
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Digit. Health , 20 January 2023
Sec. Connected Health
Volume 5 - 2023 | https://doi.org/10.3389/fdgth.2023.1064115
This article is part of the Research Topic Digital Therapeutics: Using Software to Treat, Manage, and Prevent Disease View all 12 articles
The greying of the world is leading to a rapid acceleration in both the healthcare costs and caregiver burden that are associated with dementia. There is an urgent need to develop new, easily scalable modalities of support. This perspective paper presents the theoretical background, rationale, and development plans for a music-based digital therapeutic to manage the neuropsychiatric symptoms of dementia, particularly agitation and anxiety. We begin by presenting the findings of a survey we conducted with key opinion leaders. The findings highlight the value of a music-based digital therapeutic for treating neuropsychiatric symptoms, particularly agitation and anxiety. We then consider the neural substrates of these neuropsychiatric symptoms before going on to evaluate randomized control trials on the efficacy of music-based interventions in their treatment. Finally, we present our development plans for the adaptation of an existing music-based digital therapeutic that was previously shown to be efficacious in the treatment of adult anxiety symptoms.
According to the World Health Organization (2022), there are 55 million people living with dementia worldwide with 10 million new cases annually. The same report estimates the global cost of dementia at 1.3 trillion USD (1). These costs are expected to surpass 2.8 trillion USD by 2030 as the number of people living with dementia rises. Approximately half of the global cost of dementia is attributable to the informal care provided by family members and friends who commonly shoulder tremendous physical, emotional and financial pressures (2). A sizeable minority of people living with dementia in industrialized societies will eventually be placed in long-term care (nursing or assisted care) homes. The proportion of total costs incurred in these homes that can be attributed to dementia has been estimated at 64% (3). Even before the COVID-19 pandemic, the professionals in these homes were chronically overloaded leading them to experience high levels of caregiver burden, and in some cases, moral injury, which has been defined as the perpetration, failure to prevent, or observation of morally-transgressive acts (4).
From the perspective of people living with dementia, their caregivers and the broader healthcare system, there is an urgent need to develop new, easily scalable modalities of support. Digital therapeutics (DTx) represent one such modality being considered. The focus of development in DTx for dementia has been cognitive stimulation (5), typically in the form of reminiscence therapy (6) or brainwave entrainment (7). The objective of such therapeutics is to directly slow the rate of cognitive decline (8). While we believe that interventions that directly target cognitive outcomes are clinically important, we also believe there is an urgent need to target non-cognitive outcomes. These outcomes have been less well studied in the context of DTx but they have the potential to contribute to patient and caregiver wellbeing, while lowering the costs of care (9).
Our team is currently undertaking the development of a music-based DTx that builds on core AI that we originally developed to mitigate anxiety (https://www.lucidtherapeutics.com). While the anecdotal evidence for the power of music in dementia abounds, the evidence base is still in its early days and tends to be focused on cognitive outcomes. To better understand the potential impact of a music-based DTx on cognitive and non-cognitive outcomes we started our development path by surveying key opinion leaders.
In early 2022, our team undertook a qualitative study with key opinion leaders to gauge the potential value of developing a music-based DTx for dementia. In addition to defining the value proposition, we were interested in specific outcomes that were judged to be feasible, inclusive of cognitive and non-cognitive outcomes. Participants included 7 payers and 12 health-care practitioners specializing in geriatric and dementia care. Payers included medical directors (n = 5), pharmacy directors (n = 1), and an innovation officer (n = 1) associated with health plans that are based in the United States. All payers had experience with the evaluation of DTx for coverage and reimbursement. Health-care practitioners (HCPs) included neurologists (n = 5), geriatricians (n = 4), and psychiatrists (n = 3), all of whom had significant experience in treating Alzheimer's disease (AD) and other forms of dementia (50 patients or more in the last 3 months). Most of the HCPs surveyed had experience with use of DTx in treatment (75%), and about half had some experience in recommending music therapy for patients (58.3%). Both payers and HCPs expressed the view that there was a strong clinical case for a therapy/intervention that would target non-cognitive aspects of dementia. In particular, they identified the neuropsychiatric symptoms (10) as being a non-cognitive target outcome that might be well addressed by a music-based DTx. Neuropsychiatric symptoms are extremely common in dementia, affecting as much as 97% of patients (11) and have been associated with reduced quality of life (12), as well as the progression of cognitive decline (13, 14).
In descending order of frequency, neuropsychiatric symptoms of dementia include apathy, depression, agitation, psychosis, and sleep disturbances (15). Agitation is especially frequent (80%) in residents of long-term care homes and in those who are in moderate to severe stages of the disease (16) but can also affect many individuals (60%) with mild dementia or mild cognitive impairment (MCI) (17). According to the key opinion leaders we surveyed, agitation is the most challenging symptom with respect to patient management. This perspective is consistent with prior surveys conducted with caregivers. One study of American caregivers found agitation to be more distressing than apathy or depression (18). The same conclusion was reached in a study of caregivers conducted in Japan (19). The Japanese study also found that agitation was more likely to contribute to caregiver burnout than other neuropsychiatric symptoms.
In addition to the challenge that agitation presents for caregivers it has also been associated with the progression of cognitive decline in patients (13, 14). From a biopsychosocial model of cognitive aging (20), this association may be attributable to neurotoxic factors that manifest due to stress arising from frequent bouts of agitation (see (21). In the case of patients living in long-term care homes, the association may also be due to side effects of the antipsychotic medications that are commonly prescribed to treat agitation. A meta-regression involving data from ten studies found a strong linear correlation between antipsychotic treatment duration and change in cognition, with greater declines under antipsychotic treatment compared to placebo (22).
