- 1Private Practice, Amman, Jordan
- 2Restorative Dentistry Department, School of Dentistry, University of Jordan, Amman, Jordan
- 3School of Dentistry, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom
Aim: The aim of this article is to review the scientific evidence for deep caries removal in permanent vital teeth and the choice of dentine replacement material and restoration of the teeth to maintain long-term tooth vitality and function.
Method: The two position statements, namely, the European Society of Endodontology and the American Association of Endodontists position statements on vital pulp therapy, will be scrutinized and compared with regard to the deep caries removal strategy and assessed for evidence of best practice. The properties of materials used to manage vital pulps and the best way to restore the teeth will be reviewed and guidance on the full management of vital teeth will be suggested.
Conclusions: Promoting new treatment modalities for reversible and irreversible pulpitis allowing for pulp preservation should be considered. Although debatable, cases with deep caries should be managed by complete non-selective caries removal which will allow for pulpal management if needed and a more predictable outcome can be expected when using the new materials and treatment modalities of vital pulp therapy.
Introduction
Dental caries, trauma, and tooth wear result in loss of coronal dental hard tissues. These are replaced effectively by dental restorative materials. Both the disease process and the tooth restoration compromise the vitality of the dental pulp. This review is concerned with deep caries management and its effects on dental pulp.
Changes to the dental pulp caused by disease and tooth restoration have been shown to start very early and are symptomless (1, 2). These changes disrupt the pulp tissue architecture but may not necessarily compromise the pulp enough to lead to loss of vitality. Changes to the pulp microstructure have been shown with application of fissure sealants specifically the use of acid etch where even if placed on enamel, the expression of inflammatory cytokines was upregulated while the mineralization-related genes were down regulated and cell viability was significantly reduced (3). While these changes are reversible, a more invasive treatment such as restoration of deep cavities showed more prominent inflammatory changes that, depending on the remaining sound dentine thickness, may not be reversible and lead to permanent damage of the affected part of the pulp (4–7). The histological pulp status prior to any intervention is an important variable that may affect the pulp response postoperatively but is rarely studied in the literature (8). These changes in the pulp and the disease progression are not adequately reflected in the symptoms, and thus pulp testing procedures need to be refined to adequately correlate the microstructural changes within the pulp which can be reversible or irreversible. This will have an impact on the outcome of disease management. When diagnosing teeth with deep carious lesions, the clinician must make a choice based on knowledge of the caries process and its effect on the pulp and also on existing diagnostic methods (9, 10).
After caries removal, the remaining cavity sound dentine thickness mediates a powerful influence on underlying pulp tissue vitality and inflammatory activity, but it has little effect on reactionary dentine secretion (6, 11). Following restoration, a remaining dentine thickness of 0.5 mm or greater is necessary to avoid evidence of pulp injury. When the remaining dentine thickness is less than 0.5 mm, the choice of the pulp protection material used affects the success of the treatment (5). While it is difficult to determine the remaining dentine thickness in a clinical setting, this measurement gives the clinician the urge to consider the different pulp protection materials when a deep cavity is encountered.
It has been shown that the clinical management depends on the training received by the practitioner with the cardiologists opting for selective caries removal, the operative dentists will remove all caries and the endodontists will initiate root canal therapy (12).
The effective management of the dental pulp depends on various factors which will be discussed in the rest of the article. These include:
- Proper diagnosis
- Removal of caries if the disease process is carious
- Management of the dentine substrate
- Management of the pulp
- Dressing the dentine
- Tooth restoration
The best management of vital teeth with deep caries necessitating vital pulp therapy has been addressed by both the European Society of Endodontology and the American Association of Endodontists who have issued position statements to guide the clinicians on best practice (13, 14).
Effective management of the carious tooth
Proper diagnosis
Reaching a proper diagnosis and case selection is key to success in any intended treatment. Endodontic diagnosis depends on history, symptoms, and responses to a few clinical tests assessing the pulpal and periapical status of the tooth (15, 16). Significant limitations of pulp sensibility tests have been identified and summarized in a review paper (15, 16). Most importantly, these tests measure the nerve response rather than assess the blood flow of the pulp (15, 16). In the case of young and immature teeth, lack of accuracy of electric pulp testing has been shown and is attributed to the yet incomplete innervation of the pulp (15–20). The lack of correlation between the actual histopathological status of the pulp and the responses to the pulp testing adds another major limitation of such tests (21–23). As a result, it is speculated that pulp tests can only discern a necrotic pulp from a vital one but cannot determine the severity of pulpal disease (irreversible vs. reversible) (15, 16). A comparison of pulp testing methods used to assign cases their clinical diagnosis has shown that ethyl chloride is less effective than refrigerant sprays and dry ice in eliciting pulpal responses (18, 24). A recent histopathological study showed that there is a 96.6% correlation between the histopathologic status of pulps clinically diagnosed with reversible pulpitis or considered normal pulps while about 84.4% correlation in cases of symptomatic irreversible pulpitis cases was reported (25). In this study the normal pulps and the cases with symptoms of reversible pulpitis were placed in the same category as they were treated by vital pulp therapy. Although this may have possibly skewed the correlation percentage, the treatment is the same, so it justifies the classification in one group. The types of tests for pulp sensitivity are not indicated (25). Further communication with the corresponding author (26) (Ricucci D) clarified that a number of tests were used, and the cumulative results were used to identify the sensibility test per tooth treated. The cold test used was a refrigerant spray called Pulpofluorane (Septodont, Saint Maur des Fosses, France) which, along with more robust histological preparation procedures and bacterial staining, explains the good correlation between the sensibility tests and the pulpal status (25). More studies to evaluate this assumption are needed to possibly correlate the clinical signs and symptoms to the actual histopathological status of the pulp using more accurate pulp tests than used in older studies.
There is also some debate on the diagnostic terms regarding the reversibility of pulpitis (25, 27–29). These debates are mainly driven by the recent advances in deep caries and pulpal management. The term irreversible pulpitis can be perceived as a pulp that cannot be saved, while it can be managed successfully using vital pulp therapy showing healing and maintained vitality of the remaining pulp (30–34). A suggestion of new terminology like “partial irreversible pulpitis” was made to be a closer representation of the histological status of the pulp. New classifications of pulpitis based on clinical symptoms were also introduced (27, 28) in an attempt to align the new treatment modalities more closely to pulpal condition as reflected by patients' symptoms and responses to pulp testing. While these classifications are probably very beneficial in expanding the operator's knowledge and understanding of the continuum of pulpal disease and awareness of the applications of the new procedures of vital pulp therapy, they may show some overlap from a clinical point of view and may allow for some confusion for the unexperienced, and the same limitations of our diagnostic tests and subjectivity of responses remain. One recent study (35) has evaluated the usefulness of using the new classification suggested by Wolters et al. (27). In this study, the new classification was compared to the currently used classification (36) in symptomatic cases of deep caries managed with partial pulpotomy. Cases where the pulp was not exposed were excluded from the study. The study reported a prognostic potential of the new classification as a significant difference in the outcome of mild pulpitis cases compared to severe pulpitis ones. Only 5 cases were diagnosed with severe pulpitis (new classification) out of all 23 cases diagnosed with irreversible pulpitis according to the current classification while one case of mild pulpitis category belonged to the irreversible pulpitis cases. Further studies are needed to evaluate this new classification and possibly fine-tune the categories suggested in relation to the outcome of treatments and the gold standard, the histopathological status of the pulp.
Since most studies (21–23) show no correlation of signs and symptoms to the histopathological status of the pulp so far, except for the study by Ricucci et al. (25), it may be more constructive to focus on promoting recent advances as new treatment modalities for each diagnostic category as was implemented by the recent guidelines of the American Association of Endodontists (14) with the understanding that the reversibility of pulpal disease will depend on the management and possibly removal of the affected part of the pulp, in addition to the expected status of the pulp. The publication resulting from the consensus conference committee which has been endorsed by the American Association of Endodontists (14) is currently the most relevant for nomenclature (36). This allows for standardization of case reporting in clinical studies, better comparisons in longitudinal studies, easier meta-analysis performance and easier understanding of evidence on behalf of the readers.
Removal of caries
Deep caries management poses a significant challenge to the practitioner especially if asymptomatic. The aim should be set to maintain pulpal vitality, inactivation of the caries activity, preservation of tooth structure, restoring the tooth back to function and avoiding the restorative cycle to prolong tooth survival for as long as possible (37). In an operative option of caries management where caries should be removed, the practitioner is faced with the dilemma of the extent of caries removal. Various levels of caries removal have been described in the literature, which can be classified into three groups according to a consensus report (37, 38).
• Non-selective caries removal (too hard dentine): All softened dentine is removed until only hard dentin remains in all parts of the cavity and the tooth is restored permanently.
• Selective caries removal (too firm or soft dentine): Caries is removed until hard dentin is reached in the periphery of the lesion and dentine which appears softened and still cuttable by excavators towards the pulp. After selective caries removal, a definitive restoration is placed immediately.
• Stepwise caries removal (in two visits): This treatment is performed in two sessions. In the first session, caries is removed in a selective approach and restored temporarily. After 6–12 months, the cavity is re-accessed, and residual caries is removed completely followed by definitive restoration. Therefore, this treatment approach should be considered a complete caries removal technique but in two visits (39).
In order to follow the approach of selective caries removal or stepwise caries removal; the consensus report (37, 40) recommends the use of magnification to be able to assess dentine colour, consistency and wetness, use of aids like dyes and caries detectors to help in caries excavation, use of isolation to manage any pulpal exposures should it happen having a proper pulpal diagnosis (should be asymptomatic or reversibly inflamed pulp), and use of sharp instruments for caries excavation having a good bonded restoration of the tooth to avoid leakage. In the European Society of Endodontists Guidelines, it is noted that cases amenable for selective or stepwise caries removal should present as deep caries (caries reaching the inner quarter of dentine) with a well-defined remaining rim of dentine between caries and pulp (13).
