- 1Department of Orthodontics, School of Stomatology, Peking University, Beijing, China
- 2Department of Oral Biology and Pathology, School of Dental Medicine, Stony Brook University, Stony Brook, NY, United States
- 3Department of Public Health, School of Medicine, Stony Brook University, Stony Brook, NY, United States
- 4Department of Chemistry and Pharmacological Sciences, School of Medicine, Stony Brook University, Stony Brook, NY, United States
- 5Traverse Biosciences, Inc., Stony Brook, NY, United States
- 6Division of Laboratory Animal Resources (DLAR) at Stony Brook, Stony Brook University, Stony Brook, NY, United States
- 7Department of General Dentistry, School of Dental Medicine, Stony Brook University, Stony Brook, NY, United States
Periodontitis, a destructive periodontal inflammatory disease, negatively impacts oral-health related quality of life. It's characterized by the generation of inflammatory mediators and the excess-production of collagenolytic tissue-destructive enzymes (especially matrix metalloproteinases, MMPs). Many biomarkers can be used to define/diagnose disease progression. However, there is still a critical lack of specific, fast, and reliable biomarkers that correlate well with early response to treatment, which can be used to predict/monitor disease. Here, we report that an early marker, MMP-9, was found to be sensitive in response to a 1-month systemic therapy of CMC2.24, a novel chemically-modified curcumin, in beagle dogs with naturally-occurring periodontitis. In brief, eight adult female dogs with generalized periodontitis were distributed into placebo and treatment groups (n = 4/group). After a 1-h full-mouth scaling and root planing at time 0, placebo or CMC2.24 (10 mg/kg) capsules were orally-administered once/day for 1-month. Clinical periodontal parameters were measured at time 0 and 1-month; in addition, peripheral blood samples from these dogs were collected and analyzed for the pro-, activated-, and total-forms of MMP-9 by gelatin zymography. Interestingly, we found that the 1-month systemic therapy of CMC2.24 did appear to significantly reduce both pro- and activated-MMP-9 in peripheral blood at this early stage compared to placebo, prior to apparent clinical improvements seen at a later stage in a previous study (3-months). Thus, MMP-9 may serve as an early/sensitive biomarker that can precede/predict future clinical changes in disease severity and response to treatment which we observed in the long-term study in this dog model of natural periodontitis.
Introduction
Periodontitis (periodontal disease) is comprised of a broad range of chronic inflammatory events that destroy the supporting tissue structures of the teeth (the gingiva, periodontal ligament and alveolar bone), ultimately leading to tooth loss, and also contributes to systemic inflammation (1). The initiation of this common disease is triggered by a specific oral microbial biofilm, but the characteristic tissue (including alveolar bone) destruction is mediated by the host's inflammatory/collagenolytic response (1, 2).
Traditionally, treatment strategies for periodontitis have focused on mechanically reducing the bacterial challenge, e.g., scaling and root planing (SRP), but can result in a significant loss of root surface structure, causing sensitivity, and is only partially effective (2). However, we now recognize that additional therapeutic strategies can also be considered, namely “the modulation of the excessive host inflammatory/collagenolytic response” (2). This concept has generally been referred to as “host modulation therapy (HMT),” meaning that the aim of treatment is to modify the host response by reducing those damaging aspects of the inflammatory/collagenolytic response that lead to tissue and bone destruction; HMT is an adjunct to the essential control of the microbial biofilm (dental plaque).
To this purpose, the development of novel NON-antibiotic “formulations” of tetracyclines as a first generation of HMT (based on the mechanisms of action previously unrecognized in the medical and dental fields), and NON-antibiotic “compositions” of tetracyclines as the second generation of HMT, were introduced and developed for the management of periodontitis and various related and/or relevant systemic diseases (3, 4). The former formulations were governmentally-approved in the U.S. (Food and Drug Administration, FDA), as well as Canadian and European governmental regulatory agencies. Recently, a new generation of HMTs has been developed by our group, which includes a novel series of chemically-modified curcumins (CMCs) (5, 6). A lead compound, a phenylaminocarbonyl curcumin, CMC2.24, incorporates a similar metal-ion (Ca2+, Zn2+) binding-active site, and is tri-phenolic as well as tri-ketonic (Figure 1), and has extraordinary efficacy in inhibiting pathologically-elevated inflammatory mediators and collagenolytic-destructive enzymes (especially MMPs), as well as demonstrating other pleiotropic therapeutic functions in vitro, in cell and tissue culture, and in vivo models of diseases (5–12). Compared to its parent compound natural curcumin, which did regulate expression and activity of MMP-9 (13, 14), CMC2.24 exhibited enzyme-inhibitory IC50 values, ranging from 2-8 μM against two gelatinases (MMP-2, −9) and other MMPs in vitro, much lower and more effective than curcumin (5).
