improvement of the structural and functional outcomes confirmed that R805 can treat the symptoms of OA whilst concurrently preventing disease progression by alleviating the causal destructive processes. To assess whether R805 can improve structural and functional outcomes in the TMJ OA pig model, the compound was administrated via TMJ IA injection once the TMJ OA developed at 4 weeks after the partial discectomy surgery (Figure 3). Pigs were randomly assigned into two groups: (1) R805 and (2) vehicle (1% CMC) treatment. 0.5 ml of R805 (10 mg) or 1% CMC was given via TMJ IA injection. A total of two injections were given at 4 and 7 weeks postsurgery. Pigs were euthanized 3 weeks later after the second injection followed by a CT scan, macroscopic, and histological analysis. Macroscopic TMJ condyle evaluation in both groups revealed that R805 treatment reduced irregularity, dysmorphic change, ulceration, and erosion of the condyle surface (Figures 4A,B). Histological evaluation of the TMJ condyle via Safranin O staining and macroscopic and histological scoring revealed surface irregularities (black arrows), fissures and erosion (black arrowhead) in the R805-treated group (Figure 4C) compared to the vehicle group (Figure 4D). Without R805 treatment, there was a widespread loss of cells and proteoglycan content (asterisk) and cell cluster formation (Figures 4C,D). Furthermore, R805 treatment increased the thickness of the condylar cartilage layer in TMJ (p = 0.003; Figure 4E). The contralateral TMJs within the same animal have maintained normal condyle architecture and a uniform distribution of Safranin O staining, suggesting that contralateral TMJs were not affected by the unilateral partial discectomy surgery (Figure 4F).
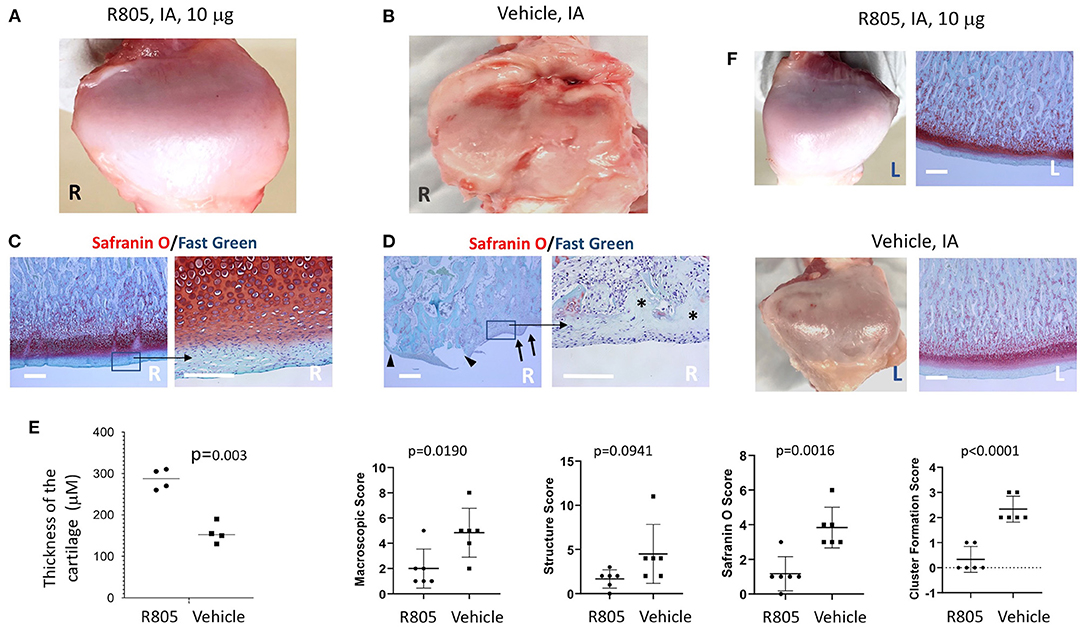
Figure 4. Intra-articular (IA) injection of R805 reduces the mandibular condyle damage in pig TMJ PTOA model. Gross morphology (A,B) and histological analysis (C,D) indicated the mandibular condyle damage was more severe in vehicle-treated group compared to the R805-treated group. (E) No significant differences were observed in contralateral (left, nonoperated joint). n = 6 animals per group. R, right; L, Left. Black arrows: surface irregularities; black arrow heads: erosion and fissure; asterisks: regional loss of chondrocytes. (F) Morphometric analysis of degenerative changes in articular cartilage. Unpaired t-test was used to determine the significant TMJ destruction following the surgery. Scale bar = 400 μm.
