- 1Department of Endodontics, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States
- 2Division of Clinical Dentistry, School of Dentistry, International Medical University, Kuala Lumpur, Malaysia
Introduction: The presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in saliva and nasopharyngeal secretions has challenged the routine practice of dentistry. Use of preprocedural mouth rinses has been recommended by several organizations to potentially reduce the transmission of SARS-CoV-2. This scoping review aimed at evaluating the available evidence on the efficacy of mouth rinses against SARS-CoV-2.
Methods: A thorough literature search on electronic databases (PubMed, Scopus, and Google Scholar) was performed by two independent reviewers and data from articles addressing the aim of this article were extracted.
Results: After exclusion of articles not addressing the end point in question, 12 articles were included in this scoping review. Of the 12 articles, seven were in vitro studies and five were in vivo human clinical studies. The in vitro studies used a standardized methodology (endpoint dilution assay) to evaluate the efficacy of antimicrobial mouth rinses against SARS-CoV-2. The in vivo studies were done utilizing polymerase chain reaction assay of samples obtained from saliva or nasopharyngeal swab or a combination of both nasopharyngeal and oropharyngeal swab. The reagents tested in these studies included povidone-iodine, chlorhexidine, hydrogen peroxide (H2O2), essential oils, and quaternary ammonium compounds and demonstrated varied efficacy against SARS-CoV-2.
Conclusion: Based on the available evidence from in vitro studies, it can be concluded that mouth rinses have a potential to reduce SARS-CoV-2 viral load; however, effectiveness in in vivo conditions is still inconclusive. Owing to the substantial heterogeneity in reporting of the anti–SARS-CoV-2 efficacy of mouth rinses, this review highlights the need to conduct future research with robust and standardized methodologies to confirm effectiveness of mouth rinses.
Introduction
Coronavirus disease 2019 (COVID-19) and its rapid spread have drastically affected the dental community worldwide. This has led to a diverse set of recommendations in which some regions had a complete lockdown of dental practices, in contrast to certain areas where dentists continued to provide care for emergency patients. However, there has been a shift toward reopening of practices and provision of routine dental care, which has led to an increase in aerosol-generating procedures. Aerosols are air-borne suspended particles with a potential to contain salivary components and microorganisms (1). This is a cause for concern as saliva and nasopharyngeal secretions can carry high viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in COVID-19 affected individuals (2). Although the main mode of transmission of SARS-CoV-2 is through respiratory droplets or close contact, transmission via aerosols is possible and has not been ruled out (3). In vitro studies have confirmed the potential of SARS-CoV-2 to be aerosolized for up to 3–16 h (4, 5). Therefore, various dental organizations responded by specifying guidelines for provision of dental care during the pandemic (6–8).
In addition to recommendations such as strict infection control practices, patient screening, and wearing appropriate personal protective equipment, use of preprocedural mouth rinse or gargle has also been suggested by numerous organizations across the world such as Centers for Disease Control and Prevention, American Dental Association, and Australian Dental Association (7–9). Use of preprocedural mouth rinse is based on the principle of reducing oral microbial load and hence mitigating the potential transmission of microbes via aerosol, splatter, or close contact. One of the most commonly used preprocedural mouth rinses in dentistry is chlorhexidine gluconate, which has been shown to be a highly effective antimicrobial agent (10). Several alternative mouth rinses such as iodine-based [povidone-iodine (PVP-I)] or essential oils-based (Listerine) or oxygenating agents [hydrogen peroxide (H2O2)] have also demonstrated comparable antimicrobial efficacy (10).
The promising results of mouth rinses against coronaviruses made the basis for recommendations supporting the use of preprocedural mouth rinses during COVID-19 pandemic (10); however, most of these guidelines were not based on efficacy of mouth rinses against SARS-CoV-2 specifically (7–9). In the past few months, reports on efficacy of topical antimicrobials against SARS-CoV-2 have been published in the literature (11–22). The aim of this scoping review is to present and critically appraise the most updated evidence on the efficacy of mouth rinses against SARS-CoV-2.
Materials and Methods
Focused Question
This scoping review was conducted according to PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews) statement (23), and the review focused on the following evidence-based question: “What is the efficacy of mouth rinses against SARS-CoV-2?”
Search Strategy
To address the aforementioned question, an exhaustive search of literature on electronic databases (PubMed, Scopus, and Google Scholar) was conducted by two independent reviewers (AA, AP) on December 8, 2020. A search strategy on PubMed was built through combination of MeSH terms using Boolean operators “AND,” “OR:” ((((Coronavirus)) OR (SARS-CoV-2 virus[MeSH terms])) OR (2019-nCoV[MeSH terms])) AND (((((((((mouth rinse[MeSH terms]) OR (mouth wash[MeSH terms])) OR (oral sprays[MeSH terms])) OR (chlorhexidine[MeSH terms])) OR (povidone iodine[MeSH terms])) OR (cetylpyridinium chloride[MeSH terms])) OR (essential oils[MeSH terms])) OR (benzalkonium compounds[MeSH terms])) OR (hydrogen peroxide[MeSH terms])) filters: from 2019 to 2020. The search strategy was then adapted for other databases. Articles published onward from December 2019 were included in the screening process. In addition, reference lists of extracted articles were further screened, and gray literature search was performed to find any missing studies. The articles were then imported into reference manager software (Mendeley Desktop, version 1.17.11; Mendeley Ltd., George Mason University, Fairfax, VA) to remove duplicates.
Study Selection Process
Inclusion Criteria
1. Original studies with in vitro or in vivo experimental design reporting on the anti–SARS-CoV-2 efficacy of mouth rinses or gargle.
2. No language restrictions were applied. Applicable articles were included regardless of languages used, as long as translation was available.
Exclusion Criteria
1) Studies reporting on topical antiseptic formulations but intended for either only nasal application or as a surface disinfectant.
2) Studies in preprint stage that have not been peer reviewed and were not intended to be utilized to make clinical recommendations.
3) Opinions, commentaries, and review articles.