Risperidone, a commonly prescribed second-generation antipsychotic with the strongest evidence base for treating agitation and anxiety appears to have no adverse effects on cognition when prescribed as indicated for short-term use (23–25). However, side effects of risperidone include an elevated risk of ischemic stroke and transient ischemic attacks (26), which elevate mortality risk. Studies of other commonly prescribed second-generation antipsychotics, such as olanzapine, have shown some level of risk for cognition, especially in the case of participants with lower cognitive functioning at baseline (27). Haloperidol, a first-generation antipsychotic that continues to be prescribed for agitation is less efficacious than risperidone (28), has potent sedative effects, and was determined to be the riskiest of all pharmacological interventions with respect to mortality (29). In summary, while chronic agitation may hasten the progression of cognitive decline, the existing pharmacological approaches have limited efficacy and can carry significant risks to physical health (30) and cognitive health (22). The healthcare practitioners we surveyed were particularly interested in the development of DTx that would serve as complementary or low-risk alternatives to pharmacological treatment in the management of agitation and anxiety.
Some researchers have characterized agitation as the external manifestation of anxiety (31, 32). Up to 80% of people living with mild-to-moderate dementia experience anxiety (15). Anxiety may even be a risk factor for developing dementia; the risk of conversion to dementia nearly doubles when anxiety symptoms are present in people living with mild cognitive impairment (33). While agitation is more of an external behavior that is readily observable, anxiety is an internal state that can be hidden from plain view. It has been conceptualized as consisting of cognitive (i.e., worry about future threats) and somatic (i.e., bodily tension) components (34). Anxiety tends to be more common in the early stages of the “dementia journey”, while agitation is more common in later stages (35). Although the anxiety does not appear to be causally related to later agitation as has often been proposed (32), there is a clear association between the two constructs across stages of disease (36).
The ‘Uncertainty and Anticipation Model of Anxiety’ (UAMA) posits that anxiety is a set of expected emotional, cognitive, and behavioural responses to the uncertainty of potential future threats, often coupled with fear (37, 38). The UAMA model proposes that activity in the frontal cortex (dorsomedial prefrontal and orbitofrontal) is responsible for generating probabilistic estimates of future events and expected costs (37). The model also proposes that the amygdala plays a central role in the transmission and interpretation of anxiety and fear. In addition to afferents from the frontal cortex, the amygdala is known to receive afferents from the thalamus, periaqueductal gray, and entorhinal cortex (38–40). People living with a diagnosis of dementia tend to experience a great deal of uncertainty because of the unknown of how their illness will progress and not knowing what threats may await them (41). Neural degradation in frontal areas supporting working memory may further predispose individuals living with dementia to experience anxiety.
In the case of AD, the most common form of dementia, anxiety is associated with damage to subcortical regions which includes atrophy in the amygdala (38, 42) and the entorhinal cortex (43). Cases of more severe anxiety are associated with hyperfusion of the anterior cingulate cortex, decreased grey matter volume in the right precuneus, inferior parietal, left parahippocampal, posterior cingulate gyrus, left insula, and bilateral putamen lobes (37, 38) and hypometabolism in the bilateral entorhinal, anterior hippocampus, left superior temporal and insula regions (38, 44). Positron emission tomography (PET) studies indicate that individuals living with AD and comorbid anxiety possess higher amyloid deposits than those without in the precuneus-posterior cingulate, frontal, parietal, and anterior cingulate cortex (45).
As shown in Figure 1, individuals living with AD that present with agitation show severe dysfunction in many of the same brain regions that are implicated in anxiety, including the amygdala, hippocampus, anterior cingulate, posterior cingulate, and insula (46). This pattern of dysfunction agrees with the pattern of disease progression wherein the propensity for anxiety is higher in earlier stages while the propensity for agitation is higher in later stages once more severe brain dysfunction, particularly dysfunction in frontal areas, has occurred (35). Although the type and onset of neural degradation that occurs in the frontal lobes will vary by type of dementia (47), at later stages of the disease, these degradations may uniformly result in the failure to downregulate autonomic arousal in response to uncertainty (37). Taken together, the available evidence suggests that anxiety and agitation are independent but related constructs whose propensity will be influenced as a function of neuropsychiatric disease progression. It stands to reason that the two types of neuropsychiatric symptoms may benefit from similar types of intervention. In recent years, music has emerged as a particularly important intervention for neuropsychiatric symptoms, especially with respect to anxiety and agitation. When used in this context, music may be regarded as a fundamental technology that can be personalized and systematically leveraged to downregulate autonomic arousal arising from uncertainty.
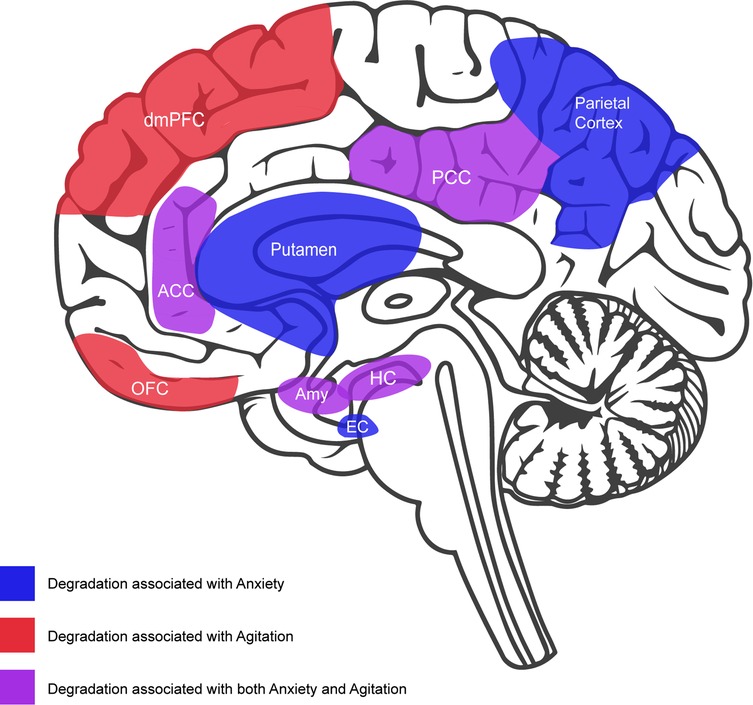
Figure 1. Midsaggital view of brain featuring neural degradation that has been associated with anxiety and agitation in dementia: anxiety (blue) = putamen, parietal cortex, and entorhinal cortex (EC); agitation (red) = orbitorfrontal cortex (OFC); dorsolateral prefrontal cortex (dmPFC); anxiety and agitation (purple) = anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), amygdala (Amy), hippocampus (HC), and insula (not depicted in this view).
