
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Digit. Health , 07 February 2025
Sec. Connected Health
Volume 7 - 2025 | https://doi.org/10.3389/fdgth.2025.1535900
 Reo Hamaguchi1*
Reo Hamaguchi1* Seiji Hongo2
Seiji Hongo2 Naoto Doi3
Naoto Doi3 Hisamitsu Ide1
Hisamitsu Ide1 Ryozo Saito4
Ryozo Saito4 Junji Kishimoto5
Junji Kishimoto5 Nobuhiro Handa1
Nobuhiro Handa1 Shigeo Horie1
Shigeo Horie1
Introduction: The increasing prevalence of dementia in aging populations necessitates effective and accessible cognitive screening tools. This study aimed to evaluate the feasibility and reliability of a newly developed mobile app for detecting and screening mild cognitive impairment (MCI).
Methods: The mobile app, developed by LifeQuest Co., Ltd. (Minato-ku, Tokyo), is an original tool inspired by the Japanese version of the Montreal Cognitive Assessment (MoCA-J). A prospective observational study was conducted with 20 participants, including healthy individuals, MCI patients, and those with mild to moderate-severe dementia. Participants completed both the mobile app and the MoCA-J in a randomized order within a two-week period, with a minimum one-day interval between tests.
Results and conclusion: The intraclass correlation coefficient (ICC) between the mobile app and the MoCA-J was 0.956 (95% CI: 0.89-0.983), demonstrating a very high level of correlation. All participants successfully completed the mobile app assessment, highlighting its feasibility across various cognitive levels. Although minor technical issues and usability challenges were identified, the results support the mobile app as a reliable and user-friendly alternative for cognitive screening. Further studies with larger sample sizes are necessary to validate these findings and refine the app for broader clinical use.
The prevalence of dementia in Japan is increasing with the advancement of an aging society. According to data from the Hisayama Study, the estimated prevalence of dementia among individuals aged 65 years and older is projected to reach approximately 20% by 2025, with an estimated 7 million individuals affected (1). While dementia treatment often combines pharmacological and non-pharmacological therapies to alleviate symptoms of cognitive decline and behavioral and psychological symptoms of dementia (BPSD), there is no established treatment to improve or halt the progression of dementia itself. However, recent developments in pharmacological treatments have shown promise. Lecanemab and donanemab, both anti–β-amyloid monoclonal antibodies, have been developed to reduce cognitive impairment and amyloid burden in patients with early Alzheimer's disease (AD) (2–4). The prevalence of mild cognitive impairment (MCI), a precursor stage to dementia, is reported to be 15%–25% among the elderly aged 65 and older (5–7). Studies indicate that 5%–30% of individuals with MCI progress to dementia annually (8, 9), while 15%–40% of MCI cases may revert to normal cognitive function each year (10–12). Therefore, early detection and intervention at the MCI stage are considered crucial for dementia prevention.
Various neuropsychological tests are conducted for the diagnosis and assessment of dementia and MCI. The Mini-Mental State Examination (MMSE) is widely used internationally in clinical practice and research for cognitive screening. In Japan, the Hasegawa's Dementia Scale-Revised (HDS-R) is also commonly utilized in clinical settings. The Montreal Cognitive Assessment-Japanese version (MoCA-J) is particularly useful for detecting mild dementia and MCI. Additionally, the Clinical Dementia Rating (CDR) scale is used as an indicator of dementia severity, and the Alzheimer's Disease Assessment Scale cognitive subscale Japanese version (ADAS-Jcog) is employed to evaluate the progression of AD symptoms. While these are established methods for assessing cognitive function, they require face-to-face evaluation by physicians or clinical psychologists, which presents a challenge in terms of convenience.
Recently, the field of digital health, utilizing smartphones and digital technology, has gained attention and is being increasingly applied to elderly individuals, including those with MCI (13). For instance, the ADAS-Jcog requires a long duration (over 45 min) and skilled examiners, but the Touch Panel-type Dementia Assessment Scale (TDAS) has been developed to allow measurement using a touchscreen on a computer, demonstrating potential as a substitute for ADAS-Jcog (14). The CogState Brief Battery, a computerized multitask test assessing attention, processing speed, memory, and executive function, has shown utility in detecting MCI (15). Similarly, Cognivue is a computerized tool for automatic cognitive assessment, conducting a 10-minute automated test measuring visuomotor, perceptual processing, and memory processing, reported to be useful as an adjunct tool for evaluating cognitive impairment (16). Moreover, simple cognitive assessment tools utilizing eye-tracking technology and short task movies and images have been developed (17). These tasks are designed to assess neurological domains such as deductive reasoning, working memory, attention, and recall, and have shown correlations with other neuropsychological test results (18).
