- 1School of Dentistry, University of Michigan, Ann Arbor, MI, United States
- 2Centre for Oral Health Outcomes and Research Translation (COHORT), Western Sydney University, Liverpool, NSW, Australia
- 3Drug Health Services, South Western Sydney Local Health District (SWSLHD), Cabramatta, NSW, Australia
This Perspective provides a brief summary of the scientific evidence for the often two-way links between hyperglycemia, including manifest diabetes mellitus (DM), and oral health. It delivers in a nutshell examples of current scientific evidence for the following oral manifestations of hyperglycemia, along with any available evidence for effect in the opposite direction: periodontal diseases, caries/periapical periodontitis, tooth loss, peri-implantitis, dry mouth (xerostomia/hyposalivation), dysbiosis in the oral microbiome, candidiasis, taste disturbances, burning mouth syndrome, cancer, traumatic ulcers, infections of oral wounds, delayed wound healing, melanin pigmentation, fissured tongue, benign migratory glossitis (geographic tongue), temporomandibular disorders, and osteonecrosis of the jaw. Evidence for effects on quality of life will also be reported. This condensed overview delivers the rationale and sets the stage for the urgent need for delivery of oral and general health care in patient-centered transdisciplinary collaboration for early detection and management of both hyperglycemia and oral diseases to improve quality of life.
Introduction
Regrettably, dentistry was separated from general health care and became an independent profession (1), leaving little education and awareness regarding oral health and its links to general health among the other health professions (2–10).
The most prevalent chronic diseases share the same “common risk factors” (11–13) (Figure 1) and hence often occur in the same patients, regardless of whether causal links and not merely associations exist. Nonetheless, rapidly emerging scientific evidence demonstrates that oral diseases and hyperglycemia (elevated blood glucose concentration), including manifest diabetes mellitus (DM), independently and mutually affect each other.
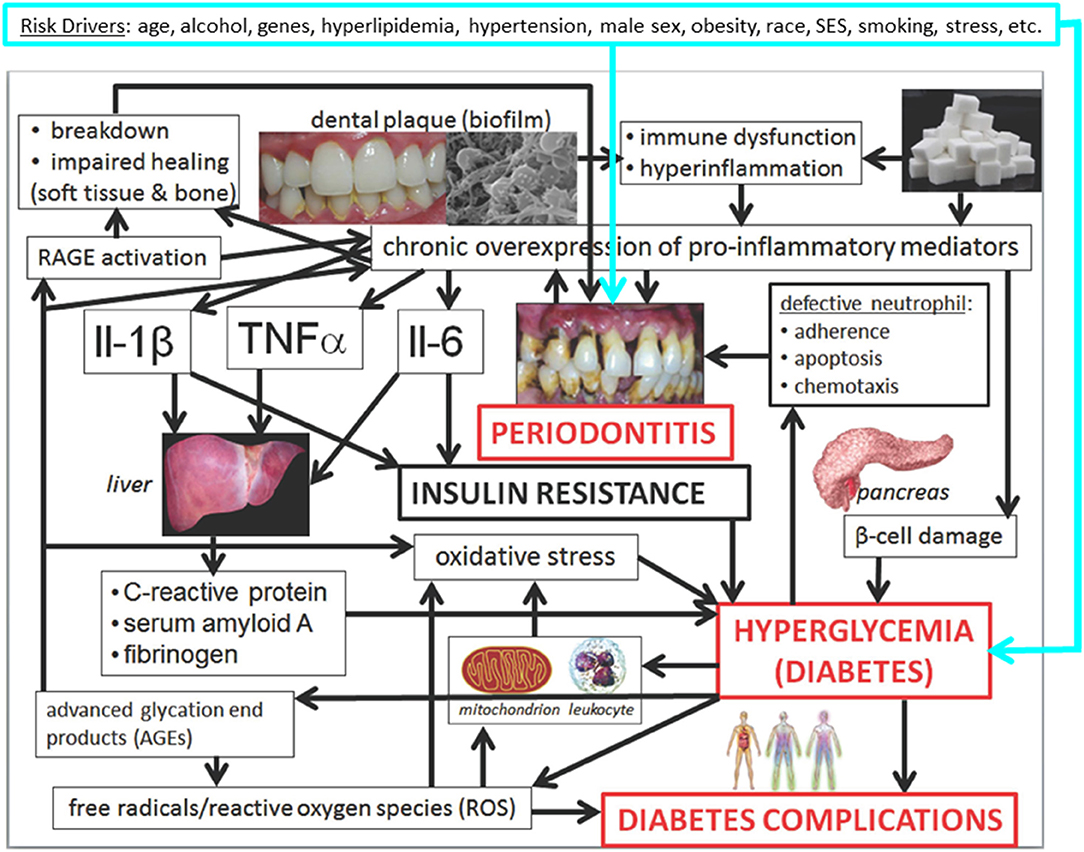
Figure 1. Mechanisms underlying the associations between periodontitis and hyperglycemia/diabetes and its complications: conceptual model (14). Slightly modified Figure 31.10 in Borgnakke et al. (14). Please see the full description and accreditations at https://www.ncbi.nlm.nih.gov/books/NBK567985/.
The term “transdisciplinary” is used to include practitioners of all health care disciplines, such as physicians, assistants of the physician, nurses, nurse practitioners, midwives, dietitians, DM educators, speech therapists, social workers, etc., in contrast to “interprofessional” that implicitly accepts the siloed approach regarding dentistry and general health care as separate professions. This research summarizes, in a nutshell, the current evidence for links between oral diseases and DM to support the need for transdisciplinary collaboration.
Hyperglycemia/Dm and Oral Health Mutually Affect Each Other
While type 1 DM (T1DM) is due to no or insufficient insulin production affecting about 5% of patients with DM, type 2 DM (T2DM) is a syndrome characterized by elevated blood glucose levels due to insufficient insulin production, insufficient insulin uptake, or both (15–17). About 463 million (9.3%) adults suffer from DM, with 700 million (10.9%) expected by the year 2045 (18, 19). An additional 374 million people have prediabetes (preDM) and are at risk of developing T2DM (18).
Systemic hyperglycemia causes complications such as retinopathy; nephropathy; neuropathy; heart, peripheral arterial, and cerebrovascular disease; obesity, cataracts; erectile dysfunction; and non-alcoholic fatty liver disease (20). Regardless of DM type, it is the hyperglycemia, not the diagnosis of DM per se, that leads to several oral complications (21) and oral health-related decreased quality of life (QoL) (22).
These oral manifestations are described, followed by any effects in the opposite direction.
For succinct brevity, DM is used for any type of diabetes or hyperglycemia; and the comparison group is non-traditionally omitted. For example, in the sentence “People with DM have greater xxxx,” the comparison “than people without DM” is implicit, but not shown.
Periodontal Diseases
Periodontal diseases affect up to 90% of adults globally, with the reversible form, gingivitis, affecting almost everybody (23). In contrast, periodontitis is chronic, irreversible destruction of soft and hard tissues around the teeth that results from an interplay between polymicrobial dysbiosis in the plaque microbiome in the gingival sulcus and the especially susceptible host (24, 25). Periodontitis affects 42.2% of US dentate adults (26), likely varies globally, and is the 12th of 291 most prevalent diseases worldwide (27), with “severe” periodontitis being the sixth most prevalent disease (28), affecting 11.2% of adults (28).
Periodontitis and hyperglycemia share the same risk factors (29, 30) and hence often occur in the same individuals with compromised immune systems or exhibiting hyperinflammatory responses; and they additionally adversely affect each other.
A much greater proportion of people with DM suffer from periodontitis (31–33), and the severity of periodontitis is much greater, especially in poorly or uncontrolled DM (31, 34, 35). Citing clinical studies from Denmark (36), Australia (37), Finland (38), Argentina (39), and the US (40, 41), including among the Pima Indians in Arizona (42–46), periodontitis was declared the sixth complication of DM in 1993 (47), but with negligible effect on the medical and dental communities.
In the opposite direction, people with periodontitis are much more likely to have T2DM (33, 48). Periodontitis, via bacteremia (49, 50) and inflammatory responses of which hyperglycemia is a normal part (Figure 1), is a risk factor for DM, that is, incident T2DM, gestational DM, poorer glycemic control in existing DM, and more severe DM complications (51, 52). Furthermore, periodontitis is increasingly regarded as an independent risk factor for the macro-vascular DM complications cardiovascular disease (CVD) (53–58) and ischemic stroke (54, 56), and is associated with the microvascular DM complications: neuropathy (54, 59), nephropathy (54, 60–62), and retinopathy (54, 60).
Cognitive Impairment in DM
Alzheimer's disease has been named “type 3 DM” (63) as a DM complication (64) partly due to glucose hypometabolism causing cognitive decline (65). Novel research supports the role of especially Porphyromonas gingivalis (Pg), a key periodontitis-associated bacterium, in Alzheimer's disease (66–72), with promising experimental treatment with gingipain inhibitors to reduce Pg brain colonization and neurodegeneration reported (73).
COVID-19
With DM being a risk factor for COVID-19, oral manifestations of the SARS-CoV-2 virus (74, 75) are reported in DM, such as painful ulcers (76, 77) and necrotizing periodontitis (78).
In COVID-19, a radiographic study found periodontitis to be associated with more intensive unit admissions, increased ventilation needs, and mortality in COVID-19 (79), and gingivitis is also reported (80). This is probably the case also in DM with its weakened immune system. Likely, periodontal pockets (81, 82), gingival crevicular fluid (83), and saliva acting as reservoirs for SARS-CoV-2 virus (81, 84–86) may even facilitate COVID-19 development (87), persistence (88), and mortality especially in people with DM (89).
Caries/Periapical Periodontitis
Untreated caries in permanent teeth is the most prevalent condition of the world affecting 2.4+ billion people (90). The evidence for links to DM is mixed, although adolescents with DM have 2- and 3-fold greater numbers of filled teeth and teeth with untreated caries, respectively (91). Patients with DM receiving hemodialysis have more caries (92); and periapical infections and their abscesses seem to be more prevalent in DM (93, 94).
Tooth Loss
Worldwide, people with DM have lost many more teeth (36, 37, 95–101), [about twice the magnitude (102)], especially if uncontrolled (103), and at an earlier age (96).
Tooth loss is a risk factor for hyperglycemia that is not usually mentioned as such, even though this has possibly the greatest immediate importance for DM management. Having loose teeth (due to periodontitis), sensitive teeth (due to deep caries lesions), few teeth left (104), or removable dentures will automatically cause problems with mastication, resulting in people not being able to eat crisp foods that need biting off or proper mastication. That is, such people are simply unable to follow the recommendations for a proper diet intended for controlling their DM by consuming appropriate amounts and kinds of healthy nutrients (105–107). In contrast, they resort to soft, processed food items with high Glycemic Index scores (108) and high Dietary Inflammatory Index scores (105, 106), typically laden with fat, sugar, and salt but deficient in fibers and vitamins (109), as opposed to fresh vegetables and fruit and whole-grain products. Alone for this reason, sincere efforts to prevent tooth loss should be invested in transdisciplinary DM management including dietitians (109).