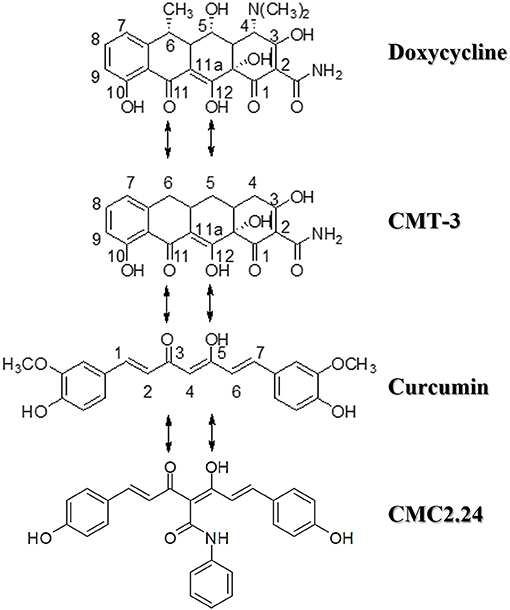
Figure 1. Structures of doxycycline (the first generation of HMT), chemically-modified tetracycline-3 (CMT-3, the second generation of HMT), and chemically-modified curcumin, CMC2.24 (the latest generation of HMT). All these bi-, tri-, and tetra-phenolic compounds possess the cation-binding β-diketone moiety. Note that CMC2.24 is tri-ketonic while the other compounds are di-ketonic.
Matrix Metalloproteinase-9 (MMP-9) is produced by polymorphonuclear neutrophils (PMNs), macrophages, and fibroblasts, and mediates (together with other MMPs, such as MMP-8) the degradation of collagen in connective tissues and alveolar bone. This destructive enzyme has been associated with the damaging phase of many inflammatory diseases (15). Research had shown a significantly higher level of expression of MMP-9 in patients with periodontitis than in gingivitis and healthy controls (16). Thus, MMP-9 can be used as an “inflammatory/collagenolytic” biomarker in periodontitis. However, there is still a lack of specific, fast, reliable, and early biomarkers that correlate well with the initial response to treatment, which can be used to predict and monitor disease, and to test the efficacy of treatment.
In this preliminary study, we describe a short-term/1-month period of therapy of CMC2.24, as an adjunct of mechanical debridement, in naturally-occurring periodontitis in beagle dogs, an animal model with characteristics similar to human periodontitis. Importantly, we also determined whether biochemical markers at an early stage, i.e., destructive MMP-9, are affected by CMC2.24 treatment before clinical changes become apparent months later.
Materials and Methods
Chemical Reagents
CMC 2.24 was synthesized and provided by Chem-Master Intl. Inc. (99.5% pure, Stony Brook, NY, USA). Carboxymethyl cellulose as vehicle (placebo) was purchased from Sigma (C-4888, St. Louis, MO, USA). All other reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Animal Studies
Protocols for animal studies were approved by Stony Brook University's Institutional Animal Care and Use Committee (IACUC #896357). Animals were housed in the Division of Laboratory Animal Resources (DLAR) at Stony Brook University, with care provided by the center's personnel. All procedures were conducted at the same location. This facility follows the Animal Welfare Act (USDA enforced), the Public Health Service Act (OLAW enforced), and NY State law (DOH enforced), and is an AAALAC International accredited facility.
Periodontitis is synonymous with the presence of periodontal pockets, and very often the clinical success of periodontal therapy is based on periodontal pocket depth reduction (17). For preliminary screening, generalized periodontitis was defined by pocket probing depth (PD), and generalized disease infers > 30% of sites were involved (all posterior teeth, six sites/per tooth). Periodontal probing was the insertion of the periodontal probe into the sulcus or the clinical pocket parallel to the long axis of the tooth and applying the force to move it apically into the tissue along the tooth surface (17, 18). The probe was inserted circumferentially around each surface of tooth to detect the areas of deepest penetration at each of six tooth surfaces: distofacial, facial, mesiofacial, distolingual, lingual and mesiolingual. The gingival margin position may be recorded as a positive number when the marginal gingiva was apical to CEJ (recession), or as a negative number. For determining severity of periodontitis, three levels were classified: Mild (3 mm < PD < 5 mm), Moderate (5 mm ≤ PD < 7 mm), and Severe (PD ≥ 7 mm) (19).
Eight adult female beagle dogs (3–5 years old, 9.5–11.5 kg) with generalized periodontitis were supplied by Marshall BioResourses (5800 Lake Bluff Rd, North Rose, NY), after preliminary screening of 49 similar dogs for significant periodontal disease in their posterior teeth. Periodontitis was determined by probing depth above 5 mm in posterior sites. A minimum disease threshold was defined by probing depth >4 mm in at least 5 posterior sites. Exclusion criteria of dogs included pregnancy, health, or laboratory abnormalities, and previously used within 3 months in another study.