Thus, various computerized cognitive tests have been developed as potential substitutes for traditional neuropsychological tests, showing promising utility. In this study, we evaluate a mobile app for MCI detection and screening developed by LifeQuest Co., Ltd. (Minato-ku, Tokyo). The app is designed for assessing cognitive function, detecting and screening for MCI and AD, and includes original tests inspired by the MoCA-J but developed as a standalone tool, allowing self-administration on a mobile device. The aim of this study is to investigate the correlation between the app's results and those from the MoCA-J.
The original MoCA is a widely used cognitive screening tool designed to detect MCI and early AD (19, 20). The MoCA-J has been adapted to account for cultural and linguistic differences, ensuring its validity and reliability in Japanese populations (21). The MoCA-J consists of various cognitive tasks that assess different domains such as memory, visuospatial abilities, executive functions, attention, concentration, language, and orientation to time and place. It is traditionally administered in a face-to-face setting by a trained examiner. The mobile app for MCI detection and screening used in this study was developed by LifeQuest Co., Ltd. (Minato-ku, Tokyo) and images of this app are shown in Figure 1. This app, which includes original tests inspired by the MoCA-J, is designed for the assessment of cognitive function, as well as the detection and screening of MCI and AD. Responses entered into the app can be viewed on a web-based results screen, and the scores are automatically calculated. The questions in the app are electronically answered and were developed inspired by the principles of the MoCA-J. This app was not developed as a digital version of the MoCA-J but rather as an original tool inspired by its principles, with minor modifications tailored for mobile use. While the response format differs and some questions have added or have variations, a detailed comparison with the MoCA-J is provided in Table 1.