R805 Reduces Degenerative Changes in the Subchondral Bone of Mandibular Condyle
CT scan analysis demonstrated more severe subchondral bone damage in the vehicle group relative to the R805-treated group (Figure 5A). However, there was no significant difference in the overall bone mineral density of the mandibular condyles from both tested experimental groups (not shown).
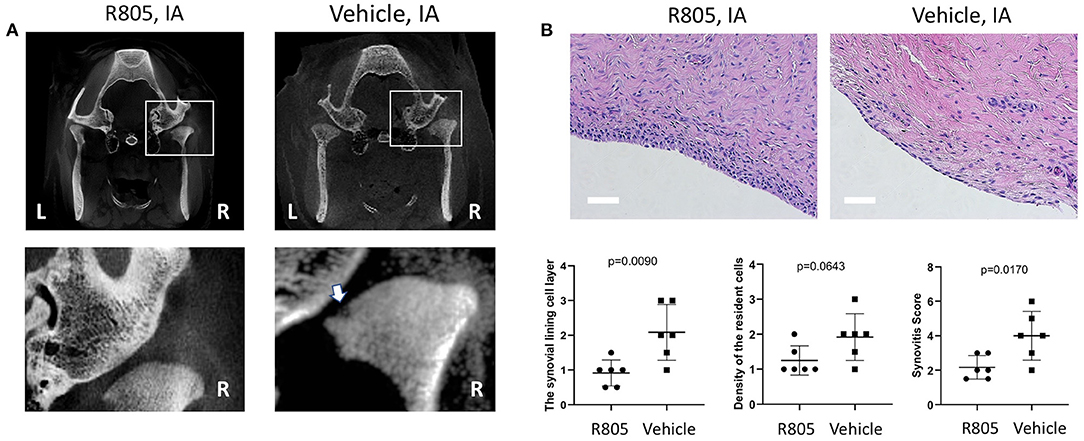
Figure 5. R805 treatment reduced the bone damage and synovial inflammation in TMJ. (A) White arrow in CT analysis indicates the mandibular condyle subchondral bone damage in vehicle-treated group. The synovitis was assessed. (B) The synovitis score was the sum of these three features (the synovial lining cell layer, density of the resident cell, and inflammatory infiltration) with 0–1, no synovitis; 2–4, low-grade synovitis; 5–9, high-grade synovitis. No leukocyte infiltration was observed in either groups. n = 6. Scale bar = 50 μm
R805 Reduces Synovial Inflammation
The grading of the synovial membranes was carried out on H&E-stained sections as described above (28). The results conveyed no significant inflammatory cell infiltrate in both drug- and vehicle-treated groups. However, following 6-week treatment with R805 at 10 mg IA injection, the enlargement of the synovial lining cell layer and synovitis score were greatly reduced relative to the vehicle-treated group (p = 0.0090 and 0.0170, respectively). The density of the resident cells in the experiment group was also reduced (Figure 5B, n = 6).
R805 Treatment Improves the Passive Maximal Mouth Opening Capacity
TMJ OA is associated with joint stiffness, joint pain that increases with movement and limitation in mouth opening (21). The mandibular range of motion is measured by maximal mouth opening capacity (MOC), which is one of the most imperative clinical diagnostic criteria and broadly conventional assessment tool for evaluation of the function of TMJ and the masticatory system (21). MOC is defined as the greatest inter-incisal distance plus the overbite when the mouth is opened as widely as possible (21, 30).