The dogs were single-housed in standard kennels with ambient temperature maintained at 18–24°C and lights automatically turned on/off at 06:00 a.m./18:00 p.m. All dogs were acclimatized to the study environment 2 weeks prior to the initiation of the experiment.
Study Design
Data collection to test the efficacy of drug was carried out at two-time points (baseline and 1-month time period) as described below. During all exams, procedures and sample collections, the dogs were administered general anesthesia. Anesthesia was performed by veterinarians in the DLAR. At each time point, food/water was removed from cages 12 h before anesthesia.
At pre-baseline, a pretreatment full-mouth exam (of all premolars and molars in both upper and lower jaws) was performed one week prior to baseline data collection. Periodontal measurements including the following clinical parameters were taken: probing depth (PD), gingival index (GI), plaque index (PI), bleeding-on-probing (BOP), clinical attachment loss (CAL), and tooth mobility. These measurements were made at all maxillary and mandibular posterior teeth using a UNC-15 periodontal probe (Hu-Friedy, Chicago, USA) as described below. Based upon the clinical periodontal measurements, dogs with generalized moderate (5 mm ≤ PD< 7 mm) to severe (PD≥7 mm) periodontitis were equally distributed into two study groups, Placebo and Treatment (n = 4/group), to ensure a similar level of disease severity in both groups.
Baseline
At time = 0, the weight of each dog was recorded, and blood samples were collected, and clinical photos were taken. Periodontal disease severity was assessed using clinical parameters: PI, GI, PD, BOP, CAL, and mobility scores, which were monitored and recorded for the sites only with PD≥5 mm at pre-baseline in both jaws. After sample and data collections, all eight dogs received a standard non-surgical periodontal treatment. Briefly, a one-hour full mouth SRP was performed with an ultrasonic scaler (Parkell Inc., Edgewood, New York) and hand instrumentation as needed for calculus and plaque removal until all deposits were clinically undetectable. After SRP, one capsule containing either CMC2.24 (10 mg/kg in the same vehicle) or placebo (vehicle-only: carboxymethylcellulose powder) was administered orally once/day to each dog for 1 month. The preliminary data showed that 10 mg/kg of CMC2.24 produced better outcomes than higher dose (e.g., 100 mg/kg) (data not shown). Food/water was removed from cages 2 h before and after systemic application.
One-Month Time Period
At 1 month, the weight for each dog was recorded and blood samples were collected at this time period. Periodontal clinical measurements were performed as described in the baseline section. All measurements were performed by double-blind technique.
Clinical Measurements
Clinical parameters (PI, GI, PD, BOP, and CAL) were measured at pre-baseline, baseline, and 1-month time periods.
Probing Depth (PD)
At pre-baseline, all posterior sites (3 buccal and 3 lingual/palatal sites per tooth, including all premolars and molars in both jaws) were measured to screen 4–8 sites with PD ≥ 5 mm as examining sites for each dog. At baseline and 1-month time period, PD of the examining sites of each dog was monitored and recorded.
Gingival Index (GI)
At pre-baseline, all posterior gingival sites (2 sites/per tooth, 1 buccal and 1 lingual/palatal sites, including all premolars and molars in both jaws) were measured on a scale of 0 to 3, based on the scoring criteria described by previous studies (20, 21). Briefly, the scale of 0 to 3 can be described as followed: GI = 0, normal gingiva; GI = 1, mild inflammation. Characterized by slight change in color and slight edema, and no bleeding when a blunt instrument (periodontal probe) is run along the soft tissue entrance of the gingival crevice; GI = 2, moderate inflammation. Characterized by redness, edema and glazing, and bleeding when a blunt instrument (periodontal probe) is run along the soft tissue entrance of the gingival crevice; GI = 3, severe inflammation. Characterized by marked redness and edema. Ulceration may be present and there is marked tendency for spontaneous bleeding (i.e., on gentle palpation of the gingiva with the side of a probe.
At baseline and 1-month time period, GI of the examining sites of each dog was monitored and recorded.
Plaque Index (PI)
At pre-baseline, 2 surfaces/per tooth (1 buccal and 1 lingual/ palatal surface) for all posterior teeth, were recorded on a scale of 0 or 1 for PI measurements, based on the scoring criteria described by previous studies (21). In brief, the scale of 0 or 1 can be described as followed: PI = 0, no visible plaque in the gingival area; PI = 1, a film of plaque adhering to the free gingival margin and adjacent area of the tooth. The plaque may only be recognized by running a probe across the tooth surface; or may be visible to the naked eye.
At baseline and 1-month time periods, PI of the examining sites was monitored and recorded for each dog.
Bleeding-on-Probing (BOP)
All posterior teeth (3 buccal and 3 lingual/palatal sites per tooth) were assessed as absence (-) or presence of bleeding (+) after probing at pre-baseline. At baseline and 1-month time periods, numbers of examining sites with bleeding (+) were recorded for each dog.