The aim of both the selective and stepwise caries removal is avoidance of pulpal exposure to avoid a perceived reduced overall survival and prognosis of the tooth (41–43). Case selection in both approaches includes cases that are asymptomatic or those with minimal symptoms of reversible pulpitis. No periapical changes or periodontal involvement are included in the cases treated. Participating patients are usually young patients with a mean age usually less than 18 years old. The depth of caries in included cases is usually >1/2 of the dentine thickness, but it varies between studies. The remaining carious dentine was managed either by calcium hydroxide lining, glass ionomer lining/base, and resin-modified glass ionomer lining followed by a restoration. The clinical success obtained in these studies is not the same as that in clinical practice due to the standard setting and specific prognostic factors in the studies reporting the success rates (44).
The stepwise caries removal aims at reducing the microbial load and removing the necrotic demineralized dentine in the first visit while avoiding pulp exposures. After providing a proper temporary seal, the remaining microbial load was reduced and shifted towards an arrested caries microbiota (45–47) and demineralized dentine remineralizes or hardens (43, 47). Tertiary dentine formation driven by the reduced microbial load and proper seal was also demonstrated in some studies aiding in reducing the risk of pulp exposure on re-entry and removal of the remaining carious dentine (47). In clinical studies comparing stepwise caries removal to non-selective (complete one step) caries removal, a higher risk of pulp exposures was noted in one-step non-selective caries removal compared to stepwise caries removal (43, 48, 49). This risk was further reduced by 70% when stepwise caries removal was to soft dentine (50). While the Bjørndal et al. (43) study showed a higher risk of loss of pulp vitality at a one-year follow-up time in the case of non-selective caries removal compared to the stepwise caries removal, the study by Leksell et al. (49) showed no difference at around 43 months follow-up time. The difference in results could be attributed to the difference in caries depth included in the studies and the remaining dentine thickness as a result. In the study by Bjørndal et al. (43), they included caries extending 75% of dentine depth with a well-defined radio-dense zone of dentine between the caries lesion and the pulp. Leksell et al. (49) described included cases as deep caries with expected pulp exposure if completely excavated. The thickness of the remaining dentine after completion of caries excavation is a key factor in pulp response (5, 6, 11, 51). Pulp vitality assessment in both studies was either vague (43) or not mentioned (49) which could also affect the result of pulpal vitality at the follow-up appointment. In addition, Bjørndal et al. (43) included patients of an older age group compared to Leksell et al. (49). Younger pulps are expected to have better healing ability than older ones. In a further follow-up study at 5 years, Bjørndal and colleagues showed higher success in maintaining pulpal vitality in the stepwise caries removal group (60.2%) compared to non-selective caries removal (46.3%) (48).
Some disadvantages are expected from the stepwise caries removal. Loss of patient compliance with multiple appointments, added discomfort, added cost and loss or failure of temporary fillings and pulpal complications have been reported in clinical studies (52–54). Based on the results of several microbiological studies showing reduced bacterial load in carious dentine following sealing the lesion (41, 46, 55–57), it was then suggested to apply partial caries removal (selective caries removal) without the need for re-entry and restoration of the tooth permanently (41, 42), thereby avoiding the disadvantages of stepwise caries removal technique.
Clinical studies comparing selective caries removal and stepwise caries removal showed less risk of pulpal exposure, less discomfort and reduced cost (53, 54, 58, 59). In a randomized clinical study comparing stepwise and selective caries removal where the operators and statistician were blinded to the approach applied, the success in maintaining pulpal vitality was higher in the case of selective caries removal compared to stepwise caries removal (53). This was attributed to the fact that a significant number of patients did not return for the second visit in case of stepwise caries removal increasing the risk of pulpal complications. When comparing only those who completed their stepwise caries removal procedure, success at a three-year follow-up was 88% compared to 91% of partial caries removal that was not statistically significant (53).
It should be noted, however, that in all studies of selective and stepwise caries removal, there is a lack of definition and standardization of the caries removal end point (and the remaining volume of residual caries) and in most studies, a clear description of techniques of determining dentine hardness was not mentioned (54), which can introduce some unaccounted bias. In fact, studies showed a moderate to high risk of bias and mostly are of low quality in several systemic reviews and meta-analysis on this topic (50, 58, 60). The limited number of cases included in these studies in relation to the very common disease (dental caries) makes the studies limited in their external validity. Bias in reporting failures and a few other design limitations undermine the study's quality. A recent Cochrane systematic review (38) evaluated the evidence regarding caries management in both permanent and primary dentition. Studies evaluating deep caries management techniques in permanent teeth showed a high risk of bias mainly due to the lack of blinding of participants and assessors. Low certainty of evidence in most studies was also reported, except in studies comparing stepwise caries removal to non-selective or complete caries removal. In these studies, the certainty of evidence was deemed moderate. Furthermore, the definition of deep caries in the included studies varied to some extent. Despite the above mentioned limitations, this Cochrane review still recommended selective caries and stepwise caries removal techniques in management of deep caries (38).
Both newly proposed approaches of deep caries removal were conducted mainly on deep caries with a remaining rim of dentine between the advancing front of caries and the pulp. Standardization of depth of caries assessment preoperatively was only mentioned in studies of Bjørndal's group (43, 48). The main goal of such approaches is to avoid pulpal exposures that are often cited as a negative prognostic factor based on the results of often-cited studies (43, 61). In the retrospective study (61), non-selective caries removal was performed by supervised dental students under rubber dam and pulp exposures capped with setting calcium hydroxide followed by a base of zinc phosphate cement or glass ionomer cement. Only 30.7% of the cases could be followed up, and about 49 out of 123 cases were not actually examined but only notes from the patient's chart/dentist showed loss of the tooth or endodontic management with no attempts to determine the reason for postoperative management. Cases of questionable pulpal responses to vitality testing and/or clinical symptoms without any radiographic changes were rated questionable. In addition, some cases did not receive the permanent filling and were only temporized. The follow-up time was 5 years and 10 years postoperatively. The failure rate reported was 44.5% at 5 years and 79.9% at 10 years. Indeed, direct pulp capping shows a declining success rate with time and its success depends on the coronal seal provided (62–66), and the material used for pulp preservation with mineral trioxide aggregate and hydraulic calcium silicates showing better outcome in direct pulp capping procedures than calcium hydroxide (63, 66, 67).
It should also be acknowledged that pulpal inflammation increases in severity the deeper the caries lesion (21, 23, 68). Usually, inflammation would be localized to the area beneath the advancing front of the caries lesion and normal and healthy pulp is not uncommon in other distant areas (2, 68, 69). This means that the cariously exposed pulp is severely inflamed and possibly shows areas of necrosis and micro-abscess formation just in the vicinity of the caries lesion. Direct pulp capping without removal of the damaged part of the pulp (the irreversibly inflamed part) is not a predictable treatment in terms of pulpal survival. Partial or complete pulpotomy may be a better option to leave healthy pulp under the pulp-capping material capable of healing and surviving (68). Leaving soft dentine covering the pulp does not necessarily avoid progression of the pulpal inflammation or allow for healing. The remaining pulpal inflammation was confirmed in cases of selective caries removal along with the remaining contamination of the leathery/firm dentine (70). The authors concluded that the presence of potentially arrested caries does not necessarily mean that the bacterial infection is absent or under control (70). This can explain the declining pulp survival rate after selective or stepwise caries removal with time (41–43, 48, 53, 71–73). The studies reported by Bjørndal's group showed pulpal success rate of 74.1% out of 143 cases at 1 year for stepwise excavation compared to 60.2% out of 118 cases at 5 years follow-up with a drop-out rate of about 24%. Selective caries removal showed a 100% success rate of 22 out of 32 cases at 14–18 months review (71) while at 36–45 months, two cases showed restorative failures necessitating endodontic management, two quit the study, and one could not be contacted, the remaining 24 reviewed cases showed normal responses to pulp cold tests (42).
Management of the dentine substrate
Depending on the caries removal strategy, the tooth will be left with either caries infected dentine, which is the type of substrate left after selective caries removal or the first stage of the stepwise caries removal or caries-affected dentine which results from the total removal of caries. If there is a breach in the dentine layer, pulp management will be necessary.
The caries removal strategy employed will determine the dentine to material interface. There is still a debate regarding caries removal approach, and excavation criteria should be validated against clinically relevant outcomes (74).
The microstructure of the dentine varies across a carious lesion as the mineral content significantly decreased across the zones with the most demineralized zone also containing considerable residual mineral (75). Another parameter that needs to be considered is the presence of bacteria in the lesion which leads to pulpal changes. There are very little opportunities for remineralization if the overlying enamel acts as a barrier (76). Most of the large carious lesions still have intact enamel which prevents mineral exchange with saliva and other sources of mineral that may help with the remineralization. Although there is a significant reduction in lactobacilli in the residual carious dentine after selective caries removal, the lesion still retains a significant volume of bacteria even after calcium hydroxide therapy (77). The metabolic activity of the biofilm in dental caries is the key to effective management (78). If the lesion is sealed, the availability of the nutrients will be reduced, and the chances of caries progression are low. Furthermore, it has been shown that the treatment received changes the microbiota. Thus, the choice of materials used to manage the carious lesion is important (77). However, the presence of bacteria is linked to pulpal pathosis.
In cases without pulpal exposures, the presence of bacteria and the specific dentine microstructure require dentine preparation prior to dressing with a suitable pulp preservation material. Both position statements (13, 14) suggest the use of sodium hypochlorite with the ESE statement (13) also suggesting the use of chlorhexidine; this was only suggested to be used over the exposed pulp rather than caries infected and affected dentine. The dentine substrate is infected thus suggesting that preparation with an antimicrobial agent prior to material placement will be beneficial.
The use of sodium hypochlorite on the caries-affected dentine improves the adhesion of hydraulic calcium silicate cements (79). The use of ethylene diamine tetra-acetic acid also enhances the interaction of the materials to the dentine at the interface (80). Bonding to sound dentine yields better results compared to caries-affected dentine (81). Etch-and-rinse adhesives performed better than self-etch adhesives when applied to caries-affected dentine (81). Sodium hypochlorite has been used extensively in endodontics to reduce the microbial load. It is assumed that it will be effective in caries management. The use of sodium hypochlorite and EDTA over caries-affected dentine prior to material placement improves the tooth to material interface and bonding (79, 80).
