- 1Department of Neurosurgery, Graduate School of Biomedical Sciences, Tokushima University, Tokushima, Japan
- 2Department of Research and Development, Beauty Life Corporation, Nagoya, Japan
- 3Parkinson’s Disease and Dystonia Research Center, Tokushima University Hospital, Tokushima, Japan
- 4Department of Advanced Brain Research, Graduate School of Biomedical Sciences, Tokushima University, Tokushima, Japan
- 5Division of Rehabilitation, Tokushima University Hospital, Tokushima, Japan
- 6Department of Neurology, Graduate School of Biomedical Sciences, Tokushima University, Tokushima, Japan
Objective: Several systematic reviews have shown that physical exercise positively affects motor function (MF) and quality of life (QoL) in patients with Parkinson's disease (PD). After the coronavirus disease (COVID-19) pandemic, numerous studies were conducted to reveal the effects of telerehabilitation for patients with PD. However, only a few empirical results of online programs for PD patients have been reported. Therefore, this study aimed to determine the effects of an online physical and cognitive training program on MF and QoL in patients with PD.
Methods: We evaluated the impact of our online program on the QoL and MF of patients with PD by comparing data at baseline and after six months of intervention. For the QoL assessment, we used the Schwab and England Activities of Daily Living scale and Parkinson's Disease Questionnaire (PDQ-39), whereas, for MF, we measured movement status using the modified 20-m walk test and timed up-and-go (TUG) test.
Results: We enrolled 20 patients for QoL and 19 for MF in this study. For PDQ-39, social support (p = 0.046, δ = 0.320) and cognitions (p = 0.028, δ = 0.268) significantly improved. Additionally, cadence (p = 0.032, g = −0.377) in the modified 20-m walk and exam duration (p = 0.003, δ = 0.296) and forward gait (p = 0.003, δ = 0.341) in the TUG test showed significant differences before and after the intervention.
Conclusion: Our results suggest that online physical and cognitive training programs positively affect MF and QoL in individuals with PD.
1 Introduction
Parkinson's disease (PD) is a neurodegenerative disorder that affects motor function (MF) and non-MF in patients (1). In addition to the cardinal symptoms, such as akinesia, bradykinesia, tremor, and rigidity, patients with PD exhibit further motor deficits, including gait disturbance, impaired handwriting, reduced grip strength, and speech impairments (2). These motor deficits substantially deteriorate the quality of life (QoL) of patients with PD. Moreover, PD encompasses non-motor symptoms, e.g., olfactory loss, sleep disturbances, autonomic dysfunction, psychiatric disorders, and cognitive impairment. These may also affect QoL in patients with PD (3). While pharmacological and device-assisted therapies are widely used for managing PD (3–7), physiotherapy also plays a crucial role (3, 8, 9). Several systematic reviews have demonstrated that physical exercise positively influences MF and QoL in patients with PD (10–13). Following the coronavirus disease pandemic, there has been a considerable proliferation of scientific literature investigating the use and implementation of digital health technology (14, 15). Concurrently, numerous studies have underscored the importance of telerehabilitation for patients with PD (16–19). However, only a few empirical results of online programs for PD patients have been reported. Therefore, in this study, we aimed to evaluate the effects of an online physical and cognitive training program on MF and QoL in patients with PD.
2 Methods
2.1 Participants
This prospective study included participants with idiopathic PD ranging from stage I to stage III on the Hoehn and Yahr (H&Y) rating scale. These participants were recruited from Tokushima University Hospital between December 2021 and December 2023. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki and the Ethics Committee of Tokushima University Hospital (approval number: 4114). Patients could choose their attendance and frequency with this program. For the sample size calculation, we estimated that 24 participants would provide 80% power (with a 5% probability of type I error) and a 60% effect size using G*Power 3.1. Therefore, we set the target sample size at 24 participants.
2.2 Interventions
The online exercise program was broadcast via the Zoom application (Zoom Video Communications, Inc., USA) for 1 h (from 9 to 10 am), Monday to Friday, excluding national holidays. Participants could also attend the exercise program in person at our salon in Tokushima University Hospital from Tuesday to Friday. Attendance was voluntary. This program aimed to improve the QoL of participants and develop functional capabilities, such as aerobic capacity, flexibility, upper and lower limb strength, motor condition, and balance. The program comprised five parts: (1) opening session: a talk about daily topics; (2) physical exercise: stretching and resistance training; (3) cognitive training: calculation, tongue twisters, quizzes, and lectures on frailty, muscle, brain and nutrition; (4) additional exercises: bicycling, aerobic exercise on a chair, dual-task training, oral training, facial yoga, and karaoke; and (5) closing session. The opening and closing sessions took approximately 10 min and included interactive conversations and discussion about today's weather, what day it is today, and other daily topics for managing social frailty. The physical exercise took approximately 15 min and included basic stretching for warming up and resistance training for the trunk and lower body. Cognitive training took approximately 15 min and focused on stimulating cognitive functions and increasing knowledge about frailty to motivate patients. Additional exercise took approximately 15 min and changed daily to keep the program fresh. The fitness bike was used for bicycling, and aerobic exercise on a chair focused on the steps and hand movement with an up-tempo rhythm. Dual-task training included cognitive training with steps, performing different movements with the right and left hands every day for 5–10 min. Sometimes, we did karaoke during aerobic exercise with the fitness bike. Oral training was designed to maintain the functions of eating, swallowing, and speaking. Facial yoga involved forming various expressions to improve facial expressions and complexion. During the karaoke sessions, we chose songs that evoked nostalgia, with the additional aim of stimulating cognitive functions. Overall, the contents of the program were carefully selected to prevent four frailties (physical, mental, social, and oral) and scheduled daily to ensure that the overall program was engaging for participants. Given the risk of falls, all participants were required to sit on a chair and maintain a seated position throughout the program.
