
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Dent. Med. , 04 June 2021
Sec. Endodontics
Volume 2 - 2021 | https://doi.org/10.3389/fdmed.2021.686701
This article is part of the Research Topic The Global Response to the SARS-CoV-2 Pandemic from Endodontists and Researchers View all 9 articles
Over the last 12 months, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2) virus has emerged as a significant global health problem with extensive repercussions for the practise of dentistry. As the principle transmission-route is via droplet-spread, aerosol-generating dental procedures (AGPs) present an exquisite challenge, which either has to be avoided or performed using strict infection-control measures, which increase the deployment of resources and cost. This new working environment necessitates the adoption of simplified, yet effective procedures that reduce intervention and minimise clinical chair time to short, single visits. Vital pulp treatment (VPT) has emerged as an attractive, technically less-complicated group of biologically-based management strategies that are aimed at maintaining pulp vitality and avoiding root canal treatment (RCT). These procedures are carried out in a strict aseptic environment using a rubber dam and have a reported high success rate, suggesting that they could be considered as effective and simple alternative therapies to relieve pain and avoid multiple visit RCT and other endodontic procedures. The relevance of promoting a simple, predictable and effective alternative to traditional, more complex dentistry has never been more compelling. In this perspective article, the latest advances in VPT are highlighted, along with an analysis of their relative success and compelling reasons why we as dentists should be adopting these treatment approaches. Thereafter, case selection, prognostic factors, techniques, limitations and future prospects of these procedures are discussed.
Since the advent of the novel coronavirus (SARS-CoV-2, COVID-19) at the end of 2019 and subsequent global spread, the fields and Medicine and Dentistry have been thrust into an uncertain and rapidly evolving new normality. Within Dentistry specifically, there has been a focus on the generation of aerosols, so called aerosol-generating procedures (AGPs), as well as concerns relating to the length of patient appointments, emergency-only treatment and reducing the invasiveness of treatment. Endodontics has been forced to deal with this situation more than most areas of Dentistry, as Endodontic procedures invariably require the use of high-speed handpieces, deal with patients in acute pain whose treatment cannot be deferred and carry out invasive time-consuming treatments. As a result, groups like the European Society of Endodontology (ESE) (https://www.e-s-e.eu/index.html), American Association of Endodontists (AAE) (https://www.aae.org/specialty/clinical-resources/covid-19-updates-resources/) and the British Endodontic Society (BES) (https://britishendodonticsociety.org.uk/) have regularly updated their websites with bulletins of the latest advice for Endodontists, General Dentists and patients on how to limit viral load during endodontic procedures.
COVID-19 is characterised by its ability to transmit from individuals who asymptomatic, through multiple transmission pathways, including respiratory droplets, skin contact, aerosol-borne and faecal-oral routes. This has created uncertainty with regard to prolonged dental procedures, particularly aerosol-generating procedures (AGPs), which are unavoidable during endodontic therapy. As a result, strategies to reduce contact time and complexity such as vital pulp treatment (VPT) (1) have been highlighted and promoted as a way of reducing the potential of virus spread and increasing simplicity. VPT has recently been advocated as a treatment not only for exposure of the pulp in cases without symptoms and reversible pulpitis (2) but also in the management of cases with more severe damage and irreversible pulpitis (3, 4). There is an increasing scope for minimally-invasive therapies such as VPT to reduce intervention and indirectly reduce aerosol in endodontics as a result the aim of this perspective article is to make a case for the promotion of VPT over traditional root canal treatment (RCT) in cases even with a diagnosis of irreversible pulpitis.
The recent promotion of regenerative endodontic techniques and the clinical development of minimally-invasive VPT presents an exciting clinical focus for the dental profession. In these biologically-based procedures, the biology of the pulp and surrounding tissues is exploited to optimise the healing following injury (5). Although, within the remit of all dentists VPT requires an understanding of the biological processes taking place if they are to achieve maximal clinical impact. In this perspective article, we highlight some of the key processes upon which clinical attention should be directed as well as considering ways in which the reparative events in the pulp-dentine-pulp complex can be maximised.
VPT can be defined as a range of therapeutic strategies aimed at maintaining the health of all or part of the pulp (2). These strategies include techniques to preserve pulp vitality in teeth with deep caries by avoiding pulp exposure (e.g., selective caries removal, indirect pulp capping) (6) or conservative management of the exposed pulp (e.g., pulp capping, pulpotomy) (7). At the core of VPT is the stimulation of the dentine-pulp complex's healing capacity, thereby defining VPT as a range of minimally invasive biologically-based wound healing strategies aimed at pulp regeneration processes (5). When considering the promotion of VPT it is critical that as clinicians we understand the nature of pulpal defence and healing, which generally involves a combination of pulpitis and reactive mineralisation in response to bacterial challenge (8, 9). In response to microbial irritation, initially, the odontoblasts and later fibroblasts, stem cells and immune cells mount a series of localised inflammatory responses (10). If uncontrolled this will lead to progressively increased pulpitis, which if unchecked can lead to pulp necrosis (11); however, if the stimulus is removed pupal healing is possible (12). If the pulpitis is allowed to continue intervention such as RCT or extraction will be required. These techniques may be unnecessary and remove tissue that could have been maintained had earlier intervention been carried out. This presents an opportunity for VPT to reverse or shift the balance of the disease toward a conservative repair of the pulpal injury.