Qualitative research that has examined the impact of music-based interventions on people living with dementia has revealed significant improvements in quality of life in patients and caregivers. Notable benefits in patients include increased social engagement and reductions in anxiety and agitation (48–54). To further understand these benefits and for whom they may accrue, we conducted a literature search focusing on randomized controlled trials (RCTs) that investigated the use of music-based treatments on anxiety or agitation symptoms in participants with MCI and/or dementia. Of the 15 RCTs found, three RCTs were excluded due to low fidelity (55) and no personalization of music/music therapy (56, 57). Of the remaining 13 RCTs, 12 of them reported a significant reduction in anxiety and/or agitation in the music/music therapy treatment arm (Table 1). The most parsimonious interpretation regarding the mechanism of action is a downregulation of autonomic arousal, owing to a shift in balance from sympathetic to parasympathetic activity over the course of music listening (70). Overall, the effect sizes (Cohen's D) trended larger in studies that recruited participants with mild to moderate dementia. It is also notable that the one study that failed to find a significant reduction in anxiety and agitation was limited to participants with severe dementia (64). Thus, based on the available evidence we may conclude that agitation and anxiety symptoms are well-indicated for music-based treatment, especially in participants with mild to moderate dementia.
Accumulating evidence demonstrates that people living with dementia enjoy music and may benefit from making music, interacting with music through movement, and passive listening to music (71, 72). It has been shown that people living with AD can retain memory for melodies and lyrics (73), and that when activated, these memories facilitate the retrieval of autobiographical memories (74). The mechanisms underlying these music and memory phenomena are not completely understood but appear to depend on the encoding of musical memories in structures and networks that are resilient to neural degeneration (75). It seems likely that these music and memory phenomena depend in part on dopaminergic activity in the ventral striatum triggered by auditory-reward network connectivity, which is well preserved in MCI but less so in AD (76).
Music and memory phenomena have sparked widespread interest in popular culture, particularly following the release of the 2014 documentary film Alive Inside. The film tells the story of MUSIC & MEMORY, a non-profit that facilitates the use of music players for people living with dementia in long-term care homes. In an often-cited highlight of the film, a long-term care home resident named Henry is jolted out of his catatonic state into a charming, articulate, and engaged lover of music. The film also chronicles the struggle that founder Dan Cohen had in convincing healthcare professionals and administrators about the value of music in care for people living with dementia. MUSIC & MEMORY program has since expanded and has been imitated with variations all over the world and validated in a number of large-scale trials (77, 78).
In community and long-term care homes, therapeutic music can be observed in various modalities from music listening to support mood and reminiscence (79), singing to promote health and social wellbeing (80), and music-making coordinated by a licensed music therapist (81). Regardless of the modality, a scoping review of the literature suggests that personalized music is systematically more effective than non-personalized music (82, 83). Personalization is likely important because familiar and preferred music leads to greater activation of dopaminergic and opioid pathways in the ventral striatum than nonfamiliar music (84, 85), and in the case of AD this reward activation is also associated with greater functional connectivity in corticocortical and corticocerebellar networks (86). Personalization is further supported by the fact that people with AD have dysfunctional dopaminergic (87) and opioid transmission (88) and stimulating production of endogenous opioids through music (85) or other means may have a beneficial impact on anxiety and agitation over the near-term and potentially slowing the progression of disease over the long-term (88).
Because personalization is so important to the effectiveness of music it stands to reason that a limiting factor in scalability of any effective music program will be the time and effort required to personalize music for a given individual. This may be especially challenging when the caregiver has limited experience with the person living with dementia and/or the individual has limited communication abilities. A licensed music therapist would be able to cultivate some level of personalization through careful interaction and observation with an individual. However, there are barriers to accessing music therapists, which limits the benefits that may be obtained from music engagement. A music-based DTx will help bridge the gap by offering the level of personalization required for optimal outcomes in the absence of a licensed music therapist.
The music based DTx is not intended to diminish or replace the benefit that trained music therapists may have in both group and individual music therapy sessions. It cannot augment or alter the benefit patients receive from the therapeutic “relationship” that results from co-engagement of both passive and active music interventions, whether those occur in the presence of music therapists or caregivers without this training. It may, however, increase the opportunities for caregivers of all types to advance the therapeutic relationship by coming to learn about their patients in new ways. Lastly, digital interventions that capture continuous physiological data reflecting patient experience with music (e.g., heart-rate variability, pupillometry) may empower caregivers with a level of insight about response to music that would not otherwise be possible, which may inform all manner of music-based interventions including traditional music therapy as well as multi-modal interventions involving music such as augmented reality applications.
To this end, the user interface of the DTx will be designed in a manner that is conducive to operation by either a caregiver in the community (e.g., family member) or a professional in a long-term care home (e.g., music or recreation therapist). There will be no expectations imposed regarding caregivers' level of experience with music, nor will there be any requirement of familiarity with the music preferences of the person they are caring for.
Our team has recently published an RCT study on the efficacy of a music-based DTx developed by LUCID (https://www.lucidtherapeutics.com) for the treatment of anxiety (89). The system incorporates an AI called Affective Music Recommendation System (AMRS) (90); based on the iso-principle from music therapy (90, 91), which is a form of personalization that is independent of experience or preference. This approach to mood regulation suggests that the mood regulating properties of music may be enhanced if the mood of the music approximates an individual's initial emotional state before it is changed to the target state (92). The iso principle has been indicated in prior research to be more effective than other musical sequences at reducing tension (93). To develop AMRS, it was necessary to begin by curating training data that labeled music with respect to the arousal and valence dimensions of the Russell's Circumplex model of emotion (94). In this model, arousal refers to the activation aspect of felt emotion, ranging from calm to excited, and valence refers to the hedonic aspect of felt emotion, ranging from pleasant to unpleasant.
