Abstract
Since their introduction in clinical dentistry, hydraulic cements have gained popularity. Their applications are diverse and their usefulness is due to their hydraulic nature. These materials require water to set and reach their optimal physical and mechanical characteristics, do not deteriorate when wet, and form calcium hydroxide as a by-product of the hydration reaction. All these characteristics are important for a number of clinical applications. The first hydraulic cement was a simple mixture of Portland cement, as used in the construction industry, with bismuth oxide to increase its radio-opacity. Regardless of being a hydraulic calcium silicate, it was initially incorrectly labeled as a phosphate cement misleadingly called “mineral trioxide aggregate.” Since then, beneficial clinical applications have led to the development of a number of materials with a different base, alternative vehicles, and incorporating modifiers of various kinds. Given the variety, and possible confusion, a rational classification of hydraulic cements used in dentistry is necessary. The classification is primarily dependent on the clinical context in which the materials are used as this informs the user about the environment and possible interactions. Secondly, a classification based on the material constitution is given as knowledge of its chemistry will help predict behavior, identify risks, and thus facilitate selection and handling.
Introduction
Hydraulic cements set by reaction with water (i.e., a hydration reaction); they can also be employed in wet environments, but drying before complete reaction is detrimental. Further reactions may occur with components of the environment (blood, tissue fluid, dentine, bone, irrigating solutions, restorative materials), modifying the chemistry, primarily at the surface, such that their interaction with that environment is altered. This environmental interaction may be of some considerable importance as it implies a change in surface chemistry, failure to set and these changes may have deleterious effects on the material.
One such material is Portland cement, as used in the construction industry. Its properties led to the suggestion that it may be used in dentistry in permanently wet contexts, such as those of root-end surgery and perforation repair (1, 2). It may be noted that its first use as a dental material in fact dates to the late nineteenth century (3) for an endodontic filler, and further developed by Schlenker (4), but it was not until Torabinejad patented it as “mineral trioxide aggregate” (“MTA”) (5) for “tooth filling” that it was taken up widely in clinical practice. Long-term stability in this wet environment is a critical requirement for this application. MTA was initially claimed to be a calcium phosphate-based cement (6), but it was identified as a hydraulic calcium silicate in 2005 (7). Since then a number of related materials have been developed and used for various applications in clinical dentistry, mostly related to endodontic procedures. A proper classification is thus believed to be necessary due to the diversity in chemistry and clinical application.
Classifications
Two classifications are being proposed for dental hydraulic cements according to
Clinical context: the environment and specific use
Constitution: chemistry and presentation.
Clinical Context
The basis of this classification is that the changes the material may undergo vary according to the specific environment (Figure 1). Thus, there are three distinct contexts:
- Intracoronal—pulp protection, barrier for regenerative endodontic procedures;
- Intraradicular—root canal sealing, apical plugs;
- Extraradicular—root-end fillers, perforation repair materials.