2.3 Assessment
For the primary study, patients were evaluated at baseline (T0) and after six months of intervention (T1). For the supplementary study, evaluations included results after 12 months of intervention (T2). QoL and MF were assessed. All assessments were conducted at the salon in Tokushima University Hospital, although some questionnaires for QoL were administered by telephone when participants could not visit the salon. The inclusion criteria were as follows: (i) a diagnosis of idiopathic PD, (ii) stages I to III on the H&Y rating scale, (iii) the ability to exercise while seated, and (iv) age older than 30 years. The exclusion criteria included the following: (i) lack of patient consent, (ii) being deemed inappropriate for participation, and (iii) having attended fewer than six practice sessions over 6 months.
2.4 Outcomes
2.4.1 Quality of life (QoL)
To evaluate the impact of our program on QoL, we employed the Schwab and England Activities of Daily Living (S&E-ADL) scale and the Japanese version of the Parkinson's Disease Questionnaire (PDQ-39) (20, 21). The S&E-ADL scale uses percentages to indicate the patient's level of independence in daily activities, with 100% representing complete independence and 0% representing full dependence. The PDQ-39 comprises a 39-item questionnaire encompassing eight distinct dimensions. Participants completed the questionnaires independently; however, responses were recorded via telephone for those unable to visit the salon.
2.4.2 Motor function (MF)
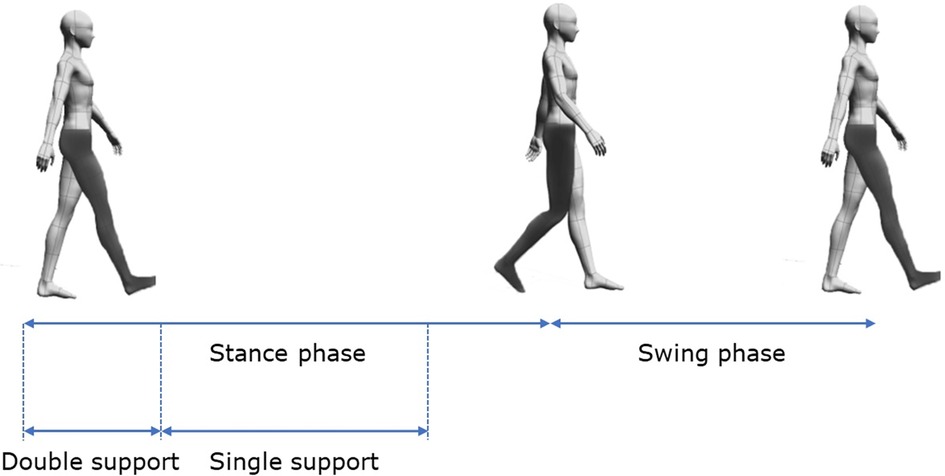
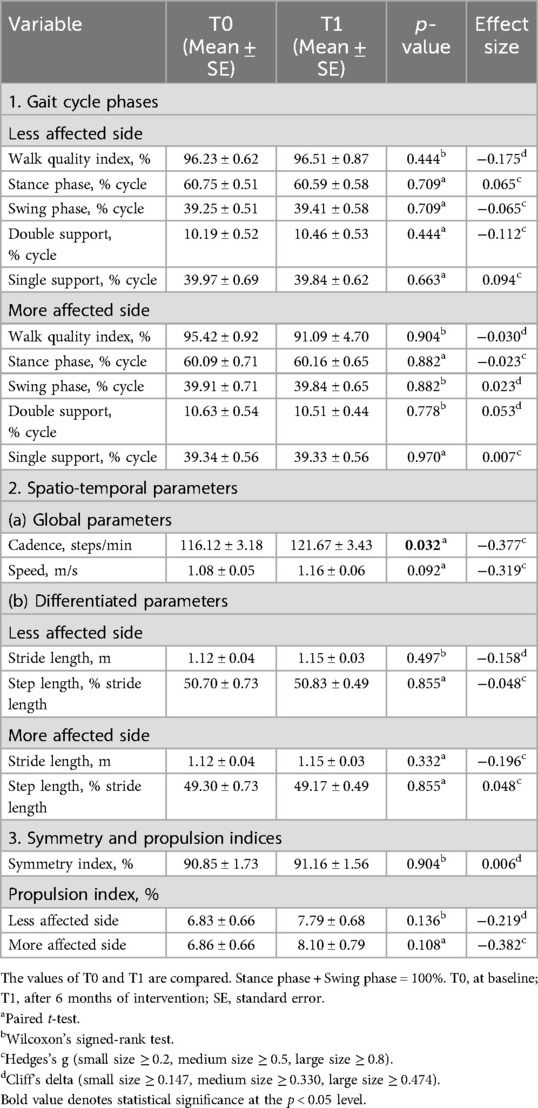