Hard tissue repair involves the production of tertiary dentine, which forms alongside inflammation beneath the area of challenge (13). There are two types of tertiary dentine depending on the severity of the irritation. Reactionary dentine is formed after mild irritation and an up-regulation of odontoblast activity, while reparative dentine is formed in response to stronger stimuli and odontoblast death. Reparative dentine formation is complex processes involving the recruitment of dental progenitor cells and their subsequent differentiation into secretory odontoblast-like cells (13). The wound healing events are regulated by the release of dentine-bound bioactive molecules, which includes growth factors (GFs) and chemokines sequestered after damage to the dentine structure (14, 15).
After pulp exposure, a positive sign of healing is the formation of a reparative dentine hard tissue bridge (16), which helps to protect the pulp tissue from further damage. However, from a clinical perspective, the hard tissue may not be visible radiographically (17) and the outcome will be evaluated by clinical exam, history, sensibility tests and radiographs (2).
The aim of the inflammatory process in the dental pulp is to clear the infection and initiate healing (18). The deep caries lesion (lesion extend to the inner quarter of dentine, but with a zone of hard or firm dentine between caries and the pulp) in which the pulp is not pathologically exposed, bacterial by-products diffuse through the dentinal tubules to initiate a protective inflammatory response that present clinically with mild symptoms mainly in response to thermal stimuli. In this situation removal of infected dentin using selective caries removal approach and sealing the pulp with restoration (indirect pulp capping), will ensure control of inflammation and maintenance of pulp vitality (2). In such teeth, the risk of pulp exposure should be avoided, but if ultimate, then direct pulp capping procedures has proved successful particularly when using calcium silicate cements (CSCs) (19, 20). However, in situations where caries is extremely deep (lesion penetrates the entire thickness of the dentine) bacteria gain access to the pulp space and here the inflammatory process takes another dimension, the severity and extent of which are determined by the level of bacterial contamination and the pulp defence capabilities. Inflammation within the dental pulp usually commences at the site of bacterial entry, and spreads circumferentially from one compartment to another (21, 22), creating different histopathological diagnosis in various parts of the same pulp (23, 24). Therefore, making a single diagnosis for the entire pulp in such cases is theoretically incorrect. Currently, such teeth are diagnosed with irreversible pulpitis indicating that the pulp lacks the capacity to heal and therefore require pulpectomy and RCT (25). This implies that the total pulp (coronal and radicular) is damaged beyond repair. However, evidence from clinical research suggests that such teeth can be successfully treated by removing the diseased and leaving the healthy part of the pulp with pulpotomy procedures. Pulpotomy, whether partial or complete has many advantages over RCT; it is not only less technically demanding, but most importantly by retaining vital pulp tissue, protect the tooth from infections and apical periodontitis (26). A high success rate for complete pulpotomy has been shown for teeth with deep caries and exposed pulp (27, 28). Even when the diagnosis is symptomatic irreversible pulpitis, complete pulpotomy has a high clinical (97, 93%) and radiographic (95, 88%%) success rate at 1 and 3 years, respectively (29). Similarly, partial pulpotomy has a high success rate of 98% at 1 year and 96% at 2 years of follow up in cariously-exposed pulp (30). The success rate for pulpotomy reported in these studies is comparable with that of root canal treatment of the vital pulp (31).
Many factors contribute to the outcome of VPT, but pulpal diagnosis is by far the most important determinant of the success of these treatments. Unfortunately, the current subjective pulpal diagnostic methods do not faithfully reflect or quantify the degree of pulp inflammation (32), and it is, therefore, likely that reported failures are associated with teeth in which inflammation is more extensive than predicted using these methods. Preoperative pain was suggested as a prognostic factor for pulpotomy (33), but high success has been reported for teeth with spontaneous lingering pain associated with symptomatic irreversible pulpitis (29). These contradicting findings only add to the limitations of our current diagnostic systems and suggest the need for a biomarker-based approach to pulpal diagnosis. The recent use of MMP9 as prognostic markers for pulpotomy outcomes (34), is a step in the right direction.
There are no known patient-related factors that are likely to affect the outcomes of VPT. The root maturity has however been shown to affect direct pulp capping (35) but not the pulpotomy. The choice of the capping material is also important. The introduction of the CSCs has contributed greatly to the success of VPT (16) and its use is recommended by ESE (2). When performing VPT for the cariously-exposed pulp, clinicians often have to deal with pulp exposure that happens through a zone of bacterial contamination. It has been suggested in this case that an enhanced operative protocol including aseptic procedure, use of magnification and disinfectant is important (2). Clinicians may consider using a rubber dam for optimal isolation and use sodium hypochlorite to wash and disinfect the pulpal wound (36).
