- 1Department of Periodontology, Faculty of Medicine, Sigmund Freud University Vienna, Vienna, Austria
- 2Division of Conservative Dentistry and Periodontology, University Dental Clinic, Medical University of Vienna, Vienna, Austria
Saliva has the potential to be used as a diagnostic and monitoring tool for various diseases if biomarkers of an adequate sensitivity and specificity could be identified. Several reviews and even meta-analyses have been performed in recent years, which have found some candidate biomarkers for periodontitis, like macrophage inflammatory protein-1 alpha, interleukin-1ß, interleukin-6, matrix metalloproteinase-8, or hemoglobin. However, none of those are currently in use to replace conventional periodontal diagnostics with a periodontal probe. For periimplantitis, to date, heterogeneity of different study protocols and implant types did not permit to discover clear biomarkers, which were able to distinguish between healthy and diseased implants. Few proinflammatory cytokines, similar to periodontitis, have been characterized as adjunct tools to clinical diagnosis. The additional determination of antimicrobial peptides, bone turnover markers, and bacteria could help to enhance sensitivity and specificity in a combined model for periodontitis and periimplantitis. Furthermore, proteomic approaches might be preferred over single biomarker determinations. A global consensus is also needed to harmonize salivary sampling methods as well as procedures of biomarker analysis to ensure future comparability.
Introduction
Periodontitis is one of the most prevalent noncommunicable diseases worldwide, affecting around 796 million people in its severe form (1). The prevalence of periimplantitis has been reported inconsistently, but according to recent data, it is about between 18 and 10% per subject and per implant, respectively, and its occurrence is linked to periodontitis (2, 3). The diagnosis of periodontitis and periimplantitis is still based on clinical evaluation using a periodontal probe and radiography and is currently defined after the combined AAP/EFP workshop on a new classification system 2017 (4). Those classical methods can reflect the current state of inflammation and attachment loss, but are limited in the detection of early tissue degradation with low predictive potential.
In the last decades, saliva came into the focus as a noninvasive diagnostic fluid for oral and systemic diseases (5, 6). Its collection is easy, at a low cost, and does not need trained medical staff (7). Since it contains, e.g., hormones, growth factors, enzymes, antibodies as well as microbes and their products, it might be useful in the early detection of systemic diseases, like cancer, autoimmune disorders, cardiovascular disease, diabetes, or virus-related diseases (7, 8). The diagnostic potential for saliva in ambulatory care and under self-collection conditions has recently been summarized in a meta-analysis for SarS-CoV-2 RT-PCR testing (9).
For periodontitis, one of the first studies about marker analysis in saliva was published by Wilton et al. (10). The conclusion of this very first study still mirrors the state of knowledge in 2021, affirming that saliva could be seen as a source of indicators for disease activity or response to treatment more than discriminating diagnostic potential. Furthermore, it was concluded in this article that the determination of markers in saliva gives no more information than one could get by direct clinical examination. In the United States, enthusiastic prospects were described in the 2010s, seeing even serum analysis for systemic diseases replaced by noninvasive saliva analysis and designating salivary diagnostic as a “game changer” for patient evaluation (5, 11–13).
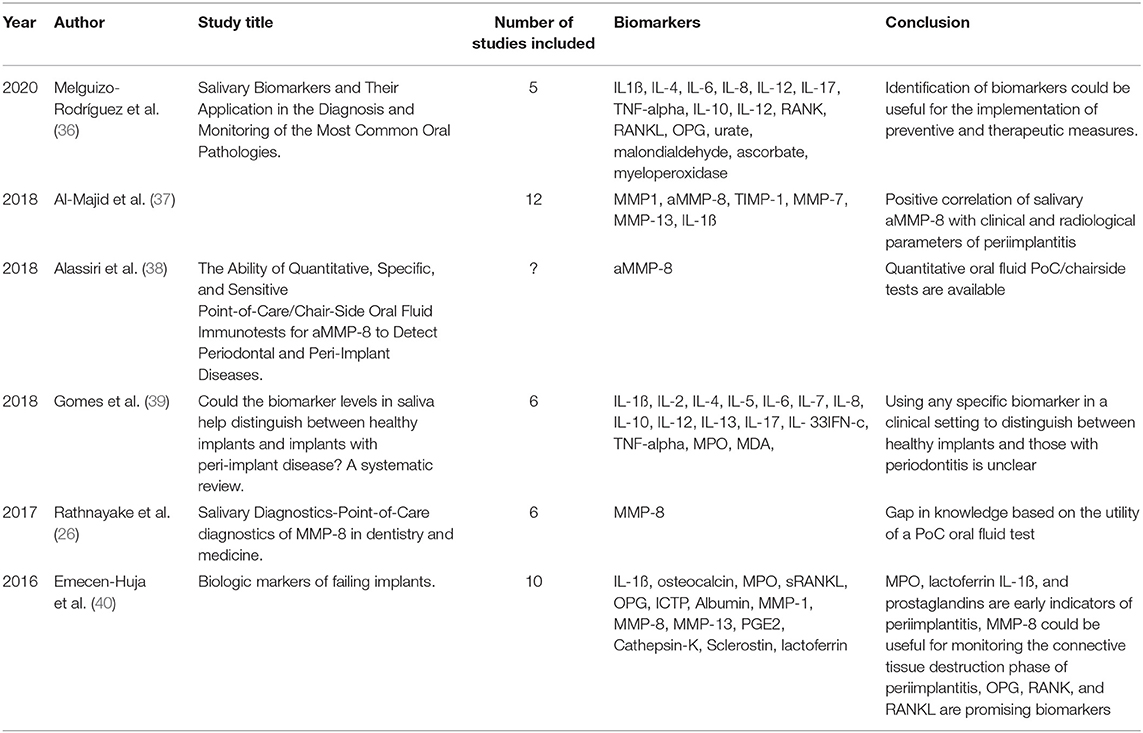
In the last few years, an increasing number of studies about saliva and periodontitis with around 70 publications per year were performed principally at universities in the United States, Europe, South America, and Asia (Figure 1). To give an overview about the current state of salivary biomarkers in connection with periodontitis and periimplantitis, a systematic research was performed in PubMed to screen articles published until December 2020, using the keywords saliva AND biomarkers AND periodontitis. Six hundred and twenty-three articles were found, including systematic reviews and meta-analyses. Of those articles, 120 were not considered, since they were animal studies, articles in foreign languages without a detailed description in the English abstract, listed twice or did not include saliva analysis, periodontitis, or periimplantitis cases (Figure 2A and Supplementary Material). The research revealed that the very first studies were conducted in Russia, Austria, Japan, Germany, and the United States. Later on, more study groups around the world discovered potential salivary biomarkers associated with periodontitis and since 2010 also with periimplantitis. Evidence is growing fast with an increasing number of studies since the 1980s (Figure 2B), which allow systematic reviews and meta-analyses for certain parameters. Periimplantitis, however, has not yet been extensively studied, and the research in PubMed for articles published until December 2020 using the keywords saliva AND biomarkers AND periimplantitis revealed only 15 articles. There exist already several systematic reviews that are summarizing the most promising diagnostic markers in different categories: bacteria-derived salivary markers, host-derived salivary biomarkers associated with inflammation, and biomarkers linked to soft or hard tissue destruction (14–16). However, a lot of studies about biomarker candidates have in part not been included in those reviews due to missing sensitivity and specificity calculations, detailed description of material and methods, or other factors that could thwart the reliability of results. This selection is sometimes distorting, since biomarkers such as MIP-1 alpha are included in a meta-analysis (16), although it was investigated by 10 studies only. However, there are already longitudinal studies including MIP-1 alpha, giving it a strong candidacy as a diagnostic marker (17).
