
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Digit. Health, 11 January 2024
Sec. Health Technology Implementation
Volume 5 - 2023 | https://doi.org/10.3389/fdgth.2023.1345814
In the domain of healthcare, most importantly pediatric healthcare, the role of artificial intelligence (AI) has significantly impacted the medical field. Congenital heart diseases represent a group of heart diseases that are known to be some of the most critical cardiac conditions present at birth. These heart diseases need a swift diagnosis as well as an intervention to ensure the wellbeing of newborns. Fortunately, with the help of AI, including the highly advanced algorithms, analytics and imaging involved, it provides us with a promising era for neonatal care. This article reviewed published data in PubMed, Science Direct, UpToDate, and Google Scholar between the years 2015–2023. To conclude The use of artificial intelligence in detecting congenital heart diseases has shown great promise in improving the accuracy and efficiency of diagnosis. Several studies have demonstrated the efficacy of AI-based approaches for diagnosing congenital heart diseases, with results indicating that the systems can achieve high levels of sensitivity and specificity. In addition, AI can help reduce the workload of healthcare professionals allowing them to focus on other critical aspects of patient care. Despite the potential benefits of using AI, in addition to detecting congenital heart disease, there are still some challenges to overcome, such as the need for large amounts of high-quality data and the requirement for careful validation of the algorithms. Nevertheless, with ongoing research and development, AI is likely to become an increasingly valuable tool for improving the diagnosis and treatment of congenital heart diseases.
In the domain of healthcare, most importantly pediatric healthcare, the role of artificial intelligence (AI) has significantly impacted the medical field. Congenital heart diseases represent a group of heart diseases that are known to be some of the most critical cardiac conditions present at birth. These heart diseases need a swift diagnosis as well as an intervention to ensure the wellbeing of newborns. Fortunately, with the help of AI, including the highly advanced algorithms, analytics and imaging involved, it provides us with a promising era for neonatal care. This article investigates the groundbreaking capability of simulated intelligence in supporting health care professionals to promptly and precisely recognize congenital heart diseases in neonates, accordingly upgrading their possibilities getting convenient clinical consideration and at last further developing results for these weak newborn children.
The published articles reviewed here were manually collected from multiple databases such as PubMed, ScienceDirect, UpToDate, and Google Scholar for research and reports published between the years 2015–2023. Keywords/combination of keywords used: “Artificial Intelligence”, “Screening tools”, “Early Diagnosis” “Neonates”, “Congenital Cardiac Defects” and “Current challenges”.
Total twenty-four published full text articles that meet the inclusion criteria were retrieved and analyzed.
According to available evidence, neonates with congenital heart defects (CHD) who are identified before birth tend to experience better outcomes compared to those whose condition is detected after they are born. This difference in outcomes can largely be attributed to the frequent delays in diagnosing ductal-dependent CHD, which often occurs when newborns arrive at the emergency department in a state of shock, many days or even weeks after their discharge from the hospital. Until now, no single test, aside from echocardiography, has been demonstrated to be an effective screening technique for identifying CHD in newborns. Recently, pulse oximetry has been suggested as a potential screening method to address the need for reducing the number of infants with serious, ductal-dependent CHD who remain undiagnosed before being sent home from the nursery (1).
Echocardiography, a diagnostic imaging technique, is a non-invasive method that employs high-frequency sound waves to create images of the heart (Table 1). This technique is capable of identifying both structural and functional anomalies in the heart. In neonates, echocardiography serves as the primary diagnostic tool for detecting congenital heart defects (CHD). It is used to confirm suspected cases of CHD based on clinical indicators such as cyanosis, heart murmurs, and feeding difficulties (2).
The utilization of echocardiography to diagnose CHD in seemingly healthy newborns with cardiac murmurs can be advantageous in earlier CHD detection, thereby enhancing the clinical outcomes for newborns with severe CHD (2).