Although high success rates have been reported for VPT, the evidence from these studies has to be interpreted with caution. Many of the interventional studies conducted are single-arm studies (case-series), which by definition are not controlled. As a result, there remains an unmet need for high-quality, adequately-powered and well-conducted randomised controlled trials to evaluate different VPT strategies both against root canal treatment and to other VPT techniques. An evaluation of the recent systematic reviews reporting on the outcomes of different VPT modalities demonstrated that the methodological quality of most of the reviews is low (Table 1), and this has mainly been attributed to either a limited number of included studies or the absence of a meta-analysis due to heterogeneity in study designs and outcomes. Future studies investigating VPT should be prospective, comparative studies using developed core outcome sets that facilitate evidence synthesis and guideline development (38).
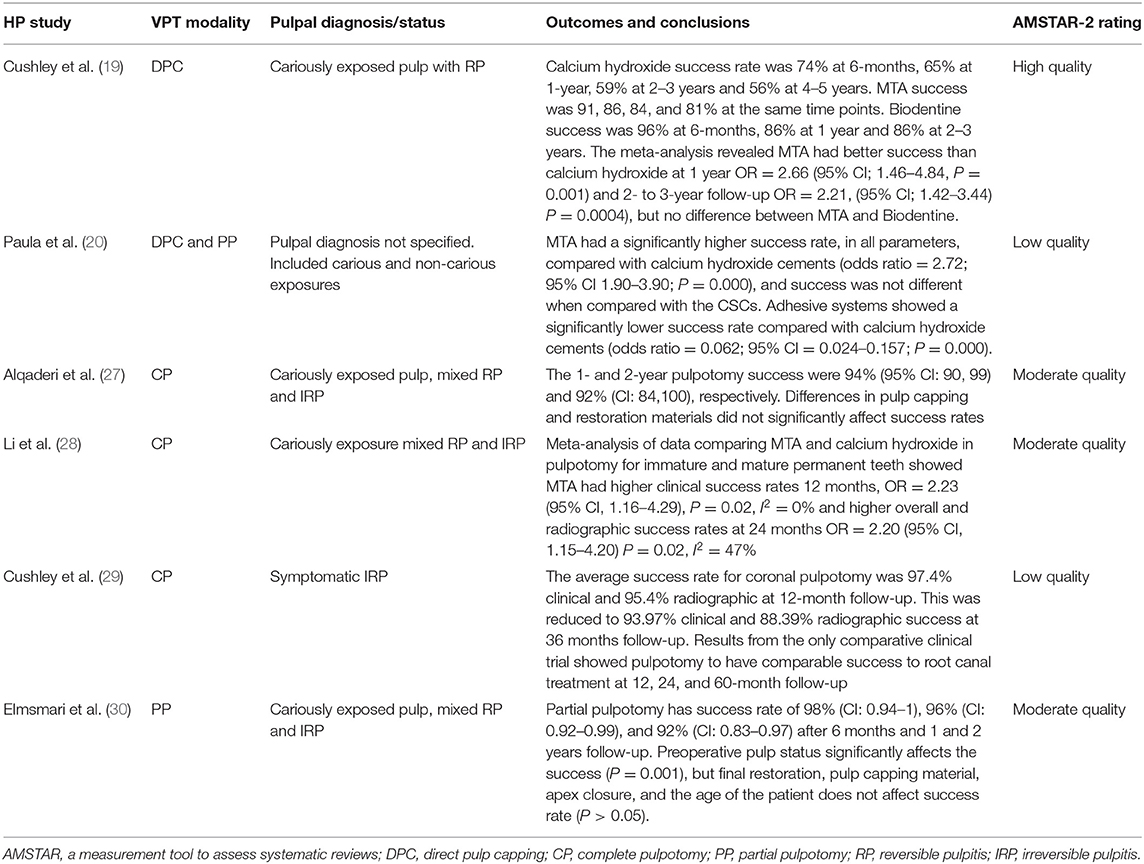
Table 1. Summary of evidence from recent systematic reviews on VPT studies on permanent teeth assessed using AMSTAR-2 (37).