In LUCID's existing DTx (VIBE), the participant is asked to input their current mood using a 2-dimensional grid representing arousal and valence dimensions. Based on this input and the user's target emotional state (e.g., calm), the machine learning algorithm within the application predicts the optimal sequence of tracks to produce mood induction in the listener from their current emotional state to the target state. This machine learning algorithm uses reinforcement learning techniques and is trained on real-world data correlating the quantitative features of musical excerpts and sequences alongside the emotional responses induced by them in listeners.
Our existing DTx (VIBE) was designed to reduce anxiety. This objective was realized through the interaction of two AI systems: BioMIR (Biological Music Information Retrieval) and AMRS (as described above). The BioMIR system extracts insights about the emotional states that are likely to be evoked by pieces of music in people living with dementia. The AMRS considers the information from the BioMIR system to generate playlists that are personalized to each user. People living with dementia often have a diminished ability to attribute mental states to music, inclusive of emotion (95). For example, a musical excerpt that may calm a healthy person down may have a different effect on a person living with dementia. Therefore, to aid in the development of a new AI for affective music recommendation in this population (AMRS-D), we had to start by obtaining training data from older participants experiencing cognitive decline. To that end, we recently completed a training database study in collaboration with the Centre for Elder Research at Sheridan College with 32 participants living with MCI or early-stage dementia.
Participants were asked to listen to and make self-report judgments of valence, arousal, and absorption. Absorption was defined as the extent to which attentional resources were allocated to the music while listening (96, 97). It is our expectation that higher levels of musical absorption will lead to increased potency of a music-based intervention for mood regulation. While this hypothesis has not yet been validated with respect to state absorption in people living with dementia, it has been validated for trait absorption in a young adult population (see (98, 99)). These subjective judgments were collected alongside a variety of biometric measures which will allow us to use industry-standard machine learning methods to develop a fully closed-loop music recommendation system that can be driven by physiological data alone independent of user input (100). Going forward, the effectiveness of the DTx may be further enhanced by embedding beat stimulation in the theta range (90) with the expectation of increasing the extent of reduction in anxiety and agitation (89).
After the development of AMRS-D and the absorption module, we will begin an exploratory trial to assess the useability of the new system, including early indications of safety and efficacy. The exploratory trial will recruit participants living with dementia in the community by way of their caregivers. The DTx will be used by caregivers on a scheduled daily basis rather than in response to anxiety or agitation in the patient. The exploratory trial will provide an opportunity to solicit qualitative feedback that will lead to product refinement and pave the way for a future clinical proof-of-concept study. In this future proof-of-concept study, adherence and clinically relevant measures of efficacy will be tracked including the Neuropsychiatric Inventory Questionnaire (NPI-Q (101);, Behavioral Pathology in Alzheimer's Disease Rating Scale (BEHAVE-AD) (102), State-Trait Inventory of Cognitive and Somatic Anxiety (STICSA) (103) and the Cohen-Mansfield Agitation Inventory (104).
In this paper we have outlined the rationale for a new music-based DTx that LUCID is developing to support the neuropsychiatric symptoms of dementia. This development represents an expansion of our prior work in adult anxiety (89) to help support a related indication in the context of persons living with dementia (i.e., agitation). We have argued that a music based DTx will be effective in mitigating both anxiety and agitation in this population. We envision our music-based intervention as having efficacy at all stages of disease but with the focus of benefits on anxiety in the early stages, and in agitation in the later stages. The DTx will be proactive rather than reactive. Scheduled daily use of the system is projected to lead to improvements in patient and caregiver outcomes and reduced costs of care. The DTx will respect individual preference for music through a personalization module that will eventually be implementable in the absence of caregiver input. The available evidence suggests that patients will find intrinsic benefits in listening to familiar music on its own, independent of the anticipated mood-regulating properties emphasized through our survey of key opinion leaders. While the primary outcome of our research on the efficacy of the DTx in our future proof of concept study will be mitigation of anxiety and agitation, the personalization module is expected to lead to dopaminergic and opioid activity in the reward system via auditory-reward network connectivity (76, 85). We envision that this music based DTx will be used and implemented by healthcare professionals or family caregivers. Special attention will be devoted to overcoming tensions that may arise due to onboarding or protocol adherence. This approach to development is expected to yield direct benefits for patients, while reducing caregiver burden and the escalating costs associated with the greying of the world.
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
FAR and AM conceptualized the manuscript and wrote the original draft. ZT and AD were responsible for the administration and formal analysis of the survey with key opinion leaders. KD, ZT, AD, and DC all made contributions to the conceptualization of the manuscript. All authors contributed to the article and approved the submitted version.
We thank KH for her contributions towards creating the Figure 1 of the manuscript. We also would like to thank Rhiannon Ueberholz for her help in proofreading the manuscript.
FR has served as an advisor for LUCID since 2018 and as Chief Science Officer since 2021. He has been granted stock options, which may qualify him to financially benefit from commercial applications of the technology considered here. AM, ZT and AR are full-time employees of LUCID and have also been granted stock options, which may qualify them to financially benefit from commercial applications of the technology considered here. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Organization WH. Dementia (2022) [updated 2022–09–20; cited 2022 2022–09–24]. Available at: https://www.who.int/news-room/fact-sheets/detail/dementia
2. Reinhard SC, Given B, Petlick NH, Bemis A. Supporting family caregivers in providing care. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. (2008.