4) Studies reporting on efficacy of topical antiseptic formulations against related coronaviruses but not specifically against SARS-CoV-2.
Study Selection
After removal of duplicate studies, two independent reviewers (AA, AP) screened the titles and abstract of all extracted articles and subjected them to the inclusion and exclusion criteria to perform preliminary elimination of ineligible studies. Further, full text of the articles was retrieved and evaluated for inclusion in the scoping review. Any duplication of data presented in studies was noted. Any disagreements in the process were resolved by consulting another reviewer (NBR).
Data Extraction
Two independent reviewers (AA, AP) performed data extraction using customized data retrieval forms. Extracted data included author, year, study design, type of SARS-CoV-2 strain, technique employed for detecting antiviral efficacy, test products or intervention, duration, key findings, details of funding (funding source), and conflict of interest.
Results
Study Selection
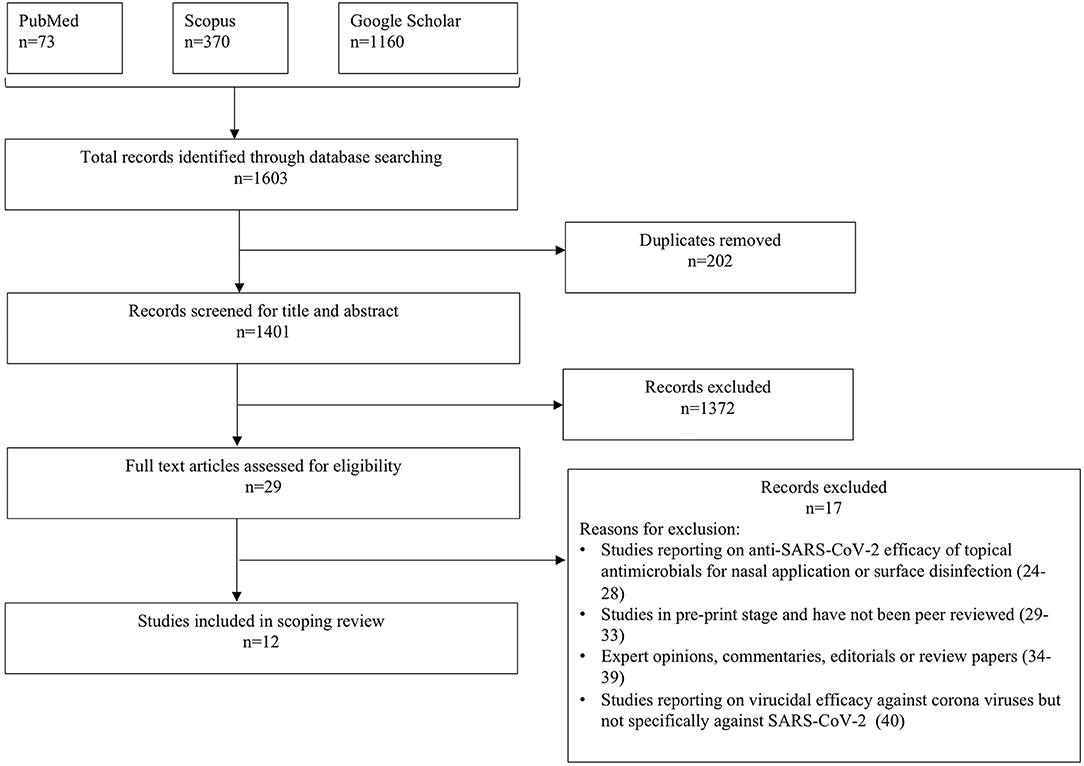
The flowchart for study selection in this review is shown in Figure 1. A total of 1,603 potentially relevant records were identified through electronic database search and gray literature search. After removing duplicates, 1,401 records were screened by reviewers (AA, AP) for title and abstract content, of which 1,372 records were excluded. Full texts of 29 articles were reviewed for eligibility assessment, and eventually 12 records (11–22) were included in the review based on the inclusion/exclusion criteria. The reasons for excluding 17 articles (24–40) are presented in Figure 1.
Study Characteristics
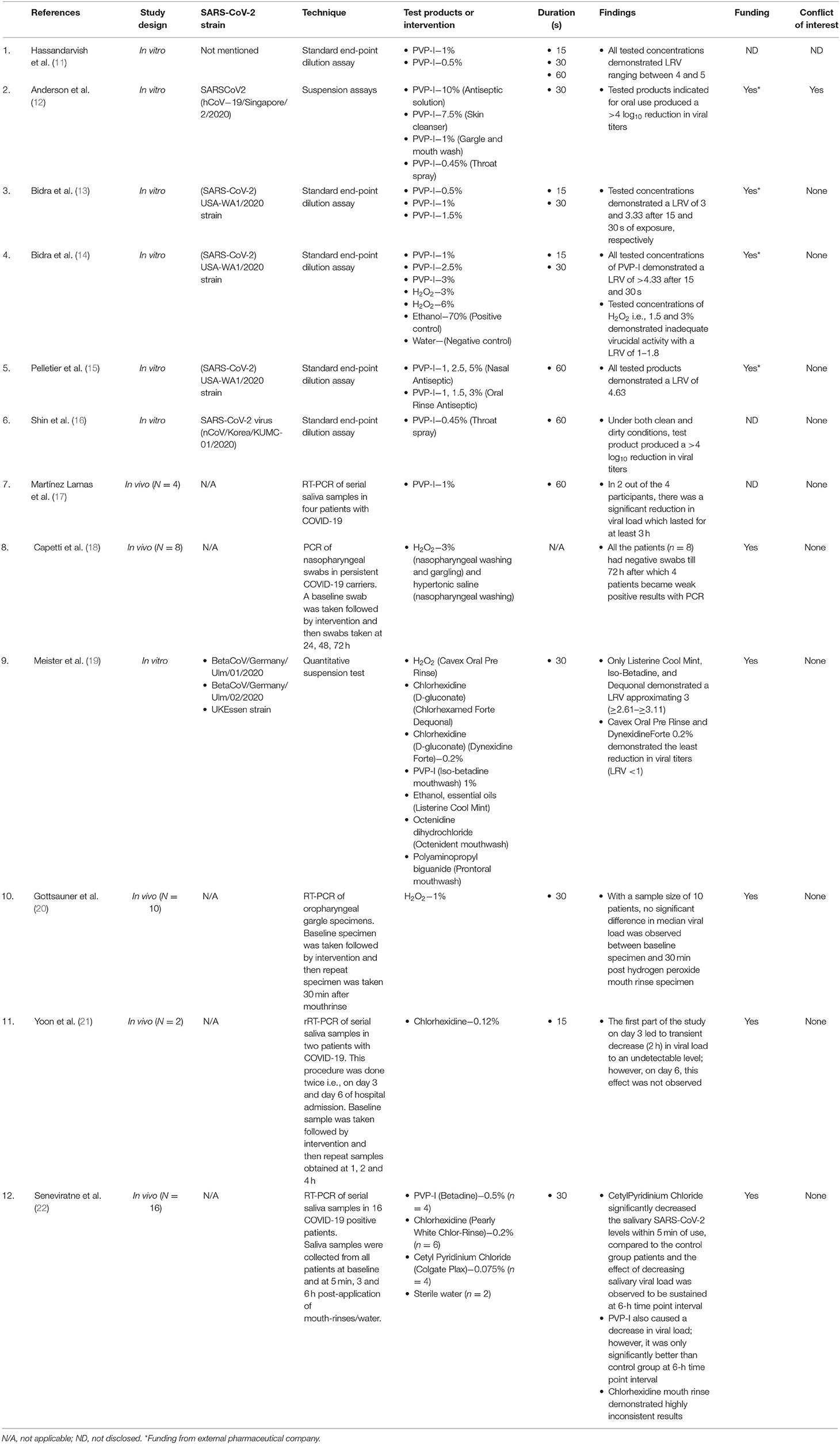
The study characteristics of the included studies are presented in Table 1. Of the 12 included articles in this review, seven were in vitro studies (11–16, 19) and five were in vivo human clinical studies (17, 18, 20–22). Among the in vivo studies, one was a randomized controlled trial (22). All in vitro studies (11–16, 19) used a standardized methodology (endpoint dilution assay) to evaluate the efficacy of antimicrobial formulations against SARS-CoV-2. Briefly, endpoint dilution assay determines the amount of virus required to kill 50% of infected hosts or to produce a cytopathic effect in 50% of inoculated tissue culture cells (TCID50) (41). Data for virucidal activity are typically reported as log10 reduction value (LRV), which denotes reduction in viral titers with experimental test group compared to virus control group (41). A log10 reduction value of >4 indicates high virucidal activity and represents 99.99% kill efficacy (42, 43). The in vivo studies were done utilizing real-time reverse transcription–polymerase chain reaction (rRT-PCR) assay of samples obtained from saliva or oropharyngeal gargle or nasopharyngeal swab or a combination of both nasopharyngeal and oropharyngeal swab (17, 18, 20–22).
The key results from the included studies are presented under the following subcategories based on the commonly used antimicrobial reagents present in mouth rinses.