Despite development in VPT biomaterials, the problem with the current diagnosis system is a major barrier to the successful development of minimally invasive biologically based therapies. When treating teeth with deep caries and diagnosis of reversible pulpitis and pulp exposure can be avoided, using stepwise excavation and indirect pulp capping is a strongly recommended approach (2). The main clinical dilemma is when the pulp is exposed during caries removal and whether practitioners should do direct pulp capping, pulpotomy or RCT? The emerging evidence suggests that in teeth with deep caries and exposed pulp, but with signs and symptoms indicative of reversible pulpitis attempts should be made to adopt a more conservative approach such as direct pulp capping using CSCs (19). Isolation is important and the provision of a good coronal seal is essential. However, for teeth with exposed pulp and signs and symptoms suggestive of irreversible pulpitis, a pulpotomy (partial or complete) should be attempted (29). In either case, the degree of pulpal inflammation is unknown, but pulpal bleeding is traditionally been used as an indicator of the severity of inflammation (39). Earlier studies demonstrated generally high success for direct pulp capping of cariously exposed pulp when bleeding is slight and can be controlled within 30 s (39). However, recent studies in which CSCs were used as capping material, a high success of DPC was reported when bleeding can be controlled within 5 min (40). Even in cases diagnosed with irreversible pulpitis studies reported control of bleeding within 6 min in over 80% of cases during partial or complete pulpotomy (3, 41). Therefore, bleeding time may not be as critical as originally reported and the ESE position statement recommended bleeding to be controlled with cotton pellet soaked in sodium hypochlorite (0.5–5%) for up to 5 min (2). There are currently no validated clinical protocols for VPT, but the evidence from emerging studies and the ESE position statement on the management of deep caries and exposed pulp (2), suggests the adoption of a conservative approach to maintain pulp vitality as possible (Figure 1). Although not advocated at present, some research reports suggest successful direct pulp capping for teeth with irreversible pulpitis (1). In the authors' opinion, the dental pulp in a tooth with symptomatic irreversible pulpitis is likely to be heavily contaminated with bacteria and to contain micro-abscesses, therefore, at least when using current biomaterials, surgical excision with partial or complete coronal pulpotomy will ensure remaining pulp tissue is healthier to recover and heal following treatment.
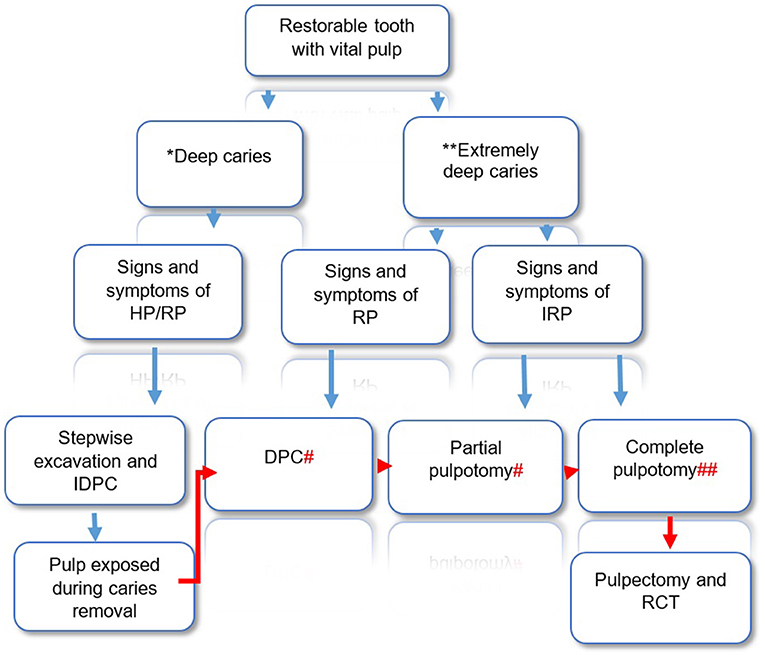
Figure 1. Decision making for VPT in restorable teeth with deep and extremely deep caries and positive response to sensibility testing. Diagram illustrating suggested clinical pathway and treatment options based on clinical findings. The suggested pathway is based on ESE position statement on the management of deep caries and exposed pulp (2), augmented with authors perspective, *Caries extend into the pulpal quarter of the dentine, but with a well-defined zone of radiopaque dentine separating the infected demineralized dentine from the pulp. **Caries involve the entire thickness of the dentine, without a radiopaque zone separating the lesion from the pulp. # bleeding can be controlled within 5 min, ## bleeding controlled within 5 min and no sign of necrosis in any canal. HP, healthy pulp; IDPC, indirect pulp capping; RP, reversible pulpitis; IRP, irreversible pulpitis.
The success of VPT is often considered based on short- and long-term outcomes. The pain relief and maintenance of pulpal and periapical health are the main outcomes often evaluated in VPT. Failure is generally considered to occur early (within 6 weeks of treatment) (33, 42). Most of the early failures are related to persistent pain and symptoms of irreversible which reflects issues with diagnosis as discussed earlier. Late failures include pulp necrosis and periapical infections and all these can be successfully managed with RCT. So, here VPT provides the opportunity to delay complex interventions like RCT and contribute to improving the life cycle of the tooth. However, optimal clinical protocols and case selection for VPT require the development of more reliable methods and better classification for pulpal diagnosis (43). Until such become available, clinician may utilise their expertise guided with the current available evidence to apply VPT in their clinical practise. Application of these therapies has numerous advantages over conventional RCT in terms of pain relief, tooth survival, aerosol-generation, time and costs as outlined in Table 2. These advantages are of particular importance at a time where both dentists and endodontists are developing strategies to reduce chair time in order to minimise viral load.