Missing teeth are also associated with DM complications, such as myocardial infarction (110, 111) and retinopathy (36, 112); and lack of proper mastication negatively impacts cognitive function (113, 114).
Peri-Implantitis
Even though dental implants can osseointegrate, albeit delayed (115), and survive in patients with poorly controlled DM (116), hyperglycemia is a risk factor for peri-implantitis (breakdown of peri-implant soft and hard tissues) that is independent of smoking (117–119).
Dry Mouth
Individuals with DM often suffer from dry mouth, meaning xerostomia (subjective feeling of mouth dryness) or hyposalivation (decreased salivary production), decreasing QoL. Patients with DM often suffer from bad breath (halitosis), foul taste, and multimorbidity with polypharmacy (22, 32, 120). All major groups of pharmaceuticals can cause mouth dryness and various periodontal complications (121). Hyposalivation also majorly impacts the oral microbiome composition (122).
Hyposalivation can lead to trouble keeping removable dentures in place, mastication, swallowing (dysphagia), and speech (104); and greater incidence of coronal and root caries and periodontitis, ultimately leading to tooth loss.
In transdisciplinary collaboration, physicians can prescribe fewer or less xerogenic medications or change the dosage and frequency. Since both hyposalivation and cancer are more prevalent in DM, they should also ensure proper protection of the (unaffected) salivary glands during radiation therapy in the head and neck region.
Microbiome Dysbiosis
Hyperglycemia causes changes (123) such as in composition (122, 124, 125) and decreased diversity (126, 127) and abundance (126, 128) of certain bacteria in the subgingival microbiome in periodontitis. Moreover, severities of DM and periodontitis are associated (129, 130). DM treatment leads to changes in the salivary microbiome (131).
Oral and gut microbiomes are closely linked (132); even a small number of periodontal bacteria predict change in glucose level in young healthy adults (133). Pg alters the gut microbiome and causes metabolic syndrome (134) and preDM (135).
Candidiasis
DM is an independent predictor of oral candidiasis (136), especially in hyposalivation (137), as DM favors the acidogenic bacteria that in turn promote the development of caries and candidiasis. Candida albicans, a commensal yeast in the oral microbiome causing candidiasis, can bind to the oral mucosa and, hence, contribute to the cumulative burden of inflammation, directly or via denture stomatitis caused by unclean dentures (138). Over 900 different species of microbes reside in biofilm adhering to dentures (139) and are an important source of sepsis that is largely unnoticed (138).
Taste Disturbances (Dysgeusia, Ageusia, and Hypogeusia)
Due to hyposalivation, neuropathy of nerves sensing taste, microangiopathy in taste buds or medications, (120), and taste impairment and disorders occur frequently in DM (59). Altered taste (gustatory changes) could be the first sign of T2DM, a useful fact for all health professionals. Ageusia is also a COVID-19 symptom (140).
Burning Mouth Syndrome
DM can contribute to the complex burning mouth syndrome that likely is caused by neuropathy and other local and systemic factors and, therefore, needs transdisciplinary treatment (32, 120, 141–143).
Cancer
Cancer is associated with inflammation and occurs more frequently in DM (144), including oral cancer that has a 4.3-fold greater risk of developing and a 2.1 times greater risk of mortality in DM than in non-DM (145). Potentially malignant oral mucosal lesions are also associated with DM, such as leukoplakia, erythroplakia, lichen planus and other lichenoid lesions, and actinic cheilitis (145).
Other Oral Mucosal Lesions and Conditions
People with DM have a greater risk of traumatic ulcer, infections of oral wounds, delayed wound healing, melanin pigmentation, fissured tongue, and benign migratory glossitis (geographic tongue), and temporomandibular disorders (21, 32).
Osteonecrosis of the Jaw (ONJ)
DM is an established risk factor for ONJ in general (146–148) and medication-related ONJ (MRONJ) (149–154). Microvascular complications (angiopathy, ischemia, endothelial cell dysfunction) impair blood circulation and hence bone nutrition and quality with reduced remodeling (155). DM also causes increased apoptosis of osteoblasts and osteocytes and changes in immune cell function, promoting inflammation (155).
Quality of Life
DM decreases QoL with a further decrease in oral health-related QoL (OHRQoL) (59, 156–160). Importantly, QoL correlates strongly with OHRQoL (161), so treating oral diseases increases QoL in DM (160, 162, 163).
Dental Treatment in DM
Non-surgical periodontal treatment (NSPT) consisting of scaling and root planing (SRP), or “deep cleaning,” home oral hygiene instruction, and maintenance follow-up visits can be performed in any dental office by dental hygienists or dentists and improves the periodontal health status also in DM (164, 165). However, advanced cases need treatment by periodontists or other especially skilled clinicians. Adults with DM and periodontitis manage to incorporate new, effective oral hygiene measures into daily life (166); and frequent tooth brushing is negatively associated with incident DM (167).
Glycated Hemoglobin Level
Non-surgical periodontal treatment can lead to a decrease in glycated hemoglobin (HbA1c) level in T2DM after 3 months, which is of clinical significance as it is of the same order of magnitude as adding a second oral antidiabetic medication to metformin (156, 168, 169). Results of meta-analyses upon systematic reviews are displayed in Table 1. Greater effect is seen with greater baseline HbA1c levels (186).
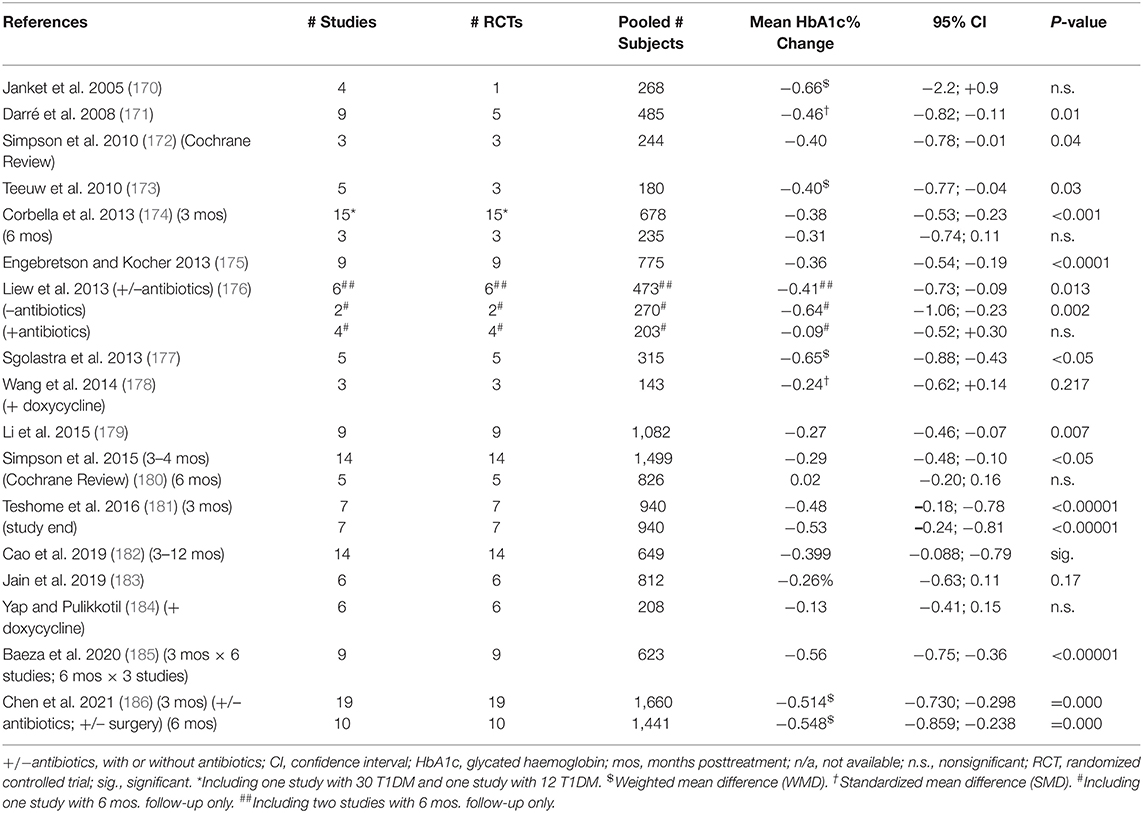
Table 1. Effect of non-surgical periodontal treatment (scaling, root planing, and oral hygiene instruction) (NSPT) on glycated hemoglobin (HbA1c) level in type 2 diabetes (T2DM) 3 and 6 months post-intervention: meta-analyses.
Few studies last longer than 3 months. Noteworthy is a definitive 12-months study (N = 133) demonstrating that intensive periodontal treatment (including surgery) reduced the crude mean HbA1c level from 8.1 (±1.7)% to 7.8 (±0.2)% (187). Upon adjustment, intensive treatment reduced the mean HbA1c value by 0.6 (95% CI; 0.3–0.9)% more than routine NSPT (187).
Inflammatory Markers
Non-surgical periodontal treatment can lead to decreased levels of inflammatory markers, such as C-reactive protein and leukocyte counts that are risk indicators for CVD (188, 189), and the subgingival periodontal biofilm is disturbed, mitigating periodontitis progression (123).
Full-mouth extraction, the ultimate treatment of terminally periodontally diseased teeth, significantly lowers systemic inflammatory markers (190).
Tooth Loss
Scaling and root planing in patients with T2DM is modeled to significantly decrease tooth loss by 34.1% overall (191) and in microvascular diseases by 20.5% in nephropathy, 17.7% in neuropathy, and 19.2% in retinopathy, respectively (191). Nonetheless, insurance data identify DM as a risk factor for tooth loss during periodontal maintenance (95).
Dental Treatment Reduces Healthcare-Related Costs in Diabetes
Dental care has been shown to reduce overall medical care costs in people with DM. Acknowledging inherent methodologic issues (192), population studies and analyses of claims data, from people with DM simultaneously insured for dental, medical outpatient care, hospitalization, and pharmacy expenses, report savings in medical care costs, hospitalization, and introduction of insulin among insureds with DM from studies in Germany (193), Japan (194, 195), the Netherlands (196), United Kingdom (197), and the US (191). A correlation between periodontitis severity and future increases in medical care costs was found among older Japanese (198).
Adults with DM consistently have fewer regular dental check-ups than their non-DM peers (22, 199–208) with between 25 and 60% having had a dental visit the last year. Nonetheless, patients with DM who do receive dental care experience incremental higher costs for more complex treatment and restoration of missing teeth rather than preventive visits (203).
