
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Dent. Med. , 15 November 2021
Sec. Periodontics
Volume 2 - 2021 | https://doi.org/10.3389/fdmed.2021.701197
This article is part of the Research Topic Biomarkers in Periodontal and Peri-Implant Diseases View all 4 articles
Recent investigations into the regulation of the inflammation in the periodontitis have revealed that chronic inflammatory diseases such as periodontitis are characterized by an imbalance in the proinflammatory and proresolution mediators and can be characterized by a failure of the resolution pathways in the late stages of the acute inflammatory response. The proresolution mediators, termed as specialized proresolving mediators (SPMs), comprise the lipoxins, resolvins, protectins, and maresins that are derived from the arachidonic acid or omega-3 polyunsaturated fatty acids. In the animal studies, treatment of the periodontitis with the topical SPMs return the inflammatory lesion to the homeostasis with the regeneration of all the components of the periodontal organ lost to the disease. In this article, the study investigates the immunomodulatory role of SPMs in the periodontal ligament stem cells (PDLSCs). Primary porcine PDLSCs (pPDLSCs) were stimulated with interleukin-1β (IL-1β) and interleukin-17 (IL-17) in vitro to simulate the periodontal inflammation in the presence or absence of SPMs. This study found that IL-1β and IL-17 synergistically activated the proinflammatory genes of pPDLSCs and altered the immune phenotype of pPDLSCs including the key signaling pathways. Addition of SPMs rescued the pPDLSCs phenotype and induced further production of the additional SPMs, which was reflected by upregulation of the requisite enzymes 12- and 15-lipoxygenase by pPDLSCs. This study interrogated the immunomodulatory actions of pPDLSCs on the monocytes/macrophages, focusing on the porcine CD14/CD16/CD163 markers by using flow cytometry. This study utilized the CD14+CD16+/CD14+CD16− ratio and CD163 on the monocytes/macrophages to differentiate between a proinflammation phenotype (lower ratio) and a resolution of the inflammation phenotype (higher ratio). This study also found that the conditioned medium from pPDLSCs treated with the cytokines and Maresin1 increased the CD14+CD16+/CD14+CD16− ratio and had the highest CD163 expression. This study concludes that in an inflammatory environment, pPDLSCs become proinflammatory and exert immunomodulatory functions. Maresin 1 resolves the inflammation by acting on pPDLSCs directly and by shifting the monocytes/macrophages phenotype to the proresolution dominance.
Periodontitis and peri-implantitis are the inflammatory diseases that are initiated by the bacteria (1). Recent investigations into the regulation of the inflammation in the periodontitis have revealed that the chronic inflammatory diseases such as periodontitis are characterized by an imbalance in the proinflammatory and proresolution mediators and can be characterized by a failure of the resolution pathways in the late stages of the acute inflammatory response (2, 3). Resolution of the inflammation is driven by a group of specialized proresolving mediators (SPMs) comprising the lipoxins, resolvins, protectins, and maresins that are derived from the arachidonic acid or omega-3 polyunsaturated fatty acids (PUFAs) (4). Resolution of the inflammation is an active process in which SPMs interact with their respective receptors on the cells to halt the neutrophil influx, skew monocytes/macrophages toward the M2-like phenotype with increased phagocytosis, and to limit the adaptive immune activation (5). These actions lead to a well-orchestrated quick return to the immune homeostasis. Importantly, administration of exogenous SPMs in pathological inflammatory lesions breaks the cycle of the chronicity and facilitates the return to the physiological form and function (5).
The repertoire of SPM biosynthesis is mainly the innate immune cells such as neutrophils, macrophages, and macrophage precursor monocytes (4). In addition, the resident stromal cells can produce SPMs under certain conditions and express SPM receptors. (3). The synthetic pathways of SPMs rely on the lipoxygenase enzymes that are processed by the various cell types (6). In humans, 5-lipoxygenase is the main enzyme for the leukotriene production (7), which creates a proinflammatory environment, whereas 12-lipoxygenase and 15-lipoxygenase are the main enzymes that drive SPM biosynthesis to promote the resolution phase (8). It is also important to note that SPMs act in an autocrine and/or paracrine fashion; SPMs further activate the lipoxygenase enzymes and accelerate SPM biosynthesis (9). Thus, regulation of the lipoxygenase enzymes in the disease is critical to understand the synthetic mechanisms of SPMs that fail in the chronic inflammatory diseases.
Resolution of the inflammation promotes the tissue regeneration, which emphasizes the role that the control of the inflammation plays in wound healing. Hasturk et al. provided the first evidence that the local application of resolvin E1 (RvE1) promoted the periodontal regeneration in a rabbit periodontitis model (10). Wang et al. later showed that Maresin1 (MaR1) accelerated the extraction socket regeneration by skewing the macrophages toward a proresolution M2-like phenotype (11). Cianci et al. has shown that the human periodontal ligament stem cells (hPDLSCs) release the abundant SPMs including lipoxin A4 (LXA4) that regulates the regenerative function of hPDLSCs (12). A recent study from our group has further demonstrated that RvE1 and MaR1 improve hPDLSCs regenerative properties by enhancing the proliferation, migration, and regulating molecular machinery to overcome the negative impact of the inflammation (13). In addition to producing SPMs and reacting to SPMs, the immunomodulatory properties of PDLSCs are also reflected in their rich synthesis of the immune markers and the impact on the immune cells. Enhancing the understanding of the immunomodulatory role of PDLSCs will provide valuable insights by further improving the periodontal regeneration.
In this study, we investigated PDLSCs from a large animal—the Yorkshire pig—to establish a large animal model to optimize translational value in regeneration related studies. The immunomodulatory properties of SPMs on pig PDLSCs (pPDLSCs) were examined to investigate the regulation of the lipoxygenase enzymes in pPDLSCs and to characterize the interaction between pPDLSCs and immune cells.