Povidone-Iodine
The search strategy yielded a total of nine studies reporting on the efficacy of PVP-I against SARS-CoV-2 (11–17, 19, 22). With the exception of two in vivo studies (17, 22), remaining of them had an in vitro study design (11–16, 19). The in vitro studies used a concentration of PVP-I ranging from 0.33 to 1.5% and a contact time varying from 15 to 60 s (11–16, 19). The collective results of these in vitro studies demonstrate that PVP-I causes a significant reduction in viral titers of SARS-CoV-2 with LRV values ranging from 2.61 to 5 (11–16, 19).
The in vivo study by Lamas et al. (17) evaluated the efficacy of 1% PVP-I mouth rinse for 1 min on the salivary viral load of SARS-CoV-2 in four patients with COVID-19. Baseline saliva samples were obtained followed by mouth rinse use for 1 min, and then serial saliva samples were collected at different time intervals to determine presence of SARS-CoV-2 using rRT-PCR assay. All the baseline samples confirmed presence of SARS-CoV-2, and post–mouth rinse saliva samples showed a significant reduction in viral load for 3 h in two patients. Interestingly, PVP-I was only effective in patients who presented with high viral loads in baseline samples.
A randomized controlled trial (22) compared anti–SARS-CoV-2 efficacy of 0.5% PVP-I (Betadine gargle and mouthwash), 0.2% chlorhexidine mouthwash (Pearly White Chlor-Rinse), 0.075% cetylpyridinium chloride (CPC) (Colgate Plax mouthwash), and sterile water (control group) in confirmed COVID-19 patients. Viral load was detected by performing RT-PCR of saliva samples obtained by passive drooling technique in 16 patients. Samples were obtained at four time point intervals: baseline, 5 min, 3 and 6 h. PVP-I rinsing caused an increase in cycle threshold value, which is an indirect inverse measure of viral load. However, when compared to control group, statistically significant difference was only observed at 6-h time point interval.
Chlorhexidine
A total of three studies were found that reported on the efficacy of chlorhexidine against SARS-CoV-2 (19–22). With the exception of one in vitro study (19), the remainder of them had an in vivo study design (21, 22). Meister et al. (19) using the in vitro TCID50 assay evaluated virucidal efficacy of two commercial preparations of chlorhexidine (Chlorhexamed Forte and Dynexidine Forte 0.2%) against three different strains of SARS-CoV-2 and demonstrated minimal benefit (LRV = 0.50–1.17).
Regarding the in vivo efficacy, Yoon et al. (21) evaluated the effectiveness of 0.12% chlorhexidine gluconate mouth rinse for 30 s on salivary viral load in two patients with confirmed COVID-19. The rRT-PCR analysis demonstrated presence of SARS-CoV-2 in baseline saliva samples of both patients and a transient (2 h) decrease in SARS-CoV-2 salivary load after chlorhexidine rinse. However, conflicting results were obtained in a randomized controlled trial that demonstrated no statistically significant difference between 0.2% chlorhexidine mouthwash (30 s) and sterile water in reducing viral load in COVID-19 patients (22).