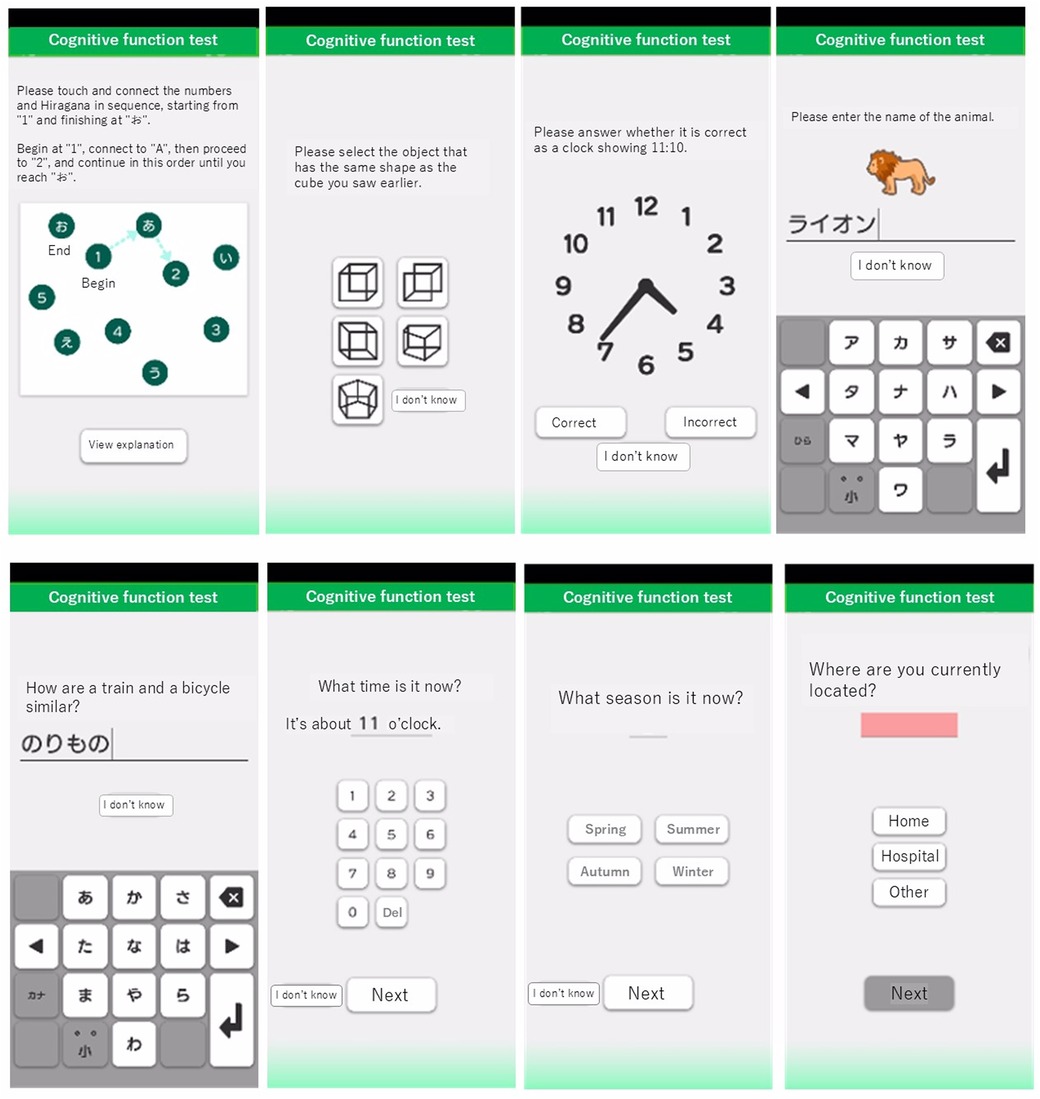
Figure 1. Screenshots of the mobile app for MCI detection and screening. Screenshots illustrating the interface of the app used for MCI detection and screening. The app includes various cognitive tasks inspired by the MoCA-J, with modified response formats and some question variations.
This study was conducted as a prospective observational study aimed to evaluate the correlation between the mobile app for MCI detection and screening, and the MoCA-J. This study has been registered at the UMIN Clinical Trials Registry as UMIN000052091 on September 4, 2023. Following the acquisition of informed consent and collection of participant background information, the cognitive assessments (both the mobile app and the MoCA-J) were randomly administered in either of the following sequences: MoCA-J followed by the mobile app, or mobile app followed by the MoCA-J. The mobile app assessments were conducted in a clinical setting under supervision. These assessments were conducted within a two-week period, with at least one day in between each test (Figure 2).
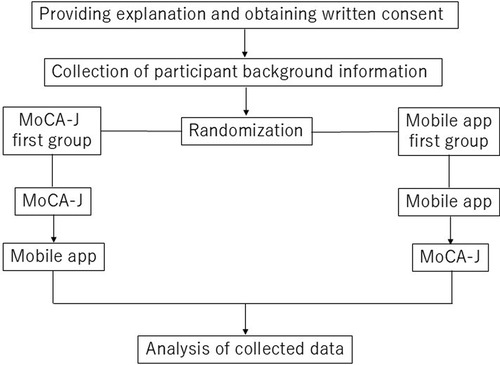
Figure 2. Flowchart of this study. Flowchart outlining the study design, including participant recruitment, randomization, and the administration of cognitive assessments.
Participants were enrolled from three institutes between September 28, 2023, and March 31, 2024. Participants included eight healthy individuals without a history of cognitive decline from LifeQuest Co., Ltd. Additionally, four participants each with MCI, mild dementia, and moderate to severe dementia were recruited from Ichigaya Himorogi Clinic, and Nanko Clinic of Psychiatry, respectively, totaling 12 participants. The total number of participants was set at 20. The classifications were based on the MMSE scores as follows; MCI: MMSE score 24–27, Mild dementia: MMSE score 21–23, Moderate to severe dementia: MMSE score ≤20.