Figure 1
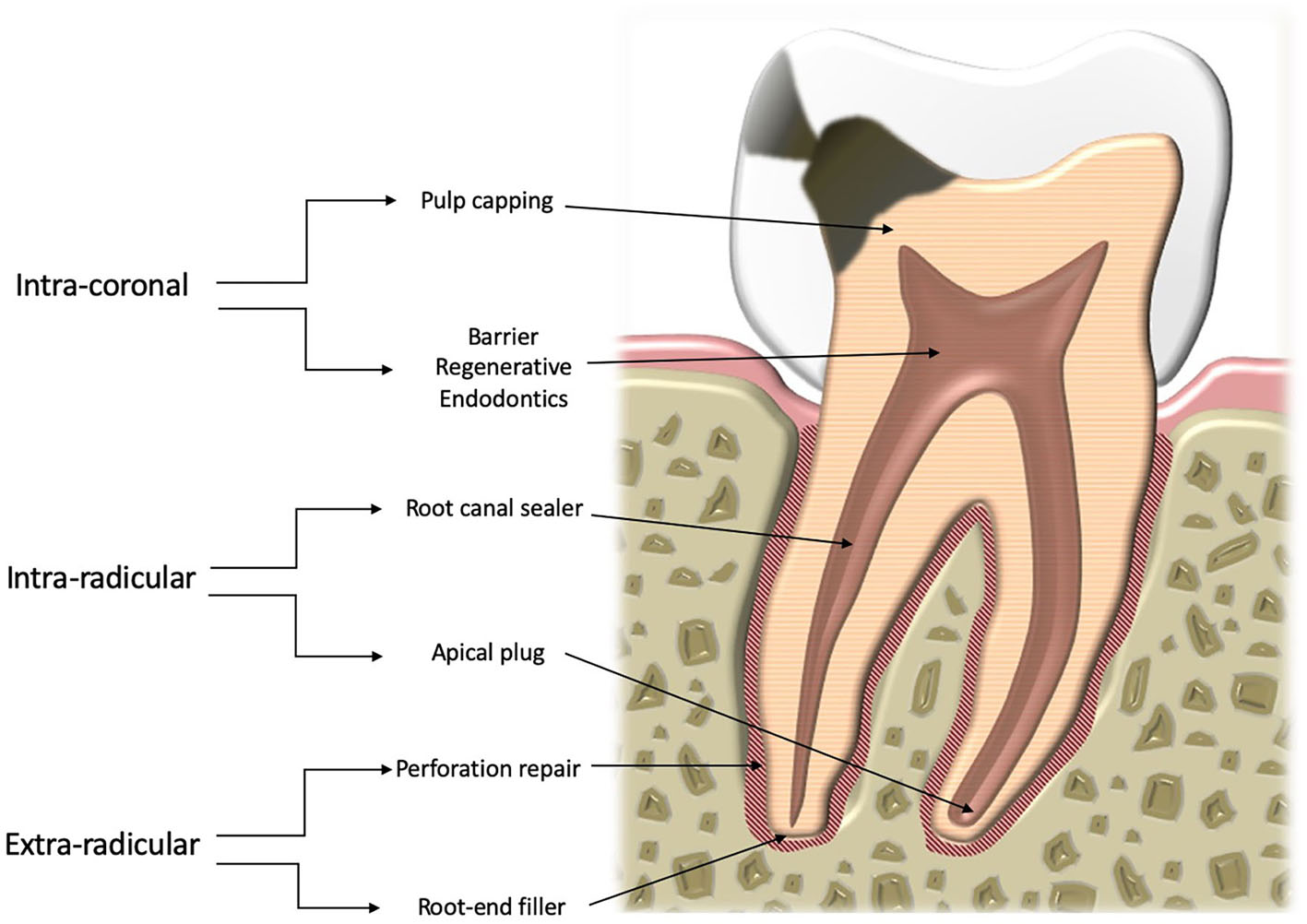
Schematic drawing of a cross section through a tooth.
Constitution
The basis of this classification is shown in Figure 2. The behavior and properties, and especially the hydration process, of a hydraulic cement depends on its chemistry. Specifically, for the hydraulic calcium silicates, these materials are all dependant on the cement chemistry, the modifiers used and whether the material is mixed with water or not. Based on this, the types of hydraulic calcium silicate cements currently available for clinical use may be identified as indicated in Table 1. The addition of a radio-opacifier is desirable as it allows post-operative detection and follow up.