Hydrogen Peroxide
Literature search yielded four studies reporting on anti–SARS-CoV-2 efficacy of hydrogen peroxide, with an equal distribution of in vitro and in vivo study designs (14, 18–20). Bidra et al. (14) demonstrated limited virucidal activity of 1.5 and 3% hydrogen peroxide when tested for either 15- or 30-s duration, with LRVs ranging from 1 to 1.8. This LRV for H2O2 was three times lower than the LRV obtained with any of the concentrations of PVP-I tested in their study (14). These findings were later corroborated by Meister et al. demonstrating LRV of <1 with commercial hydrogen peroxide–based mouth rinse (Cavex pre oral rinse) (19).
There are conflicting reports on the in vivo efficacy of hydrogen peroxide against SARS-CoV-2. Gottsauner et al. (20) demonstrated no significant difference in median viral load between the baseline oropharyngeal samples and the samples obtained 30 min after rinsing with 1% hydrogen peroxide for 30 s. On the contrary, Capetti et al. (18) reported excellent efficacy of 3% hydrogen peroxide usage by demonstrating negative PCR results in eight persistent COVID-19 carrier patients. This effect lasted for 72 h, following which four patients became weakly positive for COVID-19.
Essential Oils
Only one in vitro study was found reporting on anti–SARS-CoV-2 efficacy of essential oil–based mouth rinse (Listerine Cool Mint) (19). The study utilizing the TCID50 assay demonstrated a viral titer reduction of three orders in magnitude with Listerine in comparison to the control group (19).
Quaternary Ammonium Compounds
Literature search yielded two studies reporting on anti–SARS-CoV-2 efficacy of quaternary ammonium compounds (19, 22). An in vitro study by Meister et al. (19) evaluated a commercial preparation of benzalkonium chloride (Dequonal) for oral use and demonstrated its potent SARS-CoV-2 virucidal activity (LRV~3). A randomized controlled trial evaluated another quaternary ammonium compound, i.e., CPC, and demonstrated a statistically significant increase in fold change of cycle threshold value at 5 min and 6 h after rinsing with CPC mouth rinse compared to the sterile water group.
Discussion
Oral healthcare providers and patients are routinely exposed to aerosolized pathogens during dental treatment (44). One of the suggested measures to reduce the microbial load in aerosols is to use pre procedural mouth rinse (38). Use of preprocedural mouth rinses is not new to dentistry; however, its efficacy in reducing the transmission of infections has been a subject of debate (44). According to a survey, dentists' perceived benefits of preprocedural rinsing are to minimize microbial load and to decrease aerosolization of bacteria (44).
Dental profession has been categorized by the Occupational Safety and Health Administration to be “very high risk,” especially if it involves aerosol-generating procedures (45). Therefore, in addition to use of personal protective equipment, high-volume suction, and rubber dam, several organizations have also recommended the use of preprocedural mouth rinse as a layer of defense against aerosol microbial transmission (7–9). However, the recommendations early on during the pandemic were based on antimicrobial activity of mouth rinses that were not specific to SARS-CoV-2 (6–9). Evidence on efficacy of mouth rinses or gargles against SARS-CoV-2 has recently been published in the literature (11–22). Thus, the present scoping review provided a critical appraisal of the available evidence on anti–SARS-CoV-2 efficacy of mouth rinses. The included studies mostly focused on evaluating PVP-I, chlorhexidine, hydrogen peroxide, essential oil–based, and quaternary ammonium compounds–based mouth rinses (11–22).
PVP-I is a broad-spectrum antimicrobial typically used as a presurgical antiseptic or as a mouth rinse (46, 47). It acts by releasing free iodine, which disrupts microbial metabolic pathways and destabilizes structural components of cell membranes of pathogens (10). There have been some concerns about staining of teeth and tissues owing to the iodine content in PVP-I; however, a clinical trial has demonstrated that PVP-I causes less staining of teeth when compared to chlorhexidine-gluconate (48). The concentration of PVP-I tested in most of the studies included in this review (11, 13–17, 19, 22) is well below the recommended safe concentration of 5% for oral use (34). Ready-to-use PVP-I mouth rinse/gargle/throat spray are available in some countries; however, in the United States, PVP-I is available only as 10% topical solution (Betadine antiseptic solution, Betadine, Avrio Health L.P., USA) and 5% spray (Betadine antiseptic spray, Betadine, Avrio Health L.P., USA). Therefore, diluting them to an appropriate concentration will be needed prior to oral use (34).
The in vitro studies on PVP-I included in this review demonstrate adequate virucidal activity against SARS-CoV-2 (11–16, 19) and validate the previous recommendations made by various organizations (6–9), which were mostly based on indirect evidence. Majority of the in vitro studies on PVP-I efficacy suggest a log10 reduction value of >4 (11, 12, 14–16), which is in accordance with the European standard (EN 14776) (42) and the Robert Koch Institute guidelines for effective virucidal activity and represents 99.99% kill efficacy (43). However, data from in vivo research are currently limited to only two studies (17, 22), both with a small sample size of four patients per intervention. In the study by Lamas et al., PVP-I mouth rinse reduced viral load in 50% of the patients at 3-h time point interval; however, saliva sample obtained at 5-min time point interval did not show any significant reduction in viral load compared to baseline saliva sample (17). A similar finding was demonstrated by Seneviratne et al. wherein PVP-I was not significantly better than sterile water in reducing viral load at 5-min time point interval but fared significantly better after 6 h after rinse (22). The low viral titers obtained after 3 h (17) or 6 h (22) after rinsing in both these studies raise few questions. Is this finding a potential result of technical issues in methodology or whether PVP-I truly has a sustained virucidal effect? Substantivity with use of PVP-I has been a controversial topic; von Ohle et al. in a subgingival irrigation study demonstrated sustained antimicrobial effect of PVP-I for 31 days (49). Contrasting results were published by Macias et al., wherein PVP-I exhibited no substantivity (50). As far as anti–SARS-CoV-2 efficacy of PVP-I is concerned, data from in vitro studies look extremely promising, but clinical research still needs to corroborate these findings to draw any definitive conclusions and make clinical recommendations.
