- 1Faculty of Dentistry, Istanbul University, Istanbul, Turkey
- 2School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, United States
Certain patients, despite receiving proper treatment, still show higher failure rates of restorative dental treatments. The aim of this work was to test if MMP2 and MMP3 alleles are overrepresented in individuals with secondary caries. A total of 1,089 individuals from the University of Pittsburgh School of Dental Medicine Dental Registry and DNA Repository project were selected for this study. From this total, 341 individuals were selected for having a record of secondary caries in any type of restoration and were matched with 748 individuals by sex, age, ethnicity, and restorative work in the same teeth that did not fail. Genomic DNA extracted from saliva was used to obtain genotypes in five markers of MMP2 and MMP3 using TaqMan chemistry and end-point analysis. Chi-square was used to test if differences in allele and genotype distributions were statistically different at an alpha of 0.05. The less common allele and homozygote genotype of MMP2 rs9923304 were less commonly found among individuals with secondary caries. The less common allele of MMP2 rs2287074 was also less frequent among individuals with secondary caries. These results provide statistical evidence for the role of MMP2 in failure of restorations due to secondary caries. We can conclude that MMP2 variation impacts the risk of having secondary caries, independent of the restorative material.
Introduction
Dental caries, which is one of the most common diseases in both developing and industrialized countries, has a multifactorial etiology as well as being a chronic and complex disease (1). In a recent systematic analysis evaluating 354 different diseases and injuries, it was reported that 3.5 billion people were affected by oral diseases and the most common of these diseases was caries in permanent teeth. Dental caries in the permanent dentition affected 2.3 billion people globally. Based on these data, it was determined that untreated dental caries was the most common health condition (2).
Secondary caries is a major clinical problem defined as a carious lesion seen on the margins of the existing restoration or caries associated with sealants (3), and progress to the dentin and pulp. This form of caries has been reported in the literature as the most common reason for the replacement of many types of restorations, from the fixed partial prosthesis to fillings, as it alters the dental hard tissue, endangers the lifetime of the tooth, and reduces the longevity of the restoration (4, 5). Since the incidence of dental caries continues to be high among certain groups due to high sugar consumption and change of living conditions, there is a need to determine markers that will help identify individuals with a higher risk of caries (6). For this purpose, genetic studies have been performed in dental caries, as it has been observed that human genetic polymorphisms play a role in the susceptibility of the host to disease (7). Particularly for secondary caries, our data showed that individuals with asthma, high blood pressure, and diabetes were more likely to have secondary caries (8).
One of the genetic factors we identified that appears to impact the progression of a caries lesion into the dentin are members of the matrix metalloproteinases (MMPs) family. We showed that variants in MMP2 and MMP3 were associated of having a deep caries lesion that involved a periapical lesion formation (9). MMP2 is a gelatinase involved in mineralization and dentin degradation, and catalyzes dentine matrix degradation after demineralization (10). MMP3, also called proteoglycanase or stromelysin-1, participates in the mineralization of predentin and is detected in dentin in connection with the odontoblast process. This MMP type also contributes to the disorganization and degradation of the demineralized dentin matrix and the adhesive properties of restorative resins (11, 12). The same MMP2 genetic variant that was associated with deep caries lesions in dentin with periapical lesions was also associated with loss of extensive composite restorations not due to secondary caries (13). Therefore, a caries lesion that begins on unsealed margins of faulty restorations and sealants and progress to the dentin may progress quicker or easier in individuals that have MMP2 expressed differently due to genomic variants.
The hypothesis of the study was that individuals that express variant alleles may have lower MMP activity and secondary caries may develop in a slower pace. On the other hand, individuals with full MMP potential activity due to having wild type alleles may have secondary caries progressing quicker. We tested for statistical evidence of over-representation of alleles of MMP2 and MMP3 in individuals with secondary caries independent of the restorative material. The underlying hypothesis was that genetic variation in these MMP genes will affect the occurrence of secondary caries lesions in treated patients. Carriers of MMP2 or MMP3 fewer common alleles were expected to have secondary caries lesions less frequently. The aim of this study was to test for over-representation of alleles in MMP2 and MMP3 markers to identify statistical evidence that these genes may influence longevity of dental restorative treatments.
Method
Study Population
Participants were recruited through the Dental Registry and DNA Repository project at the University of Pittsburgh School of Dental Medicine. Starting in September of 2006, all individuals that seek treatment at the University of Pittsburgh School of Dental Medicine have been invited to be part of the registry.