Transdisciplinary Care
Transdisciplinary Care Initiated in the Medical Setting
Screening for Periodontitis in the Medical Office
Attainment of good oral health deserves the attention of medical care providers as a novel tool in DM management (209, 210). Medical care providers recognize grossly cavitated (carious) teeth and thrush (Candidiasis) and could suspect undiagnosed periodontitis based on evident signs such as having few or loose teeth, bad breath, or swollen and spontaneously bleeding gums.
Several questionnaires for assessing the risk of periodontitis by self-report exist. For example, the U.S. Centers for Disease Control and Prevention (CDC) jointly with the American Academy of Periodontology (AAP), developed a set of eight easy-to-pose-and-respond-to items that were validated and found to associate with clinically diagnosed periodontitis (211–213). These or similar questions could be included in the medical visit (22, 214). Such screening in medical practice is well-accepted by both patients (206) and medical professionals (206, 215).
Guidelines for Medical Care Providers and Their Patients
Acknowledging the importance of good oral health in DM management, several professional organizations have published guidelines for (a) medical care professionals in DM practice: AAP (156); American Diabetes Association (ADA) (20); International Diabetes Federation (IDF) (Appendix 1, available online only) (169); and the European Federation of Periodontology (EFP) (156, 169) (Appendix 1); and (b) people with DM or at risk for T2DM in medical practice: AAP (156); ADA (216); IDF (169, 217) (Appendix 1), and EFP (156, 169) (Appendix 1).
Transdisciplinary Care Initiated in the Dental Setting
Screening for T2DM in the Dental Office
Because about half the people with manifest DM and 90% of those with preDM are unaware thereof (18), the dental setting can be important for T2DM screening and referral (218–220), especially for dental patients who do not see a physician regularly (221). It is crucial to identify T2DM in its early stages during which the chances for reversal or mitigation are greatest (222–225). Periodontitis can serve as an early sign of T2DM (226), just like few teeth and recurrent periapical abscesses (93, 94). Random blood glucose or HbA1c levels can be measured chairside by quick finger-prick blood sample analysis (221, 227–241).
Interestingly, 30–54% of dental patients who denied having DM had T2DM with 1.3–5.8% having manifest T2DM as reported from studies in Denmark (227), Saudi Arabia (228), Spain (229), United Kingdom (230, 231), and the US (232–238), aided by electronic health records (239). Whereas, 7.8% of US minority elders (240), 17.2% of patients with Dutch periodontal, and, respectively, 14.6% (241) and 19.1% (221) of Indians had T2DM.
PreDM was found in 9.9% of unaware dental patients in Sweden (242), 28.7% in the US (235), and 46.6% among patients with Dutch periodontal (243).
Guidelines for Dental Care Providers and Their Patients
Acknowledging the importance of identifying undiagnosed T2DM early in dental patients, several professional organizations have published guidelines for a) dental care professionals in dental practice: AAP (156); EFP (156, 169) (Appendix 1); IDF (169) (Appendix 1); Indian Society of Periodontology (244); and Research Society for the Study of Diabetes in India (244); and b) people with DM or at risk for DM in dental practice: AAP (156); EFP (156, 169) (Appendix 1); and IDF (169) (Appendix 1).
Such screening in dental offices is well-accepted by patients (245–247), dentists (246–250) and physicians (251), and their professional organizations (252), and can lead to positive lifestyle changes and decreased HbA1c level (253, 254).
Discussion
Research to promote the understanding of mechanisms underlying reciprocal links between various aspects of oral health and DM is rapidly emerging. However, the current evidence is sufficient to act. The major causes of tooth loss are the two most common oral diseases, namely caries and periodontitis that occur in great proportions of populations globally. These diseases are associated as their respective prevalence in large population studies are not independent of each other (255). Nonetheless, both are largely preventable or treatable/manageable when developed, resulting in the survival of the teeth. Edentulism (having no natural teeth) is decreasing globally, so people keep their teeth at higher ages (28, 256). Furthermore, life expectancy also increases (28, 256); and the prevalence of DM is increasing rapidly all over the world (18). Consequently, increasing numbers of people with DM worldwide are at risk for the oral manifestations described here.
In conclusion, all health care professionals must join forces. However, such transdisciplinary patient-centered collaboration requires paradigm shifts in awareness, attitude, education, and medical and dental practice delivery systems for all health care professionals.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2021.709831/full#supplementary-material
References
1. Simon L. COVID-19 and Oral Health. In: Basu S, Alpert JL, Phillips RS, editors. Primary Care in the COVID-19 Pandemic; Improving Access to High-Quality Primary Care, Accelerating Transitions to Alternative Forms of Care Delivery, and Addressing Health Disparities. (2021). p. 89–101. Available online at: https://www.milbank.org/wp-content/uploads/2021/04/Book_Primary_Care_During_COVID_ebook_4-27-21.pdf#page=89
2. Al-Khabbaz AK, Al-Shammari KF, Al-Saleh NA. Knowledge about the association between periodontal diseases and diabetes mellitus: contrasting dentists and physicians. J Periodontol. (2011) 82:360–6. doi: 10.1902/jop.2010.100372
3. Dubar M, Delatre V, Moutier C, Sy K, Agossa K. Awareness and practices of general practitioners towards the oral-systemic disease relationship: a regionwide survey in France. J Eval Clin Pract. (2020) 26:1722–30. doi: 10.1111/jep.13343
4. Obulareddy VT, Nagarakanti S, Chava VK. Knowledge, attitudes, and practice behaviors of medical specialists for the relationship between diabetes and periodontal disease: a questionnaire survey. J Fam Med Prim Care. (2018) 7:175–8. doi: 10.4103/jfmpc.jfmpc_425_16
5. Poudel P, Griffiths R, Wong VW, Arora A, Flack JR, Khoo CL, et al. Perceptions and practices of diabetes educators in providing oral health care: a qualitative study. Diabetes Educ. (2018) 44:454–64. doi: 10.1177/0145721718796055
6. Poudel P, Griffiths R, Wong VW, Arora A, Flack JR, Khoo CL, et al. Perceptions and practices of general practitioners on providing oral health care to people with diabetes - a qualitative study. BMC Fam Pract. (2020) 21:34. doi: 10.1186/s12875-020-1102-9
7. Poudel P, Griffiths R, Wong VW, Arora A, George A. Knowledge and practices of diabetes care providers in oral health care and their potential role in oral health promotion: a scoping review. Diabetes Res Clin Pract. (2017) 130:266–77. doi: 10.1016/j.diabres.2017.06.004
8. Shimpi N, Glurich I, Panny A, Acharya A. Knowledgeability, attitude, and practice behaviors of primary care providers toward managing patients' oral health care in medical practice: Wisconsin statewide survey. J Am Dent Assoc. (2019) 150:863–72. doi: 10.1016/j.adaj.2019.05.020
9. Shimpi N, Schroeder D, Kilsdonk J, Chyou PH, Glurich I, Penniman E, et al. Medical providers' oral health knowledgeability, attitudes, and practice behaviors: an opportunity for interprofessional collaboration. J Evid Based Dent Pract. (2016) 16:19–29. doi: 10.1016/j.jebdp.2016.01.002
10. Wong G, Koo TF, Fethney J, Chen R. Assessing oral health literacy of university nursing students: a cross-sectional exploratory study. Nurse Educ Pract. (2021) 53:103066. doi: 10.1016/j.nepr.2021.103066
11. Genco RJ, Genco FD. Common risk factors in the management of periodontal and associated systemic diseases: the dental setting and interprofessional collaboration. J Evid Based Dent Pract. (2014) 14:4–16. doi: 10.1016/j.jebdp.2014.03.003
12. Sheiham A, Watt RG. The common risk factor approach: a rational basis for promoting oral health. Commun Dent Oral Epidemiol. (2000) 28:399–406. doi: 10.1034/j.1600-0528.2000.028006399.x
13. Watt RG, Serban S. Multimorbidity: a challenge and opportunity for the dental profession. Br Dent J. (2020) 229:282–6. doi: 10.1038/s41415-020-2056-y
14. Borgnakke WS, Genco RJ, Eke PI, Taylor GW. Ch 31. Oral health and diabetes. In: Cowie CCCS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, et al., editors. Diabetes in America, 3rd ed. Bethesda, MD: National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) (2017). pp. 31.01–51. NIH Pub No. 17-1468. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK567975/
15. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes - 2021. Diabetes Care. (2021) 44:S15–33. doi: 10.2337/dc21-S002
16. World Health Organization (WHO). Diabetes. Available online at: https://www.who.int/news-room/fact-sheets/detail/diabetes
17. International Diabetes Federation (IDF). What Is Diabetes. Available online at: https://www.idf.org/aboutdiabetes/what-is-diabetes.html
18. International Diabetes Federation (IDF). IDF Diabetes Atlas. 9th ed. International Diabetes Federation (IDF) (2019). Available online at: https://www.diabetesatlas.org/en/
19. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
20. American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes - 2021. Diabetes Care. (2021) 44:S40–52. doi: 10.2337/dc21-S004
21. Verhulst MJL, Loos BG, Gerdes VEA, Teeuw WJ. evaluating all potential oral complications of diabetes mellitus. Front Endocrinol. (2019) 10:56. doi: 10.3389/fendo.2019.00056
22. Verhulst MJL, Teeuw WJ, Gerdes VEA, Loos BG. Self-reported oral health and quality of life in patients with type 2 diabetes mellitus in primary care: a multi-center cross-sectional study. Diabetes Metab Syndr Obes. (2019) 12:883–99. doi: 10.2147/DMSO.S207087
23. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. (2005) 366:1809–20. doi: 10.1016/S0140-6736(05)67728-8
24. Van Dyke TE, Bartold PM, Reynolds EC. The nexus between periodontal inflammation and dysbiosis. Front Immunol. (2020) 11:511. doi: 10.3389/fimmu.2020.00511
25. Bartold PM, Van Dyke TE. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J Clin Periodontol. (2019) 46:6–11. doi: 10.1111/jcpe.13046
26. Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. (2018) 149:576–88, 588.e1–6. doi: 10.1016/j.adaj.2018.04.023
27. Nocini R, Lippi G, Mattiuzzi C. Periodontal disease: the portrait of an epidemic. J Public Health Emerg. (2020) 4:10. doi: 10.21037/jphe.2020.03.01
28. Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. (2014) 93:1045–53. doi: 10.1177/0022034514552491
29. Borgnakke WS. “Non-modifiable” risk factors for periodontitis and diabetes. Curr Oral Health Rep. (2016) 3:270–81. doi: 10.1007/s40496-016-0098-7
30. Borgnakke WS. Modifiable risk factors for periodontitis and diabetes. Curr Oral Health Rep. (2016) 3:254–69. doi: 10.1007/s40496-016-0099-6
31. Kocher T, König J, Borgnakke WS, Pink C, Meisel P. Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontol 2000. (2018) 78:59–97. doi: 10.1111/prd.12235
32. Miller A, Ouanounou A. Diagnosis, management, and dental considerations for the diabetic patient. J Can Dent Assoc. (2020) 86:k8.