To assess MF, we evaluated movement using the modified 20-m walk test and the timed up-and-go (TUG) test. Both tests used the wireless inertial sensor system BTS G-WALK (BTS Bioengineering S.p.A., Italy) to measure spatiotemporal parameters and phase durations. In the modified 20-m walk test, participants walked 10 m forward, turned around, and returned to the starting point. The BTS G-WALK assessed results using data from the forward and return walks. In the TUG test, participants began seated, stood up, walked to a target 3 m away, turned around the target, returned to the chair, turned, and sat down. The parameters measured during the modified 20-m walk test included the following: (1) gait cycle phases (walk quality index and phase percentages for stance, swing, double support, and single support, for both less affected and more affected sides); (2) spatiotemporal parameters (global parameters, such as cadence and speed, and differentiated parameters, such as stride length and step length for both less affected and more affected sides); and (3) symmetry and propulsion indices. Due to the asymmetrical nature of PD, the more affected and less affected sides were determined through a combination of patient self-reporting and clinical assessment by the physician. Gait cycle phases are illustrated in Figure 1.
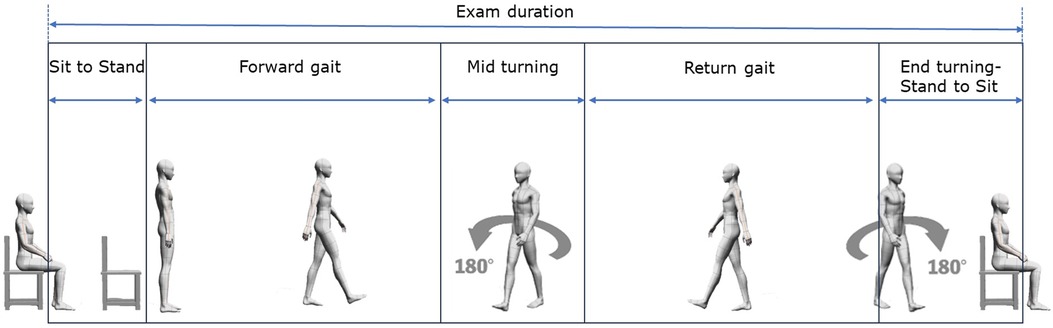
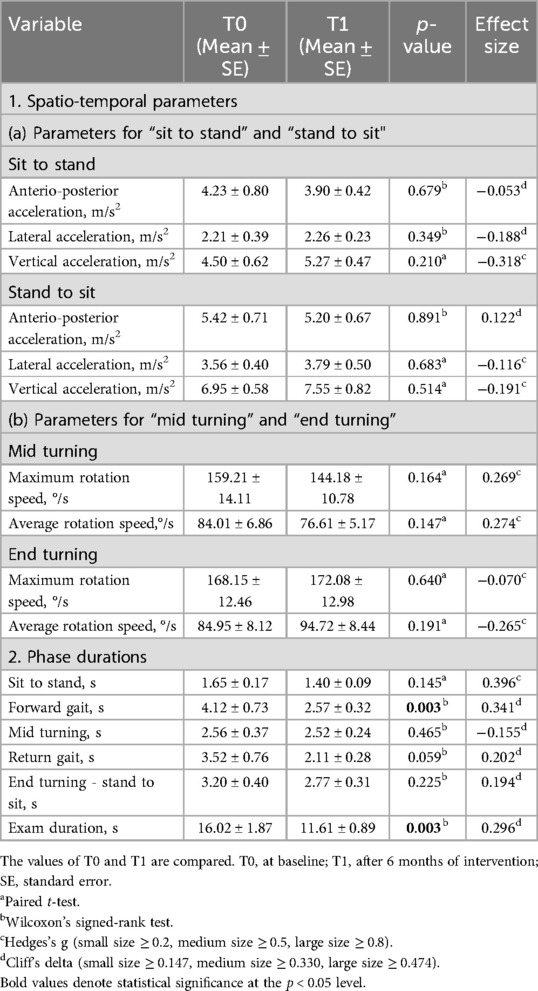
The parameters measured from the TUG test included the following: (1) spatiotemporal parameters: (a) for “sit to stand” and “stand to sit” phases, we measured phase duration, anterior-posterior acceleration, lateral acceleration, and vertical acceleration; (b) for “mid turning” and “end turning” phases, we measured phase duration, maximum rotation speed, and average rotation speed; and (2) overall phase duration for “sit to stand”, “forward gait”, “mid turning”, “return gait”, “end turning-stand to sit”, and “exam duration”. The phase durations are described in Figure 2.