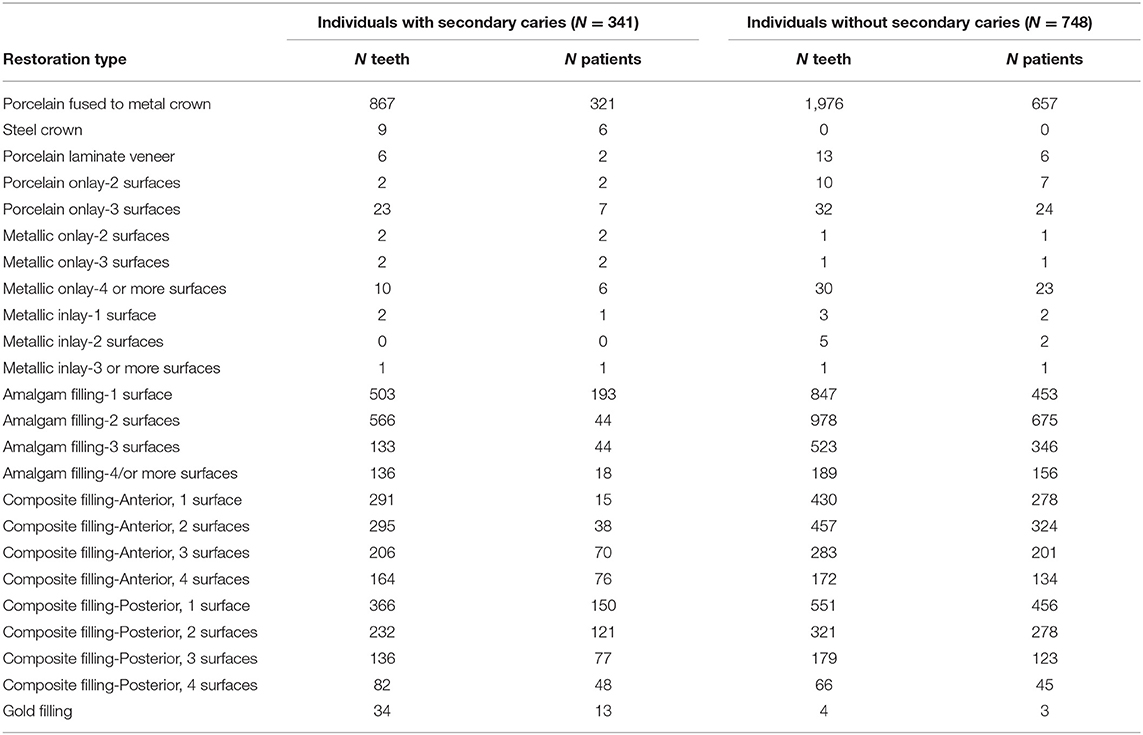
The provisions of the Declaration of Helsinki and US Federal Policy for the Protection of Human Subjects have been followed during the present study, which was approved by the University of Pittsburgh Institutional Review Board (IRB) under the protocol # CR19050020-002. All subjects gave written informed consent to participate in this study after a full explanation of the procedures of the study and admitted to providing a saliva sample as a source of genomic DNA. The sample was typically collected at the first visit, before any necessary dental treatment was performed. From 6,414 records of participants in the Dental Registry and DNA Repository project, 341 individuals 18–75 years of age with restored teeth that had an indication of presence of secondary caries were selected (these are determined by careful clinical evaluation –orted by radiographic evidence). Types of restorations included crowns, onlays, inlays, laminate veneers, amalgam, or composite fillings (Table 1). For comparison, a group of 748 individuals the same age (+/- 5 years), sex, and ethnicity with restorations of the same type but without secondary caries in the same teeth, when possible, were selected; at least two individuals were aimed for each case. Power estimates (14) suggested that this sample would provide 80% power for an alpha of 0.05 if effect size of heterozygotes was 1.5 and 3.0 for homozygotes, risk allele frequency of 0.15 and D' 1.0.
Selection of Single Nucleotide Polymorphisms
Based on results of our previous studies (9, 13) that suggested associations with phenotypes involving dentin exposure, and locations within the genes, five polymorphisms were selected marking MMP2 and MMP3 (Table 2). These SNPs were originally chosen based on their probability of having functional outcomes as tag-SNPs that were representative of the two genes. The markers were considered informative (frequency higher than 10%) and were well-characterized and recorded in public databases.