Inclusion Criteria:
(1) Individuals aged 20–90 years at the time of enrollment
(2) Participants or their families who have provided informed consent after receiving sufficient explanation about the study
Exclusion Criteria:
(1) Participants who have undergone the MoCA-J within one month prior to the scheduled study date
(2) Individuals with visual or auditory impairments, ataxia, or other disabilities that prevent the use of a smartphone
(3) Participants deemed unsuitable for the study by the investigators
Primary Endpoint:
(1) The proportion of participants who successfully complete the mobile app for MCI detection and screening
Secondary Endpoints:
(1) The correlation between the total scores and corresponding item results of the mobile app and the MoCA-J
(2) The correlation between the respective scores of the mobile app and the MoCA-J, comparing the group that completed the mobile app first with the group that completed the MoCA-J first
Descriptive statistics were conducted on participant demographics collected via questionnaire, including age, gender, body mass index (BMI), highest educational attainment, alcohol consumption, smoking habits, and current medical conditions. The correlation between the scores of the mobile app for MCI detection and screening, and the MoCA-J was evaluated using intraclass correlation coefficients (ICC), and scatter plots were generated. Additionally, the agreement between the individual item results of the mobile app and MoCA-J was assessed using the kappa coefficient. The correlation between the respective scores of the mobile app and the MoCA-J, comparing the group that completed the mobile app first with the group that completed the MoCA-J first, was analyzed using the unpaired t-test.
A total of 20 participants (11 male and 9 female), who were categorized into four groups: healthy individuals (n = 8), patients with MCI (n = 4), patients with mild dementia (n = 4), and patients with moderate to severe dementia (n = 4), were enrolled in the study period. The demographic and clinical characteristics of the study participants are summarized in Table 2. Healthy participants had a mean age of 49.8 years, while the other groups had mean ages of 73.5 years (MCI), 80.6 years (mild dementia), and 78 years (moderate to severe dementia). It should be noted that the healthy group was younger than the other groups, which may affect the comparison of mobile app usability. All healthy participants had more than 12 years of education, while most participants in the other groups had 12 years or less. Among the comorbidities, hypertension was common, being present in 37.5% of the healthy group, 25% of the MCI group, 50% of the mild dementia group, and 75% of the moderate to severe dementia group.
All 20 participants successfully completed the mobile app for MCI detection and screening, regardless of their cognitive impairment level. However, one instance of a Wi-Fi connectivity issue caused a malfunction in the repetition task. The repetition task requires participants to orally repeat a sentence after hearing it, and the accuracy is determined using speech recognition on the mobile app. Due to the Wi-Fi issue, this process failed, and no score was recorded for this task.
Table 3 presents the scores for each task and the total scores of both the mobile app and the MoCA-J, along with the kappa coefficients for each individual task. The ICC was 0.956 (95% CI: 0.89–0.983), indicating a very high degree of correlation between the mobile app for MCI detection and screening, and the MoCA-J. Additionally, Figure 3 provides a scatter plot illustrating the correlation between the two methods.
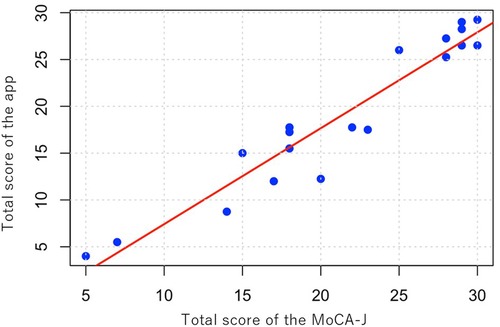
Figure 3. Scatter plot of total scores. Scatter plot illustrating the correlation between total scores obtained from the mobile app and the MoCA-J, with a trend line indicating their relationship (ICC = 0.956).
The scores of the mobile app and the MoCA-J were compared between the group that completed the mobile app first and the group that completed the MoCA-J first. An unpaired t-test showed no significant difference between the groups. For the mobile app, the first group had a mean score of 19.8 (SD 7.9), and the second group had a mean score of 18.3 (SD 8.8) (p = 0.700). For the MoCA-J, the first group had a mean score of 20.8 [standard deviation (SD) 8.0], while the second group had a mean score of 21.9 (SD 7.6) (p = 0.766). These results suggest that the order of test administration does not affect the assessment outcomes.