Management of the pulp
When treating carious lesions, the primary goal is to preserve a healthy pulp (82) as this ensures the tooth's long-term survival (37, 83). The effect of the carious process on the dental pulp has been reviewed (84–86). Once the carious process has involved the dental pulp, this may necessitate the amputation of part of the pulp with a dressing of the wound and the use of a liner material (87). The thickness of the remaining dentine influences the pulpal response (5, 6, 11, 51).
The AAE position statement for management of the vital pulp (14) contrasts starkly with the ESE statement (13) as the AAE suggests the removal of all caries to the extent of investigating the pulpal status while the ESE guidelines recommend a selective caries removal or stepwise caries removal technique. Regardless of the size of the carious lesion, pulpal changes are present (2, 68, 70) with more severe pulpal inflammation with increasing depth of caries. A study attempted to link the radiographic depth of caries to the histological pulp status in cases of deep and extremely deep caries as seen radiographically (10). Out of 68 extracted molars of 12–18 years old patients, only 46 cases were assessed histologically. Partial necrosis (indicating irreversible pulpitis changes) was noted only in extremely deep caries. The inflammatory infiltrate in radicular pulp was noted also only in extremely deep caries. No clear information was given regarding the inflammatory status of deep caries cases. In addition, it is well known that carious lesions are usually larger or deeper clinically than shown on radiographs, which should be taken into consideration when applying the radiographic caries depth assessment.
From the molecular biology point of view, the presence of metalloproteinases-9 that are expressed only when tissue breakdown in the pulp occurs seems to be a good indicator and a promising tool to assess the severity of inflammation (88, 89). Therefore, the presence of MMP-9 may be a good indicator of the level of pulpal inflammation and help in proceeding with the relevant pulpal treatment. Further research is needed for a chair-side test to help in case selection for different modalities of vital pulp therapy. It should be noted that MMP-9 levels could be measured from the blood of an exposed pulp and indicate pulpal status more reliably than MMP-9 collected from dentinal tubules (88).
Once the pulp tissue is exposed, it requires management to reduce bleeding as well as the microbial load. The use of sodium hypochlorite (13, 14) and chlorhexidine (13) to manage the pulp will lead to remnants of the solutions on the dentine and in contact with the pulp preservation material. Sodium hypochlorite is effective in the reduction of haemorrhage (90–92) but is not compatible with the use of mineral trioxide aggregate if the MTA contains bismuth oxide. Tooth discolouration has been shown to be an unwanted sequela to successful vital pulp therapy using MTA (93, 94). Although not regarded as a failure, the discolouration will necessitate further intervention. The material and tooth discoloration induced by bismuth oxide in contact with dentine (95) and sodium hypochlorite (96, 97) is well documented with the discoloured bismuth complexes migrating from the material to the tooth structure within weeks of placement (98).
The use of chlorhexidine may have potential beneficial effects in reducing the microbial load in the pulpal tissues. Chlorhexidine has been added to MTA to enhance its antimicrobial activity (99, 100) but unfortunately exhibited adverse biological effects with cell apoptosis (101) and impacting negatively on the calcific bridge formation (102). When used as a final irrigating solution in root canal therapy (103–105), chlorhexidine also exhibited an enhanced antimicrobial action but was detrimental to the cell growth and differentiation (104) and also affected the physical properties of hydraulic calcium silicate-based materials (103, 106). Due to this, chlorhexidine cannot be recommended for the management of the dental pulp.
Dressing the dentine
The dentine requires dressing with the placement of a suitable material in deep carious lesions with and without carious exposure. In cases where no exposure to the dental pulp has occurred, production of reparative dentine and remineralization of demineralized dentine occurs with the use of a cavity liner (87). The sealing of the cavity limits the nutrition of the residual bacteria (107–110) with the tertiary dentine reducing the permeability of the dentine and limiting pulpal substrates for the residual bacteria to use as nutrients (108, 111).
When the remaining dentine thickness is less than 0.5 mm, the use of a liner material is necessary and calcium hydroxide lining has been recommended (112) as at the time of these studies, hydraulic cements were not available. A clinical randomized study is being undertaken to investigate the most appropriate liner in cases managed by selective caries removal (113). Calcium hydroxide is currently not indicated for use in any of the clinical guidance, except in the British Endodontic Society Guide (114). The guidance given in the latter document is based on expert opinion using outdated scientific evidence. The clinical studies comparing MTA and calcium hydroxide (63, 115–120) indicated the better performance of MTA when compared to calcium hydroxide. Although the quality of evidence of the studies investigating the use of calcium hydroxide liner is moderate to low, it was shown that the calcium hydroxide did not influence the clinical success of treatment for deep caries lesions (110).
While glass ionomers have not been recommended for indirect pulp capping by the AAE (14), the ESE position statement (13) suggests that both glass ionomers and hydraulic calcium silicates are suitable for dressing the dentine. This is based on two clinical studies (28, 121) that have found no difference between the outcomes of indirect pulp capping using either glass ionomer (Fuji IX, GC Europe, Leuven, Belgium) or Biodentine (Septodont, Saint-Maur-des-Fosses, France). In these studies selective caries removal was employed and the outcomes of treatment were better with Biodentine when using cone beam computed tomography (121). Proper glass ionomer use necessities the use of cavity conditioning with a weak acid. Acid etching caused inflammatory changes that were more severe in deeper cavities (5, 6) and therefore cannot be undertaken in a deep cavity thus limiting the use of glass ionomers in deep carious lesions in close proximity to the dental pulp regardless of the ESE recommendations.
The AAE guidelines (14) suggest the use of hydraulic calcium silicate cements that are referred to by various names. Regardless of this and the numerous clinical studies showing the efficacy of using MTA (63, 115, 117–120), it should be noted that the use of MTA containing bismuth oxide is contraindicated for use due to the tooth discolouration as indicated earlier. Furthermore, the setting time of MTA is over 3 h, and this will compromise the adequacy of the tooth restoration as discussed later.
The research undertaken indicates that the use of fast-setting hydraulic calcium silicate cement which is bismuth oxide free such as Biodentine is the best treatment strategy. The research investigating the use of Biodentine as a pulp preservation material (93) indicates that Biodentine has a clinically acceptable setting time which is shorter than other hydraulic calcium silicate cements (122–124), high pH and calcium ion release which is initially high but drops over time (122, 123, 125, 126). Tooth discolouration has never been implicated with the use of Biodentine (127–131)- despite what is stated in the British Endodontic Society Guide (114).
If there is a breach and the dental pulp is exposed, the material used to dress the dentine in cases of indirect pulp capping needs to be effective in managing the dental pulp. Partial and full pulpotomy using calcium silicates showed very high success rates and predictable results (29–35, 64, 132). When tested in contact with human dental pulp stem cells, to assess cell proliferation, viability mineralization potential and morphology, Biodentine exhibited adequate biological characteristics (133, 134) even when employing advanced cell culture models (135). The cells adhered well to the material surface (136) and the material extracts exhibited cell proliferation and expression as well (137–139). A calcific bridge was shown in animal studies employing Biodentine as pulp preservation material (140–142).
The interface of Biodentine with the dentine has been described as having an alkaline etch with the formation of a mineral infiltration zone (143). This view has been debated in other studies where no mineral transfer has been observed at the tooth to material interface (80, 144). A recent study investigating the interaction of Biodentine and a glass ionomer cement on caries-affected dentine verifies the remineralization potential of both material types and suggests the efficacy of using Biodentine as part of a minimally invasive operative dentistry strategy in caries management (145). Although Biodentine has been tested quite extensively, a study investigating the interaction of a material with caries-affected dentine in extracted teeth without any microbiology testing and also over a short period of time can hardly be used for a clinical recommendation.
Tooth restoration
The adequate seal of the tooth is important for the success of vital pulp therapy (62, 63, 68, 132). The sealing of carious dentine results in lower levels of infection than traditional dentine caries removal thus it is important to provide an adequate restoration (146).
One of the key properties of hydraulic cements is their interaction with the clinical environment. This is more challenging when the material with a long setting time such as MTA is used when the tooth is sealed on the first visit. Such a procedure necessitates the layering with a fast-setting material such as glass ionomer cement (147). The use of glass ionomer and zinc oxide eugenol-based materials is contra-indicated over MTA due to material interaction which is detrimental to the MTA as the zinc in zinc oxide eugenol-based material interferes with the MTA setting and the acidity of the glass ionomer causes cracks at the material-to-material interface (148).
The use of fast-setting materials is thus indicated. Materials such as Biodentine are strong enough (122) to be used on their own over the pulp and serve also as a temporary filling material (149). Clinically, bonding of resin composite to Biodentine may be challenging. Biodentine has a hydrophilic surface while resin composites and their associated adhesive systems are hydrophobic, or mildly hydrophilic, respectively. Furthermore, the application of acid etch on the Biodentine surface has been shown to disrupt the material surface and result in the reduction of microhardness (150). The in vitro data showing the bonding of resin composites to Biodentine has shown weak bond strengths. Therefore, bonding to Biodentine can be considered a compromise because the interface may include gaps resulting from the difference in moisture affinity of the two materials. There are conflicting data on the best method to bond a composite resin to Biodentine with some favouring total-etch (151) and others the use of self-etching primers (152). Most of the adhesives tested were adequate (153). Reduced acid-etching times avoided the detrimental effect on the material and did not result in deterioration of the bond between the materials (154). Delaying the restoration has not shown to be of added benefit (155–157). Methacrylate-based composites were better than silorane-based composites and glass ionomer cements (158). The type of adhesive system and restoration time affect the bond performance and ultra-morphological interface between composite adhesive restoration and hydraulic calcium silicates (159).
Double layering has also been considered. While glass ionomer is not indicated as it is acidic and will disrupt the interface and result in reduced bond strength (151, 160), resin-modified glass ionomer can be considered for use over the hydraulic cement and this can be etched fully when the composite restoration is placed (161).