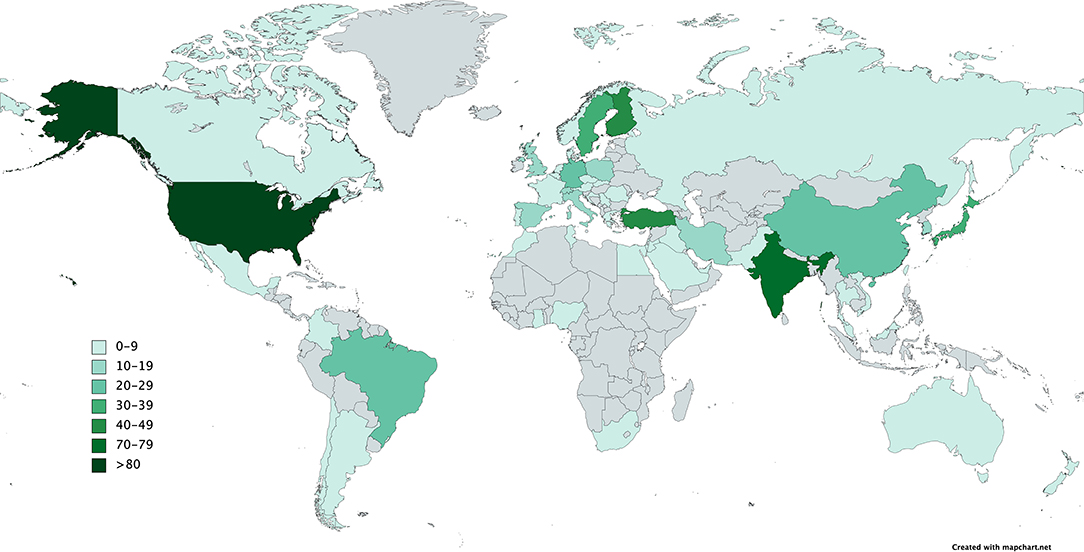
Figure 1. Number of publications per country according to the affiliations of all authors of the PubMed search about saliva, biomarkers, and periodontitis until December 2020.
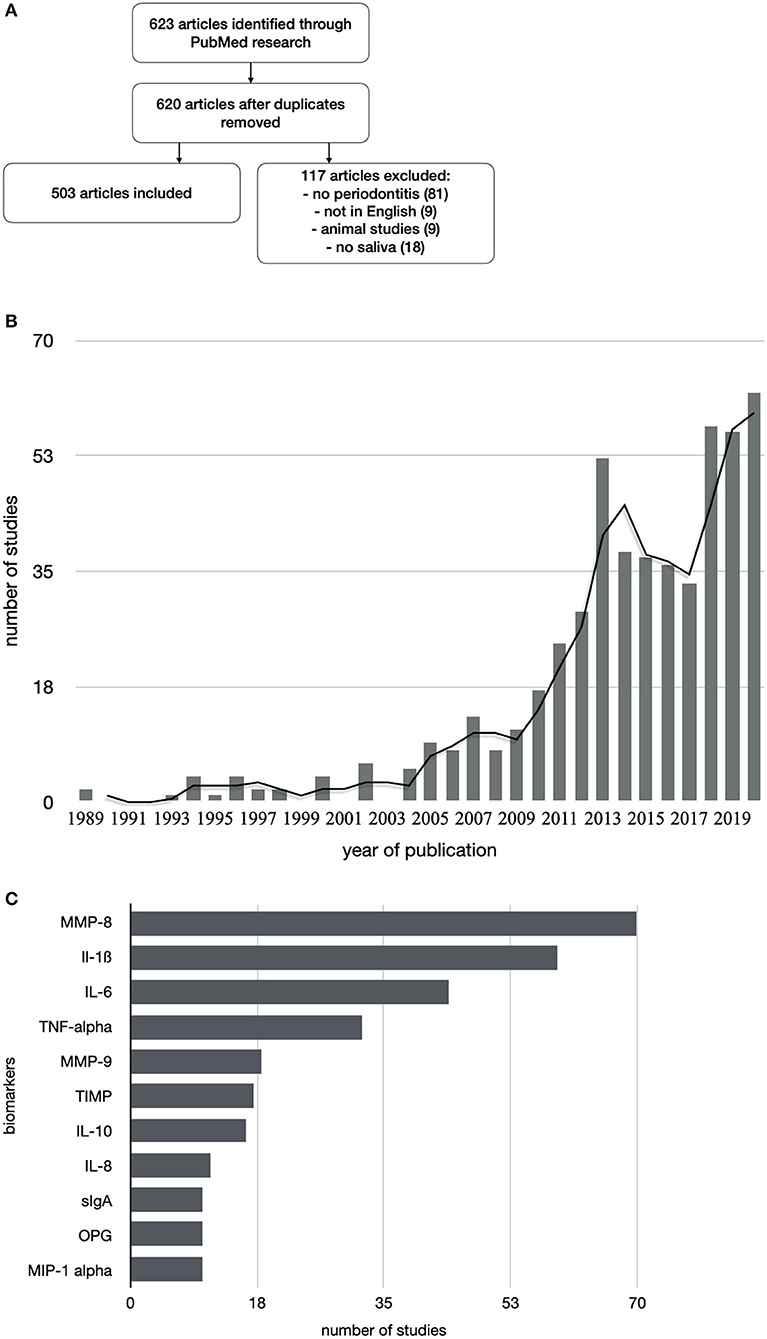
Figure 2. Flowchart of study selection (A). Increasing numbers of publications about salivary biomarkers and periodontitis over the last 30 years (B). The best investigated salivary biomarkers for periodontitis are MMP-8 with a total of 70 publications and proinflammatory cytokines (C).