This study aimed to evaluate the feasibility and correlation of the mobile app for MCI detection and screening compared to the MoCA-J. All 20 participants successfully completed the mobile app, regardless of their cognitive impairment level. This indicates that the mobile app is generally user-friendly and accessible for individuals across a range of cognitive abilities. The correlation between the mobile app and MoCA-J was found to be very high (ICC = 0.956), suggesting that the mobile app is a reliable alternative to the MoCA-J. This high correlation indicates that the mobile app can effectively measure cognitive function in a manner comparable to the MoCA-J.
Recently, lecanemab and donanemab, both anti–β-amyloid monoclonal antibodies, were approved for use in individuals with MCI and early AD (2–4). However, identifying eligible candidates for anti–β-amyloid monoclonal antibody treatment remains a significant challenge due to the rigorous eligibility criteria. A population-based study revealed that only 8% of individuals with MCI or mild dementia meet the clinical trial criteria (22). Furthermore, it is uncommon for individuals to seek medical attention during the early stages of cognitive impairment, making the detection of MCI or early AD challenging in real-world clinical settings. In this context, our mobile app for MCI detection and screening, which demonstrates a high correlation with the MoCA-J, may offer a promising solution. This app has the potential to enable effective screening for potential MCI cases through self-assessment, reducing the burden on healthcare professionals.
Specific challenges with the mobile app were noted. A Wi-Fi connectivity issue affected the repetition task for one participant, highlighting a potential technical challenge that needs addressing. Specifically, the repetition task could not be scored due to the connectivity problem. In future updates, offline capabilities and enhanced error-handling features will be considered to ensure uninterrupted functionality and allow users to seamlessly retry tasks in the event of connectivity issues. There were difficulties with abstract questions because the predetermined correct answers were insufficient, leading to cases where correct responses were not recognized as such. Addressing this issue by expanding the range of acceptable answers is necessary. Furthermore, the verbal fluency task (words starting with “ka”) was found to be more challenging when entered via the mobile app compared to answering orally. This was particularly difficult for elderly participants who were less familiar with using smartphones. To improve usability, future developments will include refining the app's interface to make it more intuitive and user-friendly for older adults and expanding the range of acceptable responses for verbal and abstract fluency tasks. Improving these aspects in future updates will enhance the app's robustness and accessibility across diverse user groups.
Mobile applications and digital tools for cognitive assessments offer promising alternatives to traditional, clinic-based methods. These technologies enable frequent, brief, and repeated assessments in natural settings, enhancing patient-centered care and detection of subtle cognitive declines. Studies have shown the feasibility, reliability, and validity of mobile cognitive assessments, with adherence rates averaging 79.2% (23). Mobile technologies enable early detection of cognitive decline and support continuous monitoring and early intervention by providing convenient and cost-effective assessments (24). Eye-tracking (ET) technology is increasingly recognized as a valuable tool for assessing cognitive impairments (17). Research indicates ET's potential in diagnosing cognitive impairments and tracking decline over time, showing modest correlations with traditional cognitive assessments (25). Spontaneous speech analysis has gained attention for cognitive testing, using linguistic and acoustic features to detect cognitive impairments (26). Studies have shown that speech tempo, articulation rate, and pause patterns significantly differ between healthy individuals and those with cognitive impairments (27). The mobile app for MCI detection and screening used in this study is an original tool inspired by the MoCA-J. It incorporates tasks familiar to clinicians, which facilitates easier adoption and integration into existing clinical workflows. Compared to other methods, the app's design ensures user-friendliness and accessibility across cognitive levels. In addition, the app may contribute to the early detection of MCI, the reduction of healthcare costs through self-administered evaluations, and the provision of accessible cognitive screening for underserved populations. However, it is important to utilize a variety of assessment methods based on the specific situation to ensure comprehensive cognitive evaluation.
We acknowledge several limitations in this study. Firstly, the sample size was small, with only 20 participants, limiting the generalizability of the findings. Secondly, familiarity and proficiency with smartphone use varied among participants, particularly older adults, which may have influenced their performance on the mobile app for MCI detection and screening. Thirdly, the healthy group had a higher frequency of alcohol consumption compared to the other groups, which may have introduced an additional demographic imbalance. Lastly, while the mobile app was inspired by the MoCA-J and incorporated tasks based on its principles, it featured differences such as tap-based input instead of oral responses. These differences may have affected participants’ performance, particularly for those unfamiliar with such input methods, and highlight opportunities for refinement to improve user-friendliness and accessibility. In particular, the healthy group was younger than the other groups in this study. This age difference may have contributed to differences in familiarity with smartphone technology, potentially affecting the usability and performance results of the app across groups. Future studies should consider recruiting age-matched healthy controls and larger, more diverse cohorts to minimize these biases and enhance the generalizability of the findings.