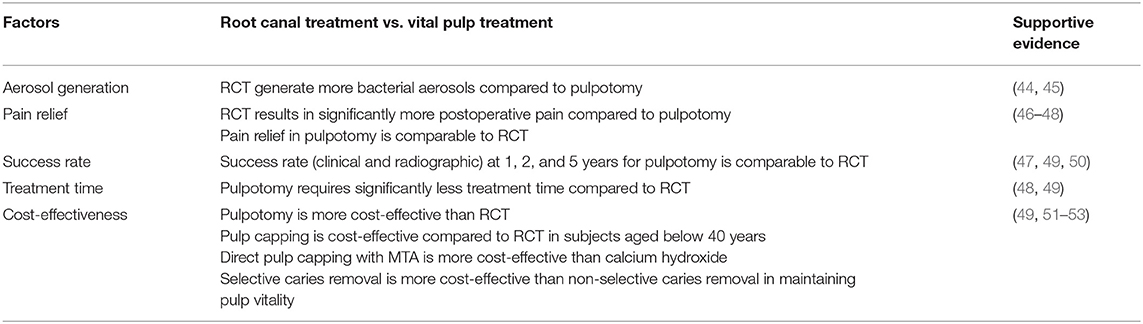
Table 2. Comparative analysis of clinical and patient related factor for root canal treatment and vital pulp treatment in permanent teeth.
The regenerative capacity and healing ability of the dental pulp is well-known, but our current clinical practise in managing the cariously exposed pulp didn't reflect this knowledge. The emerging evidence on the success of VPT needs to be consolidated with more clinical research as well as translational research to address many unmet needs. The current pulp diagnosis methods are not fit for purpose and there is a need for molecular tests to accurately diagnosis pulp status and predict treatment outcomes. The existing pulp diagnosis classification (reversible/irreversible) is also not permissive of minimally invasive treatments and with the success reported for VPT in cases of irreversible pulpitis, such classification became irrelevant. There is also a need for biomaterial development for pulpitis treatment. Although CSCs has greatly contributed to the current success of VPTs, these materials are not without limitations. The anti-inflammatory and antibacterial properties reported for these materials are controversial (54). The materials, although biocompatible, but contain heavy metals the long term effect of it is not known (55). Once placed these materials are not resorbable and therefore limit the chance of true regeneration and replacement of lost pulp tissue. Future materials that control microbial contamination and treat inflammation and at the same time allow for the regeneration of damaged pulp are required. In this regard development of functionalized scaffolds that can act as effective carriers for biological molecules, which could target inflammation and/or infection and promote the release of dentine bound growth factors and chemokines is promising (56, 57).
There is also a lack of consensus regarding the most appropriate way to manage deep caries and pulpal exposure, with recent questionnaire-based research highlighting differences in attitude influences by teaching, geographical location and dentists age, which impact clinical decision-making (58). Indeed, there is much work to be done to educate dentists, patients and other stakeholders about the advantages of VPT and its increased role in practise (59) (Table 2). Although it is challenging to alter the traditions of patient management particularly to older more experienced dentists the recent promotion of ESE-supported VPT awareness campaigns are a step in the right direction (https://www.e-s-e.eu/news/ese-awareness-campaign-on-vital-pulp-tre).