Although self-etching adhesive systems may be useful and safe when applied on dentine, in contrast, persistent inflammatory reactions as well as delay in pulpal healing and failure of dentine bridging have been observed in human pulps capped with bonding agents (162). Vital pulp therapy using acidic agents and adhesive resins is contraindicated (162).
Restoration durability, sealing ability and survival are of paramount importance for pulpal survival and tooth function. Bonding systems deteriorate over time (163–165), and this results in a decline in pulpal survival (51). This, together with coronal microleakage and additional caries challenge were not addressed in the literature. Reduced bonding strength to caries infected or affected dentine was reported (166, 167). Therefore, in selective and stepwise caries removal, caries is removed completely at the periphery of the lesion and the dentine-enamel junction with caries selectively removed at the pulpal walls. This allows for proper bonding of the restoration and provision of a proper seal. At one year follow up (43) showed no difference in restoration survival between stepwise and non-selective caries removal.
Effect of stepwise and selective caries removal on pulpal health
While there are some clinical studies showing successful results of selective caries removal, there is a clear lack of histological studies to assess the pulpal response to both stepwise and selective caries removal and their biological success. In two histopathological studies, varying degrees of pulpal inflammation were noted in asymptomatic cases managed by selective caries removal to firm dentine and restored with adhesive restoration that responded normally to pulp testing after 15 months follow up (68) or 1–9 months follow up (70). Similar inflammatory status could be discerned in treated and untreated cases of similar caries depth (70). It is speculated that this inflammation is unlikely to subside if bacterial infection is not completely removed albeit the short follow-up time (one month) in some cases (70). In contrast, healed pulps with only a reduced number of odontoblasts, tertiary dentine and some segregated calcifications in cases restored heavily but with all soft caries removed and good coronal seal provided was shown (68). The pulpal wall management in both papers mentioned was either by application of calcium hydroxide liner, a form of calcium silicate material, or just an adhesive restoration placed immediately without a base or liner. Histopathological studies of pulpal status and healing after selective caries removal to soft dentine and after stepwise caries removal are lacking. It should be mentioned that a low-grade inflammation is crucial to induce healing (168, 169). However, the threshold between “beneficial” inflammation and destructive one has never been identified in histological studies; possibly because tissue healing and destruction are driven by molecular ques secreted by the same inflammatory cells that are not reported in histopathological studies (169, 170). However, the pulp inflammation can be sustained by remaining bacteria in the dentine by leaching endotoxins preventing regeneration or healing to take place (171). Further studies using molecular biology are needed to clarify this point with regard to selective caries removal technique.
Currently most studies showed lack of correlation of pulpal symptoms to the histological condition of the pulp (21–23) with exception of one recent study (25). In addition, a good possibility of lack of symptoms in pulpitis cases was reported (172) along with clinically high success rates of calcium silicate pulpotomy procedures, it makes clinical sense to remove caries completely and assess pulpal condition and manage it accordingly once exposed. It should be mentioned that there is a lack of properly designed and randomized studies comparing selective caries removal and partial or complete pulpotomy in cases of deep caries.
Clinical significance and suggested protocol
Based on the scientific literature assessed in this narrative review it can be concluded that the ideal clinical protocol for the management of vital mature carious teeth is the following
1. Complete caries removal is recommended when managing deep caries to allow for proper assessment of pulpal status.
2. Dentine cleansing with sodium hypochlorite and ethylene diamine tetra-acetic acid
3. Management of the dental pulp if necessary. The use of sodium hypochlorite reduces bacterial load.
4. Placement of a fast setting, bismuth oxide-free hydraulic calcium silicate cement. If strong enough it can be used as a temporary restorative material
5. Selective etching with a 5 s etch of the hydraulic calcium silicate pulp preservation material and a total etch of the dentine and enamel. The enamel etch can be undertaken prior to the placement of the hydraulic cement to avoid the washing out of the material.
6. Application of a dentine bonding agent or use of a resin-modified glass ionomer over hydraulic cement and placement of a composite resin restoration.
The clinical options are illustrated in Figure 1.
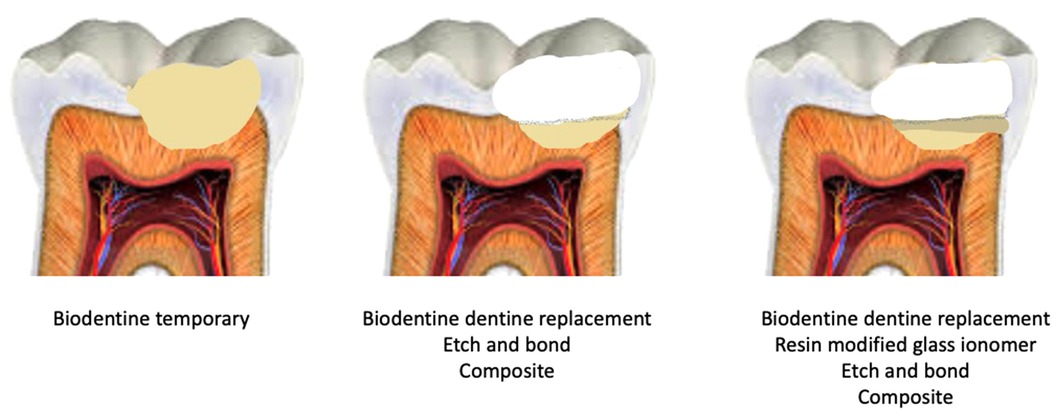
Figure 1. Different clinical management strategies for the use of Biodentine for vital pulp therapy and suggestions for relevant tooth restoration..
Conclusions of narrative review
Pulpal diagnosis remains elusive and necessitates further research and studies to improve its accuracy and to reproduce the correlation to the actual histopathological status. Consideration should be given to promote new treatment modalities for reversible and irreversible pulpitis allowing for healthy pulp preservation. Significant debate can be elicited about deep caries management. Even though selective caries removal may reduce the risk of pulpal exposure, cases with deep caries should be managed by complete non-selective caries removal which will allow for pulpal management if needed and a more predictable outcome can be expected when using the new materials and treatment modalities of vital pulp therapy. Hydraulic calcium silicate cements are the materials of choice for the management of the vital pulp. Modified versions with enhanced physical properties and bismuth oxide-free are superior to other formulations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
Both authors have contributed equally to the development, writing and finalising the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Carvalho TS, Lussi A. Age-related morphological, histological and functional changes in teeth. J Oral Rehabil. (2017) 44(4):291–8. doi: 10.1111/joor.12474
2. Ricucci D, Loghin S, Niu LN, Tay FR. Changes in the radicular pulp-e complex in healthy intact teeth and in response to deep caries or restorations: a histological and histobacteriological study. J Dent. (2018) 73:76–90. doi: 10.1016/j.jdent.2018.04.007
3. Mendes Soares IP, Anovazzi G, Anselmi C, Leite ML, Scheffel DLS, Soares DG, et al. Response of pulp cells to resin infiltration of enamel white spot-like lesions. Dent Mater. (2021) 37(6):e329–40. doi: 10.1016/j.dental.2021.01.014
4. About I, Mitsiadis TA. Molecular aspects of tooth pathogenesis and repair: in vivo and in vitro models. Adv Dent Res. (2001) 15(1):59–62. doi: 10.1177/08959374010150011501
5. About I, Murray PE, Franquin JC, Remusat M, Smith AJ. The effect of cavity restoration variables on odontoblast cell numbers and dental repair. J Dent. (2001) 29(2):109–17. doi: 10.1016/S0300-5712(00)00067-1
6. About I, Murray PE, Franquin JC, Remusat M, Smith AJ. Pulpal inflammatory responses following non-carious class V restorations. Oper Dent. (2001) 26(4):336–42. doi: 10.2341/1559-2863-26-4-1
7. Murray PE, Windsor LJ, Smyth TW, Hafez AA, Cox CF. Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies. Crit Rev Oral Biol Med. (2002) 13(6):509–20. doi: 10.1177/154411130201300607
8. Santos JM, Marques JA, Diogo P, Messias A, Sousa V, Sequeira D, et al. Influence of preoperative pulp inflammation in the outcome of full pulpotomy using a dog model. J Endod. (2021) 47(9):1417–26. doi: 10.1016/j.joen.2021.06.018
9. Bjørndal L. The caries process and its effect on the pulp: the science is changing and so is our understanding. J Endod. (2008) 34(7 Suppl):S2–S5. doi: 10.1016/j.joen.2008.02.037
10. Demant S, Dabelsteen S, Bjørndal L. A macroscopic and histological analysis of radiographically well-defined deep and extremely deep carious lesions: carious lesion characteristics as indicators of the level of bacterial penetration and pulp response. Int Endod J. (2021) 54(3):319–30. doi: 10.1111/iej.13424