The mobile app for MCI detection and screening is a feasible and reliable alternative to the MoCA-J, with high correlation in scores. Despite some technical and usability challenges, it shows promise for clinical use. Further research and improvements are needed for full validation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ichigaya Himorogi Clinic Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
RH: Writing – original draft, Writing – review & editing, Conceptualization, Investigation. SH: Writing – review & editing, Conceptualization, Data curation. ND: Writing – review & editing, Conceptualization, Data curation. HI: Writing – review & editing, Project administration. RS: Writing – review & editing, Data curation, Funding acquisition. JK: Writing – review & editing, Formal Analysis. NH: Writing – review & editing, Supervision. SH: Writing – review & editing, Project administration.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Life Quest Inc., Tokyo, Japan, which provided the app used in the research free of charge and covered the necessary expenses for conducting the research.
RS is the CEO and a shareholder of Life Quest Inc. RH holds an executive role, and NH serves as an advisor to Life Quest Inc. Additionally, RH and SHor own unlisted shares in the company. The authors declare that this study received funding from Life Quest Inc. The funder was involved in funding acquisition and data collection.
The author(s) declare that Generative AI was used in the creation of this manuscript. We utilized ChatGPT-4o, provided by OpenAI, exclusively for the purpose of English language proofreading. This usage was strictly limited to grammar and syntax checks to ensure the clarity and readability of the manuscript. We fully understand and accept the responsibility for the content of the submitted paper. The manuscript has been thoroughly reviewed and edited by the authors to guarantee the accuracy and integrity of the information presented.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ninomiya T. Japanese Legacy cohort studies: the Hisayama study. J Epidemiol. (2018) 28(11):444–51. doi: 10.2188/jea.JE20180150
2. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. (2023) 388(1):9–21. doi: 10.1056/NEJMoa2212948
3. Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. (2023) 330(6):512–27. doi: 10.1001/jama.2023.13239
4. Sato S, Hatakeyama N, Fujikoshi S, Katayama S, Katagiri H, Sims JR. Donanemab in Japanese patients with early Alzheimer’s disease: subpopulation analysis of the TRAILBLAZER-ALZ 2 randomized trial. Neurol Ther. (2024) 13(3):677–95. doi: 10.1007/s40120-024-00604-x
5. Petersen RC. Early diagnosis of Alzheimer’s disease: is MCI too late? Curr Alzheimer Res. (2009) 6(4):324–30. doi: 10.2174/156720509788929237
6. Luck T, Luppa M, Briel S, Riedel-Heller SG. Incidence of mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord. (2010) 29(2):164–75. doi: 10.1159/000272424
7. Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. (2012) 8(1):14–21. doi: 10.1016/j.jalz.2011.01.002
8. Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr. (2004) 16(2):129–40. doi: 10.1017/S1041610204000092
9. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia–meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. (2009) 119(4):252–65. doi: 10.1111/j.1600-0447.2008.01326.x
10. Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. (2013) 29(4):753–72. doi: 10.1016/j.cger.2013.07.003
11. Malek-Ahmadi M. Reversion from mild cognitive impairment to normal cognition: a meta-analysis. Alzheimer Dis Assoc Disord. (2016) 30(4):324–30. doi: 10.1097/WAD.0000000000000145
12. Shimada H, Makizako H, Doi T, Lee S, Lee S. Conversion and reversion rates in Japanese older people with mild cognitive impairment. J Am Med Dir Assoc. (2017) 18(9):808.e1–.e6. doi: 10.1016/j.jamda.2017.05.017