We extracted the deciduous incisors or canines from 3 to 6-month-old Yorkshire pigs postmortem at the Surgical Research Laboratory of the Tufts University. From the middle third of the root of the extracted teeth, we collected the periodontal ligament tissues and digested them with type I collagenase (Sigma #C0130, St. Louis, MO, USA) and dispase II (Sigma D4693) for 1 h at 37°C. After digestion, pPDLSCs were maintained in the Alpha Minimum Essential Medium (MEM) (Gibco #12-571-063 Amarillo, Texas, USA), supplemented with 10% heat-inactivated fetal bovine serum, and 1% primocin (InvivoGen #400120 San Diego, CA, USA) in a 5% carbon dioxide (CO2) incubator at 37°C. Mesenchymal stem cell markers (CAbcam #ab139364 Watham, MA, USA, CD73-R&D #AF4488 Minneapolis, MN, and CD105-R&D #AF1097) and hematopoietic stem cell markers (CD14-R&D #FAB4597N and CD45-Biorad #MCA1222GA Hercules, California, USA) were evaluated by the flow cytometry at the fourth or fifth passage to ensure the purity for the downstream experiments, which were conducted at the fifth or sixth passage. The pPDLSCs cells used in all the experiments were derived from the two pigs.
We challenged pPDLSCs with RvE1 (Cayman #10007848 Ann Arbor, MI, USA 10 nM) or MaR1 (Cayman #10878, 10 nM) for up to 48 h. We also examined that how pPDLSCs responded to the cytokine challenge with or without SPMs. We used the two proinflammatory cytokines that have negative impacts on the periodontal tissues: interleukin-1β (IL-1β) and interleukin-17 (IL-17) (14, 15). pPDLSCs were treated with either IL-1β (BioLegend #579402 San Diego, CA, USA final concentrations: 50 ng/ml), IL-17 (R&D #317-ILB, final concentration: 50 ng/ml), or in combination for 16 h, followed by RNA isolation. We also conducted a time series of the combined IL-1β and IL-17 challenge for a period of 48 h. Finally, we challenged pPDLSCs with the combined IL-1β and IL-17 in the absence or presence of SPMs (10 nM, RvE1 or MaR1) and conducted the multiple downstream experiments described below. SPM stocks were stored at −80°C under the inert nitrogen gas.
We collected the cells at the given time points for RNA isolation by using the Qiagen RNeasy Mini Kit (Cat #74106). 1.0 μg RNA was then transcribed to complementary DNA (cDNA) by using the SuperScript IV VILO Master Mix (Thermo Fisher Scientific #11756050 Waltham, MA, USA). RT-qPCR (Qiagen StepOnePlus Germantown, MD, USA) was conducted to examine the messenger RNA (mRNA) expression of multiple genes including the lipoxygenase enzyme genes−5-lipoxygenase (LOX5, Ss06909908_m1), LOX5-associated protein (FLAP, Ss03374421_m1), 12-liopoxygenase (LOX12) (Ss06825180_g1), and 15-liopoxygenase (LOX15) (Ss03392804_u1) and the immune regulatory genes—interleukin-6 (IL-6) (Ss03384604_u1), prostaglandin-endoperoxide synthase 2 (PTGS2) (Ss03394694_m1), and chemokine ligand 8 (CCL8) (Ss04245586_m1). PTGS2 and IL-6 are both the downstream inflammatory markers of IL-1β and IL-17 (16, 17). We sought to detect the genes that were critical for the immune cell activation including interferon-γ (IFNG), interleukin-4 (IL4), and CCL8 (18, 19). Whole-cell protein lysates were extracted by using the radioimmunoprecipitation assay (RIPA) buffer (Cell Signlaing #9806 Danvers, MA, USA) supplemented with the protease inhibitor (Sigma #11697498001) and the phosphoprotease inhibitor (Sigma #4906845001). Supernatants from the cell culture medium were saved at 48 h and stored at −80°C until used for the ELISA assays or saved as conditioned medium (CM) to treat the immune cells. Three cytokines were assayed by ELISA including prostaglandin E2 (PGE2) (Cayman #514010), IL-6 (MyBioSource #MBS2701081 San Diego, CA), and CCL-8 (MyBioSource #MBS2701134). We also tried to detect the indoleamine-2, 3-dioxygenase (IDO) protein by ELISA (MyBioSource #MBS269594) directly, as IDO is an important immune regulator for the mesenchymal stem cells (MSCs).
Next, we sought to evaluate the immunomodulatory effect of pPDLSCs on the monocytes/macrophage markers by using the conditioned medium (CM) from pPDLSCs. We freshly isolated peripheral blood mononuclear cells (PBMCs) from the pigs and seeded them at a density of 5 × 10∧5 cell per well in 24-well plates. Four CM treatments were applied to treat PBMCs for 24 h: control pPDLSCs treated with medium alone; pPDLSCs treated with IL-17 and IL-1β; pPDLSCs treated with IL-17, IL-1β, and RvE1; and pPDLSCs treated with IL-17, IL-1β, and MaR1. In a parallel experiment, we challenged PBMCs with 100 ng/ml phorbol 12-myristate 13-acetate (PMA) with or without RvE1 or MaR1 for 24 h. PMA is known to differentiate the monocytes to an M1-like phenotype (proinflammation) (20). Limited by the available specific antibodies to the surface markers of the pig to differentiate between an M1-like phenotype (proinflammation) and an M2-like phenotype (resolution of inflammation), we utilized a combination of CD14, CD16, and CD163 markers (R&D #FAB4597N, Bio-Rad #MCA1971PE, and Bio-Rad #MCA2311F) for the classification by the flow cytometry (Flow Cytometer, Attune NxT, Thermo Fisher Scientific, Waltham, MA, USA). An increased ratio of CD14+CD16+/CD14+CD16− with increased CD163 suggests a resolution of the inflammation phenotype; a decreased ratio of CD14+CD16+/CD14+CD16− with decreased CD163 suggests a proinflammatory phenotype (21).
The relative expression of the genes was determined by the 2−ΔΔCT method. The Kruskal–Wallis rank test was used to compare the differences in the expression levels and cell percentages between the multiple groups. Significance level was set at 0.05. All the statistics were conducted in the GraphPad Prism version 8.0 San Diego, CA, USA. For scientific rigor, a minimal of two biological replicates was used in each experiment and each experiment was repeated two to four times. In figures, we mainly presented the data from one representative experiment, unless stated otherwise.