3. Pedroza P, Miller-Petrie MK, Chen C, Chakrabarti S, Chapin A, Hay S, et al. Global and regional spending on dementia care from 2000 to 2019 and expected future health spending scenarios from 2020 to 2050: an economic modelling exercise. eClinMed. (2022) 45:101337. doi: 10.1016/j.eclinm.2022.101337
4. Litz BT, Kerig PK. Introduction to the special issue on moral injury: conceptual challenges, methodological issues, and clinical applications. J Trauma Stress. (2019) 32(3):341–9. doi: 10.1002/jts.22405
5. Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. (2012) 2:1–53. doi: 10.1002/14651858.CD005562.pub2
6. Lazar A, Thompson H, Demiris G. A systematic review of the use of technology for reminiscence therapy. Health Educ Behav. (2014) 41(1_suppl):51S–61S. doi: 10.1177/1090198114537067
7. Tichko P, Kim JC, Large E, Loui P. Integrating music-based interventions with gamma-frequency stimulation: implications for healthy ageing. Eur J Neurosci. (2022) 55(11–12):3303–23. doi: 10.1111/ejn.15059
8. Sedghizadeh MJ, Hasani H, Lahijanian M, Aghajan H, Vahabi Z. Entrainment of gamma oscillations by auditory chirp stimulation in Alzheimer's Disease patients. Alzheimer's & Dementia. (2020) 16(S5):e043198. doi: 10.1002/alz.043198
9. Abbadessa G, Brigo F, Clerico M, De Mercanti S, Trojsi F, Tedeschi G, et al. Digital therapeutics in neurology. J Neurol. (2022) 269(3):1209–24. doi: 10.1007/s00415-021-10608-4
10. Koumakis L, Chatzaki C, Kazantzaki E, Maniadi E, Tsiknakis M. Dementia care frameworks and assistive technologies for their implementation: a review. IEEE Rev Biomed Eng. (2019) 12:4–18. doi: 10.1109/RBME.2019.2892614
11. Lanctôt KL, Amatniek J, Ancoli-Israel S, Arnold SE, Ballard C, Cohen-Mansfield J, et al. Neuropsychiatric signs and symptoms of Alzheimer's Disease: new treatment paradigms. Alzheimer's & Dementia: Transl Res & Clin Interv. (2017) 3(3):440–9. doi: 10.1016/j.trci.2017.07.001
12. González-Salvador T, Lyketsos CG, Baker A, Hovanec L, Roques C, Brandt J, et al. Quality of life in dementia patients in long-term care. Int J Geriatr Psychiatry. (2000) 15(2):181–9. doi: 10.1002/(SICI)1099-1166(200002)15:2<181::AID-GPS96>3.0.CO;2-I
13. Peters ME, Schwartz S, Han D, Rabins PV, Steinberg M, Tschanz JT, et al. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer's Dementia and death: the cache county dementia progression study. Am J Psychiatry. (2015) 172(5):460–5. doi: 10.1176/appi.ajp.2014.14040480
14. Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in mci with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. (2013) 21(7):685–95. doi: 10.1016/j.jagp.2013.01.006
15. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairmentresults from the cardiovascular health study. JAMA. (2002) 288(12):1475–83. doi: 10.1001/jama.288.12.1475
16. Carrarini C, Russo M, Dono F, Barbone F, Rispoli MG, Ferri L, et al. Agitation and dementia: prevention and treatment strategies in acute and chronic conditions. Front Neurol. (2021) 12:1–18. doi: 10.3389/fneur.2021.644317
17. Van der Mussele S, Le Bastard N, Saerens J, Somers N, Mariën P, Goeman J, et al. Agitation-Associated behavioral symptoms in mild cognitive impairment and Alzheimer's Dementia. Aging Ment Health. (2015) 19(3):247–57. doi: 10.1080/13607863.2014.924900
18. Fauth EB, Gibbons A. Which behavioral and psychological symptoms of dementia are the most problematic? Variability by prevalence, intensity, distress ratings, and associations with caregiver depressive symptoms. Int J Geriatr Psychiatry. (2014) 29(3):263–71. doi: 10.1002/gps.4002
19. Hiyoshi-Taniguchi K, Becker CB, Kinoshita A. What behavioral and psychological symptoms of dementia affect caregiver burnout? Clin Gerontol. (2018) 41(3):249–54. doi: 10.1080/07317115.2017.1398797
20. Spector A, Orrell M. Using a biopsychosocial model of dementia as a tool to guide clinical practice. Int Psychogeriatr. (2010) 22(6):957–65. doi: 10.1017/S1041610210000840
21. Lupien SJ, Juster R-P, Raymond C, Marin M-F. The effects of chronic stress on the human brain: from neurotoxicity, to vulnerability, to opportunity. Front Neuroendocrinol. (2018) 49:91–105. doi: 10.1016/j.yfrne.2018.02.001
22. Wolf A, Leucht S, Pajonk F-G. Do antipsychotics lead to cognitive impairment in dementia? A meta-analysis of randomised placebo-controlled trials. Eur Arch Psychiatry Clin Neurosci. (2017) 267(3):187–98. doi: 10.1007/s00406-016-0723-4
23. Corbett A, Burns A, Ballard C. Don’t use antipsychotics routinely to treat agitation and aggression in people with dementia. BMJ: British Medical Journal. (2014) 349:g6420. doi: 10.1136/bmj.g6420
24. Rainer MK, Masching AJ, Ertl MG, Kraxberger E, Haushofer M. Effect of risperidone on behavioral and psychological symptoms and cognitive function in dementia. J Clin Psychiatry. (2001) 62(11):894–900. doi: 10.4088/JCP.v62n1110
25. Yunusa I, El Helou ML. The use of risperidone in behavioral and psychological symptoms of dementia: a review of pharmacology, clinical evidence, regulatory approvals, and off-label use. Front Pharmacol. (2020) 11:1–7. doi: 10.3389/fphar.2020.00596
26. Shin J-Y, Choi N-K, Jung S-Y, Lee J, Kwon JS, Park B-J. Risk of ischemic stroke with the use of risperidone, quetiapine and olanzapine in elderly patients: a population-based, case-crossover study. J Psychopharmacol. (2013) 27(7):638–44. doi: 10.1177/0269881113482530
27. Deberdt WG, Siegal A, Ahl J, Meyers AL, Landbloom R. Effect of olanzapine on cognition during treatment of behavioral and psychiatric symptoms in patients with dementia: a post-hoc analysis. Int J Geriatr Psychiatry. (2008) 23(4):364–9. doi: 10.1002/gps.1885
28. Suh G-H, Greenspan AJ, Choi S-K. Comparative efficacy of risperidone versus haloperidol on behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry. (2006) 21(7):654–60. doi: 10.1002/gps.1542
29. Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. (2015) 72(5):438–45. doi: 10.1001/jamapsychiatry.2014.3018
30. Ringman JM, Schneider L. Treatment options for agitation in dementia. Curr Treat Options Neurol. (2019) 21(7):30. doi: 10.1007/s11940-019-0572-3
31. Mintzer JE, Brawman-Mintzer O. Agitation as a possible expression of generalized anxiety disorder in demented elderly patients: toward a treatment approach. J Clin Psychiatry. (1996) 57(Suppl 7):55–63.; discussion 73-5.8690698
32. Twelftree H, Qazi A. Relationship between anxiety and agitation in dementia. Aging Ment Health. (2006) 10(4):362–7. doi: 10.1080/13607860600638511
33. Palmer K, Berger AK, Monastero R, Winblad B, Bäckman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. (2007) 68(19):1596–602. doi: 10.1212/01.wnl.0000260968.92345.3f
34. Ree MJ, French D, MacLeod C, Locke V. Distinguishing cognitive and somatic dimensions of state and trait anxiety: development and validation of the state-trait inventory for cognitive and somatic anxiety (sticsa). Behav Cogn Psychother. (2008) 36(3):313–32. doi: 10.1017/S1352465808004232
35. Bierman EJM, Comijs HC, Jonker C, Beekman ATF. Symptoms of anxiety and depression in the course of cognitive decline. Dement Geriatr Cogn Disord. (2007) 24(3):213–9. doi: 10.1159/000107083
36. Liu KY, Costello H, Reeves S, Howard R. The relationship between anxiety and incident agitation in Alzheimer's Disease. J Alzheimer's Dis. (2020) 78:1119–27. doi: 10.3233/JAD-200516
37. Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. (2013) 14(7):488–501. doi: 10.1038/nrn3524
38. Chen Y, Dang M, Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer's Disease: a systematic review of symptom-general and –specific lesion patterns. Mol Neurodegener. (2021) 16(1):38. doi: 10.1186/s13024-021-00456-1
39. Tagai K, Nagata T, Shinagawa S, Nemoto K, Inamura K, Tsuno N, et al. Correlation between both morphologic and functional changes and anxiety in Alzheimer's Disease. Dement Geriatr Cogn Disord. (2014) 38(3-4):153–60. doi: 10.1159/000358822
40. Nour M, Jiao Y, Teng G-J. Neuroanatomical associations of depression, anxiety and apathy neuropsychiatric symptoms in patients with Alzheimer's Disease. Acta Neurol Belg. (2021) 121(6):1469–80. doi: 10.1007/s13760-020-01349-8
41. Samsi K, Manthorpe J. Care pathways for dementia: current perspectives. Clin Interv Aging. (2014) 9:2055–63. doi: 10.2147/cia.S70628
42. Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC. Amygdala atrophy is prominent in early Alzheimer's Disease and relates to symptom severity. Psychiatry Res: Neuroimaging. (2011) 194(1):7–13. doi: 10.1016/j.pscychresns.2011.06.014
43. Mah L, Binns MA, Steffens DC. Anxiety symptoms in amnestic mild cognitive impairment are associated with medial temporal atrophy and predict conversion to Alzheimer disease. Am J Geriatr Psychiatry. (2015) 23(5):466–76. doi: 10.1016/j.jagp.2014.10.005
44. Hashimoto H, Monseratt L, Nguyen P, Feil D, Harwood D, Mandelkern MA, et al. Anxiety and regional cortical glucose metabolism in patients with Alzheimer's Disease. J Neuropsychiatry Clin Neurosci. (2006) 18(4):521–8. doi: 10.1176/jnp.2006.18.4.521
45. Bensamoun D, Guignard R, Furst AJ, Derreumaux A, Manera V, Darcourt J, et al. Associations between neuropsychiatric symptoms and cerebral amyloid deposition in cognitively impaired elderly people. J Alzheimer's Dis. (2016) 49:387–98. doi: 10.3233/JAD-150181
46. Rosenberg PB, Nowrangi MA, Lyketsos CG. Neuropsychiatric symptoms in Alzheimer's Disease: what might be associated brain circuits? Mol Asp Med. (2015) 43-44:25–37. doi: 10.1016/j.mam.2015.05.005
47. Murtha S, Cismaru R, Waechter R, Chertkow H. Increased variability accompanies frontal lobe damage in dementia. J Int Neuropsychol Soc. (2002) 8(3):360–72. doi: 10.1017/S1355617702813170
48. Lipe AW. Using music therapy to enhance the quality of life in a client with Alzheimer's Dementia: a case study. Music Ther Perspect. (1991) 9(1):102–5. doi: 10.1093/mtp/9.1.102
49. Olderog Millard KA, Smith JM. The influence of group singing therapy on the behavior of Alzheimer's Disease patients. J Music Ther. (1989) 26(2):58–70. doi: 10.1093/jmt/26.2.58
50. Shively C, Henkin L. Music and movement therapy with Alzheimer's Victims. Music Ther Perspect. (1986) 3(1):56–8. doi: 10.1093/mtp/3.1.56
51. Murphy K, Liu WW, Goltz D, Fixsen E, Kirchner S, Hu J, et al. Implementation of personalized music listening for assisted living residents with dementia. Geriatr Nurs (Minneap). (2018) 39(5):560–5. doi: 10.1016/j.gerinurse.2018.04.001
52. Sorrell JM. Music as a healing art in dementia care. J Psychosoc Nurs Ment Health Serv. (2018) 56(7):15–8. doi: 10.3928/02793695-20180619-04
53. Yao C-T, Lee B-O, Hong H, Su Y-C. Evaluation of the music therapy program interventions on agitated behavior for people with dementia in Taiwan institutional care. Educ Gerontol. (2022):1–12. doi: 10.1080/03601277.2022.2099076
54. Dahms R, Eicher C, Haesner M, Mueller-Werdan U. Influence of music therapy and music-based interventions on dementia: a pilot study. J Music Ther. (2021) 58(3):e12–36. doi: 10.1093/jmt/thab005
55. Kwak J, Anderson K, O’Connell Valuch K. Findings from a prospective randomized controlled trial of an individualized music listening program for persons with dementia. J Appl Gerontol. (2020) 39(6):567–75. doi: 10.1177/0733464818778991
56. Ceccato E, Vigato G, Bonetto C, Bevilacqua A, Pizziolo P, Crociani S, et al. Stam protocol in dementia:a multicenter, single-blind, randomized, and controlled trial. Am J Alzheimer's Dis & Other Demen®. (2012) 27(5):301–10. doi: 10.1177/1533317512452038