Figure 2
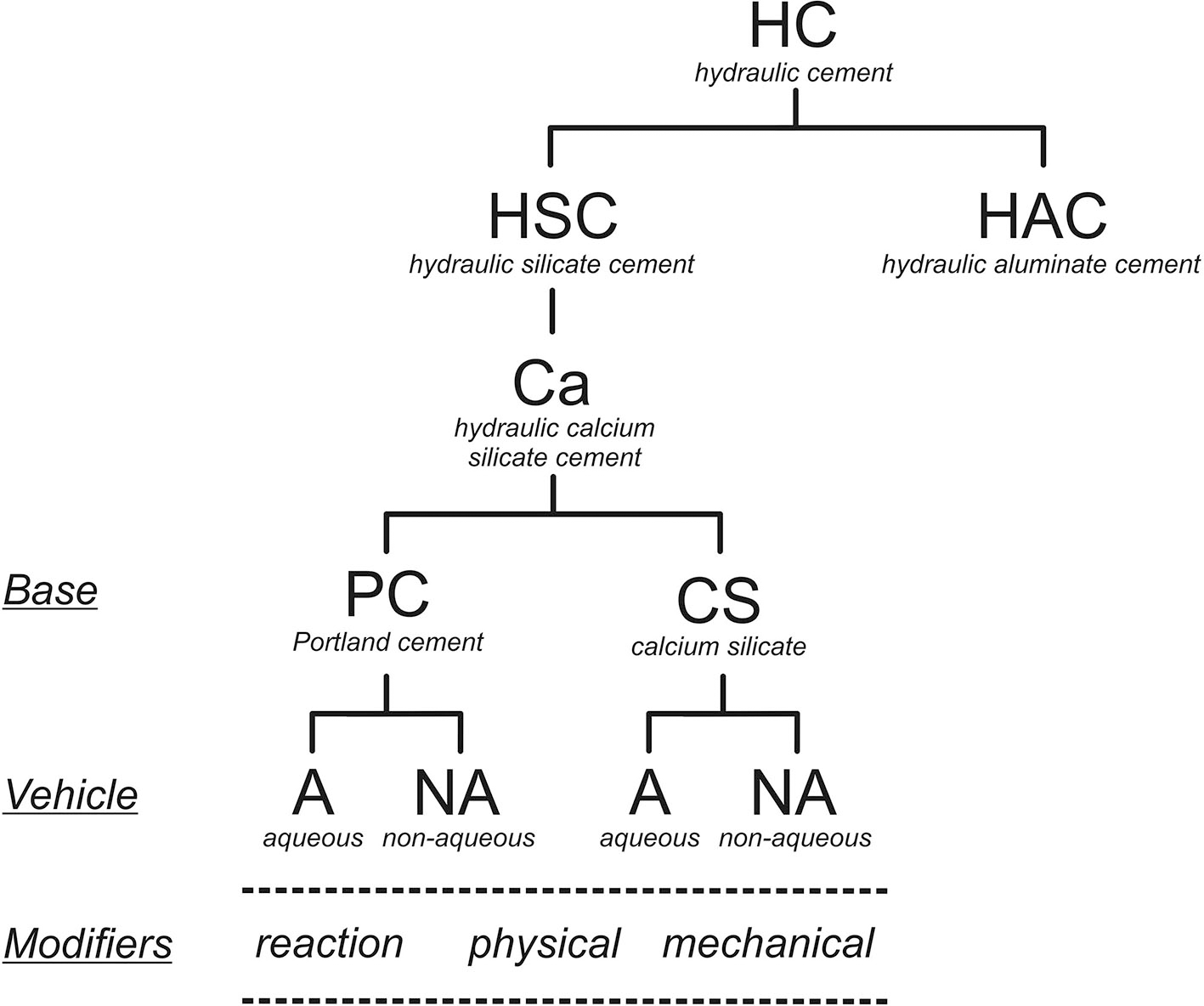
Classification of hydraulic cements based on their chemistry.
Table 1
| Type | Cement | Additives | Water | Commercial example |
|---|---|---|---|---|
| 1 | Portland cement | No | Yes | ProRoot MTA |
| 2 | Portland cement | Yes | Yes | MTA Angelus, MTA HP |
| 3 | Portland cement | Yes | No | BioC (Angelus) |
| 4 | Tricalcium/dicalcium silicate | Yes | Yes | Biodentine, BioRoot |
| 5 | Tricalcium/dicalcium silicate | Yes | No | Totalfill |
Classification of clinically available radiopacified hydraulic calcium silicate cements.