33. Wu CZ, Yuan YH, Liu HH, Li SS, Zhang BW, Chen W, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. (2020) 20:204. doi: 10.1186/s12903-020-01180-w
34. Genco RJ, Borgnakke WS. Diabetes as a potential risk for periodontitis: association studies. Periodontol 2000. (2020) 83:40–5. doi: 10.1111/prd.12270
35. Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. (2013) 62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x
36. Glavind L, Lund B, Löe H. The relationship between periodontal state and diabetes duration, insulin dosage and retinal changes. J Periodontol. (1968) 39:341–7. doi: 10.1902/jop.1968.39.6.341
37. Campbell MJ. Epidemiology of periodontal disease in the diabetic and the non-diabetic. Aust Dent J. (1972) 17:274–8. doi: 10.1111/j.1834-7819.1972.tb04931.x
38. Wolf J. Dental and periodontal conditions in diabetes mellitus; a clinical and radiographic study. Proc Finn Dent Soc. (1977) 73:1–56.
39. Sznajder N, Carraro JJ, Rugna S, Sereday M. Periodontal findings in diabetic and nondiabetic patients. J Periodontol. (1978) 49:445–8. doi: 10.1902/jop.1978.49.9.445
40. Belting CM, Hiniker JJ, Dummett CO. Influence of diabetes mellitus on the severity of periodontal disease. J Periodontol. (1964) 35:476–80. doi: 10.1902/jop.1964.35.6.476
41. Cianciola LJ, Park BH, Bruck E, Mosovich L, Genco RJ. Prevalence of periodontal disease in insulin-dependent diabetes mellitus (juvenile diabetes). J Am Dent Assoc. (1982) 104:653–60. doi: 10.14219/jada.archive.1982.0240
42. Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. (1991) 62:123–31. doi: 10.1902/jop.1991.62.2.123
43. Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol. (1978) 108:497–505. doi: 10.1093/oxfordjournals.aje.a112648
44. Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. (1990) 13:836–40. doi: 10.2337/diacare.13.8.836
45. Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc. (1990) 121:532–6. doi: 10.14219/jada.archive.1990.0211
46. Zambon JJ, Reynolds H, Fisher JG, Shlossman M, Dunford R, Genco RJ. Microbiological and immunological studies of adult periodontitis in patients with noninsulin-dependent diabetes mellitus. J Periodontol. (1988) 59:23–31. doi: 10.1902/jop.1988.59.1.23
47. Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. (1993) 16:329–34. doi: 10.2337/diacare.16.1.329
48. Ziukaite L, Slot DE, Van der Weijden FA. Prevalence of diabetes mellitus in people clinically diagnosed with periodontitis: a systematic review and meta-analysis of epidemiologic studies. J Clin Periodontol. (2018) 45:650–62. doi: 10.1111/jcpe.12839
49. Borgnakke WS. Ch 3. The traveling oral microbiome. In: Glick M, editor. The Oral-Systemic Health Connection: A Guide to Patient Care. Chicago, IL: Quintessence (2019) pp. 38–85.
50. Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. (2013) 92:485–91. doi: 10.1177/0022034513487559
51. Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol. (2013) 40:S135–52. doi: 10.1111/jcpe.12080
52. Graziani F, Gennai S, Solini A, Petrini M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes: an update of the EFP-AAP review. J Clin Periodontol. (2018) 45:167–87. doi: 10.1111/jcpe.12837
53. Ishai A, Osborne MT, El Kholy K, Takx RAP, Ali A, Yuan N, et al. Periodontal disease associates with arterial inflammation via potentiation of a hematopoietic-arterial axis. JACC Cardiovasc Imaging. (2019) 12:2271–3. doi: 10.1016/j.jcmg.2019.05.015
54. Nguyen ATM, Akhter R, Garde S, Scott C, Twigg SM, Colagiuri S, et al. The association of periodontal disease with the complications of diabetes mellitus; a systematic review. Diabetes Res Clin Pract. (2020) 165:108244. doi: 10.1016/j.diabres.2020.108244
55. Sanz M, Del Castillo AM, Jepsen S, Gonzalez-Juanatey JR, D'Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. (2020) 47:268–88. doi: 10.1111/jcpe.13189
56. Song TJ, Jeon J, Kim J. Cardiovascular risks of periodontitis and oral hygiene indicators in patients with diabetes mellitus. Diabetes Metab. (2021) 47:101252. doi: 10.1016/j.diabet.2021.101252
57. Van Dyke TE, Kholy KE, Ishai A, Takx RAP, Mezue K, Abohashem SM, et al. Inflammation of the periodontium associates with risk of future cardiovascular events. J Periodontol. (2021) 92:348–58. doi: 10.1002/JPER.19-0441
58. Wang Y, Zhen Z, Liu HN, Lai I, Pelekos G, Tse HF, et al. Periodontitis links to exacerbation of myocardial dysfunction in subjects with type 2 diabetes. J Periodontal Res. (2019) 54:339–48. doi: 10.1111/jre.12634
59. Borgnakke WS, Anderson PF, Shannon C, Jivanescu A. Is there a relationship between oral health and diabetic neuropathy? Curr Diab Rep. (2015) 15:93. doi: 10.1007/s11892-015-0673-7
60. Wu HQ, Wei X, Yao JY, Qi JY, Xie HM, Sang AM, et al. Association between retinopathy, nephropathy, and periodontitis in type 2 diabetic patients: a meta-analysis. Int J Ophthalmol. (2021) 14:141–7. doi: 10.18240/ijo.2021.01.20
61. Zhao D, Khawaja AT, Jin L, Chan KW, Tonetti M, Tang SCW, et al. Effect of non-surgical periodontal therapy on renal function in chronic kidney disease patients with periodontitis: a systematic review and meta-analysis of interventional studies. Clin Oral Investig. (2020) 24:1607–18. doi: 10.1007/s00784-019-03066-w
62. Zhao D, Khawaja AT, Jin L, Li KY, Tonetti M, Pelekos G. The directional and non-directional associations of periodontitis with chronic kidney disease: a systematic review and meta-analysis of observational studies. J Periodontal Res. (2018) 53:682–704. doi: 10.1111/jre.12565
63. de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. (2008) 2:1101–13. doi: 10.1177/193229680800200619
64. Bello-Chavolla OY, Aguilar-Salinas CA, Avila-Funes JA. The type 2 diabetes-specific dementia risk score (DSDRS) is associated with frailty, cognitive and functional status amongst Mexican community-dwelling older adults. BMC Geriatr. (2020) 20:363. doi: 10.1186/s12877-020-01776-5
65. Kuehn BM. In Alzheimer research, glucose metabolism moves to center stage. J Am Med Assoc. (2020) 323:297–9. doi: 10.1001/jama.2019.20939
66. Kamer AR, Pushalkar S, Gulivindala D, Butler T, Li Y, Annam KRC, et al. Periodontal dysbiosis associates with reduced CSF Aβ42 in cognitively normal elderly. Alzheimers Dement. (2021) 13:e12172. doi: 10.1002/dad2.12172
67. Mei F, Xie M, Huang X, Long Y, Lu X, Wang X, et al. Porphyromonas gingivalis and Its systemic impact: current status. Pathogens. (2020) 9:944. doi: 10.3390/pathogens9110944
68. Nadim R, Tang J, Dilmohamed A, Yuan S, Wu C, Bakre AT, et al. Influence of periodontal disease on risk of dementia: a systematic literature review and a meta-analysis. Eur J Epidemiol. (2020) 35:821–33. doi: 10.1007/s10654-020-00648-x
69. Norins LC. Licensed anti-microbial drugs logical for clinical trials against pathogens currently suspected in Alzheimer's disease. Antibiotics. (2021) 10:327. doi: 10.3390/antibiotics10030327
70. Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer's disease? J Oral Microbiol. (2015) 7:29143. doi: 10.3402/jom.v7.29143
71. Olsen I. Possible link between Porphyromonas gingivalis and amyloidosis in the pathogenesis of Alzheimer's and Parkinson's disease. Int J Pathol Immunol. (2020) 1:1. doi: 10.46940/ijpi.01.1001
72. Olsen I, Kell DB, Pretorius E. Is Porphyromonas gingivalis involved in Parkinson's disease? Eur J Clin Microbiol Infect Dis. (2020) 39:2013–8. doi: 10.1007/s10096-020-03944-2
73. Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. (2019) 5:eaau3333. doi: 10.1126/sciadv.aau3333
74. Iranmanesh B, Khalili M, Amiri R, Zartab H, Aflatoonian M. Oral manifestations of COVID-19 disease: a review article. Dermatol Ther. (2021) 34:e14578. doi: 10.1111/dth.14578
75. Tsuchiya H. Oral symptoms associated with COVID-19 and their pathogenic mechanisms: a literature review. Dent J. (2021) 9:32. doi: 10.3390/dj9030032
76. Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID-19). Oral Dis. (2021) 27(Suppl. 3):771–2. doi: 10.1111/odi.13465
77. Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. (2021) 27(Suppl. 3):710–2. doi: 10.1111/odi.13382
78. Patel J, Woolley J. Necrotizing periodontal disease: oral manifestation of COVID-19. Oral Dis. (2021) 27(Suppl. 3):768–9. doi: 10.1111/odi.13462
79. Marouf N, Cai W, Said KN, Daas H, Diab H, Chinta VR, et al. Association between periodontitis and severity of COVID-19 infection: a case-control study. J Clin Periodontol. (2021) 48:483–91. doi: 10.1111/jcpe.13435
80. Elibol E. Otolaryngological symptoms in COVID-19. Eur Arch Otorhinolaryngol. (2021) 278:1233–6. doi: 10.1007/s00405-020-06319-7
81. Lloyd-Jones G, Molayem S, Pontes CC, Chapple I. The COVID-19 pathway: a proposed oral-vascular-pulmonary route of SARS-CoV-2 infection and the importance of oral healthcare measures. J Oral Med Dent Res. (2021) 2:1–25.