Chlorhexidine, a broad-spectrum biocide, has been used in dentistry for several years to treat gingivitis and also as a preprocedural mouth rinse (51, 52). The cationic chlorhexidine molecule interacts with the anionic phosphate residue of the lipid molecules in the cell membrane of pathogen and causes cell membrane disruption (52). Chlorhexidine has a tendency to bind to tissues and release over an extended period of time, a beneficial antimicrobial property known as substantivity (52). Commercial preparations of chlorhexidine such as Peridex (Proctor and Gamble, Cincinnati, Ohio) usually combine 0.12% chlorhexidine with alcohol (11.6%), which can partly contribute to its microbicidal activity (53). Chlorhexidine has been demonstrated to be effective against lipid-enveloped viruses (54); however, an in vitro study reported limited to no efficacy (LRV <1) against human coronavirus, even after 10 min of exposure (55).
As far as efficacy of chlorhexidine against SARS-CoV-2 is concerned, the available evidence in literature is limited. In vitro data on two commercial preparations of chlorhexidine have shown minimal virucidal activity against SARS-CoV-2 (LRV <1) (19). The data from in vivo studies have conflicting results on anti–SARS-CoV-2 efficacy of chlorhexidine (21, 22). Yoon et al. demonstrated favorable results with use of 0.12% chlorhexidine rinse to reduce salivary SARS-CoV-2 load; however, the study was restricted to two patients and did not have a control group (21). On the other hand, the randomized controlled trial by Seneviratne et al. had inconsistent results with chlorhexidine use, which were not significantly better than sterile water group, and the authors refrained from making any firm conclusions on its virucidal efficacy (22).
Hydrogen peroxide, a widely used antiseptic in healthcare, exerts its microbicidal action by producing hydroxyl free radicals that can attack membrane lipids and other essential cell components of pathogens (10). In terms of its virucidal activity against human coronavirus, an accelerated hydrogen peroxide–based disinfectant was demonstrated to be highly effective within 1 min of contact (56). Based on these findings, use of hydrogen peroxide mouth rinse had been advocated during COVID-19 pandemic (57); however, the available in vitro studies evaluating activity of H2O2 against SARS-CoV-2 fail to demonstrate effective virucidal activity (LRV <1.8) (14, 19). The in vivo efficacy of hydrogen peroxide against SARS-CoV-2 is still inconclusive as results obtained from the two in vivo reports are contrasting. Gottsauner et al. (20) demonstrated no significant decrease in viral load after rinsing with 1% hydrogen peroxide. This indicates weak virucidal activity of hydrogen peroxide in in vivo conditions, which can also be partly attributed to the inactivation of hydrogen peroxide by catalase group of enzymes present in oral cavity (10). However, Capetti et al. (18) reported excellent efficacy of 3% hydrogen peroxide by demonstrating negative PCR results in eight persistent COVID-19 carrier patients. It is noteworthy that both the in vivo studies differ from each other in few aspects. First, the technique and concentration of hydrogen peroxide use in the study of Gottsauner et al. (20) were gargling with 1% hydrogen peroxide in contrast to the more aggressive approach in the study of Capetti et al. (18), wherein 3% H2O2 gargle and hypertonic saline nasopharyngeal wash were used. This is important as it has been shown that hypertonic saline does possess antiviral properties (58) and might have contributed to the superior efficacy of H2O2 as seen in the study of Capetti et al. (18). Second, the type of specimens obtained for PCR analysis differed between the two studies: oropharyngeal gargle (20) vs. nasopharyngeal swab (18). This can also potentially impact detection of viral RNA as it has been shown that saliva or oropharyngeal rinse can contribute to dilution of samples and lead to suboptimal detection (59). On the other hand, it has also been demonstrated that in few cases SARS-CoV-2 RNA has been detected in saliva/oropharyngeal rinse samples but was missing in the corresponding nasopharyngeal swab sample, which could be attributed to errors in obtaining the swab (60). The available evidence from in vitro and in vivo studies does not provide encouraging outcomes with use of hydrogen peroxide mouth rinse against SARS-CoV-2, and more studies are needed to evaluate its virucidal efficacy against SARS-CoV-2, especially in in vivo conditions.
Data on anti–SARS-CoV-2 efficacy of essential oil–based mouth rinse (Listerine) and quaternary ammonium compounds (benzalkonium chloride and CPC) show promising results; however, these are based on few studies (19, 22) and will need further validation.
Limitations
First, one of the limitations is that most of the evidence is based on in vitro studies wherein the viral titer reduction was evaluated in laboratory settings, which may significantly differ from a clinical scenario (61). Multiple factors such as presence of organic matter, serum proteins, and enzymes in the oral cavity can modulate effectiveness of topical antimicrobials (62). In addition, it is important to understand that virucidal activity of mouth rinses reported in in vitro studies can be a combination of their inherent virucidal efficacy along with cytotoxic effects induced by the antimicrobial agent (63). Therefore, cytotoxic effects of an antimicrobial should be evaluated separately in advance in order to establish the inherent virucidal activity of the compound being tested. Although a rigorous and standardized methodology has been used in all the in vitro studies included in this review, some of the studies have reported conflict of interest and funding from pharmaceutical companies (12–15), which can potentially bias the study outcome.