To measure the MOC of the pigs with TMJ OA, the mouth of the pig was passively opened under anesthesia. A ruler with a millimeter scale was placed between the edges of the upper and lower central incisors (Figure 6A). After administration of two TMJ IA injections of the vehicle or R805 at 10 mg/joint per injection, the MOC was measured at multiple timepoints, including (1) before surgery (baseline); (2) at 4 weeks postsurgery and before the first drug/vehicle TMJ IA injection; (3) at 7 weeks postsurgery and before the second IA injection; and (4) at 10 weeks postsurgery and before the euthanasia. No significant change in MOC was observed at baseline or at 4 weeks postsurgery. However, at 7 and 10 weeks postsurgery, there was a significant reduction of MOC in vehicle-treated groups compared to the R805-treated group (Figures 6B,C). Together, the data presents compelling evidence that R805 can improve the function of the masticatory system of pigs with TMJ OA (p = 0.0131, n = 6).
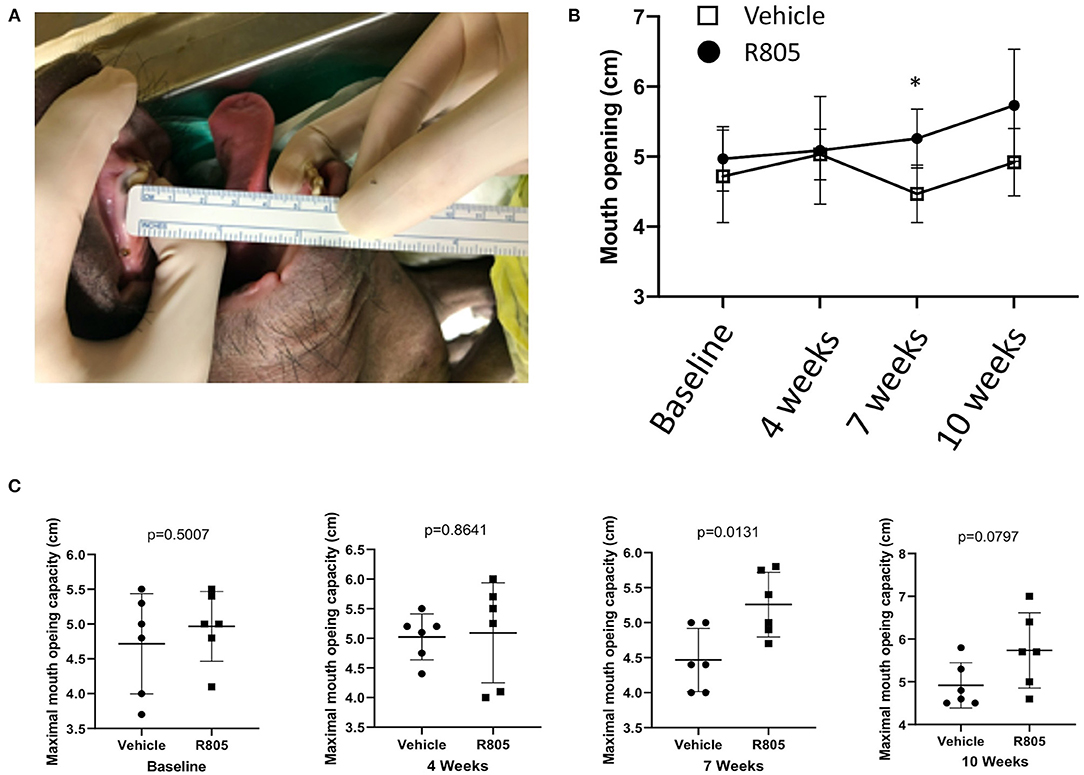
Figure 6. Functional evaluation of the TMJ following the treatment via measuring the passive maximal mouth opening capacity (MOC). (A) Ruler placed between the edges of upper and lower central incisors. (B,C) Measurements of the maximal distance between the edges of lower and upper incisors were performed on anesthetized animals (n = 6) at four different time points: baseline (before the operation) and 4, 7, and 10 weeks postsurgery. Unpaired t-test was applied to determine the statistical significance of the treatment. *p < 0.05.