Fetal echocardiography is a specialized form of echocardiography conducted during pregnancy to assess the developing fetus's heart. It can identify CHD as early as 18–22 weeks into gestation. Echocardiography can also uncover asymptomatic instances of CHD, which, if left undetected, can significantly affect long-term outcomes. Prenatal echocardiography for CHD diagnosis demonstrates moderate sensitivity and notably higher specificity (3).
It is recommended for all pregnant women with risk factors for CHD, including a family history of CHD, maternal diabetes, and exposure to certain medications or infections during pregnancy.
The fetal echocardiogram is the primary tool for evaluating and precisely diagnosing fetal cardiovascular abnormalities from the late first trimester until term. The accuracy of prenatal CHD detection via fetal echocardiography can vary significantly, with some of the variability attributed to the experience of the examiner (4). Prenatal CHD detection has the potential to enhance pregnancy outcomes for fetuses with certain types of cardiac defects. Accurate prenatal diagnosis offers valuable clinical advantages concerning infant outcomes.
Pulse oximetry screening is typically conducted on newborns within 24–48 h after birth as a non-invasive, cost-effective method for identifying critical congenital heart disease (Table 2). While this screening approach has demonstrated effectiveness in detecting critical congenital heart disease in newborns, it cannot reliably detect all types of congenital heart disease. Some forms of congenital heart disease may not lead to significant changes in oxygen saturation, and as a result, they may go unnoticed during pulse oximetry screening (5).
While echocardiography is considered the gold standard for diagnosing congenital heart disease, it typically requires more than 10 min to complete and may not be practical for every newborn, especially in resource-limited areas. In contrast, pulse oximetry provides a convenient and swift alternative, requiring only 2–3 min to analyze the results (5).
ECG (Electrocardiography) is capable of identifying irregular heart rhythms, conduction issues, and other electrical anomalies that might suggest the presence of congenital heart disease (CHD) in neonates (Table 3). In certain instances, ECG results may also offer hints regarding the specific type of heart defect present, although for a reliable diagnosis or exclusion of congenital heart disease, echocardiography remains the preferred method (6). When neonates are suspected of having CHD, an ECG is frequently employed alongside other diagnostic assessments, such as echocardiography, to confirm the diagnosis and evaluate the severity of the defect.
Alternative imaging techniques such as CT (Computed Tomography), CTA (CT Angiography), and cardiac MRI (Magnetic Resonance Imaging) can be employed to identify congenital heart disease in neonates, although they are not the primary imaging methods for this purpose (Table 4). While they have the capacity to detect congenital heart disease in neonates, echocardiography is the preferred initial imaging approach, with these alternative methods reserved for situations where more detailed information is necessary, particularly in complex cases (7).
The utilization of CT and CTA should be approached with caution, considering potential risks to the infant, including radiation exposure. CTA is generally employed where the benefits outweigh the risks, rather than for early detection of congenital heart disease in neonates (7).
On the other hand, cardiac MRI is time consuming, entails sedation or general anesthesia, which can pose risks for neonates.
While a chest x-ray can offer some insights into the heart and lungs of a neonate, it is not a dependable method for detecting congenital heart disease. Congenital heart disease may involve abnormalities in the size, shape, and positioning of heart chambers, blood vessels, and valves, which cannot be accurately identified through a chest x-ray. Consequently, chest x-rays are not routinely used as a screening tool for detecting congenital heart disease in neonates (8).
The utilization of Artificial Intelligence (AI) in various domains within the medical field has been a subject of extensive research and experimentation, aimed at enhancing healthcare and outcomes for a wide range of medical conditions. Over the past decade, there have been notable efforts to employ AI in the context of Congenital Heart Disease (CHD). In this article, we will examine the latest developments and breakthroughs in multiple aspects of machine learning that contribute to the enhancement of CHD screening and risk assessment.
The utilization of machine learning for screening Fetal Congenital Heart Disease (CHD) during gestation, facilitated by the radiation-free and cost-effective nature of echocardiograms, stands as a highly favorable area for implementation (9).