Preservation of the vital pulp is crucial for long term tooth survival. Although RCT is highly successful in managing teeth with irreversible pulpitis, its complexity and the cost could be prohibitive particularly in the situation where resources are sparse. Within the era of minimally-invasive medicine and surgery the need for Dentistry to embrace VPT is already strong; however, in light of recent calls to embrace simplicity and reduce chair time in the COVID-19 environment the opportunities for development of VPT have never been more obvious.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
IK and HD contributed equally to the conception, design and drafting and editing of the manuscript. The authors agreed to be accountable for the content of the work. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Asgary S, Hassanizadeh R, Torabzadeh H, Eghbal MJ. Treatment outcomes of 4 vital pulp therapies in mature molars. J Endod. (2018) 44:529–35. doi: 10.1016/j.joen.2017.12.010
2. Duncan HF, Galler KM, Tomson PL, Simon S, El-Karim I, Kundzina R, et al. European Society of Endodontology position statement: management of deep caries and the exposed pulp. Int Endod J. (2019) 52:923–34. doi: 10.1111/iej.13080
3. Taha NA, Khazali MA. Partial pulpotomy in mature permanent teeth with clinical signs indicative of irreversible pulpitis: a randomized clinical trial. J Endod. (2017) 43:1417–21. doi: 10.1016/j.joen.2017.03.033
4. El Karim I, McCrudden MTC, Linden GJ, Abdullah H, Curtis TM, McGahon M, et al. TNF-α-induced p38MAPK activation regulates TRPA1 and TRPV4 activity in odontoblast-like cells. Am J Pathol. (2015) 185:2994–3002. doi: 10.1016/j.ajpath.2015.07.020
5. Duncan HF, Cooper PR, Smith AJ. Dissecting dentine–pulp injury and wound healing responses: consequences for regenerative endodontics. Int Endod J. (2019) 52:261–6. doi: 10.1111/iej.13064
6. Bjørndal L, Reit C, Bruun G, Markvart M, Kjældgaard M, Näsman P, et al. Treatment of deep caries lesions in adults: randomized clinical trials comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur J Oral Sci. (2010) 118:290–7. doi: 10.1111/j.1600-0722.2010.00731.x
7. Ballal NV, Duncan HF, Rai N, Jalan P, Zehnder M. Sodium hypochlorite reduces postoperative discomfort and painful early failure after carious exposure and direct pulp capping—Initial findings of a randomized controlled trial. J Clin Med. (2020) 9:2408. doi: 10.3390/jcm9082408
8. Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S, Smith AJ. Inflammation-regeneration interplay in the dentine-pulp complex. J Dent. (2010) 38:687–97. doi: 10.1016/j.jdent.2010.05.016
9. Al Natour B, Lundy F, Moynah P, About I, Jeanneau C, Irwin C, et al. Odontoblast cell death induces NLRP3 inflammasome-dependent sterile inflammation and regulates dental pulp cell migration, proliferation and differentiation. Int Endod J. (2021) 54:941–50. doi: 10.1111/iej.13483
10. Farges JC, Alliot-Licht B, Renard E, Ducret M, Gaudin A, Smith AJ, et al. Dental pulp defence and repair mechanisms in dental caries. Mediat Inflamm. (2015) 2015:230251. doi: 10.1155/2015/230251
11. Reeves R, Stanley HR. The relationship of bacterial penetration and pulpal pathosis in carious teeth. Oral Surg Oral Med Oral Pathol. (1966) 22:59–65. doi: 10.1016/0030-4220(66)90143-5
12. Mjör IA, Tronstad L. The healing of experimentally induced pulpitis. Oral Surg Oral Med Oral Pathol. (1974) 38:115–21. doi: 10.1016/0030-4220(74)90322-3
13. Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H. Reactionary dentinogenesis. Int J Dev Biol. (1995) 39:273–80.
14. Smith AJ, Tobias RS, Cassidy N, Bégue-Kirn C, Ruch JV, Lesot H. Influence of substrate nature and immobilization of implanted dentin matrix components during induction of reparative dentinogenesis. Connect Tissue Res. (1995) 32:291–6. doi: 10.3109/03008209509013736
15. Duncan HF, Kobayashi Y, Shimizu E. Growth factors and cell homing in dental tissue regeneration. Curr Oral Heal Rep. (2018) 5:276–85. doi: 10.1007/s40496-018-0194-y
16. Nair PNR, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. (2008) 41:128–50. doi: 10.1111/j.1365-2591.2007.01329
17. Linu S, Lekshmi MS, Varunkumar VS, Sam Joseph VG. Treatment outcome following direct pulp capping using bioceramic materials in mature permanent teeth with carious exposure: a pilot retrospective study. J Endod. (2017) 43:1635–9. doi: 10.1016/j.joen.2017.06.017
18. Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. (2007) 13:151–7. doi: 10.1111/j.1601-0825.2006.01346.x
19. Cushley S, Duncan H, Lappin M, Chua P, Elamin A, Clarke M, et al. Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: a systematic review and meta-analysis. Int Endod J. (2020) 54:556–71. doi: 10.1111/iej.13449