57. Cooke ML, Moyle W, Shum DHK, Harrison SD, Murfield JE. A randomized controlled trial exploring the effect of music on agitated behaviours and anxiety in older people with dementia. Aging Ment Health. (2010) 14(8):905–16. doi: 10.1080/13607861003713190
58. Sung H-C, Lee W-L, Li T-L, Watson R. A group music intervention using percussion instruments with familiar music to reduce anxiety and agitation of institutionalized older adults with dementia. Int J Geriatr Psychiatry. (2012) 27(6):621–7. doi: 10.1002/gps.2761
59. Guétin S, Portet F, Picot MC, Pommié C, Messaoudi M, Djabelkir L, et al. Effect of music therapy on anxiety and depression in patients with Alzheimer's Type dementia: randomised, controlled study. Dement Geriatr Cogn Disord. (2009) 28(1):36–46. doi: 10.1159/000229024
60. Sánchez A, Maseda A, Marante-Moar MP, de Labra C, Lorenzo-López L, Millán-Calenti JC. Comparing the effects of multisensory stimulation and individualized music sessions on elderly people with severe dementia: a randomized controlled trial. J Alzheimer's Dis. (2016) 52:303–15. doi: 10.3233/JAD-151150
61. Vink AC, Zuidersma M, Boersma F, de Jonge P, Zuidema SU, Slaets JPJ. The effect of music therapy compared with general recreational activities in reducing agitation in people with dementia: a randomised controlled trial. Int J Geriatr Psychiatry. (2013) 28(10):1031–8. doi: 10.1002/gps.3924
62. Lin Y, Chu H, Yang C-Y, Chen C-H, Chen S-G, Chang H-J, et al. Effectiveness of group music intervention against agitated behavior in elderly persons with dementia. Int J Geriatr Psychiatry. (2011) 26(7):670–8. doi: 10.1002/gps.2580
63. Särkämö T, Laitinen S, Numminen A, Kurki M, Johnson JK, Rantanen P. Pattern of emotional benefits induced by regular singing and music listening in dementia. J Am Geriatr Soc. (2016) 64(2):439–40. doi: 10.1111/jgs.13963
64. Raglio A, Bellandi D, Baiardi P, Gianotti M, Ubezio MC, Zanacchi E, et al. Effect of active music therapy and individualized listening to music on dementia: a multicenter randomized controlled trial. J Am Geriatr Soc. (2015) 63(8):1534–9. doi: 10.1111/jgs.13558
65. Hsu MH, Flowerdew R, Parker M, Fachner J, Odell-Miller H. Individual music therapy for managing neuropsychiatric symptoms for people with dementia and their carers: a cluster randomised controlled feasibility study. BMC Geriatr. (2015) 15(1):84. doi: 10.1186/s12877-015-0082-4
66. Vink AC, Zuidersma M, Boersma F, de Jonge P, Zuidema SU, Slaets JP. Effect of music therapy versus recreational activities on neuropsychiatric symptoms in elderly adults with dementia: an exploratory randomized controlled trial. J Am Geriatr Soc. (2014) 62(2):392–3. doi: 10.1111/jgs.12682
67. Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Villani D, et al. Efficacy of music therapy in the treatment of behavioral and psychiatric symptoms of dementia. Alzheimer Dis & Associated Disorders. (2008) 22(2):158–62. doi: 10.1097/WAD.0b013e3181630b6f
68. Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Gentile S, et al. Efficacy of music therapy treatment based on cycles of sessions: a randomised controlled trial. Aging Ment Health. (2010) 14(8):900–4. doi: 10.1080/13607861003713158
69. Ridder HMO, Stige B, Qvale LG, Gold C. Individual music therapy for agitation in dementia: an exploratory randomized controlled trial. Aging Ment Health. (2013) 17(6):667–78. doi: 10.1080/13607863.2013.790926
70. Labbé E, Schmidt N, Babin J, Pharr M. Coping with stress: the effectiveness of different types of music. Appl Psychophysiol Biofeedback. (2007) 32(3):163–8. doi: 10.1007/s10484-007-9043-9
71. Baird A, Samson S. Chapter 11 - music and dementia. In: Altenmüller E, Finger S, Boller F, editors. Progress in brain research. 217. Amsterdam, Netherlands: Elsevier (2015). p. 207–35.
72. Sihvonen AJ, Särkämö T, Leo V, Tervaniemi M, Altenmüller E, Soinila S. Music-Based interventions in neurological rehabilitation. The Lancet Neurol. (2017) 16(8):648–60. doi: 10.1016/S1474-4422(17)30168-0
73. Cuddy LL, Duffin JM, Gill SS, Brown CL, Sikka R, Vanstone AD. Memory for melodies and lyrics in Alzheimer's Disease. Music Percept. (2012) 29(5):479–91. doi: 10.1525/mp.2012.29.5.479
74. Foster NA, Valentine ER. The effect of auditory stimulation on autobiographical recall in dementia. Exp Aging Res. (2001) 27(3):215–28. doi: 10.1080/036107301300208664
75. Peck KJ, Girard TA, Russo FA, Fiocco AJ. Music and memory in Alzheimer's Disease and the potential underlying mechanisms. J Alzheimers Dis. (2016) 51(4):949–59. doi: 10.3233/jad-150998
76. Wang D, Belden A, Hanser SB, Geddes MR, Loui P. Resting-State connectivity of auditory and reward systems in Alzheimer's Disease and mild cognitive impairment. Front Hum Neurosci. (2020) 14:1–10. doi: 10.3389/fnhum.2020.00280
77. Bakerjian D, Bettega K, Cachu AM, Azzis L, Taylor S. The impact of music and memory on resident level outcomes in California nursing homes. J Am Med Dir Assoc. (2020) 21(8):1045–50.e2. doi: 10.1016/j.jamda.2020.01.103
78. McCreedy EM, Yang X, Baier RR, Rudolph JL, Thomas KS, Mor V. Measuring effects of nondrug interventions on behaviors: music & memory pilot study. J Am Geriatr Soc. (2019) 67(10):2134–8. doi: 10.1111/jgs.16069
79. Cunningham S, Brill M, Whalley JH, Read R, Anderson G, Edwards S, et al. Assessing wellbeing in people living with dementia using reminiscence music with a Mobile app (memory tracks): a mixed methods cohort study. J Healthc Eng. (2019) 2019:8924273. doi: 10.1155/2019/8924273
80. Good A, Kreutz G, Choma B, Fiocco A, Russo F, Organization WH. The singwell project protocol: the road to understanding the benefits of group singing in older adults. Public Health Panorama. (2020) 6(1):141–6.