Many studies have been exploring the effectiveness of AI in prenatal ultrasounds and the aim was to aid physician's work. Given that ultrasounds are operator-dependent, inaccuracies resulting from factors like expertise and others significantly impact how the results are interpreted. It has been demonstrated that machine learning can remove these obstacles (10). Algorithms were explored that segments various cardiac ultrasound images such as apical four-chamber view and apical two-chamber view to calculate chambers sizes and stroke volume and various other cardiac indexes to assist the interpreter with labeling anatomical structures, automatized measurements, and provide provisional diagnosis with severity and outcomes. The primary objective is to enhance the efficiency of time management and the precision of diagnostic outcomes. Research has consistently demonstrated improvements in sensitivity and specificity when detecting various Congenital Heart Diseases-(CHD) early in pregnancy. These findings hold the potential to enable earlier diagnosis of fetal cardiac conditions, offering a broader timeframe for implementing interventions that can ultimately reduce mortality rates and lower associated risks (11–13).
The development of algorithms and ongoing clinical trials has highlighted a significant challenge in the deployment of these technologies - the limited availability of data. The data collected from previously diagnosed patients and the inherent anatomical variations among individuals have emerged as critical factors that contribute to elevated rates of false positives and errors in numerous trials (14).
Computer-assisted auscultation has become available to assist clinicians with physical examinations to detect congenital heart disease (CHD). In a study comparing physicians with assistance of AI and not shown that, AI-assisted auscultation demonstrated strong sensitivity, good specificity, high accuracy, and excellent agreement with the experts (15).
The electronic stethoscope not only facilitates the recording of heart sounds but also refines them to distinguish between actual and radiating sounds by detecting different frequencies. Moreover, its integration with AI technology enables the digital capture of heart sounds and intelligent identification of pathological murmurs. Clinically hearing subtle heart murmurs can be challenging even for an experienced physician, this implication helps seal that gap, and in turn makes early detection and screening of CHD possible (16).
Artificial intelligence-assisted auscultation (AI-AA) algorithms have been formed by using previous data of abnormal, normal, and subtle murmurs and further classify and form a category to make a system that's able to stratify conditions to suitable diagnosis. These test algorithms performed very well in clinical trials and potentially become used in the near future (17).
As previously discussed in prenatal diagnosis, AI-AA also is data hungry where the more data added the more accurate and less chances of falsely mixing normal with abnormal findings. Several challenges remain to be addressed in the context of AI-assisted auscultation (AI-AA). Foremost, the enhancement of AI algorithms is paramount for achieving high diagnostic accuracy in Congenital Heart Disease (CHD) cases. The clinical collection of heart sound signals often encounters various sources of noise interference, notably the cries of infants and young children (17). This presents challenges for segmentation and classification algorithms, ultimately impacting the accuracy of recognition. Nevertheless, efforts are underway to address these challenges through methods like noise filtering (18).
Pediatric cardiology, being a cognitively challenging and perceptual subfield involving complex decision-making, makes the use of AI particularly compelling for this medical field. Through simulations and computer programs, we are able to showcase the potential benefits of most AI initiatives; however, progress towards their implementation in actual clinical practice has been more limited (19).
The endless inventory of medical information consisting of diseases, diagnostic methods, biomarkers, and treatment modalities has increased in the past decade, thereby creating an additional need for even more complex decision-making using the vast amount of data available. Machine learning classifiers (MLCs) are one such algorithm that has been used in image-based diagnoses and has already exhibited great aptitude, yet the analysis of diverse and massive electronic health record (EHR) data remains challenging (20).
Some of the challenges faced include the extensive amount of data, high dimensionality, scattered data, and deviations or systematic errors in the medical data itself. These challenges make it difficult to perform accurate pattern recognition and generate predictive clinical models using machine learning techniques (20).
The quality and quantity of training data have a significant impact on how well the majority of AI algorithms perform. In addition to its expensive nature, the quality of the scanned data differs significantly. The variety in data quality might make it difficult to create a generalized network and poses a significant barrier to the commercialization of AI-based solutions. Other data variations include aberrant anatomical features, MRI machine suppliers, and hospital-specific policies (21).