Clinical Attachment Loss (CAL)
CAL is defined as CAL = PD-CEJ.GM, where CEJ.GM refers to the distance (mm) from the cementoenamel junction to the gingival margin.
Peripheral Blood
At baseline and 1-month time periods, peripheral blood samples were taken from the 8 dogs with periodontitis, which were systemically treated with CMC2.24 or placebo for one month. Ten milliliter of whole blood from each dog were drawn, then transferred to tubes coated with sodium citrate (anti-clotting) to collect plasma for later assessment of MMP-9 activity. After collection, the blood samples were mixed for 5 min to prevent coagulation, then, centrifuged for 30 min at 3,000 rpm at 4°C. The supernate (plasma) of each blood sample was taken and aliquoted to 500 μL per tube, then stored at−80°C until analyzed.
Gelatin Zymography
Assays for matrix metalloproteinase-9 (MMP-9: pro-form, 92 kDa; and activated-form, 82 kDa) in peripheral blood sample were conducted, as described by us previously (8, 22, 23).
Statistical Analysis
Measurements for clinical parameters and MMP-9 levels in both placebo and treatment groups were analyzed by Student's t-test, and also by ANOVA (two investigators carried out the data analysis, separately), with p < 0.05 taken as statistically significant. Data are expressed as the mean ± standard error of the mean (SEM). All data analyses were performed with SPSS® 22.0 software.
Results
Clinical Parameters
At the 1-month time period, there were no statistically significant changes (p>0.05) between placebo- and CMC2.24-treated groups in the clinical parameters, including GI, PI, BOP, and CAL. Although there was a slight trend of 4% reduction in probing depth (PD) measurements in the CMC2.24 treatment group at the 1-month time period, but this reduction was not statistically different at this time point, compared to placebo-treated control group (p > 0.05, data not shown).
It should be noted that the severity of clinical parameters of periodontitis was significantly reduced at the 3-month time-period with this regimen of HMT [p < 0.05, data already published (24)].
MMP Activities in Peripheral Blood
Effects of CMC2.24 on MMP-9 Activities
As shown in Figure 2, the baseline measurements showed no statistical difference between placebo and CMC2.24 treatment groups on pro- (92 kDa) and total-forms of MMP-9 levels. However, there was a statistically elevated level of activated-form of MMP-9 (82 kDa) in CMC2.24 group compared to placebo group, even before treatment was initiated.
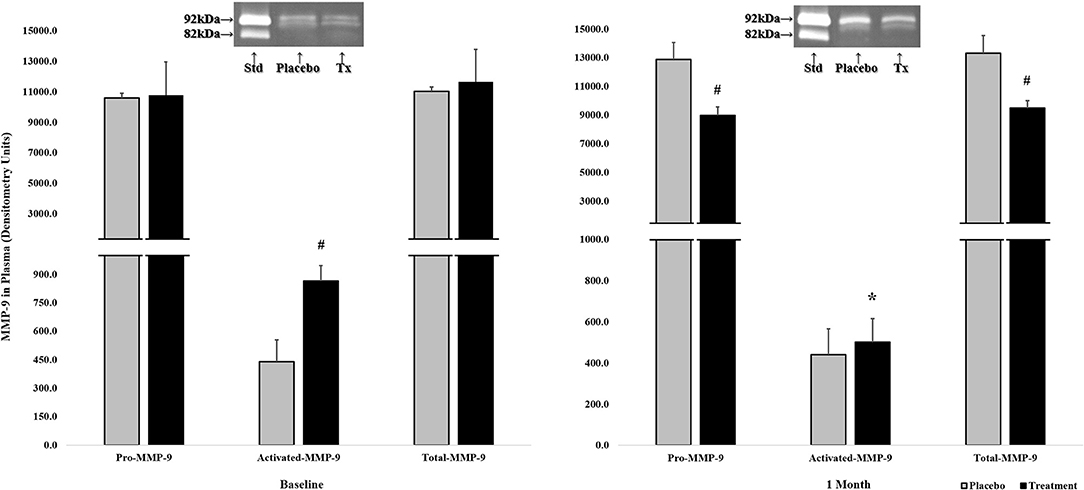
Figure 2. The effects of orally administered CMC2.24 or placebo on MMP-9 in peripheral blood (plasma), collected at baseline (left side) and 1-month (right side), measured by gelatin zymography and scanned densitometrically. Gray bar: Placebo Group; black bar: CMC2.24 Treatment Group. Each value represents the Mean (n = 4 dogs/per group) ± Standard Error of the Mean (S.E.M.). #Indicates p < 0.05, values of CMC2.24 treatment group at each time period compared to placebo group. *Indicates p < 0.05, values of CMC2.24 treatment group (activated-MMP-9) at 1-month compared to its own baseline. Std, Standard; Tx, Treatment of CMC2.24.