We confirmed the stem/stromal cell properties of the isolated pPDLSCs by the flow cytometry and differentiation assays. Flow cytometry results are presented in Supplementary Figure 1. Greater than 90% of the isolated cells expressed CD73+, CD90+, and CD105+ (MSC markers) and lacked CD45+ and CD14+ (hematopoietic stem cell markers). In response to RvE1 (10 nM) and MaR1 (10 nM) challenge for 24 h, pPDLSCs exhibited a 1.5-fold increase in LOX12 and 2-fold increase in LOX15 mRNA expression. After the second challenge of SPMs at 24 h and collection at 48 h, the protein levels LOX15 enzyme were significantly increased by 20% by the Western blotting (Figure 1). In comparison, LOX5 and Flap mRNA and protein levels were not affected by SPMs (Supplementary Figure 2).
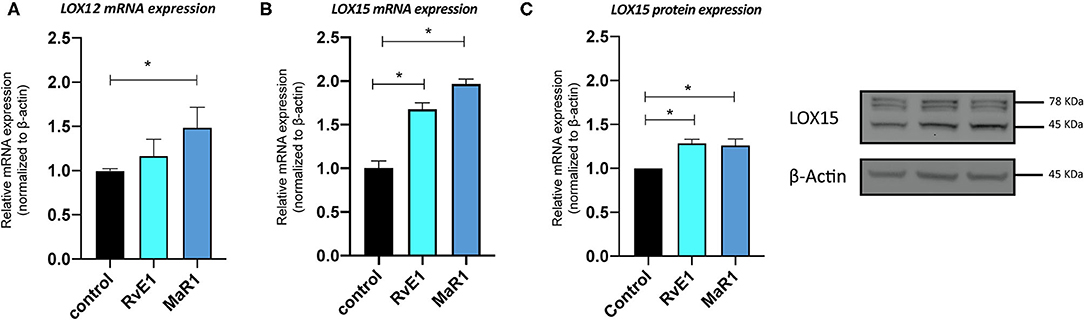
Figure 1. Specialized proresolving mediators (SPMs) elevate 12-lipoxygenase (LOX12) and 15-lipoxygenase (LOX15) enzymes in pig periodontal ligament stem cells (pPDLSCs). pPDLSCs had elevated messenger RNA (mRNA) levels of LOX12 and LOX15 after being challenged with Maresin1 (MaR1) or resolvin E1 (RvE1) (10 nM) and elevated mRNA of LOX12 after challenge by MaR1 at 24 h (A,B). At 48 h, LOX15 enzyme was slightly increased by SPMs by the Western blot (p < 0.05) (C). Densitometry was performed by using the Image J for the two independent replicates of the Western blots. Error bars represent SDs. *p < 0.05.
The mRNA expression levels of PTSG2 and IL-6 were significantly upregulated by IL-1β or IL-17; but, when administered together, the response was several folds higher than the sum of the individual treatments, indicating the synergistic actions (Figure 2). We subsequently examined PTSG2 and IL-6 gene regulation in a 48 h time-course. Interestingly, PTSG2 and IL-6 gene regulation was more elevated at 24 h and 48 h compared to the earlier time points (Figures 3A,B). We then evaluated CCL8 transcription and found that its mRNA expression reached the peak at 8 h (Supplementary Figure 3), suggesting that the induction patterns of IL-6 and PTGS2 were specific. We could not detect IFNG or IL-4 transcripts. Concomitantly, key molecules in the nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways were activated at 24 h and 48 h (Figure 3C).
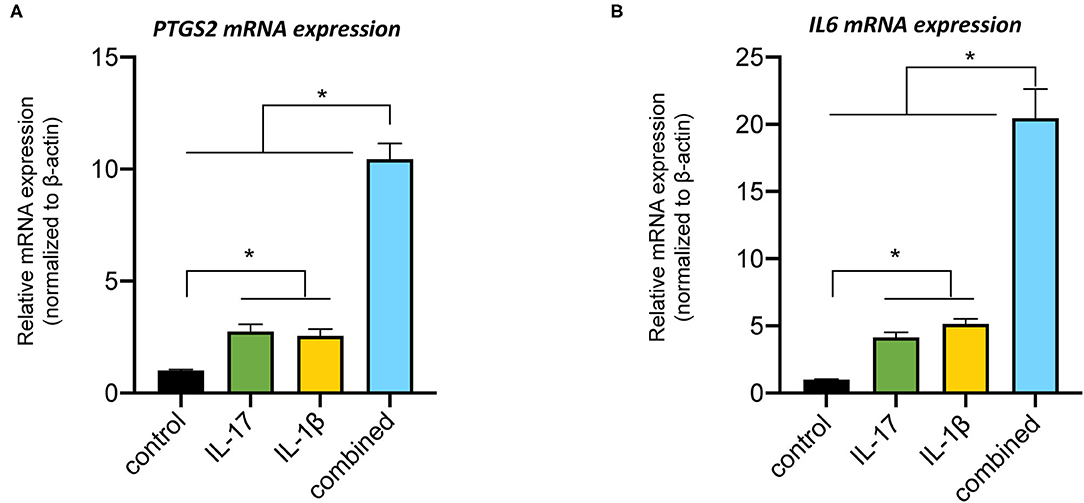
Figure 2. Synergistic actions of interleukin-1β (IL-1β) and interleukin-17 (IL-17) on the immune gene regulations in pPDLSCs. pPDLSCs were challenged with human recombinant IL-1β (50 ng/ml), recombinant IL-17 (50 ng/ml), or both combined for 16 h. Prostaglandin-endoperoxide synthase 2 (PTGS2) (A) and interleukin-6 (IL6) (B) mRNA transcripts were significantly elevated by the combined cytokine treatment indicating a synergistic effect. Error bars represent SDs. *p < 0.05.
