- 1Dentistry Graduate Program, Universidade Federal da Paraíba, João Pessoa, Brazil
- 2Cleft Lip and Palate Center, Universidade Federal da Paraíba, João Pessoa, Brazil
- 3Department of Oral Biology, University of Pittsburgh, Pittsburgh, PA, United States
Background: Oral clefts are birth defects that affect 500–1,000 livebirths depending on the geographic area. Maternal smoking increases the risk of stillbirths, prematurity, low birth weight, and oral clefts.
Methods: In this case series, we measured the cleft palate defect of 10 children born from mothers who smoked during pregnancy and compared with measurements of 36 children born from mothers who did not smoke.
Results: Palate defects tended to be larger in the group that the mother smoked during pregnancy.
Conclusion: Smoking during pregnancy aggravates the size of the cleft defect in the palate.
Introduction
Oral clefts is a relatively common birth defect that shows differences in frequency depending on geographic origin (1). Maternal smoking, including passive maternal exposure, is the only factor that conclusively associates with cleft palate in humans (2–9) increasing its risk from 4 in 10,000 live births to 96 to 128 in 10,000 live births. For the first time, one shows that the actual cleft defect of children born from mothers who smoked during pregnancy is larger. There is a question on the mechanism of how maternal cigarette smoking lead to clefts. In Xenopus laevis, E-cigarette vapors exposure reduces blood supply to the face of embryos (10). In humans, acardiac twins (11) and living above 2,000 m in relation to the sea level (12) are scenarios that suggest hypoxia is the mechanism leading to cleft palate. In both cases, lower oxygenation of the blood may stress the fusion of lip and palate processes. Nicotine may also have a direct impact in cleft palate by modifying gene expression and leading to persistence of epitelial cells in areas connective tissue should level and fuse (13). Here, we tested the hypothesis that the cleft palate defect will be larger among children whose mothers smoked during pregnancy.