Discussion
A classification of materials depending on their specific use as shown in Figure 1 is useful as it informs the user of the environment that the material is placed in, and thus the anticipated interactions. The purely chemical changes that occur on the surface of materials in contact with various clinical environments has erroneously been termed “bioactivity” (8). The clinical relevance of testing for this, primarily through the observation of the deposition of calcium phosphates, has been questioned as the use of so-called synthetic tissue fluids cannot mimic clinical conditions (9, 10). For hydraulic cements a similar effect has also been described (11), but the nature of the precipitate varies depending on where the material is placed. Calcium carbonate has been shown to form when the materials are in contact with blood (12), as are root-end fillings (13), and when used as a barrier material in regenerative endodontic procedures (14), whereas apatitic material forms in contact with dentine when the material is used as a root canal sealer (15) and in vital pulp therapy (16).
A distinction has to be made between intracoronal, intraradicular, and extraradicular materials on the basis of the nature of the environment. Pulp protection and barrier materials used for regenerative endodontic procedures are in contact with the dental pulp and coronal dentine. Intraradicular sealers and apical plug materials are used following procedures aimed at eliminating bacteria in the root canal and are thus are in contact with treated dentine but limited amounts of fluid. On the other hand, extraradicular materials are in contact with untreated dentine. Their surface is completely in contact with blood and tissue fluids. The distinction between the groups in this sense is clear.
The classification of the materials based on their chemistry is more precise and also shows how each part of the classification varies depending on specific reactions. The term “hydraulic” is used to define the setting reaction (i.e., with water), and the ability to set and then be stable in a wet environment, which is crucial. For this reason, a sub-classification of hydraulic silicate cements has been used to differentiate materials such as Portland cement, which is a silicate, from calcium aluminates that are also being proposed as dental materials (17–19). These materials are all hydraulic but have different chemistries otherwise. The term “hydraulic silicate cement” for this kind of dental materials was introduced by Darvell and Wu (20) to describe “MTA.” A further subdivision is necessary to differentiate the calcium-based materials from other cementitious silicates due to the specific application of these materials in clinical dentistry to replace the use of calcium hydroxide itself. In reacting with water, hydraulic calcium silicates produce calcium hydroxide (Equations 1, 2), which then makes the cement beneficial for several clinical uses. The reactions of di- and tricalcium silicates have been studied extensively for Portland cement for the construction industry (21–23) (the silicate hydrates are idealized formulae).
The distinction between silicates in general and calcium silicates has been made specifically because of the formation of the calcium hydroxide by the latter. Since only dental materials are of interest and radio-opacity is a pre-requisite for all dental devices, radio-opacifiers are not specified separately. The radio-opacity of Portland cement, the basis of “MTA,” has been shown to be inadequate (24). All hydraulic endodontic cements require the addition of a radio-opacifier to be able to comply with the existing standard (25). This standard is for endodontic sealers but to date there are no standards for other material types.
Overall, the hydraulic calcium silicate cements are classified according to the basic components, the presence or absence of additives, and by whether supplied as a powder to be mixed with water or suspended in a non-aqueous vehicle. All three factors change the material chemistry and modify the cement hydration. The cement components are important as the hydration process of Portland cement differs from that of just calcium silicates. Portland cement has an aluminate phase which hydrates to form ettringite and a “monosulphate,” depending on the availability of sulfate ions from the calcium sulfate that is added to the commercial product to delay setting. Materials with various amounts of calcium sulfate have been suggested for dental use. Raw cement clinker, with no added calcium sulfate, flash-sets (26, 27). A hydraulic calcium silicate mixed with a calcium aluminate results in a material with a shorter setting time. The addition of calcium sulfate to such mixtures results in expanding cements and these have poorer biocompatibility (28, 29).