82. Räisänen IT, Umeizudike KA, Pärnänen P, Heikkilä P, Tervahartiala T, Nwhator SO. Periodontal disease and targeted prevention using aMMP-8 point-of-care oral fluid analytics in the COVID-19 era. Med Hypotheses. (2020) 144:110276. doi: 10.1016/j.mehy.2020.110276
83. Gupta S, Mohindra R, Chauhan PK, Singla V, Goyal K, Sahni V, et al. SARS-CoV-2 detection in gingival crevicular fluid. J Dent Res. (2021) 100:187–93. doi: 10.1177/0022034520970536
84. Aquino-Martinez R, Hernandez-Vigueras S. Severe COVID-19 lung infection in older people and periodontitis. J Clin Med. (2021) 10:279. doi: 10.3390/jcm10020279
85. Huang N, Perez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. (2021) 27:892–903. doi: 10.1038/s41591-021-01296-8
86. Xu J, Li Y, Gan F, Du Y, Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. (2020) 99:989. doi: 10.1177/0022034520918518
87. Pitones-Rubio V, Chávez-Cortez EG, Hurtado-Camarena A, González-Rascón A, Serafín-Higuera N. Is periodontal disease a risk factor for severe COVID-19 illness? Med Hypotheses. (2020) 144:109969. doi: 10.1016/j.mehy.2020.109969
88. Liu R, Yi S, Zhang J, Lv Z, Zhu C, Zhang Y. Viral load dynamics in sputum and nasopharyngeal swab in patients with COVID-19. J Dent Res. (2020) 99:1239–44. doi: 10.1177/0022034520946251
89. Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, et al. Glycemic characteristics and clinical outcomes of covid-19 patients hospitalized in the United States. J Diabetes Sci Technol. (2020) 14:813–21. doi: 10.1177/1932296820924469
90. Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. (2015) 94:650–8. doi: 10.1177/0022034515573272
91. Beheshti M, Badner V, Shah P, Margulis KS, Yeroshalmi F. Association of diabetes and dental caries among U.S. adolescents in the NHANES Dataset. Pediatr Dent. (2021) 43:123–8.
92. Swapna LA, Koppolu P, Prince J. Oral health in diabetic and nondiabetic patients with chronic kidney disease. Saudi J Kidney Dis Transpl. (2017) 28:1099–105. doi: 10.4103/1319-2442.215123
93. Cabanillas-Balsera D, Martin-Gonzalez J, Montero-Miralles P, Sanchez-Dominguez B, Jimenez-Sanchez MC, Segura-Egea JJ. Association between diabetes and nonretention of root filled teeth: a systematic review and meta-analysis. Int Endod J. (2019) 52:297–306. doi: 10.1111/iej.13011
94. Rios-Osorio N, Munoz-Alvear HD, Montoya Canon S, Restrepo-Mendez S, Aguilera-Rojas SE, Jimenez-Pena O, et al. Association between type 2 diabetes mellitus and the evolution of endodontic pathology. Quintessence Int. (2020) 51:100–7. doi: 10.3290/j.qi.a43865
95. Raedel M, Noack B, Priess HW, Bohm S, Walter MH. Massive data analyses show negative impact of type 1 and 2 diabetes on the outcome of periodontal treatment. Clin Oral Investig. (2021) 25:2037–43. doi: 10.1007/s00784-020-03512-0
96. Suzuki S, Noda T, Nishioka Y, Imamura T, Kamijo H, Sugihara N. Evaluation of tooth loss among patients with diabetes mellitus using the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Int Dent J. (2020) 70:308–15. doi: 10.1111/idj.12561
97. Parker ML, Thornton-Evans G, Wei L, Griffin SO. Prevalence of and changes in tooth loss among adults aged >/=50 years with selected chronic conditions - United States, 1999-2004 and 2011-2016. MMWR Morb Mortal Wkly Rep. (2020) 69:641–6. doi: 10.15585/mmwr.mm6921a1
98. Lopez-Gomez SA, Gonzalez-Lopez BS, Scougall-Vilchis RJ, Pontigo-Loyola AP, Marquez-Corona ML, Villalobos-Rodelo JJ, et al. Tooth loss in patients with and without diabetes: a large-scale, cross-sectional study of Mexican adults. J Am Dent Assoc. (2020) 151:276–86. doi: 10.1016/j.adaj.2019.12.015
99. Izuora K, Yousif A, Allenback G, Gewelber C, Neubauer M. Relationship between dental loss and health outcomes among hospitalized patients with and without diabetes. J Investig Med. (2019) 67:669–73. doi: 10.1136/jim-2018-000842
100. Patel MH, Kumar JV, Moss ME. Diabetes and tooth loss: an analysis of data from the National Health and Nutrition Examination Survey, 2003-2004. J Am Dent Assoc. (2013) 144:478–85. doi: 10.14219/jada.archive.2013.0149
101. Simila T, Auvinen J, Puukka K, Keinänen-Kiukaanniemi S, Virtanen JI. Impaired glucose metabolism is associated with tooth loss in middle-aged adults: the Northern Finland Birth Cohort 1966. Diabetes Res Clin Pract. (2018) 142:110–9. doi: 10.1016/j.diabres.2018.05.035
102. Luo H, Pan W, Sloan F, Feinglos M, Wu B. Forty-year trends in tooth loss among American adults with and without diabetes mellitus: an age-period-cohort analysis. Prev Chronic Dis. (2015) 12:E211. doi: 10.5888/pcd12.150309
103. Weijdijk LPM, Ziukaite L, Van der Weijden GA, Bakker E, Slot DE. The risk of tooth loss in patients with diabetes: a systematic review and meta-analysis. Int J Dent Hyg. (2021). doi: 10.1111/idh.12512. [Epub ahead of print].
104. Lu TY, Chen JH, Du JK, Lin YC, Ho PS, Lee CH, et al. Dysphagia and masticatory performance as a mediator of the xerostomia to quality of life relation in the older population. BMC Geriatr. (2020) 20:521. doi: 10.1186/s12877-020-01901-4
105. Beaudette JR, Fritz PC, Sullivan PJ, Ward WE. Oral health, nutritional choices, and dental fear and anxiety. Dent J. (2017) 5:8. doi: 10.3390/dj5010008
106. Iwasaki M, Kimura Y, Yoshihara A, Ogawa H, Yamaga T, Takiguchi T, et al. Association between dental status and food diversity among older Japanese. Community Dent Health. (2015) 32:104–10. doi: 10.1922/CDH_3502Iwasaki07
107. Machado V, Botelho J, Viana J, Pereira P, Lopes LB, Proenca L, et al. Association between Dietary Inflammatory Index and periodontitis: a cross-sectional and mediation analysis. Nutrients. (2021) 13:1194. doi: 10.3390/nu13041194
108. Ojo O, Ojo OO, Adebowale F, Wang XH. The effect of dietary glycaemic index on glycaemia in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2018) 10:373. doi: 10.3390/nu10030373
109. Kossioni AE. The association of poor oral health parameters with malnutrition in older adults: a review considering the potential implications for cognitive impairment. Nutrients. (2018) 10:1709. doi: 10.3390/nu10111709
110. Liljestrand JM, Havulinna AS, Paju S, Männistö S, Salomaa V, Pussinen PJ. Missing teeth predict incident cardiovascular events, diabetes, and death. J Dent Res. (2015) 94:1055–62. doi: 10.1177/0022034515586352
111. Oluwagbemigun K, Dietrich T, Pischon N, Bergmann M, Boeing H. Association between number of teeth and chronic systemic diseases: a cohort study followed for 13 years. PLoS ONE. (2015) 10:e0123879. doi: 10.1371/journal.pone.0123879
112. Choi HM, Han K, Park YG, Park JB. Associations between the number of natural teeth and renal dysfunction. Medicine. (2016) 95:e4681. doi: 10.1097/MD.0000000000004681
113. Suma S, Furuta M, Yamashita Y, Matsushita K. Aging, mastication, and malnutrition and their associations with cognitive disorder: evidence from epidemiological data. Curr Oral Health Rep. (2019) 6:89–99. doi: 10.1007/s40496-019-0220-8
114. Listl S. Oral health conditions and cognitive functioning in middle and later adulthood. BMC Oral Health. (2014) 14:70. doi: 10.1186/1472-6831-14-70
115. Oates TW, Dowell S, Robinson M, McMahan CA. Glycemic control and implant stabilization in type 2 diabetes mellitus. J Dent Res. (2009) 88:367–71. doi: 10.1177/0022034509334203
116. Eskow CC, Oates TW. Dental implant survival and complication rate over 2 years for individuals with poorly controlled type 2 diabetes mellitus. Clin Implant Dent Relat Res. (2017) 19:423–31. doi: 10.1111/cid.12465
117. Lorean A, Ziv-On H, Perlis V, Ormianer Z. Marginal bone loss of dental implants in patients with type 2 diabetes mellitus with poorly controlled HbA1c values: a long-term retrospective study. Int J Oral Maxillofac Implants. (2021) 36:355–60. doi: 10.11607/jomi.8476
118. Meza Mauricio J, Miranda TS, Almeida ML, Silva HD, Figueiredo LC, Duarte PM. An umbrella review on the effects of diabetes on implant failure and peri-implant diseases. Braz Oral Res. (2019) 33:e070. doi: 10.1590/1807-3107bor-2019.vol33.0070
119. Monje A, Catena A, Borgnakke WS. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: systematic review and meta-analysis. J Clin Periodontol. (2017) 44:636–48. doi: 10.1111/jcpe.12724
120. Balakumar P, Kavitha M, Nanditha S. Cardiovascular drugs-induced oral toxicities: a murky area to be revisited and illuminated. Pharmacol Res. (2015) 102:81–9. doi: 10.1016/j.phrs.2015.09.007
121. Hughes FJ, Bartold PM. Periodontal complications of prescription and recreational drugs. Periodontol 2000. (2018) 78:47–58. doi: 10.1111/prd.12230
122. Wade WG. Resilience of the oral microbiome. Periodontol 2000. (2021) 86:113–22. doi: 10.1111/prd.12365
123. Kumar PS, Monteiro MF, Dabdoub SM, Miranda GL, Casati MZ, Ribeiro FV, et al. Subgingival host-microbial interactions in hyperglycemic individuals. J Dent Res. (2020) 99:650–7. doi: 10.1177/0022034520906842
124. Kori JA, Saleem F, Ullah S, Azim MK. Characterization of oral bacteriome dysbiosis in type 2 diabetic patients. medRxiv. (2020). doi: 10.1101/2020.04.09.20052613. [Epub ahead of print].
125. Matsha TE, Prince Y, Davids S, Chikte U, Erasmus RT, Kengne AP, et al. Oral microbiome signatures in diabetes mellitus and periodontal disease. J Dent Res. (2020) 99:658–65. doi: 10.1177/0022034520913818
126. Saeb ATM, Al-Rubeaan KA, Aldosary K, Udaya Raja GK, Mani B, Abouelhoda M, et al. Relative reduction of biological and phylogenetic diversity of the oral microbiota of diabetes and pre-diabetes patients. Microb Pathog. (2019) 128:215–29. doi: 10.1016/j.micpath.2019.01.009
127. Scannapieco FA, Dongari-Bagtzoglou A. Dysbiosis revisited; understanding the role of the oral microbiome in the pathogenesis of gingivitis and periodontitis: a critical assessment. J Periodontol. (2021). doi: 10.1002/JPER.21-0120. [Epub ahead of print].