Second, most of the included in vivo studies have inherent limitations such as small sample size (17, 21, 22) and lack of control groups (17, 20, 21). In addition, heterogeneity among the in vivo studies in terms of methodology for sample collection (oropharyngeal swab, nasopharyngeal swab, or saliva) could have affected SARS-CoV-2 RNA detection, which makes it difficult to compare the results. The studies have employed RT-PCR assay, which is currently the gold standard for SARS-CoV-2 detection (64). RT-PCR tests yield cycle threshold (Ct) values, which are a surrogate measure and are inversely proportional to the amount of target nucleic acid in the sample (65). It is worth noting that the mere presence of nucleic acid in a sample does not translate to infectivity. Therefore, it is important to conduct virus culture studies to establish the infectivity of a sample. This phenomenon was demonstrated in the study by Gottsauner et al., wherein five samples with a load of 103 SARS-CoV-2 RNA copies per milliliter were used for virus culture study, but only one sample was found to be actively replicating and infectious (20).
Lastly, several preprints could not be included in this review (29–33). Preprints are manuscripts that have not been peer reviewed, and data from the study should not be used for clinical guidance. These preprints, when peer reviewed and accepted for publication, will add to the existing literature on efficacy of mouth rinses against SARS-CoV-2.
Future Recommendations
Research on efficacy of oral mouth rinses should focus on reporting factors such as exposure time, strength, volume of mouth rinse, and SARS-CoV-2 strain, so that results can be extrapolated to clinical setting. Studies should have adequate sample size and control groups to yield more reliable conclusions and have better external validity. Factors in in vivo studies such as baseline viral titer load, patient demographics, and symptomatology should also be reported to match patient data and to provide a better understanding of the study. Viral culture technique should be employed in future in vivo research so as to establish the true potential of viral infectivity. In addition, guidelines to conduct in vitro studies, e.g., Preferred Reporting Items for Laboratory studies in Endodontology (PRILE), and clinical trials, e.g., Preferred Reporting Items for Randomized Trials in Endodontics (PRIRATE) or Consolidated Standards of Reporting Trials (CONSORT), should be followed (66–68).
Conclusion
Based on the limited evidence from in vitro studies, it can be concluded that mouth rinses have a potential to reduce SARS-CoV-2 viral load; however, the emerging evidence from in vivo studies is still inconclusive to recommend one mouth rise over another. Owing to the substantial heterogeneity in reporting of the anti–SARS-CoV-2 efficacy of mouth rinses, this review highlights the need to conduct future research with robust and standardized methodologies to confirm effectiveness of mouth rinses.
Author Contributions
AA was responsible for conceptualization, data collection, writing, editing, and finalizing the manuscript. AP and NR were responsible for data collection, editing, and finalizing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Harrel SK, and Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. (2004) 135:429–37. doi: 10.14219/jada.archive.2004.0207
2. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. (2020) 5:565−74. doi: 10.1016/S1473-3099(20)30196-1
3. Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg Infect Dis. (2020) 26:1583–91. doi: 10.3201/eid2607.200885
4. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. (2020) 382:1564–67. doi: 10.1056/NEJMc2004973
5. Fears SC, Klimstra WB, Duprex P, Hartman A, Weaver SC, Plante KS, et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis. (2020) 9:2168–71. doi: 10.3201/eid2609.201806
6. Ather A, Patel B, Ruparel NB, Diogenes A, and Hargreaves KM. Coronavirus disease 19 (COVID-19): implications for clinical dental care. J Endod. (2020) 46:584–95. doi: 10.1016/j.joen.2020.03.008
7. Guidance, for Dental Settings,. CDC. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html (accessed December 08, 2020).
8. Summary of ADA Guidance During the COVID-19 Crisis. Available online at: https://www.ada.org/en/press-room/news-releases/2020-archives/april/summary-of-ada-guidance-during-the-covid-19-crisis (accessed December 08, 2020).
9. Managing COVID-19 Guidelines. Available online at: https://www.ada.org.au/Campaign/COVID-19/Guide-to-Managing-COVID-19/ADA-Managing-COVID-19-Guide-v-2.aspx (accessed December 08, 2020).
10. O'Donnell VB, Thomas D, Stanton R, Maillard JY, Murphy RC, Jones SA, et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function. (2020) 1:zqaa002. doi: 10.1093/function/zqaa002
11. Hassandarvish P, Tiong V, Sazaly AB, Mohamed NA, Arumugam H, Ananthanarayanan A, et al. Povidone iodine gargle and mouthwash. Br Dent J. (2020) 228:900. doi: 10.1038/s41415-020-1794-1
12. Anderson DE, Sivalingam V, Kang AEZ, Ananthanarayanan A, Arumugam H, Jenkins TM, et al. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect Dis Ther. (2020) 3:669–75. doi: 10.1007/s40121-020-00316-3
13. Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, and Tessema B. Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. J Prosthodont. (2020) 6:529–33. doi: 10.1111/jopr.13209
14. Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, and Tessema B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J Prosthodont. (2020) 7:599–603. doi: 10.1111/jopr.13220
15. Pelletier JS, Tessema B, Frank S, Westover JB, Brown SM, and Capriotti JA. Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). Ear Nose Throat J. (2020). doi: 10.1101/2020.05.25.20110239. [Epub ahead of print].
16. Shin KR, Kwak K, Cui C, Bae JY, Hong W, and Park MS. In vitro virucidal effect of povidone-iodine against SARS-CoV-2. J Bacteriol Virol. (2020) 3:195–202. doi: 10.4167/jbv.2020.50.3.195
17. Martínez Lamas L, Diz Dios P, Pérez Rodríguez MT, Del Campo Pérez V, Cabrera Alvargonzalez JJ, López Domínguez AM, et al. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. (2020). doi: 10.1111/odi.13526. [Epub ahead of print].