Analysis of the Systemic R805 Pharmacokinetics After IA Injection
To explore the level of systemic exposure after IA injection of R805, we have conducted a preliminary study to measure the levels of the drug in blood plasma at 4 and 24 h after injection in six pigs using high-performance liquid chromatography—mass spectrometry (HPLC-Mass Spec) tandem. The assay development and all analytical measurements were performed by MicroConstants (Sand Diego, CA, USA). In all tested animals, no drug was detected in the systemic circulation (data not shown) at any time point above the detection limit of the assay (0.1 ng/mL).
Discussion
Currently, there is a limited number of established large animal models of TMJ OA suitable for preclinical drug development (18). Although degenerative joint disease models have been mainly performed on mice, rats, and rabbits, preclinical regeneration approaches must employ larger animal species (18). No animal model completely resembles human TMJ in all anatomical respects and function. Nevertheless, the farm pig and minipig are a close match in anatomy and physiology (18). The goat, sheep, and dog are also close to humans in terms of anatomy, but their TMJ function is somewhat different: goats and sheep are herbivores and their TMJs mainly function in translation, while the dog is a carnivore with the TMJ being a hinge joint that can only function in rotation (18).
Historically, the pig has been regarded as the gold standard for a nonprimate, large animal TMJ model based on general similarities to human anatomy. Specifically, the size of the articular TMJ structures and the shape of the disc bear significant resemblance to the human TMJ (18, 20, 31). Bermejo et al. concluded that the pig was the only suitable animal model after a comparison of dogs, cats, rabbits, rats, cows, sheep and goats (25). This was based on several criteria including that the pig is the only omnivore of these candidate mammalian models, the TMJs in the farm pig and minipig function by both rotation and translation, and the condyle and disc have a similar shape to the human TMJ disc (25). Finally, the mechanical properties of the porcine TMJ have also been shown to be similar to human (26, 32, 33). Together, these data nominate the pig as the preferred preclinical model for therapeutic development for TMJDs.
The proposed study introduces a straightforward surgical procedure designed to destabilize the TMJ joint by removing the lateral portion of the TMJ disc. A similar approach is commonly used for establishing a PTOA in the knee when a portion of the medial meniscus is surgically removed to destabilize the joint. Here, we demonstrate that partial TMJ discectomy induces highly reproducible degenerative changes observed as early as 4 weeks after the surgery. Although radiographic changes in the subchondral bone of the mandibular condyle are not detectable at 4 weeks postsurgery, the onset of degenerative changes is clearly present in the articular cartilage at this early time point and these degenerative changes evidently progress with time. Using this novel model, we have shown that IA injection of a small molecule modulator of gp130 signaling prevents the onset and progression of TMJ OA. R805 injection markedly reduced mandibular condyle cartilage loss and reduced synovitis and degenerative changes in the subchondral bone.
Our previous data suggested that IL-6 cytokines drive both regenerative and destructive outcomes in synovial joints through selective and context-specific activation of signaling residues within the intracellular domain of gp130 receptor (14, 23) and are also required for homeostatic maintenance of skeletal progenitors (34–38). Complete inhibition of gp130 signaling has been attempted previously for several therapeutic indications. Unfortunately, complete blockade of this receptor instigates major side effects prohibiting clinical development of these inhibitors. These side effects are likely caused by the highly pleotropic nature of this receptor and its multiple beneficial functions (39). We have discovered a new class of chemicals capable of selective inactivation of gp130 modules and pathways and have previously shown that modulation of gp130 signaling with small molecules protects adult articular cartilage from degeneration and promotes cartilage regeneration in rat medial meniscectomy and cartilage surface injury models (23). Most recently, we showed that local administration of a small molecule R805 that targets gp130/c-SRC/MAPK module reduced cartilage degeneration, synovial infiltration, osteophyte formation and pain in a commonly used canine model of PTOA in the knee with no safety concerns noted (14). Therapeutic assessment of R805 in the TMJ PTOA model strongly resonates with our previous studies and suggests that the fundamental drivers of OA in different synovial joints are similar.