Clinically, the change from industrial Portland cement to synthetic calcium silicate mixtures arose from concern about the leaching of aluminum ions, which have been detected in various peripheral organs and are also associated with oxidative stress in the brain (30–32). Furthermore, toxic heavy metals such as chromium, arsenic and lead are found in commercial Portland cements (33), resulting from the use of impure natural raw materials and wastes. The ISO standard for water-based cements (34) only specifies limits for acid-extractable arsenic in zinc phosphate and polycarboxylate cements, and for lead in these and glass ionomer cements; chromium is not mentioned. For hydraulic silicate cements, the acid-extractable arsenic and lead could be higher than the ISO limits (33, 35–39), while milder conditions, such as synthetic tissue fluid, gave minimal amounts (36, 40). The synthetic calcium silicates are made from purer laboratory-grade raw materials using cleaner manufacturing processes. A separate category is therefore appropriate.
The final subdivision differentiates between materials that are supplied as a powder to be mixed with water from those presented suspended in a non-aqueous vehicle and which rely on the diffusion of water from the surroundings for hydration to proceed. These latter are supplied in a syringe, but because no mixing by the user is required are called “premixed.” This term is not meaningful since the reactant water is not present in the product (8). Other setting matrices, such as polymerizing resins and salicylates, which do not involve water, cannot be considered as forming hydraulic cements (8).
A functional labeling of types from within the above classification covers essentially all hydraulic calcium silicates currently used in clinical practice (Table 1). These types are based on the type of cement used, the presence or absence of mixing water and also the use of modifiers. Only the original version of MTA (ProRoot MTA, Denstply, Tulsa, OK, USA) does not include any additive except radio-opacifier.
Various other additives have been used to modify the properties and behavior of the hydraulic calcium silicate cements under all of the above categories, but in no systematic fashion. These fall broadly into two groups: reaction and physical modifiers. Thus, reaction modifiers include fumed silica, which reacts with the calcium hydroxide formed (Equations 1, 2) to produce further hydrated calcium silicate, and calcium carbonate and calcium oxide; all of these modify the overall reaction rate (41–43). Such substances are known in the construction industry, somewhat archaically, as “mineral” additives (44). Calcium chloride can be used to reduce the setting time (45).
Reaction modifiers may also be cementitious; for example, hydroxyapatite and monobasic calcium phosphate have been used in a number of formulations supposedly to enhance the material's “bioactivity.” This claim arises from reaction of the generated calcium hydroxide with free phosphate in synthetic tissue fluids, forming calcium phosphate(s). As explained earlier, this has no clinical relevance: the reactions are purely chemical, no biology is involved. The effect of the addition of calcium phosphate depends on type and proportion, but results in reduction in the final amount of calcium hydroxide, with consequent deterioration in biological properties (46, 47).
The physical properties of the mixed aqueous materials may be altered by water-soluble polymers, which raise the viscosity of the mixture and thus may improve their fluidity and handling (48, 49), the original formulation being notoriously difficult even noted by Schlenker (4).
Mechanical modifiers can also be included in the classification and radio-opacifiers can be considered as such. However, whether they actually improve strength or hardness is unclear; this would not be expected (50). Reaction modifiers such as fumed silica and calcium carbonate could also be considered as mechanical modifiers, but this is only proper when the water-cement ratio is controlled (51, 52).
The selection of a product for a specific clinical use, the context, depends on a variety of attributes and values. The choice may arise from a combination of the base constitution and modifiers, so again does not lend itself to a systematic categorization—a dichotomous scheme of classification in this sense is not possible.
Conclusions
A clear chemical classification of hydraulic cements used in dentistry is necessary since the various materials behave differently, as reflected in their specific clinical uses.
Statements
Author contributions
JC: conceptualization, writing—review, and editing.
Acknowledgments
Prof. Brian Darvell for his mentoring and for his help in preparing this manuscript. Mr. Satnam Virdee for Figure 1.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
LeeSJMonsefMTorabinejadM. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. (1993) 19:541–4. 10.1016/S0099-2399(06)81282-3
2.