128. Farina R, Severi M, Carrieri A, Miotto E, Sabbioni S, Trombelli L, et al. Whole metagenomic shotgun sequencing of the subgingival microbiome of diabetics and non-diabetics with different periodontal conditions. Arch Oral Biol. (2019) 104:13–23. doi: 10.1016/j.archoralbio.2019.05.025
129. Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol 2000. (2020) 82:214–24. doi: 10.1111/prd.12318
130. Rodriguez-Hernandez AP, Marquez-Corona ML, Pontigo-Loyola AP, Medina-Solis CE, Ximenez-Fyvie LA. Subgingival microbiota of Mexicans with type 2 diabetes with different periodontal and metabolic conditions. Int J Environ Res Public Health. (2019) 16:3184. doi: 10.3390/ijerph16173184
131. Yang Y, Liu S, Wang Y, Wang Z, Ding W, Sun X, et al. Changes of saliva microbiota in the onset and after the treatment of diabetes in patients with periodontitis. Aging. (2020) 12:13090–114. doi: 10.18632/aging.103399
132. Schmidt TS, Hayward MR, Coelho LP, Li SS, Costea PI, Voigt AY, et al. Extensive transmission of microbes along the gastrointestinal tract. eLIFE. (2019) 8:e42693. doi: 10.7554/eLife.42693
133. Demmer RT, Trinh P, Rosenbaum M, Li G, LeDuc C, Leibel R, et al. Subgingival microbiota and longitudinal glucose change: the Oral Infections, Glucose Intolerance and Insulin Resistance Study (ORIGINS). J Dent Res. (2019) 98:1488–96. doi: 10.1177/0022034519881978
134. Watanabe K, Katagiri S, Takahashi H, Sasaki N, Maekawa S, Komazaki R, et al. Porphyromonas gingivalis impairs glucose uptake in skeletal muscle associated with altering gut microbiota. FASEB J. (2021) 35:e21171. doi: 10.1096/fj.202001158R
135. Zhou W, Sailani MR, Contrepois K, Zhou Y, Ahadi S, Leopold SR, et al. Longitudinal multi-omics of host-microbe dynamics in prediabetes. Nature. (2019) 569:663–71. doi: 10.1038/s41586-019-1236-x
136. Jhugroo C, Divakar DD, Jhugroo P, Al-Amri SAS, Alahmari AD, Vijaykumar S, et al. Characterization of oral mucosa lesions and prevalence of yeasts in diabetic patients: a comparative study. Microb Pathog. (2019) 126:363–67. doi: 10.1016/j.micpath.2018.11.028
137. Billings M, Dye BA, Iafolla T, Grisius M, Alevizos I. Elucidating the role of hyposalivation and autoimmunity in oral candidiasis. Oral Dis. (2017) 23:387–94. doi: 10.1111/odi.12626
138. Offenbacher S, Barros SP, Altarawneh S, Beck JD, Loewy ZG. Impact of tooth loss on oral and systemic health. Gen Dent. (2012) 60:494–500; quiz 501–2.
139. Glass RT, Conrad RS, Bullard JW, Goodson LB, Mehta N, Lech SJ, et al. Evaluation of microbial flora found in previously worn prostheses from the Northeast and Southwest regions of the United States. J Prosthet Dent. (2010) 103:384–9. doi: 10.1016/S0022-3913(10)60083-2
140. Daly J, Black EAM. The impact of COVID-19 on population oral health. Commun Dent Health. (2020) 37:236–8. doi: 10.1922/CDH_Dec20editorialDalyBlack03
141. Ritchie A, Kramer JM. Recent advances in the etiology and treatment of burning mouth syndrome. J Dent Res. (2018) 97:1193–9. doi: 10.1177/0022034518782462
142. Silvestre FJ, Silvestre-Rangil J, López-Jornet P. Burning mouth syndrome: a review and update. Rev Neurol. (2015) 60:457–63. doi: 10.33588/rn.6010.2014514
143. Thoppay J, Desai B. Oral burning: local and systemic connection for a patient-centric approach. EPMA J. (2019) 10:1–11. doi: 10.1007/s13167-018-0157-3
144. Miller B, Chalfant H, Thomas A, Wellberg E, Henson C, McNally MW, et al. Diabetes, obesity, and inflammation: impact on clinical and radiographic features of breast cancer. Int J Mol Sci. (2021) 22:2757. doi: 10.3390/ijms22052757
145. Ramos-Garcia P, Roca-Rodriguez MDM, Aguilar-Diosdado M, Gonzalez-Moles MA. Diabetes mellitus and oral cancer/oral potentially malignant disorders: a systematic review and meta-analysis. Oral Dis. (2021) 27:404–21. doi: 10.1111/odi.13289
146. Ito R, Huang JJ, Hsieh WC, Kao HK, Lao WW, Fang KH, et al. Identification of predisposing factors for osteonecrosis of the jaw after marginal mandibulectomy in the surgical management of oral squamous cell carcinoma. J Surg Oncol. (2018) 117:781–87. doi: 10.1002/jso.24913
147. Khan A, Morrison A, Cheung A, Hashem W, Compston J. Osteonecrosis of the jaw (ONJ): diagnosis and management in 2015. Osteoporos Int. (2016) 27:853–9. doi: 10.1007/s00198-015-3335-3
148. Kim JY, Song HC, Jee HG. Refractory healing after surgical therapy of osteonecrosis of the jaw: associated risk factors in aged patients. Clin Interv Aging. (2019) 14:797–804. doi: 10.2147/CIA.S200455
149. Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. (2016) 31:16–35. doi: 10.1002/jbmr.2708
150. Hallmer F, Bjarnadottir O, Götrick B, Malmström P, Andersson G. Incidence of and risk factors for medication-related osteonecrosis of the jaw in women with breast cancer with bone metastasis: a population-based study. Oral Surg Oral Med Oral Pathol Oral Radiol. (2020) 130:252–7. doi: 10.1016/j.oooo.2020.04.808
151. Khamaisi M, Regev E, Yarom N, Avni B, Leitersdorf E, Raz I, et al. Possible association between diabetes and bisphosphonate-related jaw osteonecrosis. J Clin Endocrinol Metab. (2007) 92:1172–5. doi: 10.1210/jc.2006-2036
152. Steybe D, Voss PJ, Ermer MA, Fuessinger MA, Schmelzeisen R, Poxleitner P. Necrotizing fasciitis as a complication of osteonecrosis of the jaw related to oral bisphosphonate application in a patient with osteoporosis: a case report. Oral Maxillofac Surg. (2019) 23:83–89. doi: 10.1007/s10006-018-0725-7
153. Valenzuela L, Alonso-Bouzon C, Manas LR. Bisphosphonate-related osteonecrosis of the jaw in an 80-year-old woman with diabetes mellitus: case report. J Am Geriatr Soc. (2015) 63:2221–2. doi: 10.1111/jgs.13689
154. Yarom N, Lazarovici TS, Whitefield S, Weissman T, Wasserzug O, Yahalom R. Rapid onset of osteonecrosis of the jaw in patients switching from bisphosphonates to denosumab. Oral Surg Oral Med Oral Pathol Oral Radiol. (2018) 125:27–30. doi: 10.1016/j.oooo.2017.09.014
155. Peer A, Khamaisi M. Diabetes as a risk factor for medication-related osteonecrosis of the jaw. J Dent Res. (2015) 94:252–60. doi: 10.1177/0022034514560768
156. Chapple IL, Genco RJ, Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. (2013) 40(Suppl. 14):S106–12. doi: 10.1111/jcpe.12077
157. Iqbal Q, Ul Haq N, Bashir S, Bashaar M. Profile and predictors of health related quality of life among type II diabetes mellitus patients in Quetta city, Pakistan. Health Qual Life Outcomes. (2017) 15:142. doi: 10.1186/s12955-017-0717-6
158. Jivanescu A, Borgnakke WS, Goguta L, Erimescu R, Shapira L, Bratu E. Effects of a hydrogel patch on denture-related traumatic ulcers; an exploratory study. J Prosthodont. (2015) 24:109–14. doi: 10.1111/jopr.12186
159. Jivanescu A, Bratu E, Goguta L, Borgnakke WS. Effect of improvement of complete dentures on quality of life in type 2 diabetes. Diabetes Stoffw Herz. (2013) 22:207–11.