However, the current study also has some limitations. More specifically, we were unable to conduct any detailed pain-related outcome measures in this model because no obvious clinical manifestation of pain and/or discomfort was observed in these animals at all tested timepoints despite major structural changes in their cartilage, bone and synovium. We also acknowledge that surgically induced animal models of OA in young healthy animals are not pathogenetically the same as idiopathic OA in humans where multiple changes in other skeletal tissues like bone and muscle are observed and the systemic homeostatic mechanisms are dysfunctional.
In summary, we have conducted a pivotal efficacy study in our newly established pig model of TMJ PTOA. IA injection of R805 has shown a highly significant reduction of articular cartilage degeneration and reduced synovitis and degenerative changes in subchondral bone in the mandibular condyle relative to the control. These data will support additional preclinical development of R805 as a first-in-class injectable therapeutic for TMJ arthritis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by USC IACUC.
Author Contributions
NL, SC, DG, JL, JZ, YL, and LL conducted surgeries. YO, RS, BV, FB, and TM performed experiments. DE and YC conceptualized the study, interpreted the data, and revised and approved the manuscript. NL and RS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Awards R01AR071734 to DE and the National Institute of Aging Award R01AG058624. Research reported in this publication was also supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number U24DE026914. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by DOD grant W81XWH-13-1-0465 to DE.
Conflict of Interest
DE and BV were co-founders and significant shareholders of Carthronix Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the Molecular Imaging Center Core in the Department of Radiology, Keck School of Medicine, University of Southern California, and Tautis Skorka for performing high-resolution microCT scanning of the samples for this study.
References
1. Liu F, Steinkeler A. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent Clin North Am. (2013) 57:465–79. doi: 10.1016/j.cden.2013.04.006
2. Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, et al. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: implications and future directions. J Pain. (2013) 14:T116–24. doi: 10.1016/j.jpain.2013.09.010
3. Johansson A, Unell L, Carlsson GE, Soderfeldt B, Halling A. Gender difference in symptoms related to temporomandibular disorders in a population of 50-year-old subjects. J Orofac Pain. (2003) 17:29–35.
4. Sodhi A, Naik S, Pai A, Anuradha A. Rheumatoid arthritis affecting temporomandibular joint. Contemp Clin Dent. (2015) 6:124–7. doi: 10.4103/0976-237X.149308
5. Kalladka M, Quek S, Heir G, Eliav E, Mupparapu M, Viswanath A. Temporomandibular joint osteoarthritis: diagnosis and long-term conservative management: a topic review. J Indian Prosthodont Soc. (2014) 14:6–15. doi: 10.1007/s13191-013-0321-3
6. Thomas AC, Hubbard-Turner T, Wikstrom EA, Palmieri-Smith RM. Epidemiology of posttraumatic osteoarthritis. J Athl Train. (2017) 52:491–6. doi: 10.4085/1062-6050-51.5.08
7. Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. (2014) 2014:561459. doi: 10.1155/2014/561459
8. Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2013-2015. Morb Mortal Wkly Rep. (2017) 66:246–53. doi: 10.15585/mmwr.mm6609e1
9. Giannakopoulos HE, Quinn PD, Granquist E, Chou JC. Posttraumatic temporomandibular joint disorders. Craniomaxillofac Trauma Reconstr. (2009) 2:91–101. doi: 10.1055/s-0029-1215872
10. Abrahamsson AK, Kristensen M, Arvidsson LZ, Kvien TK, Larheim TA, Haugen IK. Frequency of temporomandibular joint osteoarthritis and related symptoms in a hand osteoarthritis cohort. Osteoarthritis Cartilage. (2017) 25:654–7. doi: 10.1016/j.joca.2016.12.028
11. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. (2020) 29–30:100587. doi: 10.1016/j.eclinm.2020.100587
12. Wiegertjes R, van de Loo FAJ, Blaney Davidson EN. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology. (2020) 59:2681–94. doi: 10.1093/rheumatology/keaa248
13. Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, et al. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci. (2009) 29:13473–83. doi: 10.1523/JNEUROSCI.1822-09.2009
14. Shkhyan R, Flynn C, Lamoure E, Handel BV, Sarkar A, Li J, et al. Signaling modality withingp130 receptor enhances tissue regeneration. bioRxiv. (2022) 2022:475124.