This article should give an overview about the current state of knowledge not claiming to be seen as systematic reviews that are already existing in this field (16, 18, 19), but to give a reflection on how this dynamic field of research evolved and yield a tremendous potential in the diagnosis and monitoring of periodontitis and periimplantitis.
Saliva Collection Methods
Numerous saliva collection methods have been used, ranging from unstimulated whole saliva collection (20), stimulated whole saliva collection to different saliva collection systems, which could have a significant influence on biomarkers, such as proteins, in saliva (21). Proceeding of collected saliva samples, centrifugation, and storage are not overall defined and vary according to the following diagnostic method. The storage and further processing might have an important impact on the results; therefore, Henson et al. defined already in 2010 protocols for a standardized molecular analysis of salivary diagnostic constituents (22). Possible interactions of analytes with investigated biomarkers cannot be excluded, and the individual microbiome and proteome of the study population could influence the significance of the results (23). In most of the cross-sectional studies, unstimulated saliva was collected and concentrations were determined and compared between groups afterward. These procedures mostly do not take into account individual parameters, which could have a significant influence on biomarker concentrations. Individual salivary flow, gender, but mostly specific responses to inflammatory stimulus were often not included in the assessment of biomarker levels in the saliva samples. One should also be aware of the limited significance when only a single saliva sampling took place at a certain time point. Studies with stress-related biomarkers have shown, for example, that certain individuals had an immediate release of a substance, while in others, this release could be found only later, when a second sampling was performed (24).
Diagnostic Methods
The majority of the studies were using enzyme-linked immune assays for biomarker detection but more innovative methods are upcoming, such as omics technologies, which are seen as a novel and holistic approach in the management, diagnosis, prognosis, and monitoring of oral diseases (25). Recently, lateral-flow immunoassays were also shown to be suitable for the detection, prediction, or treatment outcome of periodontitis and periimplantitis (26). Furthermore, novel biodetection systems via protein fingerprinting with data processing were proposed as a convenient system for the examination of periodontal disease (27). Methods, which reduce hands-on time and easy sample preparation, like magnet-beating were shown to be suitable for preanalytic processing of saliva for automated point of care (PoC) protein analysis (28). Those PoC devices can be based on various techniques for the detection of periodontopathogens, proteins, metabolites, and small molecules (29, 30).
Another promising, rapid, and label-free diagnostic biometric tool in saliva can be provided by vibrational spectroscopy (31). Diagnostic models can eventually be constructed by combining protein and microbial profiles and computing diagnostic powers via areas under the receiver-operating characteristic (ROC) curve (32). For evaluating disease progression or stability, an individual approach was seen to be most suitable using unique patient profiles for salivary expression profiles of IL-1ß, IL-6, MMP-8, and MIP1-alpha (33, 34).
Salivary Biomarkers Discriminating Periodontal Health and Disease
Proinflammatory cytokines and proteinases have been extensively investigated in mostly cross-sectional studies. Ebersole et al. described that IL-1ß, IL-6, MMP-8, and MIP-1 alpha could be seen as suitable markers to discriminate health from gingivitis and periodontitis (19). Arias-Bujanda summarized accordingly in a recent meta-analysis that the highest values of sensitivity for periodontitis were obtained for IL-1ß, MMP-8, IL-6, and hemoglobin (15). MMP-8 is by far the best investigated biomarker for periodontitis and periimplantitis and a strong biomarker candidate for detecting alveolar bone destruction (35) (Figure 2C and Table 1). MIP-1 alpha has also great potential as a periodontitis biomarker since it showed high sensitivity and specificity and a good correlation with probing depths and the onset of bone loss (41). This biomarker is particularly interesting since it is the only one that has been used in a longitudinal study of children at risk for periodontal disease (41). Most research focused on different salivary markers, and analysis of microbes or their metabolites was scarce (42). The combination of salivary biomarkers and bacteria seems promising since periodontopathic bacteria were detectable comparably to subgingival plaque sampling (43), and a cumulative use of bacterial and host-derived biomarkers showed encouraging results (44). Furthermore, biomarkers like alanine aminotransferase levels and P. gingivalis ratio could be potential indicators for the progression of periodontitis (45). Longitudinal studies are missing to evaluate the ability of salivary copy counts of major periodontopathic bacteria predicting further periodontal breakdown (46). The combination of salivary biomarkers, MMPs, and bacterial biofilm generated ROC curves with a strong diagnostic ability (47). Commercially available tests have already been developed but there are still challenges regarding the introduction of new technologies to clinical practice and adoption by dental practitioners (11).
Salivary Biomarkers and Periimplantitis
To date, few studies exist about salivary biomarkers in periimplantitis; nevertheless, some proteinases and cytokines have been identified to possibly serve as a diagnostic or monitoring instrument for this disease. MMP-8 levels were increased in the saliva or periimplant crevicular fluid (37, 38), notably in patients who also suffered from periodontitis (48). This was also observed in patients who suffered from cardiovascular diseases, where MMP-8 was seen as a PoC biomarker (49). Increased levels of T. denticola, IL-4, IL-10 were detected in the saliva of patients with implants and type-2 diabetes (50). A list of major biomarkers for periimplantitis validated by reviews are given in Table 1.