18. Capetti AF, Borgonovo F, Morena V, Lupo A, Cossu MV, Passerini M, et al. Short-term inhibition of SARS-CoV-2 by hydrogen peroxide in persistent nasopharyngeal carriers. J Med Virol. (2020) 93:1766–9. doi: 10.1002/jmv.26485
19. Meister TL, Brüggemann Y, Todt D, Conzelmann C, Müller JA, Groß R, et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. (2020) 8:1289–92. doi: 10.1093/infdis/jiaa471
20. Gottsauner MJ, Michaelides I, Schmidt B, Scholz KJ, Buchalla W, Widbiller M, et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin Oral Investig. (2020) 10:3707–13. doi: 10.1007/s00784-020-03549-1
21. Yoon JG, Yoon J, Song JY, Yoon SY, Lim CS, Seong H, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. (2020) 35:e195. doi: 10.3346/jkms.2020.35.e195
22. Seneviratne CJ, Balan P, Ko KKK, Udawatte NS, Lai D, Ng DHL, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. (2020). doi: 10.1007/s15010-020-01563-9. [Epub ahead of print].
23. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 7:467–73. doi: 10.7326/M18-0850
24. Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen HL, Chan MCW, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. (2020) 1:e10. doi: 10.1016/S2666-5247(20)30003-3
25. Liang B, Yuan X, Wei G, Wang W, Zhang M, Peng H, et al. In-vivo toxicity studies and in-vitro inactivation of SARS-CoV-2 by povidone-iodine in-situ gel forming formulations. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.05.18.103184
26. Frank S, Brown SM, Capriotti JA, Westover JB, Pelletier JS, and Tessema B. In vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2. JAMA Otolaryngol Head Neck Surg. (2020) 11:1–5. doi: 10.1001/jamaoto.2020.3053
27. Cannon ML, Westover JB, Bleher R, Sanchez-Gonzalez MA, and Ferrer GA. In vitro analysis of the anti-viral potential of nasal spray constituents against SARS-CoV-2. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.12.02.408575
28. Gerlach M, Wolff S, Ludwig S, Schäfer W, Keiner B, Roth NJ, et al. Rapid SARS-CoV-2 inactivation by commonly available chemicals on inanimate surfaces. J Hosp Infect. (2020) 3:633–4. doi: 10.1016/j.jhin.2020.09.001
29. Davies K, Buczkowski H, Welch SR, Green N, Mawer D, Woodford N, et al. Effective in-vitro inactivation of SARS-CoV-2 by commercially available mouthwashes. bioRxiv. (2020). doi: 10.1101/2020.12.02.408047
30. Mohamed NA, Baharom N, Wan Sulaiman WS, Rashid ZZ, Wong KK, Ali UK, et al. Early viral clearance among covid-19 patients when gargling with povidone-iodine and essential oils: a pilot clinical trial. medRxiv [Preprint]. (2020). doi: 10.1101/2020.09.07.20180448
31. Steinhauer K, Meister TL, Todt D, Krawczyk A, Paßvogel L, Becker, et al. Comparison of the in vitro-efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.10.25.354571
32. Statkute E, Rubina A, O'Donnell VB, Thomas DW, and Stanton RJ. Brief report: the virucidal efficacy of oral rinse components against SARS-CoV-2 in vitro. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.11.13.381079
33. Xu C, Wang A, Hoskin ER, Cugini C, Markowitz K, Chang TL, et al. Differential effects of antiseptic mouth rinses on SARS-CoV-2 infectivity in vitro. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.12.01.405662
34. Frank S, Capriotti J, Brown SM, and Tessema B. Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era. Ear Nose Throat J. (2020) 9:586–93. doi: 10.1177/0145561320932318
35. Moosavi MS, Aminishakib P, and Ansari M. Antiviral mouthwashes: possible benefit for COVID-19 with evidence-based approach. J Oral Microbiol. (2020) 1:1794363. doi: 10.1080/20002297.2020.1794363
36. Vergara-Buenaventura A, and Castro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg. (2020) 8:924–7. doi: 10.1016/j.bjoms.2020.08.016
37. Herrera D, Serrano J, Roldán S, and Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Investig. (2020) 8:2925–30. doi: 10.1007/s00784-020-03413-2
38. Marui VC, Souto MLS, Rovai ES, Romito GA, Chambrone L, and Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review. J Am Dent Assoc. (2019) 12:1015–26.e1. doi: 10.1016/j.adaj.2019.06.024
39. de Toledo Telles-Araujo G, Caminha RDG, Kallás MS, Sipahi AM, and da Silva Santos PS. Potential mouth rinses and nasal sprays that reduce SARS-CoV-2 viral load: what we know so far?. Clinics. (2020) 75:e2328. doi: 10.6061/clinics/2020/e2328
40. Meyers C, Robison R, Milici J, Alam S, Quillen D, Goldenberg D, and Kass R. Lowering the transmission and spread of human coronavirus. J Med Virol. (2020) 93:1605–12. doi: 10.1002/jmv.26514
41. Pourianfar HR, Javadi A, and Grollo L. A colorimetric-based accurate method for the determination of enterovirus 71 titer. Indian J Virol. (2012) 23:303–10. doi: 10.1007/s13337-012-0105-0
42. Rabenau HF, Steinmann J, Rapp I, Schwebke I, and Eggers M. Evaluation of a virucidal quantitative carrier test for surface disinfectants. PLoS ONE. (2014) 9:e86128. doi: 10.1371/journal.pone.0086128
43. Rabenau HF, Schwebke I, Blümel J, Eggers M, Glebe D, Rapp I, et al. Guideline for testing chemical disinfectants regarding their virucidal activity within the field of human medicine. Bundesgesundheitsbl. (2020) 63:645–55. doi: 10.1007/s00103-020-03115-w
44. Hennessy B, and Joyce A. A survey of preprocedural antiseptic mouth rinse use in Army dental clinics. Mil Med. (2004) 8:600–3. doi: 10.7205/MILMED.169.8.600
45. COVID-19 - Control Prevention | Denstistry Workers Employers. Occupational Safety and Health Administration. Available online at: https://www.osha.gov/SLTC/covid-19/dentistry.html (accessed December 08, 2020).
46. Hemani ML, and Lepor H. Skin preparation for the prevention of surgical site infection: which agent is best? Rev Urol. (2009) 4:190–5.