2.5 Statistical analysis
All measured data are presented as mean ± standard error. For QoL analysis, Wilcoxon's signed-rank test was conducted to assess the significance between the two groups (T0 and T1), and the effect size was evaluated using Cliff's delta. For supplementary analysis, Friedman's test was applied among the three groups (T0, T1, and T2). A post hoc analysis using Wilcoxon's signed-rank test was performed when significance was found. For MF analysis, the paired t-test was used to analyze the two groups (T0 and T1) when the normality of distribution was verified by Shapiro–Wilk's test. If normality was not confirmed, Wilcoxon's signed-rank test was employed. Significance and effect size were determined using Hedges' g when normality was observed or Cliff's delta when it was not. For supplementary analysis of the three groups (T0, T1, and T2), the normality of distribution was first verified by Shapiro–Wilk's test. If normality was not observed, Friedman's test was performed. When normality was observed, sphericity was assessed with Mendoza's multi-sample sphericity test. One-way repeated measures analysis of variance (rANOVA) was performed without adjustment if sphericity was observed; if not, rANOVA was conducted with adjustment using the lower bound of epsilon (ε). Post hoc analyses were conducted using Wilcoxon's signed-rank test when Friedman's test showed significance and the paired t-test when rANOVA showed significance. Bonferroni's correction was applied for all post hoc tests. All statistical analyses were performed using R (version 4.2.1) (22), and the significance level was set at p < 0.05.
3 Results
3.1 Participant characteristics
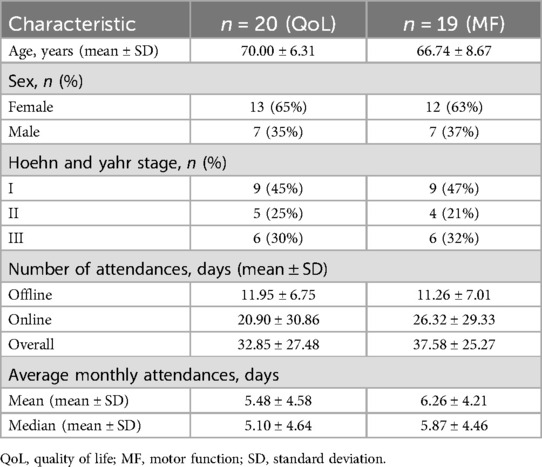
For the primary study (T0 and T1), 20 patients for QoL and 19 for MF were enrolled and evaluated on the basis of the inclusion and exclusion criteria. For QoL, 23 patients were initially recruited, but three patients with attendance of less than 6 days were excluded from the study. Similarly, 20 patients were recruited for MF, with one subsequently excluded. The number of patients, their characteristics, and attendance results in the QoL and MF groups are displayed in Table 1. For the QoL assessment, the average age was 70.00 (±6.31) years, average total attendance over 6 months was 32.85 (±27.48) days, and online attendance rate was 63.6%. The mean monthly attendance was 5.48 (±4.58), while the median monthly attendance was 5.10 (±4.64). For the MF assessment, the average age was 66.74 (±8.67) years, average total attendance over 6 months was 37.58 (±25.27) days, and online attendance rate was 70.0%. The mean monthly attendance was 6.26 (±4.21), while the median monthly attendance was 5.87 (±4.46).
For the supplementary study (T0, T1, and T2), 10 patients for QoL and nine for MF were enrolled and evaluated on the basis of the inclusion and exclusion criteria. For the QoL assessment, the average age was 67.50 (±4.79) years, average total attendance over 12 months was 88.80 (±60.16) days, and online attendance rate was 69.6%. The mean monthly attendance was 7.40 (±5.01), while the median monthly attendance was 6.55 (±5.31). For the MF assessment, the average age was 65.67 (±8.56) years, average total attendance over 12 months was 110.89 (±54.32) days, and online attendance rate was 81.9%. The mean monthly attendance was 9.24 (±4.53), while the median monthly attendance was 9.44 (±4.82).
3.2 QoL for T0 and T1
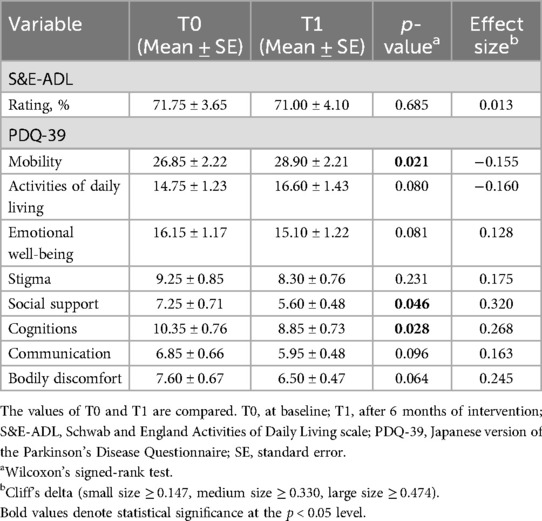
The results of the QoL assessment are presented in Table 2. No significant difference in activity of daily living (ADL) was found using the S&E-ADL. However, significant differences were observed in the PDQ-39 for the following items: mobility (p = 0.021, δ = −0.155), social support (p = 0.046, δ = 0.320), and cognitions (p = 0.028, δ = 0.268). Among these three items, social support and cognitions had positive effect sizes, indicating that participants felt better after the program despite the small effect sizes.