20. Paula AB, Laranjo M, Marto CM, Paulo S, Abrantes AM, Casalta-Lopes J, et al. Direct pulp capping: what is the most effective therapy?—Systematic review and meta-analysis. J Evid Based Dental Pract. (2018) 18:298–314. doi: 10.1016/j.jebdp.2018.02.002
21. Langeland K. Tissue response to dental caries. Dent Traumatol. (1987) 3:149–71. doi: 10.1111/j.1600-9657.1987.tb00619.x
22. Ricucci D, Siqueira JF. Fate of the tissue in lateral canals and apical ramifications in response to pathologic conditions and treatment procedures. J Endod. (2010) 36:1–15. doi: 10.1016/j.joen.2009.09.038
23. Baume LJ. Diagnosis of diseases of the pulp. Oral Surg Oral Med Oral Pathol. (1970) 29:102–16. doi: 10.1016/0030-4220(70)90416-0
24. Ricucci D, Loghin S, Siqueira JF. Correlation between clinical and histologic pulp diagnoses. J Endod. (2014) 40:1932–9. doi: 10.1016/j.joen.2014.08.010
25. Levin LG, Law AS, Holland GR, Abbott P V., Roda RS. Identify and define all diagnostic terms for pulpal health and disease states. J Endod. (2009) 35:1645–57. doi: 10.1016/j.joen.2009.09.032
26. Caplan DJ, Cai J, Yin G, White BA. Root canal filled versus non-root canal filled teeth: a retrospective comparison of survival times. J Public Health Dent. (2005) 65:90–6. doi: 10.1111/j.1752-7325.2005.tb02792.x
27. Alqaderi H, Lee CT, Borzangy S, Pagonis TC. Coronal pulpotomy for cariously exposed permanent posterior teeth with closed apices: a systematic review and meta-analysis. J Dentist. (2016) 44:1–7. doi: 10.1016/j.jdent.2015.12.005
28. Li Y, Sui B, Dahl C, Bergeron B, Shipman P, Niu L, et al. Pulpotomy for carious pulp exposures in permanent teeth: a systematic review and meta-analysis. J Dentist. (2019) 84:1–8. doi: 10.1016/j.jdent.2019.03.010
29. Cushley S, Duncan HF, Lappin MJ, Tomson PL, Lundy FT, Cooper P, et al. Pulpotomy for mature carious teeth with symptoms of irreversible pulpitis: a systematic review. J Dentist. (2019) 88:103158. doi: 10.1016/j.jdent.2019.06.005
30. Elmsmari F, Ruiz XF, Miró Q, Feijoo-Pato N, Durán-Sindreu F, Olivieri JG. Outcome of partial pulpotomy in cariously exposed posterior permanent teeth: a systematic review and meta-analysis. J Endod. (2019) 45:1296–306.e3. doi: 10.1016/j.joen.2019.07.005
31. Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature - Part 2. Influence of clinical factors. Int Endod J. (2008) 41:6–31. doi: 10.1111/j.1365-2591.2007.01323.x
32. Mejàre IA, Axelsson S, Davidson T, Frisk F, Hakeberg M, Kvist T, et al. Diagnosis of the condition of the dental pulp: a systematic review. Int Endod J. (2012) 45:597–613. doi: 10.1111/j.1365-2591.2012.02016.x
33. Tan SY, Yu VSH, Lim KC, Tan BCK, Neo CLJ, Shen L, et al. Long-term pulpal and restorative outcomes of pulpotomy in mature permanent teeth. J Endod. (2020) 46:383–90. doi: 10.1016/j.joen.2019.11.009
34. Sharma R, Kumar V, Logani A, Chawla A, Mir R, Sharma S, et al. Association between concentration of active MMP-9 in pulpal blood and pulpotomy outcome in permanent mature teeth with irreversible pulpitis – A preliminary study. Int Endod J. (2020) 54:479–89. doi: 10.1111/iej.13437
35. Lipski M, Nowicka A, Kot K, Postek-Stefańska L, Wysoczańska-Jankowicz I, Borkowski L, et al. Factors affecting the outcomes of direct pulp capping using biodentine. Clin Oral Investig. (2018) 22:2021–9. doi: 10.1007/s00784-017-2296-7
36. Munir A, Zehnder M, Rechenberg D-K. Wound lavage in studies on vital pulp therapy of permanent teeth with carious exposures: a qualitative systematic review. J Clin Med. (2020) 9:984. doi: 10.3390/jcm9040984
37. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
38. Duncan HF, Nagendrababu V, El-Karim IA, Dummer PMH. Outcome measures to assess the effectiveness of endodontic treatment for pulpitis and apical periodontitis for use in the development of European Society of Endodontology (ESE) S3 level clinical practice guidelines: a protocol. Int Endod J. (2021) 54:646–54. doi: 10.1111/iej.13501
39. Matsuo T, Nakanishi T, Shimizu H, Ebisu S. A clinical study of direct pulp capping applied to carious-exposed pulps. J Endod. (1996) 22:551–6. doi: 10.1016/S0099-2399(96)80017-3
40. Kundzina R, Stangvaltaite L, Eriksen HM, Kerosuo E. Capping carious exposures in adults: a randomized controlled trial investigating mineral trioxide aggregate versus calcium hydroxide. Int Endod J. (2017) 50:924–32. doi: 10.1111/iej.12719
41. Taha NA, Abdelkhader SZ. Outcome of full pulpotomy using biodentine in adult patients with symptoms indicative of irreversible pulpitis. Int Endod J. (2018) 51:819–28. doi: 10.1111/iej.12903