13. Bateman DR, Srinivas B, Emmett TW, Schleyer TK, Holden RJ, Hendrie HC, et al. Categorizing health outcomes and efficacy of mHealth apps for persons with cognitive impairment: a systematic review. J Med Internet Res. (2017) 19(8):e301. doi: 10.2196/jmir.7814
14. Inoue M, Jimbo D, Taniguchi M, Urakami K. Touch panel-type dementia assessment scale: a new computer-based rating scale for Alzheimer’s disease. Psychogeriatrics. (2011) 11(1):28–33. doi: 10.1111/j.1479-8301.2010.00345.x
15. de Jager CA, Schrijnemaekers AC, Honey TE, Budge MM. Detection of MCI in the clinic: evaluation of the sensitivity and specificity of a computerised test battery, the Hopkins verbal learning test and the MMSE. Age Ageing. (2009) 38(4):455–60. doi: 10.1093/ageing/afp068
16. Cahn-Hidalgo D, Estes PW, Benabou R. Validity, reliability, and psychometric properties of a computerized, cognitive assessment test (Cognivue(®)). World J Psychiatry. (2020) 10(1):1–11. doi: 10.5498/wjp.v10.i1.1
17. Liu Z, Yang Z, Gu Y, Liu H, Wang P. The effectiveness of eye tracking in the diagnosis of cognitive disorders: a systematic review and meta-analysis. PLoS One. (2021) 16(7):e0254059. doi: 10.1371/journal.pone.0254059
18. Oyama A, Takeda S, Ito Y, Nakajima T, Takami Y, Takeya Y, et al. Novel method for rapid assessment of cognitive impairment using high-performance eye-tracking technology. Sci Rep. (2019) 9(1):12932. doi: 10.1038/s41598-019-49275-x
19. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x
20. Freitas S, Simões MR, Alves L, Santana I. Montreal Cognitive assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. (2013) 27(1):37–43. doi: 10.1097/WAD.0b013e3182420bfe
21. Fujiwara Y, Suzuki H, Yasunaga M, Sugiyama M, Ijuin M, Sakuma N, et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal cognitive assessment. Geriatr Gerontol Int. (2010) 10(3):225–32. doi: 10.1111/j.1447-0594.2010.00585.x
22. Pittock RR, Aakre JA, Castillo AM, Ramanan VK, Kremers WK, Jack CR Jr., et al. Eligibility for anti-amyloid treatment in a population-based study of cognitive aging. Neurology. (2023) 101(19):e1837–e49. doi: 10.1212/WNL.0000000000207770
23. Moore RC, Swendsen J, Depp CA. Applications for self-administered mobile cognitive assessments in clinical research: a systematic review. Int J Methods Psychiatr Res. (2017) 26(4). doi: 10.1002/mpr.1562
24. Thompson LI, Harrington KD, Roque N, Strenger J, Correia S, Jones RN, et al. A highly feasible, reliable, and fully remote protocol for mobile app-based cognitive assessment in cognitively healthy older adults. Alzheimers Dement (Amst. (2022) 14(1):e12283. doi: 10.1002/dad2.12283
25. Bott N, Madero EN, Glenn J, Lange A, Anderson J, Newton D, et al. Device-embedded cameras for eye tracking-based cognitive assessment: validation with paper-pencil and computerized cognitive composites. J Med Internet Res. (2018) 20(7):e11143. doi: 10.2196/11143
26. Gosztolya G, Vincze V, Tóth L, Pákáski M, Kálmán J, Hoffmann I. Identifying mild cognitive impairment and mild Alzheimer’s disease based on spontaneous speech using ASR and linguistic features. Comput Speech Lang. (2019) 53:181–97. doi: 10.1016/j.csl.2018.07.007
Keywords: mobile app, smartphone app, neuropsychological tests, cognitive disorders, mild cognitive impairment, Alzheimer's disease, dementia
Citation: Hamaguchi R, Hongo S, Doi N, Ide H, Saito R, Kishimoto J, Handa N and Horie S (2025) Prospective observational study to evaluate the feasibility of the mobile app for mild cognitive impairment detection and screening. Front. Digit. Health 7:1535900. doi: 10.3389/fdgth.2025.1535900
Received: 28 November 2024; Accepted: 22 January 2025;
Published: 7 February 2025.
Edited by:
Premananda Indic, University of Texas at Tyler, United StatesReviewed by:
Martin Dyrba, German Center for Neurodegenerative Diseases (DZNE), GermanyCopyright: © 2025 Hamaguchi, Hongo, Doi, Ide, Saito, Kishimoto, Handa and Horie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reo Hamaguchi, cmVvaG1nQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.