However, after 1-month of daily oral administration of CMC2.24 or placebo, there were statistically significant reductions in pro- and total-forms of MMP-9 levels in the CMC2.24 treated-group (p < 0.05; p < 0.05), compared to the placebo group which still maintained unchanged high levels of both forms of MMP-9 (Figure 2). Importantly, although there was no significant difference between placebo and CMC2.24 treatment groups in the activated-form of MMP-9 at this 1-month time period, there was a significant 45% reduction of activated-MMP-9 in the CMC2.24 treated group, compared to its own baseline values (p < 0.05); whereas the placebo group exhibited no change comparing the 1-month and baseline values (Figure 2).
Discussion
Deng et al. (24) recently reported a new therapeutic strategy to treat naturally-occurring periodontitis in beagle dogs, involving oral/systemic administration of a novel chemically-modified curcumin, CMC2.24 (a phenylaminocarbonyl curcumin), for 3-months as a host-response modulator. This pleiotropic therapy, based on (but chemically-modified) a well-known natural product, was designed to reduce pathologic inflammation/collagenolysis in oral and other diseases, and includes enhanced resolution of inflammation and inhibition of bone loss.
In a previous comprehensive in vivo study of wound healing (12), CMC2.24 was either topically (1% or 3% CMC2.24 suspended in petrolatum jelly) or systemically (by the oral route; 30 mg/kg) administered to type-I diabetic rats to evaluate its efficacy on severely impaired wound-healing. CMC2.24 was extremely effective, either topically or systemically, in “normalizing” wound repair, and both regimens of CMC2.24 produced significant improvements (about 34% reduction in time-of-healing) of both the surface epithelium and the underlying connective tissue, based on biochemical and histological measurements (12). Moreover, in another previous studies, using three different rat models of periodontal disease, including (a) a locally (LPS)-induced; (b) a systemically (type I diabetes)-induced models of experimental periodontitis; and (c) a combination of both LPS and type I diabetes-induced periodontal disease, we found that CMC 2.24 has significant inhibitory effects on pro-inflammatory cytokines (IL-6 and IL-1β) and MMPs (MMP-2, −8, and−9) in these rat models of experimental periodontitis, as well as reducing local (alveolar) bone loss. Regarding molecular mechanisms, systemic treatment with CMC2.24 produces substantial inhibition of NF-κB and p38 MAPK activation in gingiva in these models of experimental periodontitis (7, 8, 11, 25).
In the current study, we demonstrated that a shorter-term therapy (1-month) with this novel compound, as an adjunct to mechanical debridement, can be effective in improving biochemical changes, prior to clinical measures. This is important because the biochemical improvements may create a better environment in the host (less inflammation/collagenolysis) which may ultimately promote better and earlier outcomes for clinical changes in response to treatment. Also the use of specific and sensitive biomarkers to detect these changes at an early stage, may precede and predict the progression of disease at later stages. This may help clinicians monitor their treatments comprehensively in advance of waiting for clinical signs to develop.
Interestingly, our results indicated that a short-term/1-month protocol, although it did not result in obvious clinical improvement at this early stage, did appear to reduce both pro- and activated-MMP-9 in peripheral blood, which might affect the systemic environment, and also serves as an early and sensitive biomarker that precedes and predicts future clinical changes in oral (and other) inflammatory diseases. In brief, although a 1-month CMC2.24 treatment produced no detectable effect on clinical parameters temporarily, these were indeed observed with longer-term/3-momth therapy (24). At baseline (Figure 2), the levels of activated-MMP-9 in CMC2.24-treated group was 100% greater/higher (p < 0.05) than in the placebo-group. However, after 1-month therapy, although the activated-MMP-9 in the placebo-group remained unchanged, the dogs treated with CMC2.24 showed a significant (p < 0.05) 45% reduction of activated-MMP-9 down to the unchanged levels in placebo-group. In addition, the total- and pro-MMP-9 values in the CMC2.24-treated group were also significantly reduced; in contrast, their baseline values were identical to placebo.
These data suggest a protection by this novel curcuminoid, early-on in the disease/inflammatory process predicting later clinical improvements. The reduction of activated-MMP-9 can be partially due to the decreased levels of total-MMP-9, or this compound promotes the EXCESSIVE degradation of pro-MMP-9 by proteinases (e.g., trypsin), during the activation process, resulting in enzymatically-inactive small molecular weight fragments. In fact, this mechanism had been proposed previously by us to explain the loss of collagenolytic activity when pro-collagenase (pro-MMP-8) and pro-gelatinase (pro-MMP-9) were activated by pre-incubation with trypsin in the presence of doxycycline (due to Ca2+/Zn2+ binding by this tetracycline) (26). This novel mechanism was also described by Smith et al. (27, 28) who stated that “doxycycline disrupts the hemopexin-like or catalytic domain of collagenase, and alters the conformation of pro-collagenase or collagenase by binding enzyme-associated Ca2+, resulting in the MMPs becoming more susceptible to proteolysis and leading to irreversible loss of enzyme protein.” Since CMC2.24 has a cation-binding site similar to (and more potent than, i.e., a triketonic rather than diketonic active site) the tetracyclines, a polyenolic assembly, it may exhibit a similar mechanism of disrupting the conformation of MMP-9, thus rendering this MMP susceptible to degradation to small-inactive fragments. Future experiments are needed to validate this proposed mechanism.