3.3 MF for T0 and T1
The results of the MF assessment are shown in Tables 3, 4. For the modified 20-m walk test, a statistically significant difference was found in cadence (p = 0.032, g = −0.377), although the effect size was small. In the TUG test, significance was found in two items: exam duration (p = 0.003, δ = 0.296) and forward gait (p = 0.003, δ = 0.341). Both items showed positive effect sizes, with forward gait demonstrating a medium-sized effect.
3.4 QoL for T0, T1, and T2
No statistically significant differences were found in ADL measured by the S&E-ADL. For the eight dimensions of the PDQ-39, significance was noted only for ADL in the PDQ-39 by Friedman's test, although post hoc analysis was not feasible.
3.5 MF for T0, T1, and T2
For the modified 20-m walk test, we found significant differences for cadence (p = 0.003), speed (p = 0.013), and symmetry index (p = 0.050) by Friedman's test, and we also found significant differences for cadence (p = 0.008, δ = −0.506) and speed (p = 0.008, δ = −0.358) by post hoc analysis of T0 and T1 and for symmetry index (p = 0.012, δ = 0.383) of T0 and T2. For TUG test results, no statistically significant difference was found.
3.6 Adverse events
No adverse events were observed among participants during the program.
4 Discussion
This is the first study to demonstrate the effects of an online physical and cognitive training program on MF and QoL in Japanese patients with PD. Our findings suggest that online programs positively impact MF and QoL in this population. Specifically, we observed a significant improvement in cadence during the modified 20-m walk and in the exam duration and forward gait phase of the TUG test over 6 months. These results align with those in previous research indicating that physical exercise enhances MF in patients with PD. Furthermore, our study revealed a statistically significant enhancement in social support and cognitions measured by the PDQ-39 over 6 months, supporting the past research findings showing that physical exercise may benefit cognitive function in patients with PD (23). Aerobic exercises, including bicycling and chair-based aerobic exercises implemented in our program, were particularly beneficial for MF and cognitive function, consistent with results of existing literature (10, 24–26). Additionally, dual-task exercises in our program likely contributed positively to motor and cognitive function improvement among the participants (27–29).
In our program, all exercises were conducted in a seated position, including those performed during in-person sessions. This approach demonstrated a notable safety profile, as we observed no adverse events throughout the study period. However, we acknowledge that this seated methodology may have imposed certain limitations on gait, mobility, and balance training. Despite this potential constraint, it is important to note that existing literature supports the efficacy of seated exercises in improving gait, mobility, and balance outcomes in older adults (30–33). In patients with PD, one study showed the effect of a sitting-based dance class for balance (34). These studies imply the possibility that seated exercises have positive effects on gait, mobility, and balance for patients with PD.
To maintain participant engagement and motivation, we incorporated various additional exercise sessions, such as oral exercises, facial yoga, karaoke, and enhanced communication among attendees (35). Educational sessions on frailty were also integrated into our program to underscore the importance of daily exercise, potentially bolstering participant motivation. Furthermore, use of incentive-based behavioral therapies is likely to be effective for PD (36). The engagement and excitement generated during dual-task training may potentially stimulate dopamine release, suggesting that this approach could be effective not only for cognitive and motor improvements but also for enhancing motivation in patients with PD. The dopaminergic activation associated with the engaging nature of dual-task exercises may contribute to increased motivation, potentially leading to improved adherence and outcomes in rehabilitation programs (37, 38). Further research is warranted to elucidate whether dual-task training influences motivation or not and quantify its effects on long-term patient engagement and therapeutic efficacy.
Although the number of patients was insufficient, worsening of the symmetry index in the modified 20-m walk test from baseline to 12 months was found. This might reflect the aggravation in asymmetric posture of PD. Axial postural abnormalities are known to aggravate in PD, with lateral abnormalities specifically recognized as Pisa syndrome (39). While the exact etiology remains unclear, there is growing evidence suggesting the involvement of impaired sensory-motor integration (40). Interventions stimulating sensory-motor integration, such as dual-task training, could be beneficial and increasing the duration and frequency of these exercises could potentially prevent the aggravation of postural asymmetry (41). Besides the symmetry index, we did not observe sustained effects of our program beyond 12 months, with improvements regressing to baseline levels after 6 months, indicating no worsening of symptoms over 12 months. It is plausible that the efficacy of exercise interventions for PD may plateau within a year. Given the progressive nature of PD, our results suggest a potential positive impact of our program.
Several studies have demonstrated the effectiveness of remotely supervised exercise and telerehabilitation for patients with PD (42–45). Telerehabilitation benefits patients with PD and enhances participation among frail older individuals by leveraging online platforms, which reduces barriers to engagement, as illustrated by our findings.