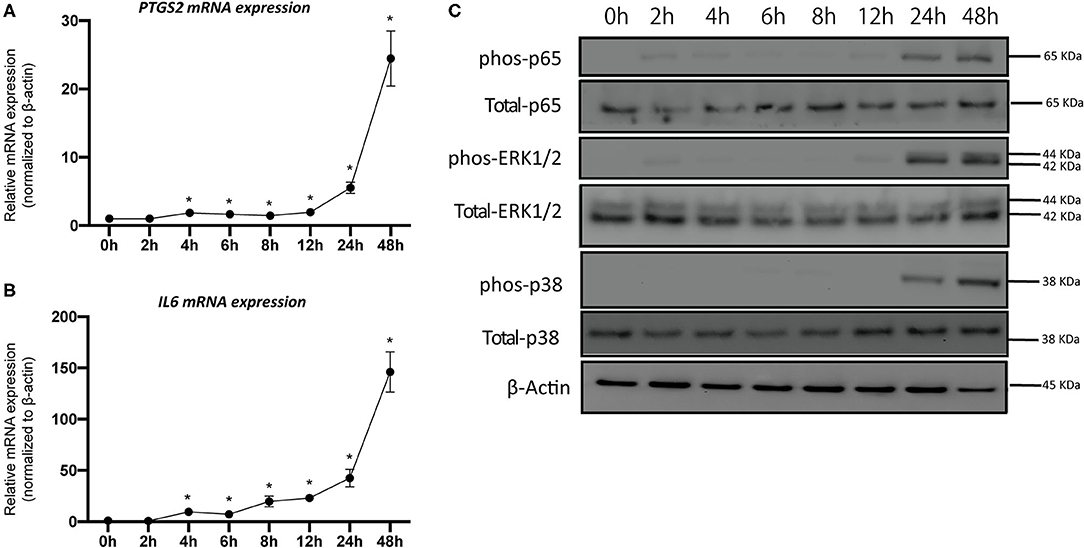
Figure 3. Combined IL-1β and IL-17 activate the immune phenotype of pPDLSCs. A time-course experiment was conducted to investigate the response of pPDLSCs to the combined cytokine treatment over 48 h. Both PTGS2 and IL-6 mRNA expression levels were significantly elevated after 4 h, but highly upregulated by 24 h and 48 h (A,B). Western blotting showed that both nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways were activated by the combined cytokine treatment (C). *p < 0.05 as compared to 0 h control.
After establishing the baseline immune gene regulation of pPDLSCs by the cytokines, we added RvE1 or MaR1 to determine their impacts on the upregulated proinflammatory cytokines. The mRNA level of PTGS2 and the levels of PGE2 were significantly lowered by the addition of MaR1 (Figures 4A,B). Likewise, the mRNA level of IL-6 gene was significantly lowered by the addition of MaR1 (Figure 4C); however, IL-6 protein levels were not significantly altered. In the ELISA assay, CCL8 protein was undetectable; cytokines or SPMs had minimal impact on the protein expression of IDO (Supplementary Figure 4). In the signaling pathway analysis, we found that phosphorylated p65 protein was inhibited by MaR1, but not RvE1 by the Western blot (Figures 4E,F), suggesting the inhibition of the classical NF-κB pathway. MAPK pathways were not affected by SPM (Supplementary Figure 5).
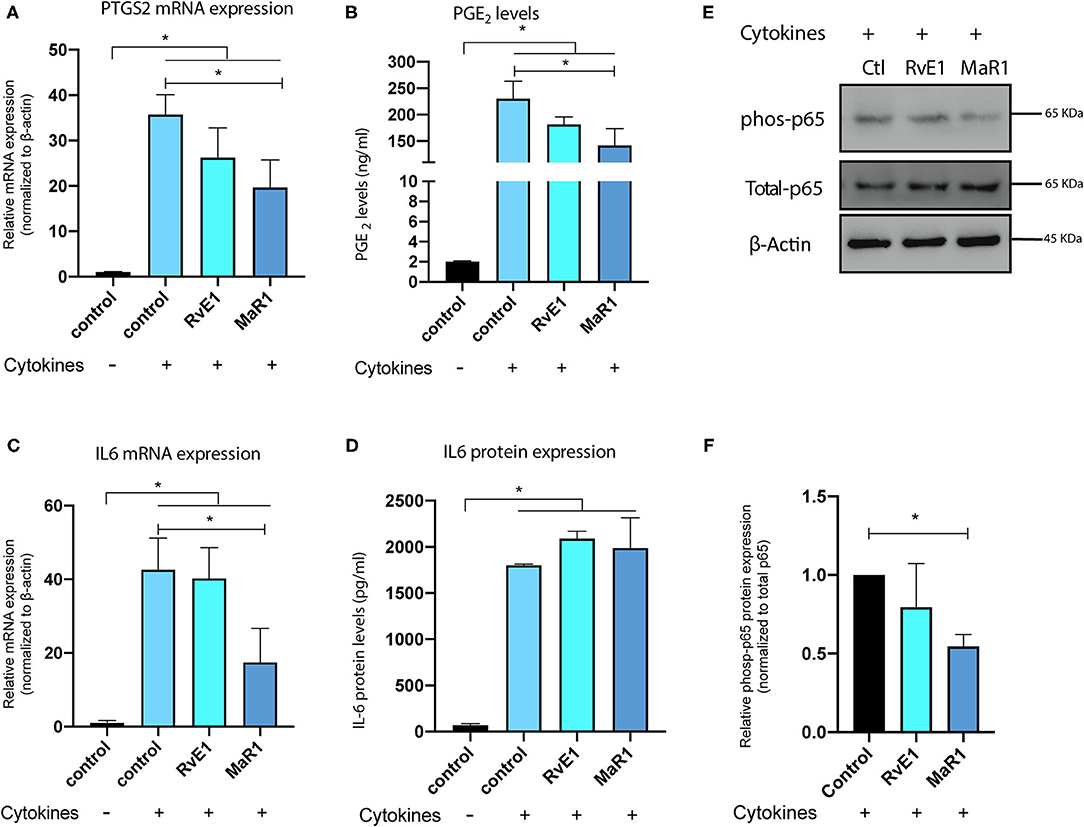
Figure 4. SPMs lower the upregulated proinflammatory cytokines induced by combined IL-1β and IL-17. PTGS2 and IL-6 mRNA levels were significantly elevated by the combined cytokine treatment after 24 h challenge, but lowered by the addition of MaR1 (*p < 0.05) (A,C). Prostaglandin E2 (PGE2) levels were significantly elevated by the combined cytokine treatment at 48 h challenge and lowered by MaR1 treatment (B). IL-6 protein levels were significantly elevated by the combined cytokine treatment, but not affected by SPM treatment (D). Western blot showed that MaR1 reduced activation of the NF-κB pathway (E). Densitometry is shown in (F).