Biomarkers Evaluating Periodontal Therapy
Besides clinical parameters such as Bleeding on Probing and Clinical Attachment Level, salivary biomarkers could also be useful to monitor the response of local tissue inflammation following periodontal therapy. A decrease in enzyme levels after scaling has been described as well as increases in anti-inflammatory biomarkers, such as melatonin (51–53). Antimicrobial peptides of the innate immune system, such as calprotectin, could also be used in oral fluid diagnosis to monitor treatment outcome (54–56), a biomarker linked to the innate immune system and first described in 2000 by Kojima et al. (57) connection with periodontitis. Salivary sTLR-2 also could have the potential as a prognostic or maintenance marker for periodontitis as well as antioxidants such as total antioxidant capacity, albumins, uric acid, superoxide dismutase, and glutathione peroxidase (58, 59). Arginase activity in saliva was also successfully reduced after nonsurgical periodontal therapy in accordance with a reduction in clinical and microbiological parameters (60). The determination of 8-hydroxy-deoxyguanosine levels also showed significant effects of periodontal therapy and could be regarded as a disease activity marker (61, 62), which was first described by Takane et al. already in 2002 (63). In patients with metabolic syndrome, successful periodontitis therapy could be mirrored in the reduction of oxidative stress markers (64). IL-1ß, MMP-8, OPG, and MIP-1 alpha, which had in part the highest sensitivity for periodontitis, are also able to reflect the response to therapy with the potential to be seen as monitoring markers for the periodontal status as well (65).
Influencing Factors on Salivary Biomarkers
The heterogeneity of evidence makes it difficult to compare and review existing studies. Biomarker analysis is dependent on many factors, like the collection system, the individual salivary flow rate (66), stimulated or unstimulated sampling, time of sampling, centrifugation and processing, the storage of samples, and the detection method. Furthermore, aggravating factors for periodontitis such as smoking (67–69) or stress, but also gender have a locally or systemically influence on the secretion of biomarkers into saliva (66, 70, 71). It was also shown that blood contamination of saliva samples could have an impact on biomarker levels (72). Proteomic analysis revealed that total protein concentration varies according to flow rate, duration of a possible stimulus, and its nature as well as circadian rhythms (73). Several systemic diseases are interconnected to periodontitis such as diabetes or cardiovascular diseases. Some biomarkers in saliva have been investigated in patients with periodontitis and atherosclerosis or diabetes, suggesting that inflammatory cytokines and biomarkers identified after metabolic profiling could be used in diagnosis and monitoring (74, 75). Rheumatoid arthritis could influence the levels of some salivary biomarkers of periodontal disease, and its therapy could significantly lower IL-1ß or TNF-alpha (76). In metabolic syndrome, dietary changes had a positive influence on inflammatory variables of periodontal disease in saliva. Nutritional intervention can therefore have a positive effect on oxidative variables as well as bacterial counts in the saliva of periodontitis patients (77).
Discussion
Due to the heterogeneity of the diagnostic approaches, an organization of an International Consortium for Biomarkers of Periodontitis has already been demanded in 2015 but has not yet been established (14). Salivary biomarkers for periodontitis can still be seen to only complement regular clinical examination (78). The harmonization of saliva sampling protocols as well as definitions of power and other calculations would tremendously help to compare studies and subsume the most promising biomarkers for periodontitis. Most of the existing studies are not able to reach the quality criteria for a meta-analysis, and therefore, their results are not taken into consideration for the worldwide search for a reliable PoC diagnostic tool for periodontitis. However, considerable progress has been made to develop as sensitive and specific salivary diagnostic devices as for blood or urine testing (79).
To identify candidate biomarkers, changes in the proteome associated with periodontitis could be analyzed in databases (80). Enhanced interactions between the host and bacteria in periodontitis might also be reflected by an altered metabolomic profile of saliva (81). Salivary concentrations of inflammatory, bone turnover, and microbiological markers alone or preferably in combination could help to replace invasive diagnostic procedures and lead to a more precise and personalized dentistry for the twenty-first century (19). Multiplex panels of combined biomarkers could serve as screening tools with continued advances in this field (82). The combination of biomarkers and salivary concentrations of periodontopathic bacteria could also be used for evaluating periodontitis risk and therefore easily be used in large population surveys (83). Higher salivary MMP-8, MMP-9, OPG, and red complex periodontopathic bacteria could be used for accurate predictions of periodontal disease category, whereas T. denticola could be used together with MMP-8 for predicting periodontal disease severity (84). A. actinomycetemcomitans in combination with MMP-8 and MPO has the potential to be a trustworthy biomarker in periodontitis patients with ischemic stroke (85).
For periimplantitis, very few articles are available. Fifty percent of the articles found in the literature research comprised reviews, which indicates that mostly studies about salivary markers for periodontitis were taken into account.
Considering the panoply of studies who tried to identify reliable markers for periodontitis, it might be disappointing that very few of the more than 100 different biomarkers withstand criteria to be finally included in meta-analyses. In 2014, a critical review described the existing literature as “infant,” which is focused on validating metrics and identifying biomarkers with diagnostic potential, and further concluded that the evidence of the literature is graded as level 3 (86). A more recent meta-analysis pointed out that many promising biomarkers could not be considered due to missing validating studies of those with substantial intergroup differences (15). It was concluded that future studies should rely on latest methodological protocols (87), standardized protocols for clinical research, and focus on clearly unbiased controls (14), which can be confirmed by the present minireview. Future studies should orientate on previous methods, preferring unstimulated saliva collection in large study populations, and include confounding factors. In periimplantitis, the current heterogeneity of studies prevents a definitive evaluation of the potential of salivary diagnostic markers, and more randomized clinical trials are needed (39). Therefore, a global effort to find salivary biomarkers with high sensitivity and specificity to discriminate periodontitis from periodontal health in terms of clearly defining the best protocols, adequate sample sizes, saliva sampling techniques, the consideration of salivary flow on total biomarker concentrations, or the consideration of influencing factors would help to improve the reliability and comparability of those biomarkers. The major challenges to current saliva-based diagnostics for oral diseases are the small number of potential and valid biomarkers, the lack of real-time assessments or existing tests which are based on microbial and inflammatory cytokines that are not exclusively specific neither to periodontitis nor to periimplantitis (88).