It is worth noting that studies have indicated that MMP-9 helps mediate connective tissue breakdown and alveolar bone loss during periodontitis (8, 29–31). Thus, treatment with MMP-inhibitors may effectively suppress tissue breakdown, and decrease severity of periodontitis, as well as complications of other chronic inflammatory diseases such as diabetes, rheumatoid arthritis, and osteoporosis (26). Previously, we mentioned that MMPs have been found to degrade the insulin receptor which would be expected to suppress normal glucose metabolism/control (8, 11, 12, 32–34). Another possible mechanism of action of MMP-inhibitors is the following. During the MMP-8 activation process either with APMA or trypsin, when doxycycline was added, the activated-MMP-8 was excessively degraded into smaller-molecular weight inactive fragments (<30 kDa) (26, 35). Thus, the reduced level of activated-MMP-9 in this 1-month study by CMC2.24 could be explained (at least in part) by this fragmentation theory; longer-term therapy with CMC2.24 could then result in additional biochemical as well as clinical improvements.
In conclusion, this preliminary 1-month study indicates that orally-administered CMC2.24 is a rapidly effective and potent inhibitor of host-derived MMPs and that this early effect, with longer periods of treatment, ultimately results in clinical improvements in periodontal and other diseases. The potential importance of the current observations are supported by previous, longer-term/3-months studies which demonstrated significant improvements in clinical measurements as well as other biomarkers of this chronic inflammatory disease. Thus MMP-9 may be used as an early, specific and sensitive biomarker in detecting early biochemical response to treatment and predicting future clinical changes effectively.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal study was reviewed and approved by Stony Brook University's Institutional Animal Care and Use Committee (IACUC #896357).
Author Contributions
JD was the principle experiment investigator, and had the substantial contributions to the acquisition, analysis and interpretation of data, and was a major contributor in writing the manuscript. LG, H-ML, and YG made substantial contributions to the conception, design of the work, acquisition, interpretation of data and substantively revised the manuscript. H-DB helped to perform experiments and contributed to the acquisition and data analysis. H-LH helped with the statistical data analysis. TZ is the chief of the Division of Laboratory Animal Resources (DLAR). TZ and his team performed the animal caring work and the general anesthesia. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by NIDCR/NIH grant (Phase II STTR Award 2R42DE024946-02).
Conflict of Interest
LG is listed as an inventor on several related patents, including CMC2.24, and these have been fully assigned to his institution, Stony Brook University, The State University of New York (SUNY). FJ is also listed as an inventor on several related patents, including CMC2.24, which have been fully assigned to Stony Brook University and to Chem-Master Int. Inc., on a shared basis. In addition, both LG and FJ are minor shareholders in Traverse Biosciences, Inc., and JS is the president and major shareholder in Traverse Biosciences Inc. Traverse Biosciences Inc. has exclusively licensed patents from the Research Foundation for the State University of New York (RF/SUNY) covering the structure and use of the chemically-modified curcumins for the purpose of commercialization in human and animal health.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study also received funding from Traverse BioSciences, Inc. The funder had the following involvement with the study: decision to publish.
Acknowledgments
Authors would like to thank Michael Lin (Oral Biology and Pathology, Stony Brook University, New York, USA), Rachel Kogan (Department of Biology, Stony Brook University, New York, USA), Akshani Patel (Department of Biology, Stony Brook University, New York, USA), Tyler Francisco (Department of Psychology, Stony Brook University, New York, USA), Kai-Xi Lin (Department of Pharmacology, Stony Brook University, New York, USA), Sandra Scherrer (Veterinary Technician, DLAR, Stony Brook University, New York, USA) for their assistances to the dog study. This manuscript has been released as a pre-print at Researchsquare, (36).