Next, we wanted to determine the immunomodulatory actions of pPDLSCs on the monocytes/macrophages by using CM derived from pPDLSCs after the cytokine and/or SPM treatment. We first verified CD14+CD16+/CD14+CD16− ratio and CD163 expression to differentiate between a proinflammation phenotype (lower ratio) and a resolution of inflammation phenotype (higher ratio). We found that the traditional M2 marker CD163 expression was higher in CD14+CD16+ cells compared to CD14+CD16− cells (Figure 5A, p < 0.05). When differentiating PBMCs toward the proinflammatory monocytes/macrophages, we observed a reduced percentage of CD14+CD16+ cells (Figure 5B). Correspondingly, CD14+CD16+/CD14+CD16− ratio was reduced in PMA-differentiated PBMCs, but partially restored by SPMs (Figure 5C). CM from the cytokine group significantly reduced the CD14+CD16+/CD14+CD16− ratio; CM from the cytokine and MaR1 group restored the ratio to baseline (control without cytokines or SPMs) (Figure 6A). Furthermore, in CD14+CD16+ cells, cytokines + MaR1 had the highest CD163 expression (Figure 6B). Overall, we show that MaR1 facilitates the resolution of inflammation through direct and indirect pathways. Cell gating strategies are presented in Supplementary Figure 6.
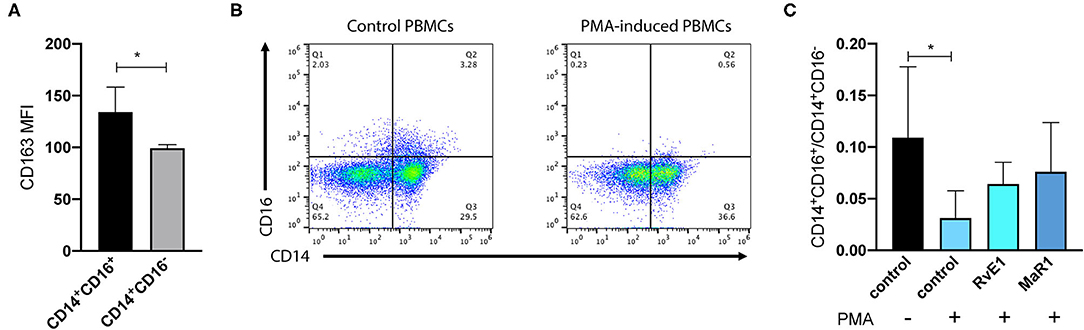
Figure 5. CD14+CD16+/CD14+CD16− ratio as an indicator for the resolution of the inflammation. We freshly isolated peripheral blood mononuclear cells (PBMCs) from the Yorkshire pigs and stained with CD14, CD16, and CD163 markers. CD163 (a traditional M2 marker) was significantly higher in CD14+CD16+ monocytes (nonclassical) compared to CD14+CD16− monocytes (classical) (A); MFI: median fluorescence intensity. For this reason, we argued that the CD14+CD16+/CD14+CD16− ratio served as an indicator for the resolution of the inflammation. To test this ratio, we differentiated PBMCs with phorbol 12-myristate 13-acetate (PMA) to the proinflammatory monocytes/macrophages and observed the reductions of CD14+CD16+ monocytes (B). CD14+CD16+/CD14+CD16− ratio was significantly reduced in PMA treatment and partially restored by SPMs (C). This figure was a composite from two independent experiments. *p < 0.05.
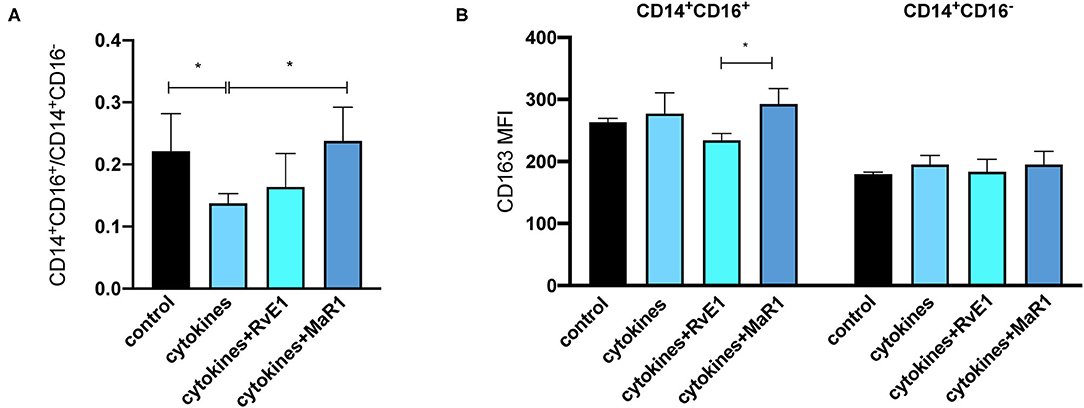
Figure 6. Impact of MaR1 on the resolution of the inflammation. We then challenged PBMCs with the conditioned medium (CM) from pPDLSCs that received the cytokines and/or SPMs treatment. CM from the cytokine groups significantly reduced the CD14+CD16+/CD14+CD16− ratio. CM from the cytokines and MaR1 group restored the ratio to baseline (control without cytokines or SPMs (A). This figure was a composite from two independent experiments. CD14+CD16+ cells expressed more CD163 compared to CD14+CD16− cells. In CD14+CD16+ cells, cytokines plus MaR1 had the highest CD163 expression (B).*p < 0.05.
In this study, we investigate the two major characteristics of PDLSCs: the stem cell function and immunomodulation. PDLSCs resemble other MSCs in their multipotency to differentiate into the fibroblasts, osteoblasts, and cementoblasts, confirming prior observations (22). Thus, PDLSCs are critical to restoring the periodontal tissues lost due to the disease. Importantly, PDLSCs play an important immunomodulatory role defined by the PDLSCs response to the inflammation. This immunomodulatory response of PDLSCs is manifested by the immune responsive actions and the influence of inflammation on the properties of the stem cells.