Methods
This analysis has Institutional Review Board approval, and parents of all participants signed a written informed consent document agreeing with their child's participation.
We used 46 pre-surgical models from consecutive cases of patients born with isolated cleft palate, without a syndrome, and a known history of maternal smoking. These children were treated from March 2012 to September 2016 at the Lauro Wanderley University Hospital Cleft Lip and Palate Center.
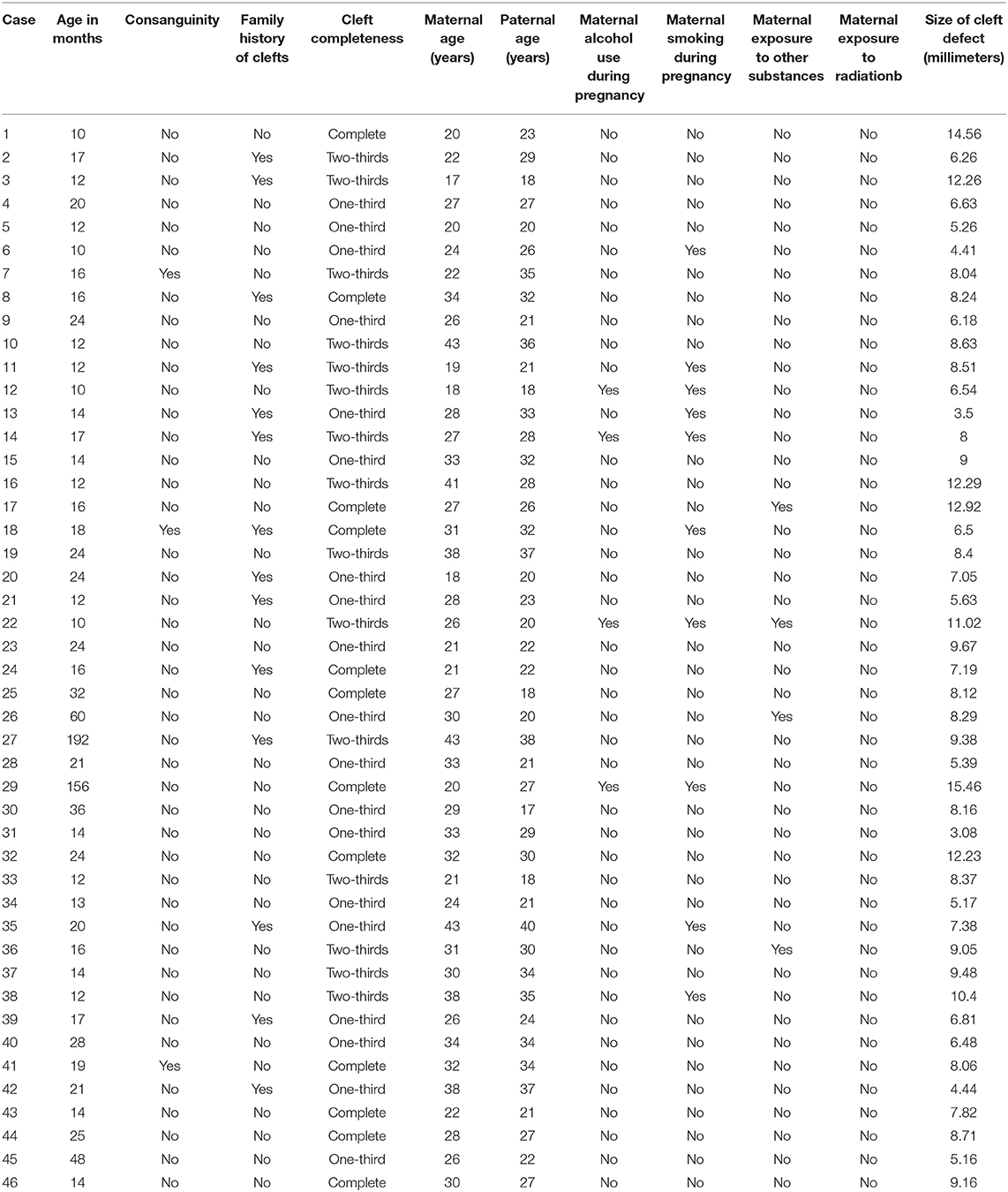
To determine the size of the palatal defect, the pre-surgical models were measured using a digital caliper positioned on the maxillary tuberosity level from left to right and registering the transversal fault (Figure 1). Antero-posterior defects were defined as involving one-third, two-thirds, or the complete palate (Figure 2) analyzed through occlusal photographs and pre-surgical models. One single examiner recorded all data. The intraexaminer agreement was assessed by a second evaluation of 10 models and photographs and after 2 weeks with a kappa of 0.90.
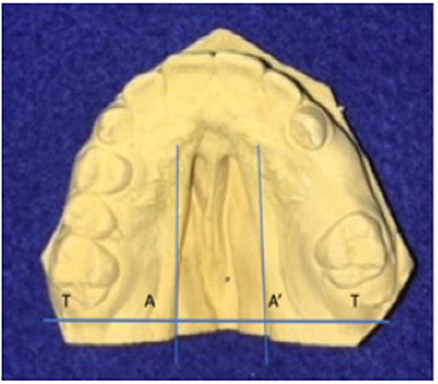
Figure 1. Width of palatal defect: distance from left to right at the level of the maxillary tuberosity. Corresponding to the distance between A and A′ at the level of T and T′, in millimeters.
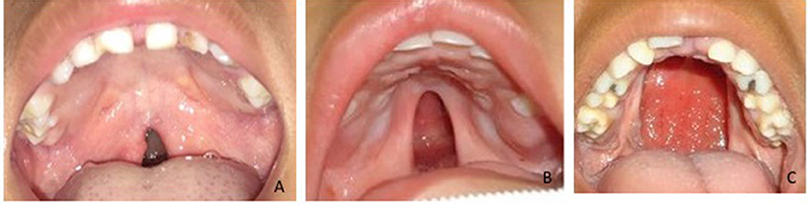
Figure 2. Anteroposterior palatal defects affecting (A) one-third, (B) two-thirds, and (C) the whole extension of the palate.
The history of maternal smoking was collected from medical records. There were no other risk factors identified, such as diabetes and use of certain medications (i.e., topiramate or valproic acid), that could be confounding these analyses (Table 1).