11. Murray PE, Smith AJ, Windsor LJ, Mjor IA. Remaining dentine thickness and human pulp responses. Int Endod J. (2003) 36(1):33–43. doi: 10.1046/j.0143-2885.2003.00609.x
12. Oen KT, Thompson VP, Vena D, Caufield PW, Curro F, Dasanayake A, et al. Attitudes and expectations of treating deep caries: a PEARL network survey. Gen Dent. (2007) 55(3):197–203.17511360
13. Duncan HF, Galler KM, Tomson PL, Simon S, El-Karim I, Kundzina R, et al. European Society of Endodontology position statement: management of deep caries and the exposed pulp. Int Endod J. (2019) 52(7):923–34. doi: 10.1111/iej.13080
14. AAE. AAE position statement on vital pulp therapy. J Endod. (2021) 47(9):1340–4. doi: 10.1016/j.joen.2021.07.015
15. Jafarzadeh H, Abbott PV. Review of pulp sensibility tests. Part I: general information and thermal tests. Int Endod J. (2010) 43(9):738–62. doi: 10.1111/j.1365-2591.2010.01754.x
16. Jafarzadeh H, Abbott PV. Review of pulp sensibility tests. Part ΙΙ: electric pulp tests and test cavities. Int Endod J. (2010) 43(11):945–58. doi: 10.1111/j.1365-2591.2010.01760.x
17. Fulling HJ, Andreasen JO. Influence of maturation status and tooth type of permanent teeth upon electrometric and thermal pulp testing. Scand J Dent Res. (1976) 84(5):286–90. doi: 10.1111/j.1600-0722.1976.tb00491.x
18. Fuss Z, Trowbridge H, Bender IB, Rickoff B, Sorin S. Assessment of reliability of electrical and thermal pulp testing agents. J Endod. (1986) 12(7):301–5. doi: 10.1016/S0099-2399(86)80112-1
19. Jespersen JJ, Hellstein J, Williamson A, Johnson WT, Qian F. Evaluation of dental pulp sensibility tests in a clinical setting. J Endod. (2014) 40(3):351–4. doi: 10.1016/j.joen.2013.11.009
20. Lin J, Chandler NP. Electric pulp testing: a review. Int Endod J. (2008) 41(5):365–74. doi: 10.1111/j.1365-2591.2008.01375.x
21. Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol. (1963) 16:969–77. doi: 10.1016/0030-4220(63)90201-9
22. Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol. (1963) 16:846–71. doi: 10.1016/0030-4220(63)90323-2
23. Dummer PM, Hicks R, Huws D. Clinical signs and symptoms in pulp disease. Int Endod J. (1980) 13(1):27–35. doi: 10.1111/j.1365-2591.1980.tb00834.x
24. Jones VR, Rivera EM, Walton RE. Comparison of carbon dioxide versus refrigerant spray to determine pulpal responsiveness. J Endod. (2002) 28(7):531–3. doi: 10.1097/00004770-200207000-00011
25. Ricucci D, Loghin S, Siqueira JF Jr. Correlation between clinical and histologic pulp diagnoses. J Endod. (2014) 40(12):1932–9. doi: 10.1016/j.joen.2014.08.010
27. Wolters WJ, Duncan HF, Tomson PL, Karim IE, McKenna G, Dorri M, et al. Minimally invasive endodontics: a new diagnostic system for assessing pulpitis and subsequent treatment needs. Int Endod J. (2017) 50(9):825–9. doi: 10.1111/iej.12793
28. Hashem D, Mannocci F, Patel S, Manoharan A, Brown JE, Watson TF, et al. Clinical and radiographic assessment of the efficacy of calcium silicate indirect pulp capping: a randomized controlled clinical trial. J Dent Res. (2015) 94(4):562–8. doi: 10.1177/0022034515571415
29. Taha NA, About I, Sedgley CM, Messer HH. Conservative management of mature permanent teeth with carious pulp exposure. J Endod. (2020) 46(9s):S33–S41. doi: 10.1016/j.joen.2020.06.025
30. Taha NA, Abdulkhader SZ. Full pulpotomy with biodentine in symptomatic young permanent teeth with carious exposure. J Endod. (2018) 44(6):932–7. doi: 10.1016/j.joen.2018.03.003
31. Taha NA, Abdelkhader SZ. Outcome of full pulpotomy using Biodentine in adult patients with symptoms indicative of irreversible pulpitis. Int Endod J. (2018) 51(8):819–28. doi: 10.1111/iej.12903
32. Taha NA, Al-Khatib H. 4-year fllow-up of full pulpotomy in symptomatic mature permanent teeth with carious pulp exposure using a stainproof calcium silicate-based material. J Endod. (2022) 48(1):87–95. doi: 10.1016/j.joen.2021.09.008
33. Taha NA, Al-Rawash MH, Imran ZA. Outcome of full pulpotomy in mature permanent molars using 3 calcium silicate-based materials: a parallel, double blind, randomized controlled trial. Int Endod J. (2022) 55(5):416–29. doi: 10.1111/iej.13707
34. Taha NA, Khazali MA. Partial pulpotomy in mature permanent teeth with clinical signs indicative of irreversible pulpitis: a randomized clinical trial. J Endod. (2017) 43(9):1417–21. doi: 10.1016/j.joen.2017.03.033
35. Careddu R, Duncan HF. A prospective clinical study investigating the effectiveness of partial pulpotomy after relating preoperative symptoms to a new and established classification of pulpitis. Int Endod J. (2021) 54(12):2156–72. doi: 10.1111/iej.13629
36. Levin LG, Law AS, Holland GR, Abbott PV, Roda RS. Identify and define all diagnostic terms for pulpal health and disease states. J Endod. (2009) 35(12):1645–57. doi: 10.1016/j.joen.2009.09.032
37. Schwendicke F, Frencken JE, Bjørndal L, Maltz M, Manton DJ, Ricketts D, et al. Managing carious lesions: consensus recommendations on carious tissue removal. Adv Dent Res. (2016) 28(2):58–67. doi: 10.1177/0022034516639271
38. Schwendicke F, Walsh T, Lamont T, Al-Yaseen W, Bjørndal L, Clarkson JE, et al. Interventions for treating cavitated or dentine carious lesions. Cochrane Database Syst Rev. (2021) 7:CD013039. doi: 10.1002/14651858.CD013039.pub2
39. Barros M, De Queiroz Rodrigues MI, Muniz F, Rodrigues LKA. Selective, stepwise, or nonselective removal of carious tissue: which technique offers lower risk for the treatment of dental caries in permanent teeth? A systematic review and meta-analysis. Clin Oral Investig. (2020) 24(2):521–32. doi: 10.1007/s00784-019-03114-5
40. Banerjee A, Frencken JE, Schwendicke F, Innes NPT. Contemporary operative caries management: consensus recommendations on minimally invasive caries removal. Br Dent J. (2017) 223(3):215–22. doi: 10.1038/sj.bdj.2017.672
41. Maltz M, de Oliveira EF, Fontanella V, Bianchi R. A clinical, microbiologic, and radiographic study of deep caries lesions after incomplete caries removal. Quintessence Int. (2002) 33(2):151–9.11890029
42. Maltz M, Oliveira EF, Fontanella V, Carminatti G. Deep caries lesions after incomplete dentine caries removal: 40-month follow-up study. Caries Res. (2007) 41(6):493–6. doi: 10.1159/000109349
43. Bjørndal L, Reit C, Bruun G, Markvart M, Kjaeldgaard M, Nasman P, et al. Treatment of deep caries lesions in adults: randomized clinical trials comparing stepwise vs. Direct complete excavation, and direct pulp capping vs. Partial pulpotomy. Eur J Oral Sci. (2010) 118(3):290–7. doi: 10.1111/j.1600-0722.2010.00731.x
44. Hilton TJ. Keys to clinical success with pulp capping: a review of the literature. Oper Dent. (2009) 34(5):615–25. doi: 10.2341/09-132-0
45. Fairbourn DR, Charbeneau GT, Loesche WJ. Effect of improved Dycal and IRM on bacteria in deep carious lesions. J Am Dent Assoc. (1980) 100(4):547–52. doi: 10.14219/jada.archive.1980.0144
46. Bjørndal L, Larsen T, Thylstrup A. A clinical and microbiological study of deep carious lesions during stepwise excavation using long treatment intervals. Caries Res. (1997) 31(6):411–7. doi: 10.1159/000262431
47. Bjørndal L, Simon S, Tomson PL, Duncan HF. Management of deep caries and the exposed pulp. Int Endod J. (2019) 52(7):949–73. doi: 10.1111/iej.13128
48. Bjørndal L, Fransson H, Bruun G, Markvart M, Kjaeldgaard M, Nasman P, et al. Randomized clinical trials on deep carious lesions: 5-year follow-up. J Dent Res. (2017) 96(7):747–53. doi: 10.1177/0022034517702620
49. Leksell E, Ridell K, Cvek M, Mejare I. Pulp exposure after stepwise versus direct complete excavation of deep carious lesions in young posterior permanent teeth. Endod Dent Traumatol. (1996) 12(4):192–6. doi: 10.1111/j.1600-9657.1996.tb00513.x
50. Schwendicke F, Dorfer CE, Paris S. Incomplete caries removal: a systematic review and meta-analysis. J Dent Res. (2013) 92(4):306–14. doi: 10.1177/0022034513477425
51. Whitworth JM, Myers PM, Smith J, Walls AWG, McCabe JF. Endodontic complications after plastic restorations in general practice. Int Endod J. (2005) 38(6):409–16. doi: 10.1111/j.1365-2591.2005.00962.x
52. Zanata RL, Navarro MF, Barbosa SH, Lauris JR, Franco EB. Clinical evaluation of three restorative materials applied in a minimal intervention caries treatment approach. J Public Health Dent. (2003) 63(4):221–6. doi: 10.1111/j.1752-7325.2003.tb03503.x
53. Maltz M, Garcia R, Jardim JJ, de Paula LM, Yamaguti PM, Moura MS, et al. Randomized trial of partial vs. stepwise caries removal:3-year follow-up. J Dent Res. (2012) 91(11):1026–31. doi: 10.1177/0022034512460403
54. Hoefler V, Nagaoka H, Miller CS. Long-term survival and vitality outcomes of permanent teeth following deep caries treatment with step-wise and partial-caries-removal: a systematic review. J Dent. (2016) 54:25–32. doi: 10.1016/j.jdent.2016.09.009