Regarding the limitations of this study, first, for MF assessment, we assessed only supervised gait and mobility which might not predict the actual real-world mobility correctly. In addition, we did not measure freezing of gait (FoG) scores, but there is a possibility that FoG could affect our results, especially parameters for forward gait, mid turning, and end turning. Moreover, for the cognitive evaluation, even though the PDQ-39 included scales about cognitions, it was subjective and we did not perform an objective one. Additionally, we set no exclusion criteria for severely cognitively impaired patients. These points should be considered when evaluating the effect shown in this research. Furthermore, we did not force patients to attend the program periodically or set the number of participations, so participation frequency varied among patients and we did not analyze the impact of participation frequency in this study, although most patients attended the program roughly evenly. We also recognize that the influence of online exercise programs is constrained and that distinguishing the effects of our program from other factors was not feasible. We did not differentiate the effects of individual exercise components and sessions. Finally, we did not distinguish between the effects of offline and online program formats. Despite these limitations, our program can positively influence participants’ motivation to exercise and their daily activity levels.
5 Conclusions
We evaluated the impact of an online program on the QoL and MF of patients with PD by comparing data at baseline and after six months of intervention. The QoL assessment was subjective, but significant differences were observed in mobility, social support, and cognitions in the PDQ-39. The MF assessment was objective, and significant differences were observed in cadence of the modified 20-m walk test and exam duration and forward gait in the TUG test. Although PD is a progressive disease and is not easy to improve, our results suggest that an online physical and cognitive training program has positive effects on MF and QoL for people with PD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Tokushima University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HN: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. RM: Conceptualization, Writing – review & editing. JF: Conceptualization, Data curation, Formal Analysis, Writing – review & editing. HO: Conceptualization, Writing – review & editing. KS: Conceptualization, Writing – review & editing. NY: Conceptualization, Writing – review & editing. YI: Supervision, Writing – review & editing. YT: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by JSPS KAKENHI (grant numbers: 23K08523 and 24K20437).
Acknowledgments
We thank Editage (www.editage.jp) for English language editing.
Conflict of interest
HN is the CEO of Beauty Life Corporation, and the Department of Advanced Brain Research is funded by Beauty Life Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Note
A correction has been made to this article. Details can be found at: 10.3389/fdgth.2025.1672261.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2024.1486662/full#supplementary-material
References
1. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primer. (2017) 3:17013. doi: 10.1038/nrdp.2017.13
2. Moustafa AA, Chakravarthy S, Phillips JR, Gupta A, Keri S, Polner B, et al. Motor symptoms in Parkinson’s disease: a unified framework. Neurosci Biobehav Rev. (2016) 68:727–40. doi: 10.1016/j.neubiorev.2016.07.010
3. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. (2021) 397:2284–303. doi: 10.1016/S0140-6736(21)00218-X
5. Fujikawa J, Morigaki R, Yamamoto N, Oda T, Nakanishi H, Izumi Y, et al. Therapeutic devices for motor symptoms in Parkinson’s disease: current progress and a systematic review of recent randomized controlled trials. Front Aging Neurosci. (2022) 14:807909. doi: 10.3389/fnagi.2022.807909
6. Fujikawa J, Morigaki R, Yamamoto N, Nakanishi H, Oda T, Izumi Y, et al. Diagnosis and treatment of tremor in Parkinson’s disease using mechanical devices. Life (Basel). (2022) 13:78. doi: 10.3390/life13010078
7. Scelzo E, Beghi E, Rosa M, Angrisano S, Antonini A, Bagella C, et al. Deep brain stimulation in Parkinson’s disease: a multicentric, long-term, observational pilot study. J Neurol Sci. (2019) 405:116411. doi: 10.1016/j.jns.2019.07.029
8. Radder DLM, Lígia Silva de Lima A, Domingos J, Keus SHJ, van Nimwegen M, Bloem BR, et al. Physiotherapy in Parkinson’s disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair. (2020) 34:871–80. doi: 10.1177/1545968320952799