When dealing with medical data, the privacy and security of the patients are the main priorities. These privacy considerations may potentially have a negative effect on the translation of AI models into clinical practice by creating trust and legal issues (21).
There is also the drawback of developing EHRs in resource-limited environments due to concerns with storage capacity as well as a lack of stable, advanced computerized systems to keep such records or data. Even now, many of these developing countries record information in paper formats, which over time may get lost or damaged beyond repair. Transitioning from paper-based records to electronic ones will require massive investments in resources, which most developing countries lack (22).
View classification represents another vital step towards building a fully automated echocardiogram interpretation system. Currently, there are additional challenges in creating a view classification model for pediatric echocardiograms compared to adult echocardiograms: multiple variations in anatomy, size, structure, and views (23).
For the development of outcome prediction in the pediatric cardiac ICU, mathematical models that evaluate continuously recorded physiologic parameters, as well as machine learning and AI technologies, are all promising. Since predictive systems are only as successful as the results they anticipate, it is crucial to continue establishing appropriate endpoints as this technology develops. Machine learning might be able to estimate the possibility of certain events, but it won't be able to surpass a physicians' clinical judgment in selecting the best course of treatment given the clinical scenario in order to prevent a clinical decompensation (24). Table 5 provides a concise overview of the advantages and disadvantages of different diagnostic methods, highlighting both traditional and AI-based approaches in diagnosing neonatal congenital heart diseases. Table 6 provides comprehensive discussion of published literature.
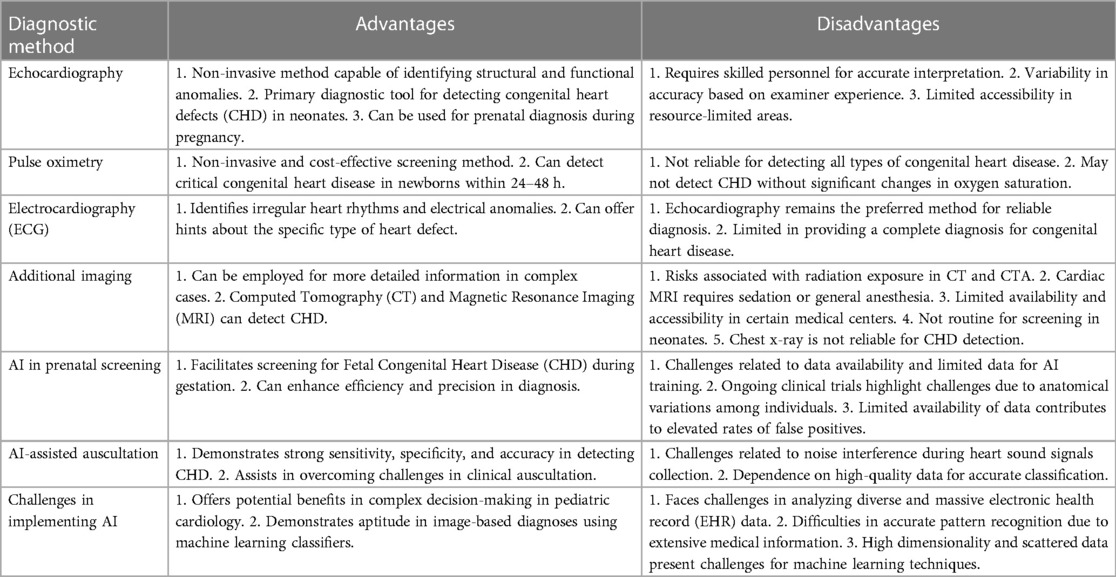
Table 5. Advantages and disadvantages of diagnostic methods in identifying neonatal congenital heart diseases.