Conclusions
No single or combination of biomarkers can so far disclose tissue destruction of periodontitis and periimplantitis effectively, and since promising biomarkers still need to be objectively demonstrated, the clinical measurements are still seen as the most reliable method of choice (89). Novel techniques of salivaomics (25), proteomics (90), peptidomics (91), metabolomics (92), and interactomics (23) might help to break the chains of single biomarker analysis and lead to a panel of biomarkers like it was already defined for IL-1ß, IL-6, MMP-8, MIP-1 alpha, and hemoglobin (16, 19) to complement or even replace invasive clinical examination.
Author Contributions
All authors contributed to the conception and correction of the manuscript and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2021.687638/full#supplementary-material
References
1. GBD 2017 Oral Disorders Collaborators, Bernabe E, Marcenes W, Hernandez CR, Bailey J, Abreu LG, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a Systematic analysis f or the global burden of disease 2017 study. J Dent Res. (2020) 99:362–73. doi: 10.1177/0022034520908533
2. Ferreira SD, Martins CC, Amaral SA, Vieira TR, Albuquerque BN, Cota LOM, et al. Periodontitis as a risk factor for peri-implantitis: systematic review and meta- analysis of observational studies. J Dent. (2018) 79:1–10. doi: 10.1016/j.jdent.2018.09.010
3. Muñoz V, Duque A, Giraldo A, Manrique R. Prevalence of peri-implant disease according to periodontal probing depth and bleeding on probing: a Systematic review and meta- analysis. Int J Oral Maxillofac Implants. (2018) 33:e89–e105. doi: 10.11607/jomi.5940
4. Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri- implant diseases and conditions-introduction and key changes from the 1999 classification. J Periodontol. (2018) 89(Suppl. 1):S1–S8. doi: 10.1002/JPER.18-0157
5. Fuentes L, Yakob M, Wong DTW. Emerging horizons of salivary diagnostics for periodontal disease. Br Dent J. (2014) 217:567–73. doi: 10.1038/sj.bdj.2014.1005
6. Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. (2009) 50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x
7. Javaid MA, Ahmed AS, Durand R, Tran SD. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofacial Res. (2016) 6:66–75. doi: 10.1016/j.jobcr.2015.08.006
8. Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem. (2011) 57:675–687. doi: 10.1373/clinchem.2010.153767
9. Tsang NNY, So HC, Ng KY, Cowling BJ, Leung GM, Ip DKM. Diagnostic performance of different sampling approaches for sARS-CoV-2 rT-PCR testing: a systematic review and meta-analysis. Lancet Infect Dis. (2021). doi: 10.1016/S1473-3099(21)00146-8. Epub ahead of print.
10. Wilton JM, Curtis MA, Gillett IR, Griffiths GS, Maiden MF, Sterne JA, et al. Detection of high-risk groups and individuals for periodontal diseases: laboratory markers from analysis of saliva. J Clin Periodontol. (1989) 16:475–83. doi: 10.1111/j.1600-051X.1989.tb02323.x
11. Giannobile WV. Salivary diagnostics for periodontal diseases. J Am Dent Assoc 1939. (2012) 143: 6S–11S. doi: 10.14219/jada.archive.2012.0341
12. Wright TA. Salivary diagnostic testing: a “game changer” for patient evaluation. Compend Contin Educ Dent Jamesburg NJ 1995. (2011) 32:28–9.
13. Brinkmann O, Zhang L, Giannobile WV, Wong DT. Salivary biomarkers for periodontal disease diagnostics. Expert Opin Med Diagn. (2011) 5:25–35. doi: 10.1517/17530059.2011.542144
14. Ji S, Choi Y. Point-of-care diagnosis of periodontitis using saliva: technically feasible but still a challenge. Front Cell Infect Microbiol. (2015) 5:65. doi: 10.3389/fcimb.2015.00065
15. Arias-Bujanda N, Regueira-Iglesias A, Balsa-Castro C, Nibali L, Donos N, Tomás I. Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: a systematic review and meta-analysis. J Clin Periodontol. (2020) 47:2–18. doi: 10.1111/jcpe.13202
16. Kc S, Wang XZ, Gallagher JE. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: systematic review. J Clin Periodontol. (2020) 47:289–308. doi: 10.1111/jcpe.13218
17. Fine DH, Markowitz K, Fairlie K, Tischio-Bereski D, Ferrandiz J, Godboley D, et al. Macrophage inflammatory protein-1α shows predictive value as a risk marker for subjects and sites vulnerable to bone loss in a longitudinal model of aggressive periodontitis. PLoS ONE. (2014) 9:e98541. doi: 10.1371/journal.pone.0098541
18. de Lima CL, Acevedo AC, Grisi DC, Taba M, Guerra E, De Luca Canto G. Host-derived salivary biomarkers in diagnosing periodontal disease: systematic review and meta-analysis. J Clin Periodontol. (2016) 43:492–502. doi: 10.1111/jcpe.12538
19. Ebersole JL, Nagarajan R, Akers D, Miller CS. Targeted salivary biomarkers for discrimination of periodontal health and disease(s). Front Cell Infect Microbiol. (2015) 5:62. doi: 10.3389/fcimb.2015.00062
20. Navazesh M, Kumar SKS, University of Southern California School of Dentistry. Measuring salivary flow: challenges and opportunities. J Am Dent Assoc 1939. (2008) 139(Suppl.3)5S–40S. doi: 10.14219/jada.archive.2008.0353
21. Topkas E, Keith P, Dimeski G, Cooper-White J, Punyadeera C. Evaluation of saliva collection devices for the analysis of proteins. Clin Chim Acta Int J Clin Chem. (2012) 413:1066–70. doi: 10.1016/j.cca.2012.02.020