9. Okada Y, Ohtsuka H, Kamata N, Yamamoto S, Sawada M, Nakamura J, et al. Effectiveness of long-term physiotherapy in Parkinson’s disease: a systematic review and meta-analysis. J Parkinsons Dis. (2021) 11:1619–30. doi: 10.3233/JPD-212782
10. Wu PL, Lee M, Huang TT. Effectiveness of physical activity on patients with depression and Parkinson’s disease: a systematic review. PLoS One. (2017) 12:e0181515. doi: 10.1371/journal.pone.0181515
11. Ernst M, Folkerts AK, Gollan R, Lieker E, Caro-Valenzuela J, Adams A, et al. Physical exercise for people with Parkinson’s disease: a systematic review and network meta-analysis. Cochrane Database Syst Rev. (2023) 1:CD013856. doi: 10.1002/14651858.CD013856.pub2
12. Zhang M, Li F, Wang D, Ba X, Liu Z. Exercise sustains motor function in Parkinson’s disease: evidence from 109 randomized controlled trials on over 4,600 patients. Front Aging Neurosci. (2023) 15:1071803. doi: 10.3389/fnagi.2023.1071803
13. Li F, Wang D, Ba X, Liu Z, Zhang M. The comparative effects of exercise type on motor function of patients with Parkinson’s disease: a three-arm randomized trial. Front Hum Neurosci. (2022) 16:1033289. doi: 10.3389/fnhum.2022.1033289
14. Mumtaz H, Riaz MH, Wajid H, Saqib M, Zeeshan MH, Khan SE, et al. Current challenges and potential solutions to the use of digital health technologies in evidence generation: a narrative review. Front Digit Health. (2023) 5:1203945. doi: 10.3389/fdgth.2023.1203945
15. Jerjes W, Harding D. Telemedicine in the post-COVID era: balancing accessibility, equity, and sustainability in primary healthcare. Front Digit Health. (2024) 6:1432871. doi: 10.3389/fdgth.2024.1432871
16. Brown EG, Chahine LM, Goldman SM, Korell M, Mann E, Kinel DR, et al. The effect of the COVID-19 pandemic on people with Parkinson’s disease. J Parkinsons Dis. (2020) 10:1365–77. doi: 10.3233/JPD-202249
17. Helmich RC, Bloem BR. The impact of the COVID-19 pandemic on Parkinson’s disease: hidden sorrows and emerging opportunities. J Parkinsons Dis. (2020) 10:351–4. doi: 10.3233/JPD-202038
18. Anghelescu BAM, Bruno V, Martino D, Roach P. Effects of the COVID-19 pandemic on Parkinson’s disease: a single-centered qualitative study. Can J Neurol Sci. (2022) 49:171–83. doi: 10.1017/cjn.2021.70
19. Papa SM, Brundin P, Fung VSC, Kang UJ, Burn DJ, Colosimo C, et al. Impact of the COVID-19 pandemic on Parkinson’s disease and movement disorders. Mov Disord Clin Pract. (2020) 35:711–5. doi: 10.1002/mds.28067
20. Schwab RS, England AC. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham FJ, Donaldson MC, editors. Third Synopsium on Parkinson's Disease. Edinburgh: Livingston (1969). p. 157–7.
21. Kohmoto J, Ohbu S, Nagaoka M, Suzukamo Y, Kihara T, Mizuno Y, et al. Validation of the Japanese version of the Parkinson’s disease questionnaire. Clin Neurol. (2003) 43:71–6.
22. Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. (1996) 5:299–314. doi: 10.2307/1390807
23. da Silva FC, Iop RDR, de Oliveira LC, Boll AM, de Alvarenga JGS, Gutierres Filho PJB, et al. Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: a systematic review of randomized controlled trials of the last 10 years. PLoS One. (2018) 13:e0193113. doi: 10.1371/journal.pone.0193113
24. Zhen K, Zhang S, Tao X, Li G, Lv Y, Yu L. A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson’s disease. NPJ Parkinsons Dis. (2022) 8:146. doi: 10.1038/s41531-022-00418-4
25. Johansson ME, Cameron IGM, Van der Kolk NM, de Vries NM, Klimars E, Toni I, et al. Aerobic exercise alters brain function and structure in Parkinson’s disease: a randomized controlled trial. Ann Neurol. (2022) 91:203–16. doi: 10.1002/ana.26291
26. Tiihonen M, Westner BU, Butz M, Dalal SS. Parkinson’s disease patients benefit from bicycling—a systematic review and meta-analysis. NPJ Parkinsons Dis. (2021) 7:86. doi: 10.1038/s41531-021-00222-6
27. Li Z, Wang T, Liu H, Jiang Y, Wang Z, Zhuang J. Dual-task training on gait, motor symptoms, and balance in patients with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil. (2020) 34:1355–67. doi: 10.1177/0269215520941142
28. Zheng Y, Meng Z, Zhi X, Liang Z. Dual-task training to improve cognitive impairment and walking function in Parkinson’s disease patients: a brief review. Sports Med Health Sci. (2021) 3:202–6. doi: 10.1016/j.smhs.2021.10.003
29. García-López H, de los Ángeles Castillo-Pintor M, Castro-Sánchez AM, Lara-Palomo IC, Obrero-Gaitán E, Cortés-Pérez I. Efficacy of dual-task training in patients with Parkinson’s disease: a systematic review with meta-analysis. Mov Disord Clin Pract. (2023) 10:1268–84. doi: 10.1002/mdc3.13823