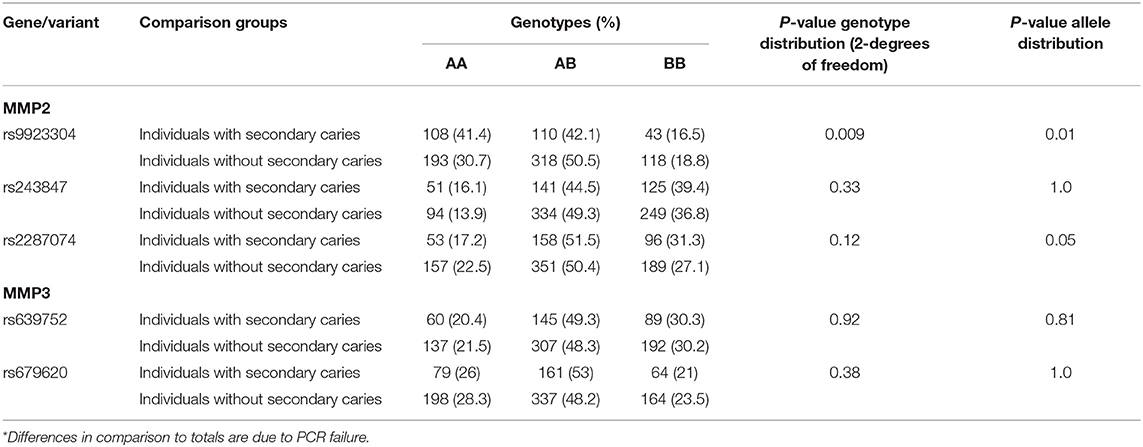
Table 2. Genotypes* and summary results of association tests between secondary caries and MMP2/MMP3.
Genotyping
Genomic DNA extraction from saliva samples was performed using PureLink™ Genomic DNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA), following the protocols of the manufacturer. Genomic DNA extraction from saliva samples was performed using the following protocol. Samples were brought to room temperature and centrifuged for 5 min at 10,000 × g. After the supernatant was removed 1 ml of extraction buffer [10 mM of hydroxymethyl aminomethane hydrochloride pH 7.8, 5 mM of ethylenediaminetetraacetic acid (EDTA), 0.5% sodium dodecyl sulfate] was added to the buccal cell pellet and thoroughly mixed. Five μl of proteinase K was added and the samples were incubated in a 56°C water bath overnight. After removal, the samples were vortexed and 500 μl of 10 M ammonium acetate was added. Samples were inverted for 3 min and centrifuged at 21,000 × g for 15 min. The supernatant was then transferred to two eppendorfs with an equal volume of cold isopropanol and shaken vigorously. Samples were then incubated at −20°C for a minimum of 30 min, after which they were centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was poured off and 1 ml of cold 70% of ethanol alcohol was added and samples were inverted 3 times. Samples were then centrifuged at 10,000 × g for 5 min at 4°C. The supernatant was poured off and samples were allowed to air dry before 100 μl of Tris-EDTA buffer was added. Samples were kept in a 4°C refrigerator for 2–3 days before ensuring the DNA was completely dissolved and reading the concentration of the samples. The extracted DNA was preserved at −20°C for further analysis.
The quality assessment and concentrations of the genomic DNA were conducted with NanoDrop 2,000 (Thermo Fisher Scientific, USA) spectrophotometer. A 260/A280 curve was applied to determine the purity of DNA.
Taqman chemistry was used for the generation of the genotypes (15). Reactions were performed in 3 μl volumes in a QuantStudio 6 Flex automatic instrument and pre-designed assays (Applied Biosystems, Foster City, CA, USA), and reagents of the same system were used. Overrepresentation of genotypes and alleles were tested using chi-square and alpha of 0.05. The distribution of genotypes (AA, AB, and BB) between the two groups were compared, as well as the number of alleles (A and B). Genotypic distributions were also tested for Hardy-Weinberg equilibrium using Pearson's chi-square test (two-tailed) based on the classical formula (p2 + 2pq + q2 = 1).
Results
Genotype distributions did not deviate from Hardy-Weinberg equilibrium, with the frequency of each genotype within the expected range based on the formula p2 + 2pq + q2 = 1 (data not shown). The less common allele and homozygotes genotype of MMP2 rs9923304 were less common among individuals with secondary caries. The less common allele of MMP2 rs2287074 was also less frequent among individuals with secondary caries (Table 2).