22. Henson BS, Wong DT. Collection, storage, and processing of saliva samples for downstream molecular applications. Methods Mol Biol Clifton NJ. (2010) 666:21–30. doi: 10.1007/978-1-60761-820-1_2
23. Rosa N, Campos B, Esteves AC, Duarte AS, Correia MJ, Silva RM, et al. Tracking the functional meaning of the human oral-microbiome protein-protein interactions. Adv Protein Chem Struct Biol. (2020) 121:199–235. doi: 10.1016/bs.apcsb.2019.11.014
24. Schubert C, Ott M, Hannemann J, Singer M, Bliem HR, Fritzsche K, et al. Dynamic effects of cAM techniques on inflammation and emotional states: an integrative single-Case study on a breast cancer survivor. Integr Cancer Ther. (2021) 20:1534735420977697. doi: 10.1177/1534735420977697
25. Tasoulas J, Patsouris E, Giaginis C, Theocharis S. Salivaomics for oral diseases biomarkers detection. Expert Rev Mol Diagn. (2016) 16:285–95. doi: 10.1586/14737159.2016.1133296
26. Rathnayake N, Gieselmann D-R, Heikkinen AM, Tervahartiala T, Sorsa T. Salivary diagnostics-Point-of-Care diagnostics of mMP-8 in dentistry and medicine. Diagn Basel Switz. (2017) 7:7. doi: 10.3390/diagnostics7010007
27. Tominaga Y, Usui K, Hirata A, Ito H-O, Nokihara K. Applications of a novel biodetection system to saliva using protein fingerprints with data processing. Bioorg Med Chem. (2018) 26:3210–6. doi: 10.1016/j.bmc.2018.04.049
28. Johannsen B, Müller L, Baumgartner D, Karkossa L, Früh SM, Bostanci N, et al. Automated pre-analytic processing of whole saliva using magnet-Beating for point-of-Care protein biomarker analysis. Micromachines. (2019) 10:833. doi: 10.3390/mi10120833
29. Song Y, Huang Y-Y, Liu X, Zhang X, Ferrari M, Qin L. Point-of-care technologies for molecular diagnostics using a drop of blood. Trends Biotechnol. (2014) 32:132–9. doi: 10.1016/j.tibtech.2014.01.003
30. Su W, Gao X, Jiang L, Qin J. Microfluidic platform towards point-of-care diagnostics in infectious diseases. J Chromatogr A. (2015) 1377:13–26. doi: 10.1016/j.chroma.2014.12.041
31. Derruau S, Robinet J, Untereiner V, Piot O, Sockalingum GD, Lorimier S. Vibrational spectroscopy saliva profiling as biometric tool for disease diagnostics: a Systematic literature. Mol Basel Switz. (2020) 25:4142. doi: 10.3390/molecules25184142
32. Lee J, Lee J-B, Song H-Y, Son MJ, Li L, Rhyu I-C, et al. Diagnostic models for screening of periodontitis with inflammatory mediators and microbial profiles in saliva. Diagn Basel Switz. (2020) 10: doi: 10.3390/diagnostics10100820
33. Nagarajan R, Miller CS, Dawson D, Al-Sabbagh M, Ebersole JL. Patient-Specific variations in biomarkers across gingivitis and periodontitis. PLoS ONE. (2015) 10:e0136792. doi: 10.1371/journal.pone.0136792
34. Al-Sabbagh M, Alladah A, Lin Y, Kryscio RJ, Thomas MV, Ebersole JL, et al. Bone remodeling-associated salivary biomarker mIP-1α distinguishes periodontal disease from health. J Periodontal Res. (2012) 47:389–95. doi: 10.1111/j.1600-0765.2011.01445.x
35. Gursoy UK, Könönen E, Huumonen S, Tervahartiala T, Pussinen PJ, Suominen AL, et al. Salivary type i collagen degradation end-products and related matrix metalloproteinases in periodontitis. J Clin Periodontol. (2013) 40:18–25. doi: 10.1111/jcpe.12020
36. Melguizo-Rodríguez L, Costela-Ruiz VJ, Manzano-Moreno FJ, Ruiz C, Illescas-Montes R. Salivary biomarkers and their application in the diagnosis and monitoring of the most common oral pathologies. Int J Mol Sci. (2020) 21:5173. doi: 10.3390/ijms21145173
37. Al-Majid A, Alassiri S, Rathnayake N, Tervahartiala T, Gieselmann D-R, Sorsa T. Matrix metalloproteinase-8 as an inflammatory and prevention biomarker in periodontal and peri- implant diseases. Int J Dent. (2018) 2018:7891323. doi: 10.1155/2018/7891323
38. Alassiri S, Parnanen P, Rathnayake N, Johannsen G, Heikkinen A-M, Lazzara R, et al. The ability of quantitative, specific, and sensitive point-of-Care/Chair-Side oral fluid immunotests for aMMP-8 to detect periodontal and peri-Implant diseases. Dis Markers. (2018) 2018:1306396. doi: 10.1155/2018/1306396
39. Gomes AM, Douglas-de-Oliveira DW, Oliveira Costa F. Could the biomarker levels in saliva help distinguish between healthy implants and implants with peri-implant disease? A systematic review. Arch Oral Biol. (2018) 96:216–222. doi: 10.1016/j.archoralbio.2018.09.008
40. Emecen-Huja P, Hasan I, Miller CS. Biologic markers of failing implants. Dent Clin North Am. (2015) 59:179–94. doi: 10.1016/j.cden.2014.08.007
41. Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, et al. Macrophage inflammatory protein-1alpha: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J Periodontol. (2009) 80:106–13. doi: 10.1902/jop.2009.080296
42. Bregy L, Müggler AR, Martinez-Lozano Sinues P, García-Gómez D, Suter Y, Belibasakis GN, et al. Differentiation of oral bacteria in in vitro cultures and human saliva by secondary electrospray ionization - mass spectrometry. Sci Rep. (2015) 5:15163. doi: 10.1038/srep15163
43. Haririan H, Andrukhov O, Bertl K, Lettner S, Kierstein S, Moritz A, et al. Microbial analysis of subgingival plaque samples compared to that of whole saliva in patients with periodontitis. J Periodontol. (2014) 85:819–28. doi: 10.1902/jop.2013.130306
44. Gursoy UK, Könönen E. Editorial: use of saliva in diagnosis of periodontitis: cumulative use of bacterial and host-Derived biomarkers. Front Cell Infect Microbiol. (2016) 6:196. doi: 10.3389/fcimb.2016.00196