Periodontal ligament stem cells are conveniently harvested from the deciduous or permanent teeth, providing a reliable and reproducible cell source for the study of the periodontal regeneration. In this study, we used PDLSCs isolated from the deciduous teeth of the Yorkshire pig. We chose the porcine model, since they share the physiological and anatomical similarities to the humans in their teeth and tooth-supporting structures; pigs have been widely used in the periodontal regeneration studies (23–25). Indeed, in this study, pPDLSCs exhibited similar MSC markers to human PDLSCs (Supplementary Figure 1) as previously reported (13). Thus, the Yorkshire pig provides an excellent large animal model to guide the ongoing regeneration research leading up to human clinical trials.
Periodontal regeneration occurs in an inflammatory environment and PDLSCs sense the inflammation cues and produce the inflammatory cytokines (26). We demonstrate that there are synergistic actions of IL-1β and IL-17 that regulate PTGS2 and IL-6 gene expression by pPDLSCs (Figure 2). Individual actions of IL-1β or IL-17 on MSCs including PDLSCs have been well documented. Carrero et al. reported that in response to IL-1β challenge, bone marrow MSCs exhibited the major changes in the immune phenotype with increased production of the multiple inflammatory cytokines including cytokines critical for the recruitment of the leukocytes (27). IL-1β appears to have dual functions on PDLSCs. Mao et al. showed that the lower doses of IL-1β enhanced the osteogenic properties of PDLSCs, while the higher doses of IL-1β inhibited the osteogenic properties of PDLSCs (28). A recent study support this observation showing that IL-1β and tumor necrosis factor-α (TNF-α) together inhibit hPDLSCs proliferation, migration, and inhibit osteogenesis and cementogenesis (13).
Interleukin-17 is mainly released by T-helper 17 (Th17) cells during the inflammation with contribution from the other resident cells (29). Opinions on the impact of IL-17 on MSCs are divergent. In some studies, IL-17 was shown to cooperate with the other inflammatory cytokines to promote the immunosuppressive functions of MSCs (30, 31). In other studies, IL-17 enhanced proinflammatory cytokine production by MSCs that positively influenced osteogenesis (32). There are few reports of the direct impact of IL-17 on PDLSCs. Dordević et al. reported that IL-17 negatively regulated osteogenesis by PDLSCs and inhibited PDLSCs proliferation and migration (33). IL-1β and IL-17 together inhibit the proliferation of PDLSCs, agreeing with the negative regulatory role of IL-17. At the same time, the synergistic actions of IL-1β and IL-17 suggest that the stem cell niche in PDL tissues is profoundly affected by the inflammation; thus, understanding how to predictably regulate the local inflammatory environment will be important for the periodontal regeneration.
In the time-course experiments, it is interesting to note that PTSG2 and IL-6 were significantly more upregulated at 24 h and 48 h (Figures 3A,B). This mRNA induction pattern was specific to PTGS2 and IL-6, as the transcription of CCL8 peaked at an earlier time point and was almost depleted at 48 h (Supplementary Figure 3). It is possible that after a long exposure to the inflammatory cues, PDLSCs partially lose stemness properties and behave like a resident periodontal ligament fibroblast and are, thus, more immune sensitive. Fibroblasts are known to be the major source of PGE2 in the periodontal tissues (34). In Figure 3C, we also show activation of the corresponding NF-κB and MAPK pathways. Both the pathways are the immune response pathways downstream of IL-1β or IL-17; their activation plays an important role in defining the stem cell fate and in shaping the stem cell immune properties (27, 28, 35). The immunomodulatory role of MaR1 in lowering the proinflammatory cytokine production of PDLSCs was evident (Figure 4). However, we found inconsistency between the mRNA and protein data for the detection of IL-6. The levels of PGE2 were an order of greater magnitude. The level of PGE2 was hundreds of nanograms (~200 ng/ml), but ~2 ng/ml for IL-6 and ~20 pg/ml for CCL8. This suggests that the immunomodulatory properties of PDLSCs have specific patterns and some mediators are more dominant in the case of PGE2. Ern et al. demonstrated that PGE2 inhibits the osteogenic differentiation of PDLSCs (36). MaR1-hampered PGE2 production may be biologically important for two reasons: to alleviate the osteogenesis inhibition by PGE2 or change the dynamics of the crosstalk between PDLSCs and immune cells.
An additional objective of this study was to investigate the regulations of the lipoxygenase enzymes that mediate the production of SPMs including LOX5 and its activating protein FLAP, LOX12, and LOX15. These lipoxygenases catalyze the oxygenation of PUFAs to form SPM precursors (6). The SPM family includes lipoxins derived from arachidonic acids; E-series resolvins derived from eicosapentaenoic acid (EPA); and D-series resolvins, protectins, and maresins derived from docosahexaenoic acid (DHA) (37). LOX15 is predominantly expressed in the leukocytes and resident epithelium and LOX12 is predominantly expressed in the platelets (6, 38). In addition to participating in SPM biosynthesis, LOX5 and its activating protein FLAP induce the inflammation catalyzing the synthesis of the leukotrienes derived from arachidonic acid (39). In this study, we found that neither LOX5 nor FLAP expression was affected by SPM challenge of pPDLSCs (Supplementary Figure 2). However, under the same conditions, LOX12 and LOX15 were significantly elevated (Figure 1). These findings suggest that SPMs shift PDLSCs toward the resolution of the inflammation by activating LOX12 and LOX15, while maintaining baseline levels of LOX5.