TorabinejadMWatsonTFPitt FordTR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. (1993) 19:591–5. 10.1016/S0099-2399(06)80271-2
3.
Witte. The filling of a root canal with Portland cement. German Q Dent J Central Assoc German Dent. (1878) 18:153–4.
4.
SchlenkerM. Fuellen der Wurzelkanaele mit Portland-Cement nach Dr Witte classification of clinically available hydraulic calcium silicate cements. Deutsche Vrtljschr. F. Zahnh. (1880) 20:277–83.
5.
TorabinejadMWhiteJD. Tooth Filling Material and Method of Use. Patent No. 5,415,547 (1993).
6.
TorabinejadMHongCUMcDonaldFPitt FordTR. Physical and chemical properties of a new root-end filling material. J Endod. (1995) 21:349–53. 10.1016/S0099-2399(06)80967-2
7.
CamilleriJMontesinFEBradyKSweeneyRCurtisRVPitt FordTR. The constitution of mineral trioxide aggregate. Dental Mater. (2005) 21:297–303. 10.1016/j.dental.2004.05.010
8.
DarvellBW. Materials chemistry as a means to an end(o): the invisible foundation. In: CamilleriJ, editor. Chapter 1: Introduction in Endodontic Materials and Their Clinical Application.Wiley Blackwell (2020).
9.
PanHZhaoXDarvellBWLuWW. Apatite-formation ability–predictor of “bioactivity?” Acta Biomater. (2010) 6:4181–8. 10.1016/j.actbio.2010.05.013
10.
BohnerMLemaitre can bioactivity be tested in vitro with SBF solution?J Biomater. (2009) 30:2175–9. 10.1016/j.biomaterials.2009.01.008
11.
NiuLNJiaoKWangTDZhangWCamilleriJBergeronBEet al. A review of the bioactivity of hydraulic calcium silicate cements. J Dent. (2014) 42:517–33. 10.1016/j.jdent.2013.12.015
12.
Schembri WismayerPLungCYRappaFCappelloFCamilleriJ. Assessment of the interaction of Portland cement-based materials with blood and tissue fluids using an animal model. Sci Rep. (2016) 6:34547. 10.1038/srep34547
13.
MoinzadehATAznar PortolesCSchembri WismayerPCamilleriJ. Bioactivity potential of EndoSequence BC RRM putty. J Endod. (2016) 42:615–21. 10.1016/j.joen.2015.12.004
14.
MeschiNLiXVan GorpGCamilleriJVan MeerbeekBLambrechtsP. Bioactivity potential of Portland cement in regenerative endodontic procedures: from clinic to lab. Dent Mater. (2019) 35:1342–50. 10.1016/j.dental.2019.07.004
15.
XuerebMVellaPDamidotDSammutCVCamilleriJ. In situ assessment of the setting of tricalcium silicate-based sealers using a dentin pressure model. J Endod. (2015) 41:111–24. 10.1016/j.joen.2014.09.015
16.
LiXPongprueksaPVan LanduytKChenZPedanoMVan MeerbeekBet al. Correlative micro-Raman/EPMA analysis of the hydraulic calcium silicate cement interface with dentin. Clin Oral Investig. (2016) 20:1663–73. 10.1007/s00784-015-1650-x
17.
Castro-RaucciLMSTeixeiraLNBarbosaAFSFernandesRRRaucci-NetoWJacobovitzMet al. Calcium chloride-enriched calcium aluminate cement promotes in vitro osteogenesis. Int Endod J. (2018) 51:674–83. 10.1111/iej.12883
18.
OliveiraIRAndradeTLJacobovitzMPandolfelliVC. Bioactivity of calcium aluminate endodontic cement. J Endod. (2013) 39:774–8. 10.1016/j.joen.2013.01.013
19.
AguilarFGRoberti GarciaLFPanzeriPires-de-Souza FC. Biocompatibility of new calcium aluminate cement (EndoBinder). J Endod. (2012) 38:367–71. 10.1016/j.joen.2011.11.002
20.