45. Nomura Y, Shimada Y, Hanada N, Numabe Y, Kamoi K, Sato T, et al. Salivary biomarkers for predicting the progression of chronic periodontitis. Arch Oral Biol. (2012) 57:413–20. doi: 10.1016/j.archoralbio.2011.09.011
46. Saygun I, Nizam N, Keskiner I, Bal V, Kubar A, Açikel C, et al. Salivary infectious agents and periodontal disease status. J Periodontal Res. (2011) 46:235–39. doi: 10.1111/j.1600-0765.2010.01335.x
47. Lee A, Ghaname CB, Braun TM, Sugai JV, Teles RP, Loesche WJ, et al. Bacterial and salivary biomarkers predict the gingival inflammatory profile. J Periodontol. (2012) 83:79–89. doi: 10.1902/jop.2011.110060
48. Teixeira MKS, Lira-Junior R, Lourenço EJV, Telles DM, Boström EA, Figueredo CM, et al. The modulation of the tREM-1/PGLYRP1/MMP-8 axis in peri-implant diseases. Clin Oral Investig. (2020) 24:1837–44. doi: 10.1007/s00784-019-03047-z
49. Sorsa T, Tervahartiala T, Leppilahti J, Hernandez M, Gamonal J, Tuomainen AM, et al. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol Res. (2011) 63:108–13. doi: 10.1016/j.phrs.2010.10.005
50. Tatarakis N, Kinney JS, Inglehart M, Braun TM, Shelburne C, Lang NP, et al. Clinical, microbiological, and salivary biomarker profiles of dental implant patients with type 2 diabetes. Clin Oral Implants Res. (2014) 25:803–12. doi: 10.1111/clr.12139
51. Keles Yucel ZP, Keles GC, Avci B, Cetinkaya BO. Nonsurgical periodontal therapy reduces salivary and gingival crevicular fluid yKL-40 and iL-6 levels in chronic periodontitis. Oral Health Prev Dent. (2020) 18:815–22. doi: 10.3290/j.ohpd.a45086
52. Bertl K, Schoiber A, Haririan H, Laky M, Steiner I, Rausch WD, et al. Non-surgical periodontal therapy influences salivary melatonin levels. Clin Oral Investig. (2013) 17:1219–25. doi: 10.1007/s00784-012-0801-6
53. Yoshie H, Tai H, Kobayashi T, Oda-Gou E, Nomura Y, Numabe Y, et al. Salivary enzyme levels after scaling and interleukin-1 genotypes in japanese patients with chronic periodontitis. J Periodontol. (2007) 78:498–503. doi: 10.1902/jop.2007.060216
54. Gorr S-U. Antimicrobial peptides in periodontal innate defense. Front Oral Biol. (2012) 15:84–98. doi: 10.1159/000329673
55. Güncü GN, Yilmaz D, Könönen E, Gürsoy UK. Salivary antimicrobial peptides in early detection of periodontitis. Front Cell Infect Microbiol. (2015) 5:99. doi: 10.3389/fcimb.2015.00099
56. Haririan H, Andrukhov O, Pablik E, Neuhofer M, Moritz A, Rausch-Fan X. Comparative analysis of calcium-Binding myeloid-Related protein-8/14 in saliva and serum of patients with periodontitis and healthy individuals. J Periodontol. (2016) 87:184–92. doi: 10.1902/jop.2015.150254
57. Kojima T, Andersen E, Sanchez JC, Wilkins MR, Hochstrasser DF, Pralong WF, et al. Human gingival crevicular fluid contains mRP8 (S100A8) and mRP14 (S100A9), two calcium-binding proteins of the s100 family. J Dent Res. (2000) 79:740–47. doi: 10.1177/00220345000790020701
58. Prakasam S, Srinivasan M. Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral Dis. (2014) 20:171–177. doi: 10.1111/odi.12085
59. Novakovic N, Todorovic T, Rakic M, Milinkovic I, Dozic I, Jankovic S, et al. Salivary antioxidants as periodontal biomarkers in evaluation of tissue status and treatment outcome. J Periodontal Res. (2014) 49:129–36. doi: 10.1111/jre.12088
60. Pereira AL, Cortelli SC, Aquino DR, Franco GCN, Cogo K, Rodrigues E, et al. Reduction of salivary arginine catabolic activity through periodontal therapy. Quintessence Int Berl Ger 1985. (2012) 43:777–87.
61. Dede FÖ, Ozden FO, Avci B. 8-hydroxy-deoxyguanosine levels in gingival crevicular fluid and saliva in patients with chronic periodontitis after initial periodontal treatment. J Periodontol. (2013) 84:821–828. doi: 10.1902/jop.2012.120195
62. Sezer U, Ciçek Y, Canakçi CF. Increased salivary levels of 8-hydroxydeoxyguanosine may be a marker for disease activity for periodontitis. Dis Markers. (2012) 32:165–72. doi: 10.1155/2012/215430
63. Takane M, Sugano N, Iwasaki H, Iwano Y, Shimizu N, Ito K. New biomarker evidence of oxidative dNA damage in whole saliva from clinically healthy and periodontally diseased individuals. J Periodontol. (2002) 73:551–54. doi: 10.1902/jop.2002.73.5.551
64. Torumtay G, Kirzioglu FY, Öztürk Tonguç M, Kale B, Calapoglu M, Orhan H. Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome. J Periodontal Res. (2016) 51:489–98. doi: 10.1111/jre.12328
65. Sexton WM, Lin Y, Kryscio RJ, Dawson DR, Ebersole JL, Miller CS. Salivary biomarkers of periodontal disease in response to treatment. J Clin Periodontol. (2011) 38:434–41. doi: 10.1111/j.1600-051X.2011.01706.x
66. Han D-H, Kim M-S, Shin H-S, Park KP, Kim H-D. Association between periodontitis and salivary nitric oxide metabolites among community elderly koreans. J Periodontol. (2013) 84:776–84. doi: 10.1902/jop.2012.120237
67. Heikkinen AM, Sorsa T, Pitkäniemi J, Tervahartiala T, Kari K, Broms U, et al. Smoking affects diagnostic salivary periodontal disease biomarker levels in adolescents. J Periodontol. (2010) 81:1299–307. doi: 10.1902/jop.2010.090608
68. Lahdentausta L, Paju S, Mäntylä P, Buhlin K, Pietiäinen M, Tervahartiala T, et al. Smoking confounds the periodontal diagnostics using saliva biomarkers. J Periodontol. (2019) 90:475–83. doi: 10.1002/JPER.18-0545