References
1. Kinane DF, Stathopoulou PG, and Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. (2017) 3:17038. doi: 10.1038/nrdp.2017.38
2. Preshaw PM. Host modulation therapy with anti-inflammatory agents. Periodontology. (2018) 76:131–49. doi: 10.1111/prd.12148
3. Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, et al. Minocycline reduces gingival collagenolytic activity during diabetes. Preliminary observations and a proposed new mechanism of action. J Periodontal Res. (1983) 18:516–26. doi: 10.1111/j.1600-0765.1983.tb00388.x
4. Golub LM, and Lee HM. Periodontal therapeutics: Current host-modulation agents and future directions. Periodontol 2000. (2020) 82:186–204. doi: 10.1111/prd.12315
5. Zhang Y, Gu Y, Lee HM, Hambardjieva E, Vrankova K, Golub LM, et al. Design, synthesis and biological activity of new polyenolic inhibitors of matrix metalloproteinases: a focus on chemically-modified curcumins. Curr Med Chem. (2012) 19:4348–58. doi: 10.2174/092986712802884295
6. Zhang Y, Golub LM, Johnson F, and Wishnia A. pKa, zinc- and serum albumin-binding of curcumin and two novel biologically- active chemically-modified curcumins. Curr Med Chem. (2012) 19:4367–75. doi: 10.2174/092986712802884240
7. Elburki MS, Rossa C, Guimaraes MR, Goodenough M, Lee HM, Curylofo FA, et al. A novel chemically modified curcumin reduces severity of experimental periodontal disease in rats: initial observations. Mediators Inflamm. (2014) 2014:959471. doi: 10.1155/2014/959471
8. Elburki MS, Moore DD, Terezakis NG, Zhang Y, Lee HM, Johnson F, et al. A novel chemically modified curcumin reduces inflammation-mediated connective tissue breakdown in a rat model of diabetes: periodontal and systemic effects. J Periodontal Res. (2017) 52:186–200. doi: 10.1111/jre.12381
9. Deng J, Gu Y, Lee HM, Raja V, Johnson F, and Golub LM. Novel modified-curcumin: resolution of cytokines and MMPs in cell culture. J Dent Res. (2018) 97(Special Issue A):0129.
10. Gu Y, Deng J, Lee HM, Raja V, Yang P, Johnson F, et al. Chemically-modified-curcumin: resolvin activity in experimental diabetes. J Dent Res. (2017) 96(Special Issue A):1173.
11. Elburki MS, Rossa C Jr, Guimaraes-Stabili MR, Lee HM, Curylofo-Zotti FA, et al. A chemically modified curcumin (CMC 2.24) inhibits nuclear factor kappaB activation and inflammatory bone loss in murine models of LPS-induced experimental periodontitis and diabetes-associated natural periodontitis. Inflammation. (2017) 40:1436–49. doi: 10.1007/s10753-017-0587-4
12. Zhang Y, McClain SA, Lee HM, Elburki MS, Yu H, Gu Y, et al. A novel chemically modified curcumin “normalizes” wound-healing in rats with experimentally induced type i diabetes: initial studies. J Diabetes Res. (2016) 2016:5782904. doi: 10.1155/2016/5782904
13. Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P, and Sharma AV. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J Biol Chem. (2005) 280:9409–15. doi: 10.1074/jbc.M413398200
14. Cao J, Han Z, Tian L, Chen K, Fan Y, Ye B, et al. Curcumin inhibits EMMPRIN and MMP-9 expression through AMPK-MAPK and PKC signaling in PMA induced macrophages. J Transl Med. (2014) 12:266. doi: 10.1186/s12967-014-0266-2
15. Escalona LA, Mastromatteo-Alberga P, and Correnti M. Cytokine and metalloproteinases in gingival fluid from patients with chronic periodontitis. Invest Clin. (2016) 57:131–42.
16. Lazăr L, Loghin A, Bud ES, Cerghizan D, Horváth E, and Nagy EE. Cyclooxygenase-2 and matrix metalloproteinase-9 expressions correlate with tissue inflammation degree in periodontal disease. Rom J Morphol Embryol. (2015) 56:1441–6.
18. Bosshardt DD. The periodontal pocket: pathogenesis, histopathology and consequences. Periodontology. (2018) 76:43–50. doi: 10.1111/prd.12153
19. American Academy of Periodontology Task Force Report on the Update to the 1999. Classification of Periodontal Diseases and Conditions. J Periodontol. (2015) 86:835–8. doi: 10.1902/jop.2015.157001
20. Hefti AF, and Preshaw PM. Examiner alignment and assessment in clinical periodontal research. Periodontology. (2012) 59:41–60. doi: 10.1111/j.1600-0757.2011.00436.x
21. Yimam M, Brownell L, Do SG, Lee YC, Kim DS, Seo K, et al. Protective effect of UP446 on ligature-induced periodontitis in beagle dogs. Dent J (Basel) (2019) 7:33. doi: 10.3390/dj7020033
22. Golub LM, Ramamurthy NS, Llavaneras A, Ryan ME, Lee HM, Liu Y, et al. A chemically modified nonantimicrobial tetracycline (CMT-8) inhibits gingival matrix metalloproteinases, periodontal breakdown, and extra-oral bone loss in ovariectomized rats. Ann N Y Acad Sci. (1999) 878:290–310. doi: 10.1111/j.1749-6632.1999.tb07691.x
23. Lee HM, Golub LM, Cao J, Teronen O, Laitinen M, Salo T, et al. CMT-3, a non-antimicrobial tetracycline (TC), inhibits MT1-MMP activity: relevance to cancer. Curr Med Chem. (2001) 8:257–60. doi: 10.2174/0929867013373660
24. Deng J, Golub LM, Lee HM, Lin MC, Bhatt HD, Hong HL, et al. Chemically-modified curcumin 2.24: a novel systemic therapy for natural periodontitis in dogs. J Exp Pharmacol. (2020) 12:47–60. doi: 10.2147/JEP.S236792
25. Elburki MS, Goren AD, Lee HM, and Golub LM. Chemically-modified curcumins and alveolar bone loss in diabetic rats. J Dent Res. (2011) 90(Special Issue A):2295.
26. Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, and Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. (1998) 12:12–26. doi: 10.1177/08959374980120010501
27. Smith GN Jr, Brandt KD, and Hasty KA. Activation of recombinant human neutrophil procollagenase in the presence of doxycycline results in fragmentation of the enzyme and loss of enzyme activity. Arthritis Rheum. (1996) 39:235–44. doi: 10.1002/art.1780390209
28. Smith GN Jr, Mickler EA, Hasty KA, and Brandt KD. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: relationship to structure of the enzyme. Arthr Rheum. (1999) 42:1140–6. doi: 10.1002/1529-0131(199906)42:6<1140::AID-ANR10>3.0.CO;2-7
29. Thompson DM, Lee HM, Stoner JA, Golub LM, Nummikoski PV, and Payne JB. Loss of alveolar bone density in postmenopausal, osteopenic women is associated with circulating levels of gelatinases. J Periodontal Res. (2019) 54:525–32. doi: 10.1111/jre.12656
30. Liu X, Zhang Z, Pan S, Shang S, and Li C. Interaction between the Wnt/beta-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis. J Periodontal Res. (2018) 53:842–52. doi: 10.1111/jre.12574
31. Martinho FC, Teixeira FF, Cardoso FG, Ferreira NS, Nascimento GG, Carvalho CA, et al. Clinical investigation of matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, and matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase complexes and their networks in apical periodontitis. J Endod. (2016) 42:1082–8. doi: 10.1016/j.joen.2016.04.001
32. Golub LM, Elburki MS, Walker C, Ryan M, Sorsa T, Tenenbaum H, et al. Non-antibacterial tetracycline formulations: host-modulators in the treatment of periodontitis and relevant systemic diseases. Int Dent J. (2016) 66:127–35. doi: 10.1111/idj.12221
33. Derosa G, Ferrari I, D'Angelo A, Tinelli C, Salvadeo SA, Ciccarelli L, et al. Matrix metalloproteinase-2 and−9 levels in obese patients. Endothelium. (2008) 15:219–24. doi: 10.1080/10623320802228815
34. Frankwich K, Tibble C, Torres-Gonzalez M, Bonner M, Lefkowitz R, Tyndall M, et al. Proof of Concept: Matrix metalloproteinase inhibitor decreases inflammation and improves muscle insulin sensitivity in people with type 2 diabetes. J Inflamm (Lond). (2012) 9:35. doi: 10.1186/1476-9255-9-35
35. Griffin MO, Ceballos G, and Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. (2011) 63:102–7. doi: 10.1016/j.phrs.2010.10.004
Keywords: host-modulation therapy, blood, inflammation, matrix metalloproteinase 9, chemically-modified curcumin, periodontitis
Citation: Deng J, Golub LM, Lee H-M, Bhatt H-D, Hong H-L, Johnson F, Scaduto J, Zimmerman T and Gu Y (2021) A Novel Chemically-Modified Curcumin 2.24: Short-Term Systemic Therapy for Natural Periodontitis in Dogs. Front. Dent. Med. 2:609795. doi: 10.3389/fdmed.2021.609795
Received: 03 October 2020; Accepted: 05 February 2021;
Published: 01 March 2021.
Edited by:
Moritz Kebschull, University of Birmingham, United KingdomReviewed by:
Nurcan Buduneli, Ege University, TurkeyRenato Correa Viana Casarin, Campinas State University, Brazil
Jorge Antonio Gamonal, University of Chile, Chile
Copyright © 2021 Deng, Golub, Lee, Bhatt, Hong, Johnson, Scaduto, Zimmerman and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Deng, amllLmRlbmc5MTdAZ21haWwuY29t
 Jie Deng
Jie Deng Lorne M. Golub
Lorne M. Golub Hsi-Ming Lee
Hsi-Ming Lee Heta-Dinesh Bhatt
Heta-Dinesh Bhatt Hou-Lin Hong
Hou-Lin Hong Francis Johnson
Francis Johnson Joseph Scaduto5
Joseph Scaduto5