Out of the 46 individuals studied, 10 had history of maternal smoking. Power estimates with the sample size available (10 vs. 36), assuming similar standard deviations in each group, suggested the ability to detect an effect size of Cohen's D of 0.8 (a large effect size) (14).
Results
Ten out of the 46 cases studied were born from mothers who smoked while pregnant. The size of the defect among these 10 children ranged from 3.5 to 15.46 mm (mean, 8.17 mm) in comparison to 3.08 to 14.56 mm (mean, 8.09 mm) among the 36 children born from mothers who did not smoke. Nineteen had one-third of the palate affected (three were born from mothers who smoked while pregnant), 15 had two-thirds of the palate affected (five born from mothers who smoked while pregnant), and 12 had a complete defect in the palate (two were born from mothers who smoked while pregnant).
No difference was found between the groups in the transverse measurement (p = 0.33). There was a statistical significant difference for the anteroposterior measurements (p < 0.05).
Discussion
We speculate that if it is the case that nicotine is the main factor that leads to cleft palate, there should not be a difference in the sizes of defects of children born from mothers who smoked vs. mothers who did not smoke while pregnant. On the other hand, if hypoxia is the main reason for which maternal smoking increases the chance for a child to be born with cleft palate, we believe we should see that the defects of the children born from mothers who smoked while pregnant are larger. If the mother smoked while pregnant, children with two-thirds or complete palate defects had larger clefts (p = 0.000003, Student's t-test) with a difference of 2.88 standard deviations (Cohen's D test) in comparison to children born from mothers who did not smoke. This evidence suggests that maternal smoking causes cleft palate through hypoxia. This inference is compatible to the suggestion that hypoxia is one of the mechanisms underlying clefts and may be the reason for the findings related to associations with maternal cigarette smoking during pregnancy, differences in frequency of clefts depending on altitude, and the frequency of occurrence of clefts in acardiac twins. Frequency of clefts in high altitude places and acardiac twins is not compatible with the idea that nicotine is the reason risks for cleft lip and palate are increased among mothers who smoked during pregnancy. It is true that genetic variation in detoxification genes appears to modulate risks for clefts (4, 15), but activation of these mechanisms could also be in response to stress consequence of low oxygenation. Despite the perceived limitation of a small sample, our study showed for the first time that maternal cigarette smoking leads to larger palatal cleft defects. Another perceived limitation of our study aside from the sample size was not obtaining measurements using computational approaches. However, the use of digital models in comparison to plaster dental casts, although saving clinical steps, showed that the accuracy of the software for space analysis on digital models is comparable with traditional plaster study model analyses (16).
We suggest that future cleft research needs to incorporate more sophisticated definitions beyond just calling individuals affected or not affected, if the goal is to unveil the etiological mechanisms underlying multifactorial cleft lip and palate.
Conclusion
Mothers who smoked while pregnant were more likely to have children with defects involving two-thirds of the palate completely in comparison to mothers who did not smoke.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Comitê de Ética do Hospital Universitário Lauro Wanderley-UFPB. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
RL and PF obtained data and critically revised the manuscript. AV proposed the concept and design, interpreted and analyzed data, and wrote first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors are indebted to the participants of the study and to Smile Train to support cleft care.