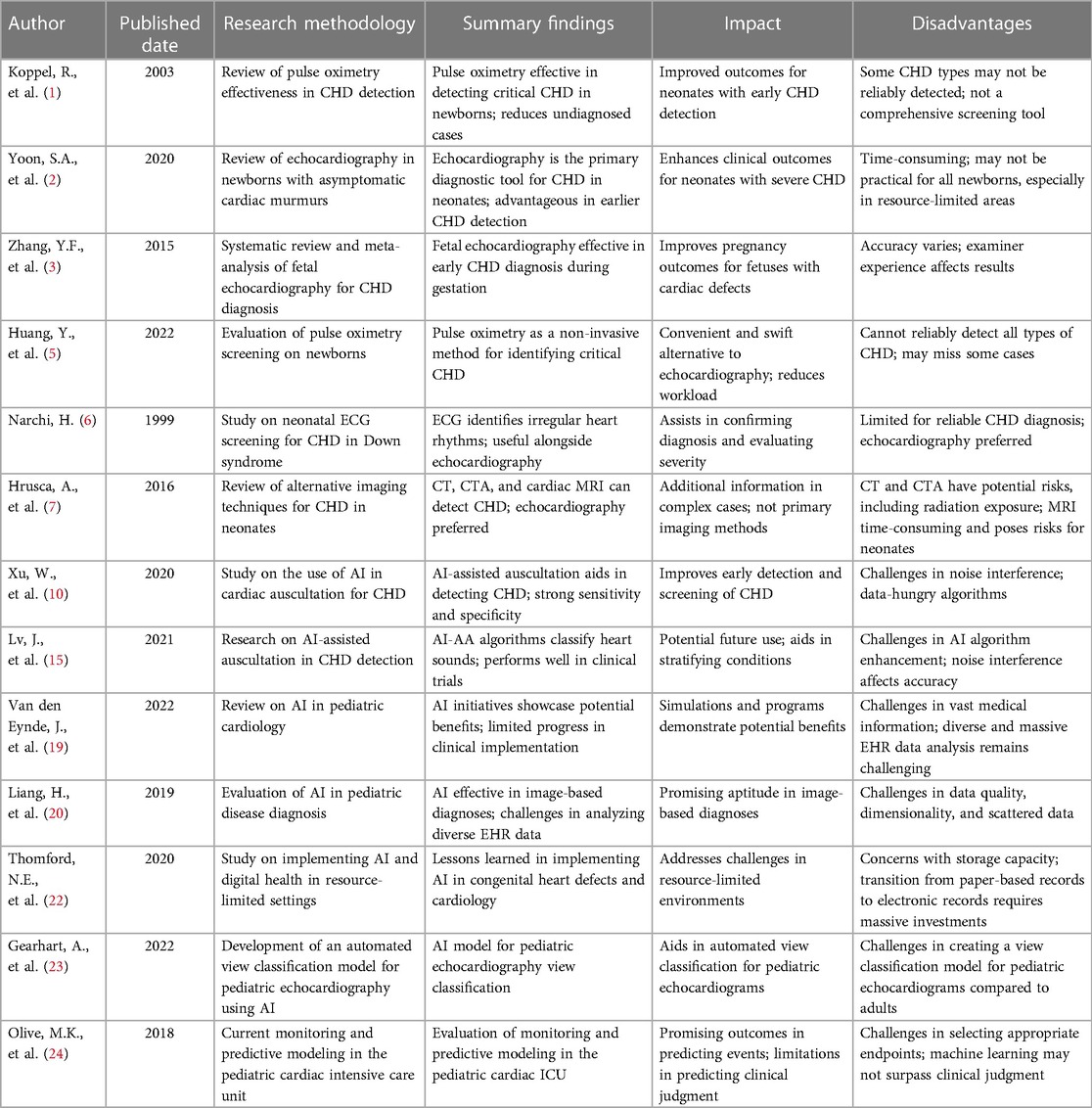
Table 6. Utility of traditional and AI based diagnostic methods in detecting congenital heart diseases in neonates.