The crosstalk between PDLSCs and immune cells is an important component of PDLSCs immune-modulatory properties. Overall, MSCs have the ability to recruit the leukocytes to the local tissues for the immune regulation (27). PDLSCs promote the polarization of the monocytes/macrophages toward a resolution phenotype (M2-like phenotype) to enhance the regeneration (40). Due to the limited markers to detect the conventional M1-like (proinflammation) or M2-like (resolution of inflammation) phenotype macrophages in the pigs, we utilized CD14, CD16, and CD163 to differentiate the phenotype macrophages. In humans, CD14+CD16− monocytes (classical) contribute more to proinflammation and CD14+CD16+ monocytes (non-classical) are viewed as anti-inflammatory (21). CD163, a conventional M2 marker, has divergent expression patterns within CD14+CD16− or CD14+CD16+ cells in different diseases (41). We demonstrate the feasibility of using CD14+CD16+/CD14+CD16− ratio to differentiate between the proinflammation and resolution of inflammation phenotype macrophages (Figure 5). CM from pPDLSCs treated with the cytokines (IL-1β and IL-17) drives monocytes/macrophages toward a proinflammatory phenotype compared to the control pPDLSC CM; MaR1 skews the system toward the resolution of inflammation (Figure 6). CM was collected 48 h after pPDLSCs were challenged with the cytokines and/or SPMs. By this time, pPDLSCs already exhibit the major immune gene regulatory changes and secrete many proteins to influence the expression of CD14/CD16/CD163 markers. It is not within the scope of this project, but worthwhile to further investigate what specific molecules contribute to the immune marker changes within the CM. MaR1 has already been shown to skew macrophages toward a proresolution M2-like phenotype (11). Because in PMA-differentiated PBMCs, MaR1 demonstrates the direct actions on promoting the resolution of inflammation, we conclude that MaR1 skews the inflammation toward the resolution through the direct and indirect mechanisms via pPDLSCs.
Specialized proresolving mediators have shown promise in the preclinical studies for the promotion of the periodontal regeneration (10). The principle that control of the inflammation is critical to the regeneration appears to be universal to all the tissues and evolutionarily conserved. Recently, the Serhan group explored the function of SPM/protein conjugates in planarian regeneration further linking the resolution of the inflammation and regeneration (42). Likewise, Norling et al. clearly demonstrate the role of SPMs in the wound healing and regeneration (43).
In conclusion, it is becoming clear that PDLSCs function cannot be separated in situ from the local inflammatory environment. PDLSCs function is negatively impacted by the curtailed local inflammation and regeneration. The role of SPMs in PDLSCs function is critical to their function mechanistically operating by the regulation of the mediator and the cellular surrounding environment including cytokine production and function and the modulation of the phenotype macrophages. This is further evidenced by the previously described production of SPMs by PDLSCs (12) and the response of PDLSCs to exogenously provided SPMs. Thus, continued elucidation of the actions and mechanisms of SPMs in stem cell-mediated periodontal and peri-implant regeneration will likely lead to improved regeneration outcomes in the future human therapies.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
NY, AR, and AD contributed to the design of the study, performing experiments, and writing of the manuscript. TVD contributed to the design of the study, discussions, writing, and critical review of the manuscript.
This study is supported by the NIDCR R01DE025020 (TVD) and NIDCR T90DE026110 (AR).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Ms Courtney Bogins from the Surgical Research Laboratory of the Tufts University for her assistance in acquiring the samples of the porcine. We thank Ms Michele Patel and Danielle Stephens from the Forsyth multiplex core facility for assisting the ELISA assay.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2021.701197/full#supplementary-material
1. Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol. (2018) 45:1–8. doi: 10.1111/jcpe.12935
2. Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, Van Dyke TE. Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1. PLoS ONE. (2011) 6:e24422. doi: 10.1371/journal.pone.0024422
3. Ferguson B, Bokka NR, Maddipati KR, Ayilavarapu S, Weltman R, Zhu L, et al. Distinct profiles of specialized pro-resolving lipid mediators and corresponding receptor gene expression in periodontal inflammation. Front Immunol. (2020) 11:1307. doi: 10.3389/fimmu.2020.01307
4. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. (2018) 128:2657–69. doi: 10.1172/JCI97943
5. Serhan CN, Chiang N, Dalli J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med. (2018) 64:1–17. doi: 10.1016/j.mam.2017.08.002
6. Mashima R, Okuyama T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. (2015) 6:297–310. doi: 10.1016/j.redox.2015.08.006
7. Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. (2007) 32:332–41. doi: 10.1016/j.tibs.2007.06.002
8. Freedman C, Tran A, Tourdot BE, Kalyanaraman C, Perry S, Holinstat M, et al. Biosynthesis of the maresin intermediate, 13S,14S-Epoxy-DHA, by Human 15-Lipoxygenase and 12-Lipoxygenase and its regulation through negative allosteric modulators. Biochemistry. (2020) 59:1832–44. doi: 10.1021/acs.biochem.0c00233
9. Chatterjee A, Komshian S, Sansbury BE, Wu B, Mottola G, Chen M, et al. Biosynthesis of proresolving lipid mediators by vascular cells and tissues. FASEB journal. (2017) 31:3393–402. doi: 10.1096/fj.201700082R
10. Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. (2007) 179:7021–9. doi: 10.4049/jimmunol.179.10.7021
11. Wang CW, Yu SH, Fretwurst T, Larsson L, Sugai JV, Oh J, et al. Maresin 1 promotes wound healing and socket bone regeneration for alveolar ridge preservation. J Dent Res. (2020) 99:930–7. doi: 10.1177/0022034520917903
12. Cianci E, Recchiuti A, Trubiani O, Diomede F, Marchisio M., Miscia S, et al. Human periodontal stem cells release specialized proresolving mediators and carry immunomodulatory and prohealing properties regulated by lipoxins stem cells. Transl Med. (2016) 5:20–32. doi: 10.5966/sctm.2015-0163