DarvellBWWuRC. “MTA” -an hydraulic silicate cement: review update and setting reaction. Dent Mater. (2011) 27:407–22. 10.1016/j.dental.2011.02.001
21.
TaylorHFW. Cement Chemistry. London: Thomas Telford (1997).
22.
OdlerI. Hydration, setting and hardening of Portland cement. In: HewlettPC, editor. Lea's Chemistry of Cement and Concrete.London: Arnold (1998). p. 241–84.
23.
MoirGK. Cements. In: NewmanJChooBS, editors. Advanced Concrete Technology; Constituent Materials. Oxford: Elsevier Butterworth Heinemann (2003). p. 3–45.
24.
ShahPMChongBSSidhuSKFordTR. Radiopacity of potential root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1996) 81:476–9. 10.1016/S1079-2104(96)80028-X
25.
International Standards Organization (ISO). Dentistry — Root Canal Sealing Materials (2012).
26.
CamilleriJMontesinFEDi SilvioLPitt FordTR. The constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endodontic J. (2005) 38:834–42. 10.1111/j.1365-2591.2005.01028.x
27.
CamilleriJ. The physical properties of accelerated Portland cement for endodontic use. Int Endod J. (2008) 41:151–7. 10.1111/j.1365-2591.2007.01330.x
28.
CamilleriJ. Characterization and chemical activity of the Portland cement and two experimental cements with potential for use in dentistry. Int Endod J. (2008) 41:791–9. 10.1111/j.1365-2591.2008.01439.x
29.
CamilleriJ. The biocompatibility of modified experimental Portland cement with potential for use in dentistry. Int Endod J. (2008) 41:1107–14. 10.1111/j.1365-2591.2008.01483.x
30.
DemirkayaKCanDemirdögen BÖncel TorunZErdemOÇetinkayaSAkayC. In vivo evaluation of the effects of hydraulic calcium silicate dental cements on plasma and liver aluminium levels in rats. Eur J Oral Sci. (2016) 124:75–81. 10.1111/eos.12238
31.
DemirkayaKDemirdögenBCTorunZÖErdemOÇirakETuncaYM. Brain aluminium accumulation and oxidative stress in the presence of calcium silicate dental cements. Hum Exp Toxicol. (2017) 36:1071–80. 10.1177/0960327116679713
32.
SimsekNBulutETAhmetogluFAlanH. Determination of trace elements in rat organs implanted with endodontic repair materials by ICP-MS. J Mater Sci Mater Med. (2016) 27:46. 10.1007/s10856-015-5663-4
33.
SchembriMPeplowGCamilleriJ. Analyses of heavy metals in mineral trioxide aggregate and Portland cement. J Endod. (2010) 36:1210–5. 10.1016/j.joen.2010.02.011
34.
International Standards Organization. Dentistry—Water-Based Cements—Part 1: Powder/Liquid Acid-Base Cements. ISO 9917-1 (2007).
35.
ChangSWShonWJLeeWKumKYBaekSHBaeKS. Analysis of heavy metal contents in gray and white MTA and 2 kinds of Portland cement: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2010) 109:642–6. 10.1016/j.tripleo.2009.12.017
36.
CamilleriJKraljPVeberMSinagraE. Characterization and analyses of acid-extractable and leached trace elements in dental cements. Int Endod J. (2012) 45:737–43. 10.1111/j.1365-2591.2012.02027.x
37.
Monteiro BramanteCDemarchiACde MoraesIGBernadineliNGarciaRBSpångbergLSet al. Presence of arsenic in different types of MTA and white and gray Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2008) 106:909–13. 10.1016/j.tripleo.2008.07.018
38.
De-DeusGde SouzaMCSergio FidelRAFidelSRde CamposRCLunaAS. Negligible expression of arsenic in some commercially available brands of Portland cement and mineral trioxide aggregate. J Endod. (2009) 35:887–90. 10.1016/j.joen.2009.03.003
39.