The use of artificial intelligence in detecting congenital heart diseases has shown great promise in improving the accuracy and efficiency of diagnosis. By leveraging machine learning algorithms, AI systems can analyze vast amounts of medical data, including images and patient records, to identify potential abnormalities that may indicate a congenital heart condition. Several studies have demonstrated the efficacy of AI-based approaches for diagnosing congenital heart diseases, with results indicating that the systems can achieve high levels of sensitivity and specificity. In addition, AI can help reduce the workload of healthcare professionals allowing them to focus on other critical aspects of patient care. Despite the potential benefits of using AI, in addition to detecting congenital heart disease, there are still some challenges to overcome, such as the need for large amounts of high-quality data and the requirement for careful validation of the algorithms. Nevertheless, with ongoing research and development, AI is likely to become an increasingly valuable tool for improving the diagnosis and treatment of congenital heart diseases.
HE: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft. TT: Conceptualization, Methodology, Project administration, Writing – review & editing. AI: Writing – review & editing, Conceptualization, Formal Analysis, Investigation, Methodology, Project administration. AN: Investigation, Project administration, Writing – review & editing. JM: Writing – review & editing, Data curation, Supervision, Validation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Koppel RI, Druschel CM, Carter T, Goldberg BE, Mehta PN, Talwar R, et al. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics. (2003) 111:451–5. doi: 10.1542/peds.111.3.451
2. Yoon SA, Hong WH, Cho HJ. Congenital heart disease diagnosed with echocardiogram in newborns with asymptomatic cardiac murmurs: a systematic review. BMC Pediatr. (2020) 20(1):322. doi: 10.1186/s12887-020-02212-8
3. Zhang YF, Zeng XL, Zhao EF, Lu HW. Diagnostic value of fetal echocardiography for congenital heart disease: a systematic review and meta-analysis. Medicine. (2015) 94(42):e1759. doi: 10.1097/MD.0000000000001759
4. DeVore GR, Medearis AL, Bear MB, Horenstein J, Platt LD. Fetal echocardiography: factors that influence imaging of the fetal heart during the second trimester of pregnancy. J Ultrasound. (1993) 12:659–63. doi: 10.7863/jum.1993.12.11.659
5. Huang Y, Zhong S, Zhang X, Kong L, Wu W, Yue S, et al. Large scale application of pulse oximeter and auscultation in screening of neonatal congenital heart disease. BMC Pediatr. (2022) 22(1):483. doi: 10.1186/s12887-022-03540-7
6. Narchi H. Neonatal ECG screening for congenital heart disease in down syndrome. Ann Trop Paediatr. (1999) 19(1):51–4. doi: 10.1080/02724939992635
7. Hrusca A, Rachisan AL, Gach P, Pico H, Sorensen C, Bonello B, et al. Detection of pulmonary and coronary artery anomalies in tetralogy of fallot using non-ECG-gated CT angiography. Diagn Interv Imaging. (2016) 97(5):543–8. doi: 10.1016/j.diii.2016.03.010
8. Fonseca B, Chang RK, Senac M, Knight G, Sklansky MS. Chest radiography and the evaluation of the neonate for congenital heart disease. Pediatr Cardiol. (2005) 26:367–72. doi: 10.1007/s00246-005-8649-z
9. Mcleod G, Shum K, Gupta T, Chakravorty S, Kachur S, Bienvenu L, et al. Echocardiography in congenital heart disease. Prog Cardiovasc Dis. (2018) 61(5–6):468–75. doi: 10.1016/j.pcad.2018.11.004
10. Sakai A, Komatsu M, Komatsu R, Matsuoka R, Yasutomi S, Dozen A, et al. Medical professional enhancement using explainable artificial intelligence in fetal cardiac ultrasound screening. Biomedicines. (2022) 10(3):551. doi: 10.3390/biomedicines10030551