55. Handelman SL, Leverett DH, Solomon ES, Brenner CM. Use of adhesive sealants over occlusal carious lesions: radiographic evaluation. Community Dent Oral Epidemiol. (1981) 9(6):256–9. doi: 10.1111/j.1600-0528.1981.tb00341.x
56. Handelman SL, Washburn F, Wopperer P. Two-year report of sealant effect on bacteria in dental caries. J Am Dent Assoc. (1976) 93(5):967–70. doi: 10.14219/jada.archive.1976.0007
57. Bjørndal L, Larsen T. Changes in the cultivable flora in deep carious lesions following a stepwise excavation procedure. Caries Res. (2000) 34(6):502–8. doi: 10.1159/000016631
58. Ricketts DN, Kidd EA, Innes N, Clarkson J. Complete or ultraconservative removal of decayed tissue in unfilled teeth. Cochrane Database Syst Rev. (2006) 19(3):CD003808. doi: 10.1002/14651858.CD003808.pub2
59. Thompson V, Craig RG, Curro FA, Green WS, Ship JA. Treatment of deep carious lesions by complete excavation or partial removal: a critical review. J Am Dent Assoc. (2008) 139(6):705–12. doi: 10.14219/jada.archive.2008.0252
60. Li T, Zhai X, Song F, Zhu H. Selective versus non-selective removal for dental caries: a systematic review and meta-analysis. Acta Odontol Scand. (2018) 76(2):135–40. doi: 10.1080/00016357.2017.1392602
61. Barthel CR, Rosenkranz B, Leuenberg A, Roulet J-F. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. (2000) 26(9):525–8. doi: 10.1097/00004770-200009000-00010
62. Trope M. Regenerative potential of dental pulp. J Endod. (2008) 34(7 Suppl):S13–7. doi: 10.1016/j.joen.2008.04.001
63. Mente J, Geletneky B, Ohle M, Koch MJ, Friedrich Ding PG, Wolff D, et al. Mineral trioxide aggregate or calcium hydroxide direct pulp capping: an analysis of the clinical treatment outcome. J Endod. (2010) 36(5):806–13. doi: 10.1016/j.joen.2010.02.024
64. Aguilar P, Linsuwanont P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: a systematic review. J Endod. (2011) 37(5):581–7. doi: 10.1016/j.joen.2010.12.004
65. Kunert GG, Kunert IR, da Costa Filho LC, de Figueiredo JAP. Permanent teeth pulpotomy survival analysis: retrospective follow-up. J Dent. (2015) 43(9):1125–31. doi: 10.1016/j.jdent.2015.06.010
66. Cushley S, Duncan HF, Lappin MJ, Chua P, Elamin AD, Clarke M, et al. Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: a systematic review and meta-analysis. Int Endod J. (2021) 54(4):556–71. doi: 10.1111/iej.13449
67. Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. (2008) 41(2):128–50. doi: 10.1111/j.1365-2591.2007.01329.x
68. Ricucci D, Siqueira JF Jr, Li Y, Tay FR. Vital pulp therapy: histopathology and histobacteriology-based guidelines to treat teeth with deep caries and pulp exposure. J Dent. (2019) 86:41–52. doi: 10.1016/j.jdent.2019.05.022
69. Ricucci D, Siqueira JF Jr, Loghin S, Lin LM. Pulp and apical tissue response to deep caries in immature teeth: a histologic and histobacteriologic study. J Dent. (2017) 56:19–32. doi: 10.1016/j.jdent.2016.10.005
70. Ricucci D, Siqueira JF Jr, Rocas IN, Lipski M, Shiban A, Tay FR. Pulp and dentine responses to selective caries excavation: a histological and histobacteriological human study. J Dent. (2020) 100:103430. doi: 10.1016/j.jdent.2020.103430
71. Oliveira EF, Carminatti G, Fontanella V, Maltz M. The monitoring of deep caries lesions after incomplete dentine caries removal: results after 14-18 months. Clin Oral Investig. (2006) 10(2):134–9. doi: 10.1007/s00784-006-0033-8
72. Maltz M, Koppe B, Jardim JJ, Alves LS, de Paula LM, Yamaguti PM, et al. Partial caries removal in deep caries lesions: a 5-year multicenter randomized controlled trial. Clin Oral Investig. (2018) 22(3):1337–43. doi: 10.1007/s00784-017-2221-0
73. Maltz M, Jardim JJ, Mestrinho HD, Yamaguti PM, Podesta K, Moura MS, et al. Partial removal of carious dentine: a multicenter randomized controlled trial and 18-month follow-up results. Caries Res. (2013) 47(2):103–9. doi: 10.1159/000344013
74. Schwendicke F, Paris S, Tu YK. Effects of using different criteria for caries removal: a systematic review and network meta-analysis. J Dent. (2015) 43(1):1–15. doi: 10.1016/j.jdent.2014.10.004
75. Pugach MK, Strother J, Darling CL, Fried D, Gansky SA, Marshall SJ, et al. Dentin caries zones: mineral, structure, and properties. J Dent Res. (2009) 88(1):71–6. doi: 10.1177/0022034508327552
76. Kidd EA, Fejerskov O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res. (2004) 83(Spec No C):C35–8. doi: 10.1177/154405910408301s07
77. Dame-Teixeira N, Ev LD, Bitello-Firmino L, Soares VK, Dalalba RS, Rup AG, et al. Characterization of Lactobacilli isolated from carious dentin after selective caries removal and cavity sealing. Arch Oral Biol. (2021) 121:104988. doi: 10.1016/j.archoralbio.2020.104988
78. Nyvad B, Crielaard W, Mira A, Takahashi N, Beighton D. Dental caries from a molecular microbiological perspective. Caries Res. (2013) 47(2):89–102. doi: 10.1159/000345367
79. Meraji N, Nekoofar MH, Yazdi KA, Sharifian MR, Fakhari N, Camilleri J. Bonding to caries affected dentine. Dent Mater. (2018) 34(9):e236–45. doi: 10.1016/j.dental.2018.05.017
80. Hadis M, Wang J, Zhang ZJ, Di Maio A, Camilleri J. Interaction of hydraulic calcium silicate and glass ionomer cements with dentine. Materialia. (2020) 9:100515. doi: 10.1016/j.mtla.2019.100515
81. Isolan CP, Sarkis-Onofre R, Lima GS, Moraes RR. Bonding to sound and caries-affected dentin: a systematic review and meta-analysis. J Adhes Dent. (2018) 20(1):7–18. doi: 10.3290/j.jad.a39775
82. Innes NP, Frencken JE, Bjørndal L, Maltz M, Manton DJ, Ricketts D, et al. Managing carious lesions: consensus recommendations on terminology. Adv Dent Res. (2016) 28(2):49–57. doi: 10.1177/0022034516639276
83. Byers MR, Narhi MV, Mecifi KB. Acute and chronic reactions of dental sensory nerve fibers to cavities and desiccation in rat molars. Anat Rec. (1988) 221(4):872–83. doi: 10.1002/ar.1092210412
84. Larmas M, Sandor GK. Enzymes, dentinogenesis and dental caries: a literature review. J Oral Maxillofac Res. (2014) 5(4):e3. doi: 10.5037/jomr.2014.5403
85. Farges JC, Alliot-Licht B, Renard E, Ducret M, Gaudin A, Smith AJ, et al. Dental pulp defence and repair mechanisms in dental caries. Mediators Inflamm. (2015) 2015:230251. doi: 10.1155/2015/230251
86. Widbiller M, Weiler R, Knuttel H, Galler KM, Buchalla W, Scholz KJ. Biology of selective caries removal: a systematic scoping review protocol. BMJ Open. (2022) 12(2):e061119. doi: 10.1136/bmjopen-2022-061119
87. Mickenautsch S, Yengopal V, Banerjee A. Pulp response to resin-modified glass ionomer and calcium hydroxide cements in deep cavities: a quantitative systematic review. Dent Mater. (2010) 26(8):761–70. doi: 10.1016/j.dental.2010.03.021
88. Ballal V, Rao S, Bagheri A, Bhat V, Attin T, Zehnder M. MMP-9 in dentinal fluid correlates with caries lesion depth. Caries Res. (2017) 51(5):460–5. doi: 10.1159/000479040
89. Mente J, Petrovic J, Gehrig H, Rampf S, Michel A, Schurz A, et al. A prospective clinical pilot study on the level of matrix metalloproteinase-9 in dental pulpal blood as a marker for the state of inflammation in the pulp tissue. J Endod. (2016) 42(2):190–7. doi: 10.1016/j.joen.2015.10.020
90. Ballal NV, Duncan HF, Wiedemeier DB, Rai N, Jalan P, Bhat V, et al. MMP-9 levels and NaOCl lavage in randomized trial on direct pulp capping. J Dent Res. (2022) 101(4):414–9. doi: 10.1177/00220345211046874
91. Matsuo T, Nakanishi T, Shimizu H, Ebisu S. A clinical study of direct pulp capping applied to carious-exposed pulps. J Endod. (1996) 22(10):551–6. doi: 10.1016/S0099-2399(96)80017-3
92. Hafez AA, Cox CF, Tarim B, Otsuki M, Akimoto N. An in vivo evaluation of hemorrhage control using sodium hypochlorite and direct capping with a one- or two-component adhesive system in exposed nonhuman primate pulps. Quintessence Int. (2002) 33(4):261–72.11989375
93. Camilleri J, Atmeh A, Li X, Meschi N. Present status and future directions: hydraulic materials for endodontic use. Int Endod J. (2022) 55(Suppl 3):710–77. doi: 10.1111/iej.13709
94. Palma PJ, Marques JA, Santos J, Falacho RI, Sequeira D, Diogo P, et al. Tooth discoloration after regenerative endodontic procedures with calcium silicate-based cements—an ex vivo study. Appl Sci. (2020) 10(17):5793. doi: 10.3390/app10175793
95. Marciano MA, Costa RM, Camilleri J, Mondelli RF, Guimaraes BM, Duarte MA. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J Endod. (2014) 40(8):1235–40. doi: 10.1016/j.joen.2014.01.044
96. Camilleri J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J Endod. (2014) 40(3):436–40. doi: 10.1016/j.joen.2013.09.040