References
1. Vanderas AP. Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. Cleft Palate J. (1987) 24:216–25.
2. Wyszynski DF, Duffy DL, Beaty TH. Maternal cigarette smoking and oral clefts: a meta-analysis. Cleft Palate Craniofac J. (1997) 34:206–10. doi: 10.1597/1545-1569_1997_034_0206_mcsaoc_2.3.co_2
3. Xuan Z, Zhongpeng Y, Yanjun G, Jiaqi D, Yuchi Z, Bing S, et al. Maternal cigarette smoking and oral clefts: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. (2016) 122:680–90. doi: 10.1016/j.oooo.2016.08.007
4. Shi M, Christensen K, Weinberg CR, Romitti P, Bathem L, Lozada A, et al. Orofacial cleft risk is increased with maternal smoking and specific detoxification-gene variants. Am J Hum Genet. (2007) 80:76–90. doi: 10.1086/510518
5. Martelli DRB, Coletta RD, Oliveira EA, Swerts MSO, Rodrigues LAM, Oliveira MC, et al. Association between maternal smoking, gender, and cleft lip and palate. Braz J Otorhinolaryngol. (2015) 81:514–9. doi: 10.1016/j.bjorl.2015.07.011
6. Raut JR, Simeone RM, Tinker SC, Canfield MA, Day RS, Agopian AJ. Proportion of orofacial clefts attributable to recognized risk factors. Cleft Palate Craniofac J. (2019) 56:151–8. doi: 10.1177/1055665618774019
7. Li Z, Liu J, Ye R, Zhang L, Zheng X, Ren A. Maternal passive smoking and risk of cleft lip with or without cleft palate. Epidemiology. (2010) 21:240–2. doi: 10.1097/EDE.0b013e3181c9f941
8. Gunnerbeck A, Bonamy A-KE, Wikström A-K, Granath F, Wickström R, Cnattingius S. Maternal snuff use and smoking and the risk of oral cleft malformations - a population-based cohort study. PLoS ONE. (2014) 9:e84715. doi: 10.1371/journal.pone.0084715
9. Leite M, Albieri V, Kjaer SK, Jensen A. Maternal smoking in pregnancy and risk for congenital malformations: results of a Danish register-based cohort study. Acta Obstet Gynecol Scand. (2014) 93:825–34. doi: 10.1111/aogs.12433
10. Kennedy AE, Kandalam S, Olivares-Navarrete R, Dickinson AJG. E-cigarette aerosol exposure can cause craniofacial defects in Xenopus laevis embryos and mammalian neural crestal cells. PLoS ONE. (2017) 12:e0185729. doi: 10.1371/journal.pone.0185729
11. Jones KL, Webster WS, Vaux KK. Acardiac fetus: evidence in support of a vascular/hypoxia pathogenesis for isolated oral clefting. Birth Defects Res Part A Clin Mol Teratol. (1998) 82:597–600. doi: 10.1002/bdra.20473
12. Castilla EE, Lopez-Camelo JS, Campaña H. Altitude as a risk factor for congenital anomalies. Am J Med Genet. (1999) 86:9–14.
13. Kang P, Svoboda KK. Nicotine inhibits palatal fusion and modulates nicotinic receptors and the PI-3 kinase pathway in medial edge epithelia. Orthod Craniofac Res. (2003) 6:129–42. doi: 10.1034/j.1600-0544.2003.02236.x
14. Stangroom J. Effect Size Calculator for T-test. Social Science Statistics. Available online at: https://www.socscistatistics.com/tests/chisquare2/default2.aspx (accessed December 17, 2020).
15. Lie RT, Wilcox AJ, Taylor J, Gjesing HK, Saugstad OD, Aabyholm F, et al. Maternal smoking and oral clefts: the role of detoxification pathway genes. Epidemiology. (2008) 19:606–15. doi: 10.1097/EDE.0b013e3181690731
Keywords: cleft palate, smoking, nicotine, altitude (MeSH), acardiac anomaly
Citation: Lacerda RHW, Furtado PGC and Vieira AR (2021) Maternal Smoking Leads to Larger Cleft Palate Defects. Front. Dent. Med. 1:632037. doi: 10.3389/fdmed.2020.632037
Received: 21 November 2020; Accepted: 18 December 2020;
Published: 27 January 2021.
Edited by:
Mohammad Khursheed Alam, Al Jouf University, Saudi ArabiaReviewed by:
Anand Marya, University of Puthisastra, CambodiaAdith Venugopal, University of Puthisastra, Cambodia
Copyright © 2021 Lacerda, Furtado and Vieira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandre Rezende Vieira, YXJ2MTFAcGl0dC5lZHU=
 Rosa Helena Wanderley Lacerda
Rosa Helena Wanderley Lacerda Paulo Germano Cavalcanti Furtado2
Paulo Germano Cavalcanti Furtado2 Alexandre Rezende Vieira
Alexandre Rezende Vieira