160. Machado V, Botelho J, Proença L, Alves R, Oliveira MJ, Amaro L, et al. Periodontal status, perceived stress, diabetes mellitus and oral hygiene care on quality of life: a structural equation modelling analysis. BMC Oral Health. (2020) 20:229. doi: 10.1186/s12903-020-01219-y
161. Sekulić S, John MT, Davey C, Rener-Sitar K. Association between oral health-related and health-related quality of life. Zdr Varst. (2020) 59:65–74. doi: 10.2478/sjph-2020-0009
162. Mizuno H, Ekuni D, Maruyama T, Kataoka K, Yoneda T, Fukuhara D, et al. The effects of non-surgical periodontal treatment on glycemic control, oxidative stress balance and quality of life in patients with type 2 diabetes: a randomized clinical trial. PLoS ONE. (2017) 12:e0188171. doi: 10.1371/journal.pone.0188171
163. Vergnes JN, Canceill T, Vinel A, Laurencin-Dalicieux S, Maupas-Schwalm F, Blasco-Baque V, et al. The effects of periodontal treatment on diabetic patients: the DIAPERIO randomized controlled trial. J Clin Periodontol. (2018) 45:1150–63. doi: 10.1111/jcpe.13003
164. Pedroso JF, Lotfollahi Z, Albattarni G, Arrruda Schulz M, Monteiro A, Sehnem AL, et al. Influence of periodontal disease on cardiovascular markers in diabetes mellitus patients. Sci Rep. (2019) 9:16138. doi: 10.1038/s41598-019-52498-7
165. Zhang H, Li C, Shang S, Luo Z. Scaling and root planing with enhanced root planing on healthcare for type 2 diabetes mellitus: a randomized controlled clinical trial. J Dent Sci. (2013) 8:272–80. doi: 10.1016/j.jds.2012.10.009
166. Jaedicke KM, Bissett SM, Finch T, Thornton J, Preshaw PM. Exploring changes in oral hygiene behaviour in patients with diabetes and periodontal disease: a feasibility study. Int J Dent Hyg. (2019) 17:55–63. doi: 10.1111/idh.12365
167. Chang Y, Lee JS, Lee KJ, Woo HG, Song TJ. Improved oral hygiene is associated with decreased risk of new-onset diabetes: a nationwide population-based cohort study. Diabetologia. (2020) 63:924–33. doi: 10.1007/s00125-020-05112-9
168. Borgnakke WS. IDF Diabetes Atlas: diabetes and oral health - a two-way relationship of clinical importance. Diabetes Res Clin Pract. (2019) 157:107839. doi: 10.1016/j.diabres.2019.107839
169. Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the Joint Workshop on Periodontal Diseases and Diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. (2018) 45:138–49. doi: 10.1111/jcpe.12808
170. Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res. (2005) 84:1154–9. doi: 10.1177/154405910508401212
171. Darré L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: a meta-analysis of interventional studies. Diabetes Metab. (2008) 34:497–506. doi: 10.1016/j.diabet.2008.03.006
172. Simpson TC, Needleman I, Wild SH, Moles DR, Mills EJ. Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database Syst Rev. (2010) CD004714. doi: 10.1002/14651858.CD004714.pub2
173. Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. (2010) 33:421–7. doi: 10.2337/dc09-1378
174. Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD. Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J Diabetes Investig. (2013) 4:502–9. doi: 10.1111/jdi.12088
175. Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Clin Periodontol. (2013) 40(Suppl. 14):S153–63. doi: 10.1111/jcpe.12084
176. Liew AK, Punnanithinont N, Lee YC, Yang J. Effect of non-surgical periodontal treatment on HbA1c: a meta-analysis of randomized controlled trials. Aust Dent J. (2013) 58:350–7. doi: 10.1111/adj.12091
177. Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol. (2013) 84:958–73. doi: 10.1902/jop.2012.120377
178. Wang TF, Jen IA, Chou C, Lei YP. Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta-analysis. Med. (2014) 93:e292. doi: 10.1097/MD.0000000000000292
179. Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials. (2015) 16:291. doi: 10.1186/s13063-015-0810-2
180. Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. (2015) 11:CD004714. doi: 10.1002/14651858.CD004714.pub3
181. Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. (2016) 17:31. doi: 10.1186/s12903-016-0249-1
182. Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health. (2019) 19:176. doi: 10.1186/s12903-019-0829-y
183. Jain A, Gupta J, Bansal D, Sood S, Gupta S, Jain A. Effect of scaling and root planing as monotherapy on glycemic control in patients of Type 2 diabetes with chronic periodontitis: a systematic review and meta-analysis. J Indian Soc Periodontol. (2019) 23:303–10. doi: 10.4103/jisp.jisp_417_18
184. Yap KCH, Pulikkotil SJ. Systemic doxycycline as an adjunct to scaling and root planing in diabetic patients with periodontitis: a systematic review and meta-analysis. BMC Oral Health. (2019) 19:209. doi: 10.1186/s12903-019-0873-7
185. Baeza M, Morales A, Cisterna C, Cavalla F, Jara G, Isamitt Y, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis [see COMMENT by Shelswell 2021]. J Appl Oral Sci. (2020) 28:e20190248. doi: 10.1590/1678-7757-2019-0248
186. Chen YF, Zhan Q, Wu CZ, Yuan YH, Chen W, Yu FY, et al. Baseline HbA1c level influences the effect of periodontal therapy on glycemic control in people with type 2 diabetes and periodontitis: a systematic review on randomized controlled trails. Diabetes Ther. (2021) 12:1249–78. doi: 10.1007/s13300-021-01000-6
187. D'Aiuto F, Gkranias N, Bhowruth D, Khan T, Orlandi M, Suvan J, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. (2018) 6:954–65. doi: 10.1016/S2213-8587(18)30038-X
188. Roca-Millan E, Gonzalez-Navarro B, Sabater-Recolons MM, Mari-Roig A, Jane-Salas E, Lopez-Lopez J. Periodontal treatment on patients with cardiovascular disease: systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. (2018) 23:e681–90. doi: 10.4317/medoral.22725
189. Vidal F, Cordovil I, Figueredo CM, Fischer RG. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J Clin Periodontol. (2013) 40:681–7. doi: 10.1111/jcpe.12110
190. Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, Carter TR, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. (2006) 85:74–8. doi: 10.1177/154405910608500113
191. Choi SE, Sima C, Pandya A. Impact of treating oral disease on preventing vascular diseases: a model-based cost-effectiveness analysis of periodontal treatment among patients with type 2 diabetes. Diabetes Care. (2020) 43:563–71. doi: 10.2337/dc19-1201
192. Blaschke K, Seitz MW, Schubert I, Listl S. Methodological approaches for investigating links between dental and chronic diseases with claims data: a scoping study. J Public Health Dent. (2019) 79:334–42. doi: 10.1111/jphd.12335
193. Blaschke K, Hellmich M, Samel C, Listl S, Schubert I. The impact of periodontal treatment on healthcare costs in newly diagnosed diabetes patients: evidence from a German claims database. Diabetes Res Clin Pract. (2021) 172:108641. doi: 10.1016/j.diabres.2020.108641
194. Iwasaki M, Sato M, Yoshihara A, Miyazaki H. Effects of periodontal diseases on diabetes-related medical expenditure. Curr Oral Health Rep. (2016) 3:7–13. doi: 10.1007/s40496-016-0076-0
195. Shin JH, Takada D, Kunisawa S, Imanaka Y. Effects of periodontal management for patients with type 2 diabetes on healthcare expenditure, hospitalization and worsening of diabetes: an observational study using medical, dental and pharmacy claims data in Japan. J Clin Periodontol. (2021) 48:774–84. doi: 10.1111/jcpe.13441
196. Smits KPJ, Listl S, Plachokova AS, Van der Galien O, Kalmus O. Effect of periodontal treatment on diabetes-related healthcare costs: a retrospective study. BMJ Open Diabetes Res Care. (2020) 8:e001666. doi: 10.1136/bmjdrc-2020-001666
197. Solowiej-Wedderburn J, Ide M, Pennington M. Cost-effectiveness of non-surgical periodontal therapy for patients with type 2 diabetes in the UK. J Clin Periodontol. (2017) 44:700–7. doi: 10.1111/jcpe.12746
198. Sato M, Iwasaki M, Yoshihara A, Miyazaki H. Association between periodontitis and medical expenditure in older adults: a 33-month follow-up study. Geriatr Gerontol Int. (2016) 16:856–64. doi: 10.1111/ggi.12569
199. Allen EM, Ziada HM, O'Halloran D, Clerehugh V, Allen PF. Attitudes, awareness and oral health-related quality of life in patients with diabetes. J Oral Rehabil. (2008) 35:218–23. doi: 10.1111/j.1365-2842.2007.01760.x
200. Baccaglini L, Kusi Appiah A, Ray M, Yu F. US adults with diabetes mellitus: variability in oral healthcare utilization. PLoS ONE. (2021) 16:e0251120. doi: 10.1371/journal.pone.0251120
201. Burton WN, Chen CY, Li X, Schultz AB. Association between employee dental claims, health risks, workplace productivity, and preventive services compliance. J Occup Environ Med. (2017) 59:721–26. doi: 10.1097/JOM.0000000000001069
202. Chaudhari M, Hubbard R, Reid RJ, Inge R, Newton KM, Spangler L, et al. Evaluating components of dental care utilization among adults with diabetes and matched controls via hurdle models. BMC Oral Health. (2012) 12:20. doi: 10.1186/1472-6831-12-20
203. Chen Y, Zhang P, Luman ET, Griffin SO, Rolka DB. Incremental dental expenditures associated with diabetes among noninstitutionalized U.S. adults aged >/=18 years old in 2016-2017. Diabetes Care. (2021) 44:1317–23. doi: 10.2337/dc20-2744
204. Luo H, Bell RA, Wright W, Wu Q, Wu B. Trends in annual dental visits among US dentate adults with and without self-reported diabetes and prediabetes, 2004-2014. J Am Dent Assoc. (2018) 149:460–9. doi: 10.1016/j.adaj.2018.01.008
205. Macek MD, Tomar SL. Dental care visits among dentate adults with diabetes and periodontitis. J Public Health Dent. (2009) 69:284–9. doi: 10.1111/j.1752-7325.2009.00136.x
206. McGowan K, Phillips T, Gielis E, Dover T, Mitchell G, Mutch A, et al. Developing a prototype for integrated dental and diabetes care: understanding needs and priorities. Aust Dent J. (2021) 66:41–8. doi: 10.1111/adj.12804
207. Myers-Wright N, Lamster IB, Jasek JP, Chamany S. Evaluation of medical and dental visits in New York City: opportunities to identify persons with and at risk for diabetes mellitus in dental settings. Community Dent Oral Epidemiol. (2018) 46:102–8. doi: 10.1111/cdoe.12334
208. Reid J, Koopu P, Burkhardt N, Stewart T, Anderson A, Harwood M. Oral and dental health and health care for Maori with type 2 diabetes: a qualitative study. Community Dent Oral Epidemiol. (2020) 48:101–8. doi: 10.1111/cdoe.12501
209. Darling-Fisher CS, Borgnakke WS, Haber J. Oral health and diabetes; gain the confidence to discuss this important topic with your patients. Am Nurse Today. (2017) 12:22–5.
210. Darling-Fisher CS, Kanjirath PP, Peters MC, Borgnakke WS. Oral health: an untapped resource in managing glycemic control in diabetes and promoting overall health. J Nurse Pract. (2015) 11:889–96. doi: 10.1016/j.nurpra.2015.08.001
211. Miller K, Eke PI, Schoua-Glusberg A. Cognitive evaluation of self-report questions for surveillance of periodontitis. J Periodontol. (2007) 78:1455–62. doi: 10.1902/jop.2007.060384
212. Eke PI, Dye B. Assessment of self-report measures for predicting population prevalence of periodontitis. J Periodontol. (2009) 80:1371–9. doi: 10.1902/jop.2009.080607
213. Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Beck JD, et al. Self-reported measures for surveillance of periodontitis. J Dent Res. (2013) 92:1041–7. doi: 10.1177/0022034513505621
214. Verhulst MJL, Teeuw WJ, Bizzarro S, Muris J, Su N, Nicu EA, et al. A rapid, non-invasive tool for periodontitis screening in a medical care setting. BMC Oral Health. (2019) 19:87. doi: 10.1186/s12903-019-0784-7
215. Northridge ME, Kumar A, Kaur R. Disparities in access to oral health care. Annu Rev Public Health. (2020) 41:513–35. doi: 10.1146/annurev-publhealth-040119-094318
216. American Diabetes Association. Diabetes and Oral Health. Available online at: https://www.diabetes.org/diabetes/complications/keeping-your-mouth-healthy (accessed July 05, 2021).