97. Camilleri J, Borg J, Damidot D, Salvadori E, Pilecki P, Zaslansky P, et al. Colour and chemical stability of bismuth oxide in dental materials with solutions used in routine clinical practice. PLoS One. (2020) 15(11):e0240634. doi: 10.1371/journal.pone.0240634
98. Marciano MA, Duarte MA, Camilleri J. Dental discoloration caused by bismuth oxide in MTA in the presence of sodium hypochlorite. Clin Oral Investig. (2015) 19(9):2201–9. doi: 10.1007/s00784-015-1466-8
99. Mittag SG, Eissner C, Zabel L, Wrbas KT, Kielbassa AM. The influence of chlorhexidine on the antibacterial effects of MTA. Quintessence Int. (2012) 43(10):901–6.23115769
100. Ghatole K, Patil A, Giriyappa RH, Singh TV, Jyotsna SV, Rairam S. Evaluation of antibacterial efficacy of MTA with and without additives like silver zeolite and chlorhexidine. J Clin Diagn Res. (2016) 10(6):ZC11–4. doi: 10.7860/JCDR/2016/18014.7913
101. Hernandez EP, Botero TM, Mantellini MG, McDonald NJ, Nor JE. Effect of ProRoot MTA mixed with chlorhexidine on apoptosis and cell cycle of fibroblasts and macrophages in vitro. Int Endod J. (2005) 38(2):137–43. doi: 10.1111/j.1365-2591.2004.00922.x
102. Manochehrifar H, Parirokh M, Kakooei S, Oloomi MM, Asgary S, Eghbal MJ, et al. The effect of mineral trioxide aggregate mixed with chlorhexidine as direct pulp capping agent in dogs teeth: a histologic study. Iran Endod J. (2016) 11(4):320–4. doi: 10.22037/iej.2016.12
103. Kapralos V, Rukke HV, Orstavik D, Koutroulis A, Camilleri J, Sunde PT. Antimicrobial and physicochemical characterization of endodontic sealers after exposure to chlorhexidine digluconate. Dent Mater. (2021) 37(2):249–63. doi: 10.1016/j.dental.2020.11.011
104. Kapralos V, Sunde PT, Camilleri J, Morisbak E, Koutroulis A, Orstavik D, et al. Effect of chlorhexidine digluconate on antimicrobial activity, cell viability and physicochemical properties of three endodontic sealers. Dent Mater. (2022) 38(6):1044–59. doi: 10.1016/j.dental.2022.04.013
105. Kapralos V, Valen H, Koutroulis A, Camilleri J, Orstavik D, Sunde PT. The dentine-sealer interface: modulation of antimicrobial effects by irrigation. Int Endod J. (2022) 55(5):544–60. doi: 10.1111/iej.13692
106. Lindblad RM, Lassila LVJ, Vallittu PK, Tjaderhane L. The effect of chlorhexidine and dimethyl sulfoxide on long-term sealing ability of two calcium silicate cements in root canal. Dent Mater. (2021) 37(2):328–35. doi: 10.1016/j.dental.2020.11.031
107. Hayashi M, Fujitani M, Yamaki C, Momoi Y. Ways of enhancing pulp preservation by stepwise excavation--a systematic review. J Dent. (2011) 39(2):95–107. doi: 10.1016/j.jdent.2010.10.012
108. Ricketts D, Lamont T, Innes NP, Kidd E, Clarkson JE. Operative caries management in adults and children. Cochrane Database Syst Rev. (2013) 28(3):CD003808. doi: 10.1002/14651858.CD003808.pub3
109. Schwendicke F, Meyer-Lueckel H, Dorfer C, Paris S. Failure of incompletely excavated teeth--a systematic review. J Dent. (2013) 41(7):569–80. doi: 10.1016/j.jdent.2013.05.004
110. da Rosa WLO, Lima VP, Moraes RR, Piva E, da Silva AF. Is a calcium hydroxide liner necessary in the treatment of deep caries lesions? A systematic review and meta-analysis. Int Endod J. (2019) 52(5):588–603. doi: 10.1111/iej.13034
111. Schwendicke F, Tu YK, Hsu LY, Gostemeyer G. Antibacterial effects of cavity lining: a systematic review and network meta-analysis. J Dent. (2015) 43(11):1298–307. doi: 10.1016/j.jdent.2015.07.001
112. Murray PE, About I, Lumley PJ, Franquin JC, Remusat M, Smith AJ. Cavity remaining dentin thickness and pulpal activity. Am J Dent. (2002) 15(1):41–6.12074229
113. Stafuzza TC, Vitor LLR, Lourenco Neto N, Rios D, Cruvinel T, Sakai VT, et al. Pulp liner materials in selective caries removal: study protocol for a randomised controlled trial. BMJ Open. (2021) 11(1):e029612. doi: 10.1136/bmjopen-2019-029612
114. Society BE. A Guide to Good Endodontic Practice 2022 accessed on 14th Sept. 2022]. Available from: https://britishendodonticsociety.org.uk/_userfiles/pages/files/a4_bes_guidelines_2022_hyperlinked_final.pdf
115. Petrou MA, Alhamoui FA, Welk A, Altarabulsi MB, Alkilzy M, Splieth CH. A randomized clinical trial on the use of medical Portland cement, MTA and calcium hydroxide in indirect pulp treatment. Clin Oral Investig. (2014) 18(5):1383–9. doi: 10.1007/s00784-013-1107-z
116. Abarajithan M, Dham S, Velmurugan N, Valerian-Albuquerque D, Ballal S, Senthilkumar H. Comparison of Endovac irrigation system with conventional irrigation for removal of intracanal smear layer: an in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2011) 112(3):407–11. doi: 10.1016/j.tripleo.2011.02.024
117. Mente J, Hufnagel S, Leo M, Michel A, Gehrig H, Panagidis D, et al. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: long-term results. J Endod. (2014) 40(11):1746–51. doi: 10.1016/j.joen.2014.07.019
118. Leye Benoist F, Gaye Ndiaye F, Kane AW, Benoist HM, Farge P. Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (dycal((R))) in the formation of a dentine bridge: a randomised controlled trial. Int Dent J. (2012) 62(1):33–9. doi: 10.1111/j.1875-595X.2011.00084.x
119. Kundzina R, Stangvaltaite L, Eriksen HM, Kerosuo E. Capping carious exposures in adults: a randomized controlled trial investigating mineral trioxide aggregate versus calcium hydroxide. Int Endod J. (2017) 50(10):924–32. doi: 10.1111/iej.12719
120. Hilton TJ, Ferracane JL, Mancl L, Northwest Practice-based Research Collaborative in Evidence-based D. Comparison of CaOH with MTA for direct pulp capping: a PBRN randomized clinical trial. J Dent Res. (2013) 92(7 Suppl):16S–22S. doi: 10.1177/0022034513484336
121. Hashem D, Mannocci F, Patel S, Manoharan A, Watson TF, Banerjee A. Evaluation of the efficacy of calcium silicate vs. Glass ionomer cement indirect pulp capping and restoration assessment criteria: a randomised controlled clinical trial-2-year results. Clin Oral Investig. (2019) 23(4):1931–9. doi: 10.1007/s00784-018-2638-0
122. Grech L, Mallia B, Camilleri J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent Mater. (2013) 29(2):e20–8. doi: 10.1016/j.dental.2012.11.007
123. Kang TY, Choi JW, Seo KJ, Kim KM, Kwon JS. Physical, chemical, mechanical, and biological properties of four different commercial root-end filling materials: a comparative study. Materials (Basel). (2021) 14(7):1693. doi: 10.3390/ma14071693
124. Kaup M, Schafer E, Dammaschke T. An in vitro study of different material properties of Biodentine compared to ProRoot MTA. Head Face Med. (2015) 11:16. doi: 10.1186/s13005-015-0074-9
125. Koutroulis A, Kuehne SA, Cooper PR, Camilleri J. The role of calcium ion release on biocompatibility and antimicrobial properties of hydraulic cements. Sci Rep. (2019) 9(1):19019. doi: 10.1038/s41598-019-55288-3
126. Atmeh AR. Investigating the effect of bicarbonate ion on the structure and strength of calcium silicate-based dental restorative material-biodentine. Clin Oral Investig. (2020) 24(12):4597–606. doi: 10.1007/s00784-020-03328-y
127. Kang SH, Shin YS, Lee HS, Kim SO, Shin Y, Jung IY, et al. Color changes of teeth after treatment with various mineral trioxide aggregate-based materials: an ex vivo study. J Endod. (2015) 41(5):737–41. doi: 10.1016/j.joen.2015.01.019
128. Felman D, Parashos P. Coronal tooth discoloration and white mineral trioxide aggregate. J Endod. (2013) 39(4):484–7. doi: 10.1016/j.joen.2012.11.053
129. Keskin C, Demiryurek EO, Ozyurek T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J Endod. (2015) 41(3):409–11. doi: 10.1016/j.joen.2014.11.013
130. Valles M, Roig M, Duran-Sindreu F, Martinez S, Mercade M. Color stability of teeth restored with biodentine: a 6-month in vitro study. J Endod. (2015) 41(7):1157–60. doi: 10.1016/j.joen.2015.03.014
131. Al-Hiyasat AS, Ahmad DM, Khader YS. The effect of different calcium silicate-based pulp capping materials on tooth discoloration: an in vitro study. BMC Oral Health. (2021) 21(1):330. doi: 10.1186/s12903-021-01677-y
132. Taha NA, Ahmad MB, Ghanim A. Assessment of Mineral Trioxide Aggregate pulpotomy in mature permanent teeth with carious exposures. Int Endod J. (2017) 50(2):117–25. doi: 10.1111/iej.12605
133. Tomas-Catala CJ, Collado-Gonzalez M, Garcia-Bernal D, Onate-Sanchez RE, Forner L, Llena C, et al. Biocompatibility of new pulp-capping materials NeoMTA plus, MTA repair HP, and biodentine on human dental pulp stem cells. J Endod. (2018) 44(1):126–32. doi: 10.1016/j.joen.2017.07.017
134. Collado-Gonzalez M, Garcia-Bernal D, Onate-Sanchez RE, Ortolani-Seltenerich PS, Alvarez-Muro T, Lozano A, et al. Cytotoxicity and bioactivity of various pulpotomy materials on stem cells from human exfoliated primary teeth. Int Endod J. (2017) 50(Suppl 2):e19–e30. doi: 10.1111/iej.12751