15. Petrigliano FA, Liu NQ, Lee S, Tassey J, Sarkar A, Lin Y, et al. Long-term repair of porcine articular cartilage using cryopreservable, clinically compatible human embryonic stem cell-derived chondrocytes. NPJ Regen Med. (2021) 6:77. doi: 10.1038/s41536-021-00187-3
16. Liu NQ, Lin Y, Li L, Lu J, Geng D, Zhang J, et al. gp130/STAT3 signaling is required for homeostatic proliferation and anabolism in postnatal growth plate and articular chondrocytes. Commun Biol. (2022) 5:64. doi: 10.1038/s42003-021-02944-y
17. Wu L, Zhang S, Shkhyan R, Lee S, Gullo F, Eliasberg CD, et al. Kappa opioid receptor signaling protects cartilage tissue against posttraumatic degeneration. JCI Insight. (2017) 2:e88553. doi: 10.1172/jci.insight.88553
18. Almarza AJ, Brown BN, Arzi B, Angelo DF, Chung W, Badylak SF, et al. Preclinical animal models for temporomandibular joint tissue engineering. Tissue Eng B Rev. (2018) 24:171–8. doi: 10.1089/ten.teb.2017.0341
19. Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage. (2010) 18(Suppl.3):S53–65. doi: 10.1016/j.joca.2010.05.029
20. Berg R. Contribution to the applied and topographical anatomy of the temporomandibular joint of some domestic mammals with particular reference to the partial resp. total resection of the articular disc. Folia Morphol. (1973) 21:202–4.
21. Muller L, van Waes H, Langerweger C, Molinari L, Saurenmann RK. Maximal mouth opening capacity: percentiles for healthy children 4-17 years of age. Pediatr Rheumatol Online J. (2013) 11:17. doi: 10.1186/1546-0096-11-17
22. Embree MC, Iwaoka GM, Kong D, Martin BN, Patel RK, Lee AH, et al. Soft tissue ossification and condylar cartilage degeneration following TMJ disc perforation in a rabbit pilot study. Osteoarthritis Cartilage. (2015) 23:629–39. doi: 10.1016/j.joca.2014.12.015
23. Shkhyan R, Van Handel B, Bogdanov J, Lee S, Yu Y, Scheinberg M, et al. Drug-induced modulation of gp130 signalling prevents articular cartilage degeneration and promotes repair. Ann Rheum Dis. (2018) 77:760–9. doi: 10.1136/annrheumdis-2017-212037
24. Lei J, Chen S, Jing J, Guo T, Feng J, Ho TV, et al. Inhibiting Hh signaling in Gli1(+) osteogenic progenitors alleviates TMJOA. J Dent Res. (2022) 2022:220345211059079. doi: 10.1177/00220345211059079
25. Bermejo A, Gonzalez O, Gonzalez JM. The pig as an animal model for experimentation on the temporomandibular articular complex. Oral Surg Oral Med Oral Pathol. (1993) 75:18–23. doi: 10.1016/0030-4220(93)90399-O
26. Vapniarsky N, Aryaei A, Arzi B, Hatcher DC, Hu JC, Athanasiou KA. The yucatan minipig temporomandibular joint disc structure-function relationships support its suitability for human comparative studies. Tissue Eng C Methods. (2017) 23:700–9. doi: 10.1089/ten.tec.2017.0149
27. Dai LY, Zhou WJ. Ectopic ossification following total hip replacement. Zhonghua Wai Ke Za Zhi. (1992) 30:599–602.
28. Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. (2006) 49:358–64. doi: 10.1111/j.1365-2559.2006.02508.x
29. Ouanounou A, Goldberg M, Haas DA. Pharmacotherapy in temporomandibular disorders: a review. J Can Dent Assoc. (2017) 83:h7.