30. Niemelä K, Väänänen I, Leinonen R, Laukkanen P. Benefits of home-based rocking-chair exercise for physical performance in community-dwelling elderly women: a randomized controlled trial—a pilot study. Aging Clin Exp Res. (2011) 23:279–87. doi: 10.1007/BF03337754
31. Baum EE, Jarjoura D, Polen AE, Faur D, Rutecki G. Effectiveness of a group exercise program in a long-term care facility: a randomized pilot trial. J Am Med Dir Assoc. (2003) 4:74–80. doi: 10.1097/01.JAM.0000053513.24044.6C
32. Yao CT, Tseng CH. Effectiveness of chair yoga for improving the functional fitness and well-being of female community-dwelling older adults with low physical activities. Top Geriatr Rehabil. (2019) 35:248–54. doi: 10.1097/TGR.0000000000000242
33. Kim SG, Goo M, Park JH. Comparison of the effectiveness of balance training using a reaching task between a sitting position and a standing position in the elderly. J Phys Ther Sci. (2015) 27:2337–9. doi: 10.1589/jpts.27.2337
34. Ooshio Y, Iwamura M, Okamoto Y, Higashiimuta M, Fujimoto S, Sugino M. Immediate effect of a sitting-based Parkinson’s dance class on Parkinson’s disease patients. Rigakuryoho Kagaku. (2021) 36:505–9. doi: 10.1589/rika.36.505
35. Grannell A, Hallson H, Gunlaugsson B, Jonsson H. Exercise therapy as a digital therapeutic for chronic disease management: consideration for clinical product development. Front Digit Health. (2023) 5:1250979. doi: 10.3389/fdgth.2023.1250979
36. Renfroe JB, Bradley MM, Okun MS, Bowers D. Motivational engagement in Parkinson’s disease: preparation for motivated action. Int J Psychophysiol. (2016) 99:24–32. doi: 10.1016/j.ijpsycho.2015.11.014
37. Bäckman L, Waris O, Johansson J, Andersson M, Rinne JO, Alakurtti K, et al. Increased dopamine release after working-memory updating training: neurochemical correlates of transfer. Sci Rep. (2017) 7:7160. doi: 10.1038/s41598-017-07577-y
38. Takeuchi H, Magistro D, Kotozaki Y, Motoki K, Nejad KK, Nouchi R, et al. Effects of simultaneously performed dual-task training with aerobic exercise and working memory training on cognitive functions and neural systems in the elderly. Neural Plast. (2020) 2020:3859824. doi: 10.1155/2020/3859824
39. Castrioto A, Piscicelli C, Pérennou D, Krack P, Debû B. The pathogenesis of Pisa syndrome in Parkinson’s disease. Mov Disord. (2014) 29:1100–7. doi: 10.1002/mds.25925
40. Barone P, Santangelo G, Amboni M, Pellecchia MT, Vitale C. Pisa Syndrome in Parkinson’s disease and parkinsonism: clinical features, pathophysiology, and treatment. Lancet Neurol. (2016) 15:1063–74. doi: 10.1016/S1474-4422(16)30173-9
41. Strobach T. The dual-task practice advantage: empirical evidence and cognitive mechanisms. Psychon Bull Rev. (2020) 27:3–14. doi: 10.3758/s13423-019-01619-4
42. Gandolfi M, Geroin C, Dimitrova E, Boldrini P, Waldner A, Bonadiman S, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized, controlled trial. BioMed Res Int. (2017) 2017:7962826. doi: 10.1155/2017/7962826
43. Bianchini E, Onelli C, Morabito C, Alborghetti M, Rinaldi D, Anibaldi P, et al. Feasibility, safety, and effectiveness of telerehabilitation in mild-to-moderate Parkinson’s disease. Front Neurol. (2022) 13:909197. doi: 10.3389/fneur.2022.909197
44. van der Kolk NM, de Vries NM, Kessels RPC, Joosten H, Zwinderman AH, Post B, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. (2019) 18:998–1008. doi: 10.1016/S1474-4422(19)30285-6
Keywords: Parkinson’s disease, telerehabilitation, online system, cognitive training, physical exercise, motor function, quality of life, frailty
Citation: Nakanishi H, Morigaki R, Fujikawa J, Ohmae H, Shinohara K, Yamamoto N, Izumi Y and Takagi Y (2024) Online training program maintains motor functions and quality of life in patients with Parkinson's disease. Front. Digit. Health 6:1486662. doi: 10.3389/fdgth.2024.1486662
Received: 26 August 2024; Accepted: 28 October 2024;
Published: 13 November 2024;
Corrected: 5 August 2025.
Edited by:
Ben Singh, University of South Australia, AustraliaReviewed by:
Edoardo Bianchini, Sapienza University of Rome, ItalyFrancesco Lena, Mediterranean Neurological Institute Neuromed (IRCCS), Italy
Copyright: © 2024 Nakanishi, Morigaki, Fujikawa, Ohmae, Shinohara, Yamamoto, Izumi and Takagi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryoma Morigaki, bW9yaWdha2kucml5b21hLjFAdG9rdXNoaW1hLXUuYWMuanA=
 Hiroshi Nakanishi
Hiroshi Nakanishi Ryoma Morigaki
Ryoma Morigaki Joji Fujikawa
Joji Fujikawa Hiroshi Ohmae
Hiroshi Ohmae Keisuke Shinohara
Keisuke Shinohara Nobuaki Yamamoto
Nobuaki Yamamoto Yuishin Izumi
Yuishin Izumi Yasushi Takagi
Yasushi Takagi