47. Al-Saeed MY, and Babay N. The use of povidone–iodine and hydrogen peroxide mixture as an adjunct to non- surgical treatment of slight to moderate chronic periodontitis. Saudi Dent J. (2009) 3:127–33. doi: 10.1016/j.sdentj.2009.10.004
48. Fine PD. A clinical trial to compare the effect of two antiseptic mouthwashes on gingival inflammation. J Hosp Infect. (1985) 6(Suppl. A):189–93. doi: 10.1016/S0195-6701(85)80067-0
49. von Ohler C, Weiger R, Decker E, Schlagenhauf U, and Brecx M. The efficacy of a single pocket irrigation on subgingival microbial vitality. Clin Oral Investig. (1998) 2:84–90. doi: 10.1007/s007840050050
50. Macias JH, Arreguin V, Munoz JM, Alvarez JA, Mosqueda JL, and Macias AE. Chlorhexidine is a better antiseptic than povidone iodine and sodium hypochlorite because of its substantive effect. Am J Infect Control. (2013) 7:634–7. doi: 10.1016/j.ajic.2012.10.002
51. Jones CG. Chlorhexidine: is it still the gold standard? Periodontol 2000. (1997) 15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x
52. Horner C, Mawer D, and Wilcox M. Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter? J Antimicrob Chemother. (2012) 11:2547–59. doi: 10.1093/jac/dks284
53. Herrera D, Roldán S, Santacruz I, Santos S, Masdevall M, and Sanz M. Differences in antimicrobial activity of four commercial 0.12% chlorhexidine mouthrinse formulations: an in vitro contact test and salivary bacterial counts study. J Clin Periodontol. (2003) 4:307–14. doi: 10.1034/j.1600-051X.2003.00341.x
54. Bernstein D, Schiff G, Echler G, Prince A, Feller M, and Briner W. In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse. J Dent Res. (1990) 3:874–6. doi: 10.1177/00220345900690030901
55. Saknimit M, Inatsuki I, Sugiyama Y, and Yagami K. Virucidal efficacy of physico-chemical treatments against coronaviruses and parvoviruses of laboratory animals. Jikken Dobutsu. (1988) 3:341–5. doi: 10.1538/expanim1978.37.3_341
56. Omidbakhsh N, and Sattar SA. Broad-spectrum microbicidal activity, toxicologic assessment, and materials compatibility of a new generation of accelerated hydrogen peroxide-based environmental surface disinfectant. Am J Infect Control. (2006) 5:251–7. doi: 10.1016/j.ajic.2005.06.002
57. Peng X, Xu X, Li Y, Cheng L, Zhou X, and Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. (2020) 1:9. doi: 10.1038/s41368-020-0075-9
58. Ramalingam S, Graham C, Dove J, Morrice L, and Sheikh A. A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci Rep. (2019) 1:1015. doi: 10.1038/s41598-018-37703-3
59. Babady NE, McMillen T, Jani K, Viale A, Robilotti EV, Aslam A, et al. Performance of severe acute respiratory syndrome coronavirus 2 real-time RT-PCR tests on oral rinses and saliva samples. J Mol Diagn. (2021) 1:3–9. doi: 10.1016/j.jmoldx.2020.10.018
60. Sakanashi D, Asai N, Nakamura A, Miyazaki N, Kawamoto Y, Ohno T, et al. Comparative evaluation of nasopharyngeal swab and saliva specimens for the molecular detection of SARS-CoV-2 RNA in Japanese patients with COVID-19. J Infect Chemother. (2021) 1:126–9. doi: 10.1016/j.jiac.2020.09.027
61. Fan J, Zhang X, Liu J, Yang Y, Zheng N, Liu Q, et al. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients. Clin Infect Dis. (2020). 71:3232–6. doi: 10.1093/cid/ciaa623
62. Ross NM, Charles CH, and Dills SS. Long-term effects of Listerine antiseptic on dental plaque and gingivitis. J Clin Dent. (1989) 1:92–5.
63. Müller H-D, Eick S, Moritz A, Lussi A, and Gruber R. Cytotoxicity and antimicrobial activity of oral rinses in vitro. BioMed Res Int. (2017). doi: 10.1155/2017/4019723. [Epub ahead of print].
64. D'Cruz RJ, Currier AW, and Sampson VB. Laboratory testing methods for novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Front Cell Dev Biol. (2020) 8:468. doi: 10.3389/fcell.2020.00468
65. Tom MR, and Mina MJ. To Interpret the SARS-CoV-2 test, consider the cycle threshold value. Clin Infect Dis. (2020) 16:2252–4. doi: 10.1093/cid/ciaa619
66. Nagendrababu V, Murray PE, Ordinola-Zapata R, Peters OA, Rôças IN, Siqueira JF Jr, et al. A protocol for developing reporting guidelines for laboratory studies in endodontology. Int Endod J. (2019) 8:1090–5. doi: 10.1111/iej.13123
67. Nagendrababu V, Duncan HF, Bjørndal L, Kvist T, Priya E, Jayaraman J, et al. PRIRATE 2020 guidelines for reporting randomized trials in endodontics: a consensus-based development. Int Endod J. (2020) 6:764–73. doi: 10.1111/iej.13294
Keywords: SARS-CoV-2, oral, mouth rinse, COVID-19, aerosols
Citation: Ather A, Parolia A and Ruparel NB (2021) Efficacy of Mouth Rinses Against SARS-CoV-2: A Scoping Review. Front. Dent. Med. 2:648547. doi: 10.3389/fdmed.2021.648547
Received: 31 December 2020; Accepted: 05 February 2021;
Published: 09 March 2021.
Edited by:
Johnah Galicia, University of the Pacific, United StatesReviewed by:
Sameer D. Jain, Virginia Commonwealth University, United StatesJulian G. Leprince, Catholic University of Louvain, Belgium
Copyright © 2021 Ather, Parolia and Ruparel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amber Ather, YXRoZXJAbGl2ZW1haWwudXRoc2NzYS5lZHU=
 Amber Ather
Amber Ather Abhishek Parolia
Abhishek Parolia Nikita B. Ruparel
Nikita B. Ruparel