69. Bertl K, Haririan H, Laky M, Matejka M, Andrukhov O, Rausch-Fan X. Smoking influences salivary histamine levels in periodontal disease. Oral Dis. (2012) 18:410–16. doi: 10.1111/j.1601-0825.2011.01891.x
70. Haririan H, Andrukhov O, Böttcher M, Pablik E, Wimmer G, Moritz A, et al. Salivary neuropeptides, stress, and periodontitis. J Periodontol. (2018) 89:9–18. doi: 10.1902/jop.2017.170249
71. Rai B, Kaur J, Anand SC, Jacobs R. Salivary stress markers, stress, and periodontitis: a pilot study. J Periodontol. (2011) 82:287–92. doi: 10.1902/jop.2010.100319
72. Kamodyová N, Banasová L, Janšáková K, Koborová I, Tóthová L, Stanko P, et al. Blood contamination in saliva: impact on the measurement of salivary oxidative stress markers. Dis Markers. (2015) 2015:479251. doi: 10.1155/2015/479251
73. Siqueira WL, Dawes C. The salivary proteome: challenges and perspectives. Proteomics Clin Appl. (2011) 5:575–79. doi: 10.1002/prca.201100046
74. Kosaka T, Kokubo Y, Ono T, Sekine S, Kida M, Kikui M, et al. Salivary inflammatory cytokines may be novel markers of carotid atherosclerosis in a japanese general population: the suita study. Atherosclerosis. (2014) 237:123–8. doi: 10.1016/j.atherosclerosis.2014.08.046
75. Barnes VM, Kennedy AD, Panagakos F, Devizio W, Trivedi HM, Jönsson T, et al. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS ONE. (2014) 9:e105181. doi: 10.1371/journal.pone.0105181
76. Mirrielees J, Crofford LJ, Lin Y, Kryscio RJ, Dawson DR, Ebersole JL, et al. Rheumatoid arthritis and salivary biomarkers of periodontal disease. J Clin Periodontol. (2010) 37:1068–74. doi: 10.1111/j.1600-051X.2010.01625.x
77. Jenzsch A, Eick S, Rassoul F, Purschwitz R, Jentsch H. Nutritional intervention in patients with periodontal disease: clinical, immunological and microbiological variables during 12 months. Br J Nutr. (2009) 101:879–85. doi: 10.1017/S0007114508047776
78. Front E, Laster Z, Unis R, Gavish M, Nagler RM. Salivary biomarker analysis complementing regular clinical examination. Biomark Med. (2013) 7:701–8. doi: 10.2217/bmm.13.76
79. Giannobile WV, McDevitt JT, Niedbala RS, Malamud D. Translational and clinical applications of salivary diagnostics. Adv Dent Res. (2011) 23:375–80. doi: 10.1177/0022034511420434
80. Rosa N, Correia MJ, Arrais JP, Lopes P, Melo J, Oliveira JL, et al. From the salivary proteome to the oralOme: comprehensive molecular oral biology. Arch Oral Biol. (2012) 57:853–64. doi: 10.1016/j.archoralbio.2011.12.010
81. Barnes VM, Ciancio SG, Shibly O, Xu T, Devizio W, Trivedi HM, et al. Metabolomics reveals elevated macromolecular degradation in periodontal disease. J Dent Res. (2011) 90:1293–7. doi: 10.1177/0022034511416240
82. Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, et al. Current developments in salivary diagnostics. Biomark Med. (2010) 4:171–89. doi: 10.2217/bmm.09.68
83. Gursoy UK, Könönen E, Pussinen PJ, Tervahartiala T, Hyvärinen K, Suominen AL, et al. Use of host- and bacteria-derived salivary markers in detection of periodontitis: a cumulative approach. Dis Markers. (2011) 30:299–305. doi: 10.1155/2011/621484
84. Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CA, et al. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. (2009) 80:436–46. doi: 10.1902/jop.2009.080480
85. Palm F, Lahdentausta L, Sorsa T, Tervahartiala T, Gokel P, Buggle F, et al. Biomarkers of periodontitis and inflammation in ischemic stroke: a case-control study. Innate Immun. (2014) 20:511–18. doi: 10.1177/1753425913501214
86. Nový BB. Saliva and biofilm-based diagnostics: a critical review of the literature concerning sialochemistry. J Evid-Based Dent Pract. (2014) 14(Suppl.):27–32. doi: 10.1016/j.jebdp.2014.04.004
87. Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. (2015) 162:W1–73. doi: 10.7326/M14-0698
88. Kim JJ, Kim CJ, Camargo PM. Salivary biomarkers in the diagnosis of periodontal diseases. J Calif Dent Assoc. (2013) 41:119–24.
89. Buduneli N, Kinane DF. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol. (2011) 38(Suppl. 11):85–105. doi: 10.1111/j.1600-051X.2010.01670.x
90. Salazar MG, Jehmlich N, Murr A, Dhople VM, Holtfreter B, Hammer E, et al. Identification of periodontitis associated changes in the proteome of whole human saliva by mass spectrometric analysis. J Clin Periodontol. (2013) 40:825–32. doi: 10.1111/jcpe.12130
91. Zhang J, Zhou S, Li R, Cao T, Zheng H, Wang X, et al. Magnetic bead-based salivary peptidome profiling for periodontal-orthodontic treatment. Proteome Sci. (2012) 10:63. doi: 10.1186/1477-5956-10-63
Keywords: saliva, periodontitis, periimplantitis, biomarkers, diagnostics
Citation: Haririan H, Andrukhov O, Laky M and Rausch-Fan X (2021) Saliva as a Source of Biomarkers for Periodontitis and Periimplantitis. Front. Dent. Med. 2:687638. doi: 10.3389/fdmed.2021.687638
Received: 29 March 2021; Accepted: 25 May 2021;
Published: 24 June 2021.
Edited by:
Priscila Casado, Fluminense Federal University, BrazilReviewed by:
Alessandro Polizzi, University of Catania, ItalyMia Rakic, Complutense University of Madrid, Spain
Copyright © 2021 Haririan, Andrukhov, Laky and Rausch-Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hady Haririan, aGFkeS5oYXJpcmlhbkBtZWQuc2Z1LmFjLmF0
 Hady Haririan
Hady Haririan Oleh Andrukhov
Oleh Andrukhov Markus Laky
Markus Laky Xiaohui Rausch-Fan
Xiaohui Rausch-Fan