KumKYZhuQSafaviKGuYBaeKSChangSW. Analysis of six heavy metals in Ortho mineral trioxide aggregate and ProRoot mineral trioxide aggregate by inductively coupled plasma-optical emission spectrometry. Aust Endod J. (2013) 39:126–30. 10.1111/j.1747-4477.2012.00349.x
40.
DuarteMADe Oliveira DemarchiACYamashitaJCKugaMCDe Campos FragaS. Arsenic release provided by MTA and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2005) 99:648–50. 10.1016/j.tripleo.2004.09.015
41.
CamilleriJMontesinFECurtisRVFordTR. Characterization of Portland cement for use as a dental restorative material. Dent Mater. (2006) 22:569–75. 10.1016/j.dental.2005.06.005
42.
CamilleriJSorrentinoFDamidotD. Characterization of un-hydrated and hydrated BioAggregate™ and MTA Angelus™. Clin Oral Investig. (2015) 19:689–98. 10.1007/s00784-014-1292-4
43.
CamilleriJSorrentinoFDamidotD. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater. (2013) 29:580–93. 10.1016/j.dental.2013.03.007
44.
PaillereAM. Admixtures in Concrete (RILEM Report) 1. Oxford: CRC Press; Taylor Francis (1994).
45.
BortoluzziEABroonNJBramanteCMFelippeWTTanomaru FilhoMEsberardRM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. (2009) 35:550–4. 10.1016/j.joen.2008.12.018
46.
Schembri-WismayerPCamilleriJ. Why biphasic? Assessment of the effect on cell proliferation and expression. J Endod. (2017) 43:751–9. 10.1016/j.joen.2016.12.022
47.
KoutroulisAKuehneSACooperPRCamilleriJ. The role of calcium ion release on biocompatibility and antimicrobial properties of hydraulic cements. Sci Rep. (2019) 9:19019. 10.1038/s41598-019-55288-3
48.
GalarçaADDa RosaWLODa SilvaTMda Silveira LimaGCarreñoNLVPereiraTMet al. Physical and biological properties of a high-plasticity tricalcium silicate cement. Biomed Res Int. (2018) 8063262. [Epub ahead of print]. 10.1155/2018/8063262
49.
GuimarãesBMVivanRRPiazzaBAlcaldeMPBramanteCMDuarteMAH. Chemical-physical properties and apatite-forming ability of mineral trioxide aggregate flow. J Endod. (2017) 43:1692–6. 10.1016/j.joen.2017.05.005
50.
DarvellBW. Materials Science for Dentistry. 10th ed. Cambridge: Woodhead (2018).
51.
CutajarAMalliaBAbelaSCamilleriJ. Replacement of radiopacifier in mineral trioxide aggregate; characterization and determination of physical properties. Dent Mater. (2011) 27:879–91. 10.1016/j.dental.2011.04.012
52.
KoutroulisABatchelorHKuehneSACooperPRCamilleriJ. Investigation of the effect of the water to powder ratio on hydraulic cement properties. Dent Mater. (2019) 35:1146–54. 10.1016/j.dental.2019.05.011
Summary
Keywords
hydraulic cements, classification, material use, environment, hydration reaction
Citation
Camilleri J (2020) Classification of Hydraulic Cements Used in Dentistry. Front. Dent. Med. 1:9. doi: 10.3389/fdmed.2020.00009
Received
22 June 2020
Accepted
05 August 2020
Published
08 September 2020
Volume
1 - 2020
Edited by
Vesna Miletic, University of Belgrade School of Dental Medicine, Serbia
Reviewed by
Mariano Simon Pedano, KU Leuven, Belgium
Updates
Copyright
© 2020 Camilleri.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Josette Camilleri J.Camilleri@bham.ac.uk
This article was submitted to Dental Materials, a section of the journal Frontiers in Dental Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.