Discussion
Dental caries progresses differently into dentin for different people. In some cases, lesions may be devastating to the point that teeth cannot be saved, due to extreme loss of structure. In others, however, the lesions stay confined to dentin without affecting the pulp. Evidence suggests that despite the same environmental conditions and the same time, not everyone will have the same fate regarding dental caries lesions progression (16). One approach that can be used to help elucidate the reasons why these differences occur is to test for evidence of over-representation of alleles, which would suggest a genetic basis for the differences in outcome seen in the clinics. In this study, we expected to see that people without variant alleles of MMP2 or MMP3 markers were more likely to have secondary caries since MMP activity was expected to be in its full potential. The presence of variant alleles was expected to imply that MMP activity was slightly decreased and these carriers were less likely to have secondary caries lesions. The rationale of these assumptions is based on the idea that more hydrolytic degradation of dentin by MMPs activated when pH is lower and expression of MMP2 and MMP3 is at their full potential. The presence of genetic variants (hypomorphic alleles) would decrease MMP activity making it less likely that these carriers of less common alleles to show secondary caries lesions.
We originally reported associations between MMP2 and MMP3, and individuals with deep caries lesions in dentin that were accompanied by periapical pathology (9). The MMP2 marker rs9923304, that was associated with periapical pathology of teeth with deep carious lesions in the original work, was also associated with failure of extensive composite restorations that were not due to secondary caries (13). In this report, we showed that markers in MMP2, including rs9923304 and rs2287074, were also associated with secondary caries, independent of the type of restoration. These data, in sum, provide evidence that MMP2 contributes to failed outcomes resulting from restorative treatment and/or pulp involvement. The association of these two particular MMP2 variants, rs9923304 and rs2287074, is due to the increased number of wild type homozygotes among individuals with failed restorations. Since it is expected that MMP2 acts by degrading components of the extracellular matrix and non-matrix proteins (mainly type IV collagen), the result fits perfectly with our hypothesis that individuals that show a higher MMP2 activity may be prone to more failures of extensive restorations. These individuals with higher MMP2 activity would carry zero copies of the variant allele, and individuals carrying zero copies are presumed to have less MMP2 activity and be less prone to secondary caries. It is possible that these markers are the ones providing direct information on MMP2 activity and could be considered as genomic markers for future clinical management.
The pattern of MMP2 expression in dentin suggests that treating the dentin to minimize the effects of metalloproteinases action may be useful. Determining an individual's MMP2 rs9923304 variant genomic status may be a useful tool in proactively implementing dentin crosslinking to prevent restoration failure. Future clinical research should be designed to incorporate genomic analysis in the evaluation of dentin crosslinking outcomes to determine the profiles of patients who will benefit most from these approaches. Dental practitioners would find value in understanding the genetic contribution to dental caries for at least two reasons: First, they would be able to explain to patients that some forms of decay are more strongly associated with inherited risk. This would help explain for both the patient and dentist why persons with similar behavioral risks (e.g., tooth brushing frequency or dietary habits) have different caries rates, thus avoiding the “blaming the victim” that sometimes accompanies preventive health messages in dentistry. Second, future technological developments may make it possible to someday identify patients who are genetically at higher risk for caries. These patients then could be monitored more closely and provided with more aggressive prevention programs (16).
We and others (7, 17) have shown that genes involved in enamel formation (tuftelin, amelogenin, enamelin, kallikrein 4, ameloblastin, MMP20) are associated with dental caries. Although most of the existing data are on primary lesions or models of dental caries initiation in enamel, it is not possible to exclude the potential effect of these genes in the occurrence of secondary caries lesions.
The use of a registry and existing data may be seen as a limitation, since no reassessments of the cases studied could be proposed and possible recording errors may exist. Information used was recorded by different professionals over the years and analysis per type of restoration was not performed.
In conclusion, MMP2 rs9923304 and rs2287074 are associated with failure of dental restorative treatments and have the potential to be used for determination of risks impacting longevity of dental treatments.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Pittsburgh Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LF and MB generated data extraction and organization. KD processed samples and supported recruitment. AM supported recruitment and obtaining data. AV designed the project, created all infra-structure, supervised all aspects of the project, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Data for this project was obtained from the Dental Registry and DNA Repository project, which is supported by the University of Pittsburgh School of Dental Medicine. MB was supported by the Fulbright Postdoctoral program (FY-2020-TR-PD-02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2021.735535/full#supplementary-material
References
1. United Nations General Assembly. Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Noncommunicable Diseases. Resolution A_66_L-1. New York, NY: Unted Nations (2011).
2. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
3. Askar H, Krois J, Göstemeyer G, Bottenberg P, Zero D, Banerjee A, et al. Secondary caries: what is it, and how it can be controlled, detected, and managed? Clin Oral Invest. (2020) 24:1869–76. doi: 10.1007/s00784-020-03268-7
4. Zoellner A, Brägger U, Fellmann V, Gaengler P. Correlation between clinical scoring of secondary caries at crown margins and histologically assessed extent of the lesions. Int J Prosthodont. (2000) 13:453–9.
5. Arnold WH, Sonkol T, Zoellner A, Gaengler P. Comparative study of in vitro caries-like lesions and natural caries lesions at crown margins. J Prosthodont. (2007) 16:445–51. doi: 10.1111/j.1532-849X.2007.00220.x
6. Weber M, Bogstad Søvik J, Mulic A, Deeley K, Tveit AB, Forella J, et al. Redefining the phenotype of dental caries. Caries Res. (2018) 52:263–71. doi: 10.1159/000481414
7. Vieira AR, Modesto A, Marazita ML. Caries: review of human genetics research. Caries Res. (2014) 48:491–506. doi: 10.1159/000358333
8. Johnston L, Vieira AR. Caries experience and overall health status. Oral Health Prev Dent. (2014) 12:163–70. doi: 10.3290/j.ohpd.a31670
9. Menezes-Silva R, Khaliq S, Deeley K, Letra A, Vieira AR. Genetic susceptibility to periapical disease: conditional contribution of MMP2 and MMP3 genes to the development of periapical lesions and healing response. J Endod. (2012) 38:604–7. doi: 10.1016/j.joen.2012.02.009
10. Niu LN, Zhang L, Jiao K, Li F, Ding YX, Wang DY, et al. Localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in human coronal dentine. J Dent. (2011) 39:536–42. doi: 10.1016/j.jdent.2011.05.004
11. Boukpessi T, Menashi S, Camoin L, TenCate JM, Goldberg M, Chaussain-Miller C. The effect of stromelysin-1 (MMP-3) on non-collagenous extracellular matrix proteins of demineralized dentin and the adhesive properties of restorative resins. Biomaterials. (2008) 29:4367–73. doi: 10.1016/j.biomaterials.2008.07.035
12. Mazzoni A, Papa V, Nato F, Carrilho M, Tjäderhane L, Ruggeri Jr. A, et al. Immunohistochemical and biochemical assay of MMP-3 in human dentine. J Dent. (2011) 39:231–7. doi: 10.1016/j.jdent.2011.01.001
13. Vieira AR, Silva MB, Souza KKA, Filho AVA, Rosenblatt A, Modesto A. A pragmatic study shows failure of dental composite fillings is genetically determined: a contribution to the discussion on dental amalgams. Front Med. (2017) 4:186. doi: 10.3389/fmed.2017.00186
14. Purcell S, Cherny SS, Sham PC. Genetic power calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. (2003) 19:149–50. doi: 10.1093/bioinformatics/19.1.149
15. Ranade K, Chang MS, Ting CT, Pei D, Hsiao CF, Olivier M, et al. High-throughput genotyping with single nucleotide polymorphisms. Genome Res. (2001) 11:1262–8. doi: 10.1101/gr.157801
16. Leonardi DP, Vieira AR. From caries progression and restoration failures to periapical lesions in the era of precision. JDR Clin Trans Res. (2020) 5:10–2. doi: 10.1177/2380084419846436
Keywords: dental caries, dental materials, composite resin, amalgam, porcelain
Citation: Benli M, Frota de Souza LA, Deeley K, Modesto A and Vieira AR (2021) Matrix Metalloproteinase 2 Is Associated With Secondary Caries Independent From the Restorative Material. Front. Dent. Med. 2:735535. doi: 10.3389/fdmed.2021.735535
Received: 02 July 2021; Accepted: 09 September 2021;
Published: 06 October 2021.
Edited by:
Ziad N. Al-Dwairi, Jordan University of Science and Technology, JordanReviewed by:
Lidiany Karla Azevedo Rodrigues, Federal University of Ceara, BrazilSatyawan Gangaramji Damle, Maharishi Markandeshwar University, India
Copyright © 2021 Benli, Frota de Souza, Deeley, Modesto and Vieira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandre R. Vieira, YXJ2MTFAcGl0dC5lZHU=
†These authors have contributed equally to this work
 Merve Benli
Merve Benli Luiza Arieta Frota de Souza2†
Luiza Arieta Frota de Souza2† Adriana Modesto
Adriana Modesto Alexandre R. Vieira
Alexandre R. Vieira