42. Zanini M, Hennequin M, Cousson PY. A review of criteria for the evaluation of pulpotomy outcomes in mature permanent teeth. J Endod. (2016) 42:1167–74. doi: 10.1016/j.joen.2016.05.008
43. Wolters WJ, Duncan HF, Tomson PL, Karim IE, McKenna G, Dorri M, et al. Minimally invasive endodontics: a new diagnostic system for assessing pulpitis and subsequent treatment needs. Int Endod J. (2017) 50:825–9. doi: 10.1111/iej.12793
44. Bahador M, Alfirdous RA, Alquria TA, Griffin IL, Tordik PA, Martinho FC. Aerosols generated during endodontic treatment: a special concern during the coronavirus disease 2019 pandemic. J Endod. (2021) 47:732–9 doi: 10.1016/j.joen.2021.01.009
45. Manarte-Monteiro P, Carvalho A, Pina C, Oliveira H, Manso MC. Air quality assessment during dental practice: aerosols bacterial counts in an universitary clinic. Rev Port Estomatol Med Dentária Cirurgia Maxilofacial. (2013) 54:2–7. doi: 10.1016/j.rpemd.2012.10.002
46. Asgary S, Eghbal MJ. The effect of pulpotomy using a calcium-enriched mixture cement versus one-visit root canal therapy on postoperative pain relief in irreversible pulpitis: a randomized clinical trial. Odontology. (2010) 98:126–33. doi: 10.1007/s10266-010-0127-2
47. Galani M, Tewari S, Sangwan P, Mittal S, Kumar V, Duhan J. Comparative evaluation of postoperative pain and success rate after pulpotomy and root canal treatment in cariously exposed mature permanent molars: a randomized controlled trial. J Endod. (2017) 43:1953–62. doi: 10.1016/j.joen.2017.08.007
48. Eghbal MJ, Haeri A, Shahravan A, Kazemi A, Moazami F, Mozayeni MA, et al. Postendodontic pain after pulpotomy or root canal treatment in mature teeth with carious pulp exposure: a multicenter randomized controlled trial. Pain Res Manag. (2020) 2020:5853412. doi: 10.1155/2020/5853412
49. Asgary S, Eghbal MJ, Ghoddusi J, Yazdani S. One-year results of vital pulp therapy in permanent molars with irreversible pulpitis: an ongoing multicenter, randomized, non-inferiority clinical trial. Clin Oral Investig. (2013) 17:431–9. doi: 10.1007/s00784-012-0712-6
50. Asgary S, Eghbal MJ, Fazlyab M, Baghban AA, Ghoddusi J. Five-year results of vital pulp therapy in permanent molars with irreversible pulpitis: a non-inferiority multicenter randomized clinical trial. Clin Oral Investig. (2015) 19:335–41. doi: 10.1007/s00784-014-1244-z
51. Schwendicke F, Stolpe M. Direct pulp capping after a carious exposure versus root canal treatment: a cost-effectiveness analysis. J Endod. (2014) 40:1764–70. doi: 10.1016/j.joen.2014.07.028
52. Schwendicke F, Brouwer F, Stolpe M. Calcium hydroxide versus mineral trioxide aggregate for direct pulp capping: a cost-effectiveness analysis. J Endod. (2015) 41:1969–74. doi: 10.1016/j.joen.2015.08.019
53. Emara R, Krois J, Schwendicke F. Maintaining pulpal vitality: cost-effectiveness analysis on carious tissue removal and direct pulp capping. J Dent. (2020). doi: 10.1016/j.jdent.2020.103330
54. Careddu R, Duncan HF. How does the pulpal response to biodentine and proroot mineral trioxide aggregate compare in the laboratory and clinic? Br Dent J. (2018) 225:743–9. doi: 10.1038/sj.bdj.2018.864
55. Camilleri J. Staining potential of Neo MTA Plus, MTA plus, and biodentine used for pulpotomy procedures. J Endod. (2015) 41:1139–45. doi: 10.1016/j.joen.2015.02.032
56. Colombo JS, Moore AN, Hartgerink JD, D'Souza RN. Scaffolds to control inflammation and facilitate dental pulp regeneration. J Endod. (2014) 40(4 Suppl.):S6–12. doi: 10.1016/j.joen.2014.01.019
57. Piva E, Silva AF, Nör JE. Functionalized scaffolds to control dental pulp stem cell fate. J Endod. (2014) 40(4 Suppl.) S33–40. doi: 10.1016/j.joen.2014.01.013
58. Careddu R, Plotino G, Cotti E, Duncan HF. The management of deep carious lesions and the exposed pulp amongst members of two European endodontic societies: a questionnaire-based study. Int Endod J. (2020) 54:366–76. doi: 10.1111/iej.13418
Keywords: VPT, pulpitis, irreversible pulpitis, vital pulp therapy, endodontics
Citation: El karim IA and Duncan HF (2021) Reducing Intervention in the COVID-19 Era: Opportunities for Vital Pulp Treatment. Front. Dent. Med. 2:686701. doi: 10.3389/fdmed.2021.686701
Received: 27 March 2021; Accepted: 12 May 2021;
Published: 04 June 2021.
Edited by:
Bruno Cavalcanti, University of Michigan, United StatesReviewed by:
Julián Balanta-Melo, University of Valle, ColombiaCopyright © 2021 El karim and Duncan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ikhlas A. El karim, aS5lbGthcmltQHF1Yi5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.