135. Rodrigues NS, Franca CM, Tahayeri A, Ren Z, Saboia VPA, Smith AJ, et al. Biomaterial and biofilm interactions with the pulp-dentin Complex-on-a-chip. J Dent Res. (2021) 100(10):1136–43. doi: 10.1177/00220345211016429
136. Widbiller M, Lindner SR, Buchalla W, Eidt A, Hiller KA, Schmalz G, et al. Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clin Oral Investig. (2016) 20(2):237–46. doi: 10.1007/s00784-015-1515-3
137. Kang S. Mineralization-inducing potentials of calcium silicate-based pulp capping materials in human dental pulp cells. Yeungnam Univ J Med. (2020) 37(3):217–25. doi: 10.12701/yujm.2020.00248
138. Manaspon C, Jongwannasiri C, Chumprasert S, Sa-Ard-Iam N, Mahanonda R, Pavasant P, et al. Human dental pulp stem cell responses to different dental pulp capping materials. BMC Oral Health. (2021) 21(1):209. doi: 10.1186/s12903-021-01544-w
139. Paula A, Laranjo M, Marto CM, Abrantes AM, Casalta-Lopes J, Goncalves AC, et al. Biodentine() boosts, WhiteProRoot((R))MTA increases and life((R)) suppresses odontoblast activity. Materials (Basel). (2019) 12(7):1184. doi: 10.3390/ma12071184
140. Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, et al. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res. (2012) 91(12):1166–71. doi: 10.1177/0022034512460833
141. Pedano MS, Li X, Camargo B, Hauben E, De Vleeschauwer S, Yoshihara K, et al. Injectable phosphopullulan-functionalized calcium-silicate cement for pulp-tissue engineering: an in-vivo and ex-vivo study. Dent Mater. (2020) 36(4):512–26. doi: 10.1016/j.dental.2020.01.011
142. Amin LE, Montaser M. Comparative evaluation of pulpal repair after direct pulp capping using stem cell therapy and biodentine: an animal study. Aust Endod J. (2021) 47(1):11–9. doi: 10.1111/aej.12463
143. Atmeh AR, Chong EZ, Richard G, Festy F, Watson TF. Dentin-cement interfacial interaction: calcium silicates and polyalkenoates. J Dent Res. (2012) 91(5):454–9. doi: 10.1177/0022034512443068
144. Li X, Pongprueksa P, Van Landuyt K, Chen Z, Pedano M, Van Meerbeek B, et al. Correlative micro-Raman/EPMA analysis of the hydraulic calcium silicate cement interface with dentin. Clin Oral Investig. (2016) 20(7):1663–73. doi: 10.1007/s00784-015-1650-x
145. Sajini S, Atmeh AR, Banerjee A, Festy F, Cook RJ, Andiappan M, et al. Glass-ionomer and calcium silicate-based cements interactions with human dentine in health and disease: two-photon fluorescence microscopy and Raman spectroscopy analysis. Dent Mater. (2022) 38(11):1710–20. doi: 10.1016/j.dental.2022.09.001
146. Maltz M, Henz SL, de Oliveira EF, Jardim JJ. Conventional caries removal and sealed caries in permanent teeth: a microbiological evaluation. J Dent. (2012) 40(9):776–82. doi: 10.1016/j.jdent.2012.05.011
147. Ali AH, Koller G, Foschi F, Andiappan M, Bruce KD, Banerjee A, et al. Self-limiting versus conventional caries removal: a randomized clinical trial. J Dent Res. (2018) 97(11):1207–13. doi: 10.1177/0022034518769255
148. Camilleri J. Scanning electron microscopic evaluation of the material interface of adjacent layers of dental materials. Dent Mater. (2011) 27(9):870–8. doi: 10.1016/j.dental.2011.04.013
149. Hashem DF, Foxton R, Manoharan A, Watson TF, Banerjee A. The physical characteristics of resin composite-calcium silicate interface as part of a layered/laminate adhesive restoration. Dent Mater. (2014) 30(3):343–9. doi: 10.1016/j.dental.2013.12.010
150. Camilleri J. Investigation of Biodentine as dentine replacement material. J Dent. (2013) 41(7):600–10. doi: 10.1016/j.jdent.2013.05.003
151. Meraji N, Camilleri J. Bonding over dentin replacement materials. J Endod. (2017) 43(8):1343–9. doi: 10.1016/j.joen.2017.03.025
152. Nekoofar MH, Motevasselian F, Mirzaei M, Yassini E, Pouyanfar H, Dummer PM. The micro-shear bond strength of various resinous restorative materials to aged biodentine. Iran Endod J. (2018) 13(3):356–61. doi: 10.22037/iej.v13i3.20880
153. Colak H, Tokay U, Uzgur R, Uzgur Z, Ercan E, Hamidi MM. The effect of different adhesives and setting times on bond strength between biodentine and composite. J Appl Biomater Funct Mater. (2016) 14(2):e217–22. doi: 10.5301/jabfm.5000266
154. Shafiei F, Doozandeh M, Gharibpour F, Adl A. Effect of reducing acid-etching duration time on compressive strength and bonding of a universal adhesive to calcium silicate cements. Int Endod J. (2019) 52(4):530–9. doi: 10.1111/iej.13026
155. Palma PJ, Marques JA, Falacho RI, Vinagre A, Santos JM, Ramos JC. Does delayed restoration improve shear bond strength of different restorative protocols to calcium silicate-based cements? Materials (Basel). (2018) 11(11):2216. doi: 10.3390/ma11112216
156. Sultana N, Nawal RR, Chaudhry S, Sivakumar M, Talwar S. Effect of acid etching on the micro-shear bond strength of resin composite-calcium silicate interface evaluated over different time intervals of bond aging. J Conserv Dent. (2018) 21(2):194–7. doi: 10.4103/JCD.JCD_167_17
157. Schmidt A, Schafer E, Dammaschke T. Shear bond strength of lining materials to calcium-silicate cements at different time intervals. J Adhes Dent. (2017) 19(2):129–35. doi: 10.3290/j.jad.a38100
158. Cantekin K, Avci S. Evaluation of shear bond strength of two resin-based composites and glass ionomer cement to pure tricalcium silicate-based cement (Biodentine(R)). J Appl Oral Sci. (2014) 22(4):302–6. doi: 10.1590/1678-775720130660
159. Xavier MT, Costa AL, Caramelo FJ, Palma PJ, Ramos JC. Evaluation of the interfaces between restorative and regenerative biomaterials used in vital pulp therapy. Materials (Basel). (2021) 14(17):5055. doi: 10.3390/ma14175055
160. Cengiz E, Ulusoy N. Microshear bond strength of tri-calcium silicate-based cements to different restorative materials. J Adhes Dent. (2016) 18(3):231–7. doi: 10.3290/j.jad.a35934
161. Tulumbaci F, Almaz ME, Arikan V, Mutluay MS. Shear bond strength of different restorative materials to mineral trioxide aggregate and Biodentine. J Conserv Dent. (2017) 20(5):292–6. doi: 10.4103/JCD.JCD_97_17
162. Costa CA, Hebling J, Hanks CT. Current status of pulp capping with dentin adhesive systems: a review. Dent Mater. (2000) 16(3):188–97. doi: 10.1016/S0109-5641(00)00008-7
163. Foxton RM, Melo L, Stone DG, Pilecki P, Sherriff M, Watson TF. Long-term durability of one-step adhesive-composite systems to enamel and dentin. Oper Dent. (2008) 33(6):651–7. doi: 10.2341/07-166
164. Takahashi A, Inoue S, Kawamoto C, Ominato R, Tanaka T, Sato Y, et al. In vivo long-term durability of the bond to dentin using two adhesive systems. J Adhes Dent. (2002) 4(2):151–9.12236644
165. Haak R, Hahnel M, Schneider H, Rosolowski M, Park KJ, Ziebolz D, et al. Clinical and OCT outcomes of a universal adhesive in a randomized clinical trial after 12 months. J Dent. (2019) 90:103200. doi: 10.1016/j.jdent.2019.103200
166. Doi J, Itota T, Yoshiyama M, Tay FR, Pashley DH. Bonding to root caries by a self-etching adhesive system containing MDPB. Am J Dent. (2004) 17(2):89–93.15151333
167. Yoshiyama M, Tay FR, Torii Y, Nishitani Y, Doi J, Itou K, et al. Resin adhesion to carious dentin. Am J Dent. (2003) 16(1):47–52.12744413
168. Cooper PR, Holder MJ, Smith AJ. Inflammation and regeneration in the dentin-pulp complex: a double-edged sword. J Endod. (2014) 40(4 Suppl):S46–S51. doi: 10.1016/j.joen.2014.01.021
169. Goldberg M, Njeh A, Uzunoglu E. Is pulp inflammation a prerequisite for pulp healing and regeneration? Mediators Inflamm. (2015) 2015:347649. doi: 10.1155/2015/347649
170. Cooper PR, Smith AJ. Molecular mediators of pulp inflammation and regeneration. Endod Topics. (2013) 28(1):90–105. doi: 10.1111/etp.12036
171. Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S, Smith AJ. Inflammation-regeneration interplay in the dentine-pulp complex. J Dent. (2010) 38(9):687–97. doi: 10.1016/j.jdent.2010.05.016
Keywords: vital pulp therapy, indirect pulp capping, materials choice, deep caries, caries-affected dentine (CAD), caries removal, tooth restoration, guidelines
Citation: Al-Ali M and Camilleri J (2022) The scientific management of deep carious lesions in vital teeth using contemporary materials—a narrative review. Front. Dent. Med 3:1048137. doi: 10.3389/fdmed.2022.1048137
Received: 19 September 2022; Accepted: 4 November 2022;
Published: 24 November 2022.
Edited by:
Vesna Miletic, The University of Sydney, AustraliaReviewed by:
Lars Bjørndal, University of Copenhagen, DenmarkPaulo Jorge Palma, University of Coimbra, Portugal
Copyright: © 2022 Al-Ali and Camilleri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Camilleri, ai5jYW1pbGxlcmlAYmhhbS5hYy51aw==
Specialty Section: This article was submitted to Dental Materials, a section of the journal Frontiers in Dental Medicine
 M. Al-Ali
M. Al-Ali J. Camilleri
J. Camilleri