13. Albuquerque-Souza E, Schulte F, Chen T, Hardt M, Hasturk H, Van Dyke TE, et al. Maresin-1 and resolvin E1 promote regenerative properties of periodontal ligament stem cells under inflammatory conditions. Front Immunol. (2020) 11:585530. doi: 10.3389/fimmu.2020.585530
14. Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, et al. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. (2009) 24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x
15. Cheng R, Wu Z, Li M, Shao M, Hu T. Interleukin-1beta is a potential therapeutic target for periodontitis: a narrative review. Int J Oral Sci. (2020) 12:2. doi: 10.1038/s41368-019-0068-8
16. Liu W, Reinmuth N, Stoeltzing O, Parikh AA, Tellez C, Williams S, et al. Cyclooxygenase-2 is up-regulated by interleukin-1 beta in human colorectal cancer cells via multiple signaling pathways. Cancer Res. (2003) 63:3632–6. Available online at: https://cancerres.aacrjournals.org/content/63/13/3632.long
17. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
18. Silva-Filho JL, Caruso-Neves C, Pinheiro AAS. IL-4: an important cytokine in determining the fate of T cells. Biophys Rev. (2014) 6:111–8. doi: 10.1007/s12551-013-0133-z
19. Tewari K, Nakayama Y, Suresh M. Role of direct effects of IFN-gamma on T cells in the regulation of CD8 T cell homeostasis. J Immunol. (2007) 179:2115–25. doi: 10.4049/jimmunol.179.4.2115
20. Starr T, Bauler TJ, Malik-Kale P, Steele-Mortimer O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS ONE. (2018) 13:e0193601. doi: 10.1371/journal.pone.0193601
21. Narasimhan PB, Marcovecchio P, Hamers AAJ, Hedrick CC. Nonclassical monocytes in health and disease. Annu Rev Immunol. (2019) 37:439–56. doi: 10.1146/annurev-immunol-042617-053119
22. Roguljic H, Matthews BG, Yang W, Cvija H, Mina M, Kalajzic I. In vivo identification of periodontal progenitor cells. J Dent Res. (2013) 92:709–15. doi: 10.1177/0022034513493434
23. Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. (2008) 26:1065–73. doi: 10.1634/stemcells.2007-0734
24. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. (2006) 1:e79. doi: 10.1371/journal.pone.0000079
25. Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, et al. Proresolving nanomedicines activate bone regeneration in periodontitis. J Dental Res. (2015) 94:148–56. doi: 10.1177/0022034514557331
26. Thomas MV, Puleo DA. Infection, inflammation, and bone regeneration: a paradoxical relationship. J Dent Res. (2011) 90:1052–61. doi: 10.1177/0022034510393967
27. Carrero R, Cerrada I, Lledo E, Dopazo J, Garcia-Garcia F, Rubio MP, et al. IL1beta induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-kappaB. Stem Cell Rev Rep. (2012) 8:905–16. doi: 10.1007/s12015-012-9364-9
28. Mao CY, Wang YG, Zhang X, Zheng XY, Tang TT, Lu EY. Double-edged-sword effect of IL-1beta on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-kappaB, MAPK and BMP/Smad signaling pathways. Cell Death Dis. (2016) 7:e2296. doi: 10.1038/cddis.2016.204
29. Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. (2008) 223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x
30. Han X, Yang Q, Lin L, Xu C, Zheng C, Chen X, et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. (2014) 21:1758–68. doi: 10.1038/cdd.2014.85
31. Ma T, Wang X, Jiao Y, Wang H, Qi Y, Gong H, et al. Interleukin 17 (IL-17)-Induced mesenchymal stem cells prolong the survival of allogeneic skin grafts. Ann Transplant. (2018) 23:615–21. doi: 10.12659/AOT.909381
32. Liao C, Zhang C, Jin L, Yang Y. IL-17 alters the mesenchymal stem cell niche towards osteogenesis in cooperation with osteocytes. J Cell Physiol. (2020) 235:4466–80. doi: 10.1002/jcp.29323
33. Dordevic IO, Kukolj T, Krstic J, Trivanovic D, Obradovic H, Santibanez JF, et al. The inhibition of periodontal ligament stem cells osteogenic differentiation by IL-17 is mediated via MAPKs. Int J Biochem Cell Biol. (2016) 71:92–101. doi: 10.1016/j.biocel.2015.12.007
34. Bage T, Kats A, Lopez BS, Morgan G, Nilsson G, Burt I, et al. Expression of prostaglandin E synthases in periodontitis immunolocalization and cellular regulation. Am J Pathol. (2011) 178:1676–88. doi: 10.1016/j.ajpath.2010.12.048
35. Lin D, Li L, Sun Y, Wang W, Wang X, Ye Y, et al. IL-17 regulates the expressions of RANKL and OPG in human periodontal ligament cells via TRAF6/TBK1-JNK/NF-kappaB pathways. Immunology. (2014) 144:472–85. doi: 10.1111/imm.12395
36. Ern C, Berger T, Frasheri I, Heym R, Hickel R, Folwaczny M. Differentiation of hMSC and hPDLSC induced by PGE2 or BMP-7 in 3D models. Prostaglandins Leukot Essent Fatty Acids. (2017) 122:30–7. doi: 10.1016/j.plefa.2017.06.005
37. Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M, Norris PC, et al. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun. (2018) 9:59. doi: 10.1038/s41467-017-02538-5
38. Snodgrass RG, Brune B. Regulation and functions of 15-Lipoxygenases in human macrophages. Front Pharmacol. (2019) 10:719. doi: 10.3389/fphar.2019.00719
39. Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. (1990) 323:645–55. doi: 10.1056/NEJM199009063231006
40. Liu J, Chen B, Bao J, Zhang Y, Lei L, Yan F. Macrophage polarization in periodontal ligament stem cells enhanced periodontal regeneration. Stem Cell Res Ther. (2019) 10:320. doi: 10.1186/s13287-019-1409-4
41. Glezeva N, Voon V, Watson C, Horgan S, McDonald K, Ledwidge M, et al. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: evidence of M2 macrophage activation in disease pathogenesis. J Card Fail. (2015) 21:167–77. doi: 10.1016/j.cardfail.2014.11.004
42. Chiang N, de la Rosa X, Libreros S, Pan H, Dreyfuss JM, Serhan CN. Cysteinyl-specialized proresolving mediators link resolution of infectious inflammation and tissue regeneration via TRAF3 activation. Proc Nation Acad Sci. (2021) 118:e2013374118. doi: 10.1073/pnas.2013374118
Keywords: periodontitis, stem cell, immunology, regeneration, omega-3 fatty acids
Citation: Yu N, Rakian A, Dean A and Van Dyke TE (2021) Specialized Proresolving Mediators Facilitate the Immunomodulation of the Periodontal Ligament Stem Cells. Front. Dent. Med. 2:701197. doi: 10.3389/fdmed.2021.701197
Received: 27 April 2021; Accepted: 20 September 2021;
Published: 15 November 2021.
Edited by:
Jamil Awad Shibli, Guarulhos University, BrazilReviewed by:
Oleh Andrukhov, University Dental Clinic Vienna, AustriaCopyright © 2021 Yu, Rakian, Dean and Van Dyke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas E. Van Dyke, dHZhbmR5a2VAZm9yc3l0aC5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.