30. Fatima J, Kaul R, Jain P, Saha S, Halder S, Sarkar S. Clinical measurement of maximum mouth opening in children of Kolkata and its relation with different facial types. J Clin Diagn Res. (2016) 10:ZC01–5. doi: 10.7860/JCDR/2016/21232.8217
31. Embree MC, Chen M, Pylawka S, Kong D, Iwaoka GM, Kalajzic I, et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat Commun. (2016) 7:13073. doi: 10.1038/ncomms13073
32. Sun Z, Liu ZJ, Herring SW. Movement of temporomandibular joint tissues during mastication and passive manipulation in miniature pigs. Arch Oral Biol. (2002) 47:293–305. doi: 10.1016/S0003-9969(02)00004-3
33. Herring SW, Decker JD, Liu ZJ, Ma T. Temporomandibular joint in miniature pigs: anatomy, cell replication, and relation to loading. Anat Rec. (2002) 266:152–66. doi: 10.1002/ar.10049
34. Ferguson GB, Van Handel B, Bay M, Fiziev P, Org T, Lee S, et al. Mapping molecular landmarks of human skeletal ontogeny and pluripotent stem cell-derived articular chondrocytes. Nat Commun. (2018) 9:3634. doi: 10.1038/s41467-018-05573-y
35. Liu NQ, Lin Y, Li L, Lu J, Geng D, Zhang J, et al. gp130/STAT3 signaling is required for homeostatic proliferation and anabolism in postnatal growth plate and articular chondrocytes. Commun Biol. (2021) 2021:464120. doi: 10.1101/2021.10.12.464120
36. Tassey J, Sarkar A, Van Handel B, Lu J, Lee S, Evseenko D, et al. Single-cell culture system for dissecting microenvironmental signaling in development and disease of cartilage tissue. Front Cell Dev Biol. (2021) 9:725854. doi: 10.3389/fcell.2021.725854
37. Sarkar A, Lee S, Shkhyan R, Liu NQ, Van Handel B, Tassey J, et al. STAT3 as the regulatory determinant of epigenetic landscape in articular chondrocytes. Biorxiv [Preprint]. (2021).
38. Petrigliano FA, Liu NQ, Lee S, Tassey J, Sarkar A, Lin Y, et al. Long-term repair of porcine articular cartilage using cryopreservable, clinically compatible human embryonic stem cell-derived chondrocytes. NPG Regener Med. (2021) 2021:446024. doi: 10.1101/2021.05.27.446024
Keywords: TMJ, osteoarthritis, drug, intra-articular injection, pig model
Citation: Liu NQ, Chen S, Geng D, Lei J, Zhang J, Li L, Lin Y, Ouyang Y, Shkhyan R, Van Handel B, Bian F, Mkaratigwa T, Chai Y and Evseenko D (2022) Local Drug-Induced Modulation of gp130 Receptor Signaling Delays Disease Progression in a Pig Model of Temporo-Mandibular Joint Osteoarthritis. Front. Dent. Med. 3:937819. doi: 10.3389/fdmed.2022.937819
Received: 06 May 2022; Accepted: 10 June 2022;
Published: 11 July 2022.
Edited by:
Yan Liu, Peking University Hospital of Stomatology, ChinaReviewed by:
Danqing He, Peking University Hospital of Stomatology, ChinaXiaoxing Kou, Sun Yat-sen University, China
Copyright © 2022 Liu, Chen, Geng, Lei, Zhang, Li, Lin, Ouyang, Shkhyan, Van Handel, Bian, Mkaratigwa, Chai and Evseenko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Denis Evseenko, ZXZzZWVua29AdXNjLmVkdQ==; Yang Chai, eWNoYWlAdXNjLmVkdQ==
†These authors have contributed equally to this work
 Nancy Q. Liu
Nancy Q. Liu Shuo Chen
Shuo Chen Dawei Geng
Dawei Geng Jie Lei
Jie Lei Jiankang Zhang
Jiankang Zhang Liangliang Li
Liangliang Li Yucheng Lin
Yucheng Lin Yuxin Ouyang1
Yuxin Ouyang1 Fangzhou Bian
Fangzhou Bian Tadiwanashe Mkaratigwa
Tadiwanashe Mkaratigwa Yang Chai
Yang Chai Denis Evseenko
Denis Evseenko