217. IDF Clinical Guidelines Task Force. IDF Guideline on Oral Health for People With Diabetes. Brussels: International Diabetes Federation (2009). Available online at: http://www.idf.org/guidelines/diabetes-and-oral-health/guideline
218. Greenberg BL, Glick M. Assessing systemic disease risk in a dental setting: a public health perspective. Dent Clin North Am. (2012) 56:863–74. doi: 10.1016/j.cden.2012.07.011
219. Hein C. Scottsdale revisited: the role of dental practitioners in screening for undiagnosed diabetes and the medical co-management of patients with diabetes or those at risk for diabetes. Compend Contin Educ Dent. (2008) 29:538–40, 542–4, 546–53.
220. Lalla E, Kunzel C, Burkett S, Cheng B, Lamster IB. Identification of unrecognized diabetes and pre-diabetes in a dental setting. J Dent Res. (2011) 90:855–60. doi: 10.1177/0022034511407069
221. Shetty S, Kohad R, Yeltiwar R, Shetty K. Gingival blood glucose estimation with reagent test strips: a method to detect diabetes in a periodontal population. J Periodontol. (2011) 82:1548–55. doi: 10.1902/jop.2011.110009
222. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. (2020) 18:117–24. doi: 10.2174/1570161117666190502103733
223. Herman WH, Ye W, Griffin SJ, Simmons RK, Davies MJ, Khunti K, et al. Early detection and treatment of type 2 diabetes reduce cardiovascular morbidity and mortality: a simulation of the results of the Anglo-Danish-Dutch study of intensive treatment in people with screen-detected diabetes in primary care (ADDITION-Europe). Diabetes Care. (2015) 38:1449–55. doi: 10.2337/dc14-2459
224. Selph S, Dana T, Blazina I, Bougatsos C, Patel H, Chou R. Screening for type 2 diabetes mellitus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. (2015) 162:765–76. doi: 10.7326/M14-2221
225. Yonel Z, Batt J, Jane R, Cerullo E, Gray LJ, Dietrich T, et al. The role of the oral healthcare team in identification of type 2 diabetes mellitus: a systematic review. Curr Oral Health Rep. (2020) 7:87–97. doi: 10.1007/s40496-020-00250-w
226. Teeuw WJ, Kosho MX, Poland DC, Gerdes VE, Loos BG. Periodontitis as a possible early sign of diabetes mellitus. BMJ Open Diabetes Res Care. (2017) 5:e000326. doi: 10.1136/bmjdrc-2016-000326
227. Holm NCR, Belstrøm D, Østergaard JA, Schou S, Holmstrup P, Grauballe MCB. Identification of individuals with undiagnosed diabetes and pre-diabetes in a danish cohort attending dental treatment. J Periodontol. (2016) 87:395–402. doi: 10.1902/jop.2016.150266
228. AlGhamdi AST, Merdad K, Sonbul H, Bukhari SMN, Elias WY. Dental clinics as potent sources for screening undiagnosed diabetes and prediabetes. Am J Med Sci. (2013) 345:331–4. doi: 10.1097/MAJ.0b013e318287c96c
229. Montero E, Matesanz P, Nobili A, Luis Herrera-Pombo J, Sanz M, Guerrero A, et al. Screening of undiagnosed hyperglycaemia in the dental setting: the DiabetRisk Study; a field trial. J Clin Periodontol. (2021) 48:378–88. doi: 10.1111/jcpe.13408
230. Thomas MC, Walker MK, Emberson JR, Thomson AG, Lawlor DA, Ebrahim S, et al. Prevalence of undiagnosed type 2 diabetes and impaired fasting glucose in older British men and women. Diabet Med. (2005) 22:789–93. doi: 10.1111/j.1464-5491.2005.01516.x
231. Wright D, Muirhead V, Weston-Price S, Fortune F. Type 2 diabetes risk screening in dental practice settings: a pilot study. Br Dent J. (2014) 216:E15. doi: 10.1038/sj.bdj.2014.250
232. Estrich CG, Araujo MWB, Lipman RD. Prediabetes and diabetes screening in dental care settings: NHANES 2013 to 2016. JDR Clin Trans Res. (2019) 4:76–85. doi: 10.1177/2380084418798818
233. Franck SD, Stolberg RL, Bilich LA, Payne LE. Point-of-care HbA1c screening predicts diabetic status of dental patients. J Dent Hyg. (2014) 88:42–52.
234. Genco RJ, Schifferle RE, Dunford RG, Falkner KL, Hsu WC, Balukjian J. Screening for diabetes mellitus in dental practices: a field trial. J Am Dent Assoc. (2014) 145:57–64. doi: 10.14219/jada.2013.7
235. Herman WH, Taylor GW, Jacobson JJ, Burke R, Brown MB. Screening for prediabetes and type 2 diabetes in dental offices. J Public Health Dent. (2015) 75:175–82. doi: 10.1111/jphd.12082
236. Kalladka M, Greenberg BL, Padmashree SM, Venkateshaiah NT, Yalsangi S, Raghunandan BN, et al. Screening for coronary heart disease and diabetes risk in a dental setting. Int J Public Health. (2014) 59:485–92. doi: 10.1007/s00038-013-0530-x
237. Lalla E, Cheng B, Kunzel C, Burkett S, Lamster IB. Dental findings and identification of undiagnosed hyperglycemia. J Dent Res. (2013) 92:888–92. doi: 10.1177/0022034513502791
238. Philips KH, Zhang S, Moss K, Ciarrocca K, Beck JD. Periodontal disease, undiagnosed diabetes, and body mass index: implications for diabetes screening by dentists. J Am Dent Assoc. (2021) 152:25–35. doi: 10.1016/j.adaj.2020.09.002
239. Acharya A, Cheng B, Koralkar R, Olson B, Lamster IB, Kunzel C, et al. Screening for diabetes risk using integrated dental and medical electronic health record data. JDR Clin Trans Res. (2018) 3:188–94. doi: 10.1177/2380084418759496
240. Marshall SE, Cheng B, Northridge ME, Kunzel C, Huang C, Lamster IB. Integrating oral and general health screening at senior centers for minority elders. Am J Public Health. (2013) 103:1022–5. doi: 10.2105/AJPH.2013.301259
241. Jadhav AN, Tarte PR, Puri SK. Dental clinic: potential source of high-risk screening for prediabetes and type 2 diabetes. Indian J Dent Res. (2019) 30:851–4. doi: 10.4103/ijdr.IJDR_80_18
242. Engström S, Berne C, Gahnberg L, Svärdsudd K. Effectiveness of screening for diabetes mellitus in dental health care. Diabet Med. (2013) 30:239–45. doi: 10.1111/dme.12009
243. Su N, Teeuw WJ, Loos BG, Kosho MXF, van der Heijden G. Development and validation of a screening model for diabetes mellitus in patients with periodontitis in dental settings. Clin Oral Investig. (2020) 24:4089–100. doi: 10.1007/s00784-020-03281-w
244. Jain A, Chawla M, Kumar A, Chawla R, Grover V, Ghosh S, et al. Management of periodontal disease in patients with diabetes- good clinical practice guidelines: a joint statement by Indian Society of Periodontology and Research Society for the Study of Diabetes in India. J Indian Soc Periodontol. (2020) 24:498–524. doi: 10.4103/jisp.jisp_688_20
245. Bin Mubayrik A, Al Dosary S, Alshawaf R, Alduweesh R, Alfurayh S, Alojaymi T, et al. Public attitudes toward chairside screening for medical conditions in dental settings. Patient Prefer Adher. (2021) 15:187–95. doi: 10.2147/PPA.S297882
246. Greenberg BL, Glick M. Providing health screenings in a dental setting to enhance overall health outcomes. Dent Clin North Am. (2018) 62:269–78. doi: 10.1016/j.cden.2017.11.006
247. Rosedale MT, Strauss SM. Diabetes screening at the periodontal visit: patient and provider experiences with two screening approaches. Int J Dent Hyg. (2012) 10:250–8. doi: 10.1111/j.1601-5037.2011.00542.x
248. Esmeili T, Ellison J, Walsh MM. Dentists' attitudes and practices related to diabetes in the dental setting. J Public Health Dent. (2010) 70:108–14. doi: 10.1111/j.1752-7325.2009.00150.x
249. Greenberg BL, Glick M, Frantsve-Hawley J, Kantor ML. Dentists' attitudes toward chairside screening for medical conditions. J Am Dent Assoc. (2010) 141:52–62. doi: 10.14219/jada.archive.2010.0021
250. Yonel Z, Yahyouche A, Jalal Z, James A, Dietrich T, Chapple ILC. Patient acceptability of targeted risk-based detection of non-communicable diseases in a dental and pharmacy setting. BMC Public Health. (2020) 20:1576. doi: 10.1186/s12889-020-09649-7
251. Greenberg BL, Thomas PA, Glick M, Kantor ML. Physicians' attitudes toward medical screening in a dental setting. J Public Health Dent. (2015) 75:225–33. doi: 10.1111/jphd.12093
252. Friman G, Hultin M, Nilsson GH, Wårdh I. Medical screening in dental settings: a qualitative study of the views of authorities and organizations. BMC Res Notes. (2015) 8:580. doi: 10.1186/s13104-015-1543-8
253. Lalla E, Lamster IB. Assessment and management of patients with diabetes mellitus in the dental office. Dent Clin North Am. (2012) 56:819–29. doi: 10.1016/j.cden.2012.07.008
254. Lalla E, Cheng B, Kunzel C, Burkett S, Ferraro A, Lamster IB. Six-month outcomes in dental patients identified with hyperglycaemia: a randomized clinical trial. J Clin Periodontol. (2015) 42:228–35. doi: 10.1111/jcpe.12358
255. Haworth S, Shungin D, Kwak SY, Kim HY, West NX, Thomas SJ, et al. Tooth loss is a complex measure of oral disease: determinants and methodological considerations. Commun Dent Oral Epidemiol. (2018) 46:555–62. doi: 10.1111/cdoe.12391
Keywords: diabetes mellitus, early diagnosis, health care costs, interdisciplinary communication, interprofessional relations, periodontal diseases, prevention and control, referral and consultation
Citation: Borgnakke WS and Poudel P (2021) Diabetes and Oral Health: Summary of Current Scientific Evidence for Why Transdisciplinary Collaboration Is Needed. Front. Dent. Med. 2:709831. doi: 10.3389/fdmed.2021.709831
Received: 14 May 2021; Accepted: 22 June 2021;
Published: 29 July 2021.
Edited by:
Martha J. Somerman, National Institutes of Health (NIH), United StatesReviewed by:
Paul Rosen, University of Maryland, Baltimore, United StatesGuo-Hao Lin, University of Michigan, United States
Copyright © 2021 Borgnakke and Poudel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenche Sylling Borgnakke, d3NiQHVtaWNoLmVkdQ==
 Wenche Sylling Borgnakke
Wenche Sylling Borgnakke Prakash Poudel
Prakash Poudel