11. Han G, Jin T, Zhang L, Guo C, Gui H, Na R, et al. Adoption of compound echocardiography under artificial intelligence algorithm in fetal congenital heart disease screening during gestation. Appl Bionics Biomech. (2022) 2022:6410103. doi: 10.1155/2022/6410103
12. de Vries IR, van Laar JOEH, van der Hout-van der Jagt MB, Clur SB, Vullings R. Fetal electrocardiography and artificial intelligence for prenatal detection of congenital heart disease. Acta Obstet Gynecol Scand. (2023) 102(11):511–20. doi: 10.1111/aogs.14623
13. Chang Junior J, Binuesa F, Caneo LF, Turquetto ALR, Arita ECTC, Barbosa AC, et al. Improving preoperative risk-of-death prediction in surgery congenital heart defects using artificial intelligence model: a pilot study. PLoS One. (2020) 15(9):e0238199. doi: 10.1371/journal.pone.0238199
14. Xu W, Yu K, Xu J, Ye J, Li H, Shu Q. Artificial intelligence technology in cardiac auscultation screening for congenital heart disease: present and future. Zhejiang Da Xue Xue Bao Yi Xue Ban. (2020) 49(5):548–55. doi: 10.3785/j.issn.1008-9292.2020.10.01
15. Lv J, Dong B, Lei H, Shi G, Wang H, Zhu F, et al. Artificial intelligence-assisted auscultation in detecting congenital heart disease. Eur Heart J Digit Health. (2021) 2(1):119–24. doi: 10.1093/ehjdh/ztaa017
16. Thompson WR, Reinisch AJ, Unterberger MJ, Schriefl AJ. Artificial intelligence-assisted auscultation of heart murmurs: validation by virtual clinical trial. Pediatr Cardiol. (2019) 40:623–9. doi: 10.1007/s00246-018-2036-z
17. Deng MQ, Meng TT, Cao JW, Wang SM, Zhang J, Fan HJ. Heart sound classification based on improved MFCC features and convolutional recurrent neural networks. Neural Netw. (2020) 130:22–32. doi: 10.1016/j.neunet.2020.06.015
18. Ou Y. Can artificial intelligence-assisted auscultation become the heimdallr for diagnosing congenital heart disease? Eur Heart J Digit Health. (2021) 2(1):117–8. doi: 10.1093/ehjdh/ztab016
19. Van den Eynde J, Kutty S, Danford DA, Manlhiot C. Artificial intelligence in pediatric cardiology: taking baby steps in the big world of data. Curr Opin Cardiol. (2022) 37(1):130–6. doi: 10.1097/HCO.0000000000000927
20. Liang H, Tsui BY, Ni H, Valentim CCS, Baxter SL, Liu G, et al. Evaluation and accurate diagnoses of pediatric diseases using artificial intelligence. Nat Med. (2019) 25:433–8. doi: 10.1038/s41591-018-0335-9
21. Arafati A, Hu P, Finn JP, Rickers C, Cheng AL, Jafarkhani H, et al. Artificial intelligence in pediatric and adult congenital cardiac MRI: an unmet clinical need. Cardiovasc Diagn Ther. (2019) 9(Suppl 2):S310–25. doi: 10.21037/cdt.2019.06.09
22. Thomford NE, Bope CD, Agamah FE, Dzobo K, Ateko RO, Chimusa E, et al. Implementing artificial intelligence and digital health in resource-limited settings? Top 10 lessons we learned in congenital heart defects and cardiology. OMICS. (2020) 24(5):264–77. doi: 10.1089/omi.2019.0142
23. Gearhart A, Goto S, Deo RC, Powell AJ. An automated view classification model for pediatric echocardiography using artificial intelligence. J Am Soc Echocardiogr. (2022) 35(12):1238–46. doi: 10.1016/j.echo.2022.08.009
Keywords: artificial intelligence, screening tools, early diagnosis, neonates, congenital cardiac defects, current challenges
Citation: Ejaz H, Thyyib T, Ibrahim A, Nishat A and Malay J (2024) Role of artificial intelligence in early detection of congenital heart diseases in neonates. Front. Digit. Health 5:1345814. doi: 10.3389/fdgth.2023.1345814
Received: 28 November 2023; Accepted: 29 December 2023;
Published: 11 January 2024.
Edited by:
Giovanni Ferrara, University of Alberta, CanadaReviewed by:
Norawit Kijpaisalratana, Massachusetts General Hospital and Harvard Medical School, United States© 2024 Ejaz, Thyyib, Ibrahim, Nishat and Malay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jhancy Malay amhhbmN5QHJha21oc3UuYWMuYWU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.