81. Rio R. A community-based music therapy support group for people with Alzheimer's Disease and their caregivers: a sustainable partnership model. Front Med (Lausanne). (2018) 5:1–7. doi: 10.3389/fmed.2018.00293
82. Leggieri M, Thaut MH, Fornazzari L, Schweizer TA, Barfett J, Munoz DG, et al. Music intervention approaches for Alzheimer's Disease: a review of the literature. Front Neurosci. (2019) 13:1–8. doi: 10.3389/fnins.2019.00132
83. Tomaino CM. Music on their minds: A qualitative study of the effects of using familiar music to stimulate preserved memory function in persons with dementia. New York, New York, USA: New York University (1998).
84. Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. (2011) 14(2):257–62. doi: 10.1038/nn.2726
85. Mallik A, Chanda ML, Levitin DJ. Anhedonia to music and mu-opioids: evidence from the administration of naltrexone. Sci Rep. (2017) 7:41952. doi: 10.1038/srep41952
86. King JB, Jones KG, Goldberg E, Rollins M, MacNamee K, Moffit C, et al. Increased functional connectivity after listening to favored music in adults with Alzheimer dementia. J Prev Alzheimer's Dis. (2019) 6(1):56–62. doi: 10.14283/jpad.2018.19
87. Engelborghs S, Vloeberghs E, Le Bastard N, Van Buggenhout M, Mariën P, Somers N, et al. The dopaminergic neurotransmitter system is associated with aggression and agitation in frontotemporal dementia. Neurochem Int. (2008) 52(6):1052–60. doi: 10.1016/j.neuint.2007.10.018
88. Cai Z, Ratka A. Opioid system and Alzheimer's Disease. NeuroMol Med. (2012) 14(2):91–111. doi: 10.1007/s12017-012-8180-3
89. Mallik A, Russo FA. The effects of music & auditory beat stimulation on anxiety: a randomized clinical trial. PLOS ONE. (2022) 17(3):e0259312. doi: 10.1371/journal.pone.0259312
90. Labbé A, McMahon Z, Thomson Z. Music as Medicine: Lucid Science+Technology White Paper. [White Paper]. In press (2021)).
91. Altshuler IM. A Psychiatrist's Experience with music as a therapeutic agent. In: Schullian DM Schoen, M., editor. Music and medicine. New York: Schuman, Inc. (1948). p. 69–76.
92. Heiderscheit A, Madson A. Use of the iso principle as a central method in mood management: a music psychotherapy clinical case study. Music Ther Perspect. (2015) 33(1):45–52. doi: 10.1093/mtp/miu042%J
93. Rider MS. Entrainment mechanisms are involved in pain reduction, muscle relaxation, and music-mediated imagery. J Music Ther. (1985) 22(4):183–92. doi: 10.1093/jmt/22.4.183
94. Russell JA. A circumplex model of affect. J Pers Soc Psychol. (1980) 39(6):1161–78. doi: 10.1037/h0077714
95. Downey LE, Blezat A, Nicholas J, Omar R, Golden HL, Mahoney CJ, et al. Mentalising music in frontotemporal dementia. Cortex. (2013) 49(7):1844–55. doi: 10.1016/j.cortex.2012.09.011
96. Lange EB, Zweck F, Sinn P. Microsaccade-Rate indicates absorption by music listening. Conscious Cogn. (2017) 55:59–78. doi: 10.1016/j.concog.2017.07.009
97. Hall SE, Schubert E, Wilson SJ. The role of trait and state absorption in the enjoyment of music. PLOS ONE. (2016) 11(11):e0164029. doi: 10.1371/journal.pone.0164029
98. Sandstrom GM, Russo FA. Absorption in music: development of a scale to identify individuals with strong emotional responses to music. Psychol Music. (2013) 41(2):216–28. doi: 10.1177/0305735611422508
99. Dvorak AL, Hernandez-Ruiz E. Comparison of music stimuli to support mindfulness meditation. Psychol Music. (2021) 49(3):498–512. doi: 10.1177/0305735619878497
100. Labbé A, Russo FA. Inventors; Method and System for Measuring, Calibrating and Training Psychological Absorption. United States of America (2022)).
101. Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the npi-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. (2000) 12(2):233–9. doi: 10.1176/jnp.12.2.233
102. Reisberg B, Borenstein J, Franssen E, Salob S, Steinberg G, Shulman E, et al. Behave-Ad: a clinical rating scale for the assessment of pharmacologically remediable behavioral symptomatology in Alzheimer's Disease. In: Altman HJ, editor. Alzheimer's disease: problems, prospects, and perspectives. Boston, MA: Springer US (1987). p. 1–16.
103. Grös DF, Antony MM, Simms LJ, McCabe RE. Psychometric properties of the state-trait inventory for cognitive and somatic anxiety (sticsa): comparison to the state-trait anxiety inventory (stai). Psychol Assess. (2007) 19(4):369. doi: 10.1037/1040-3590.19.4.369
Keywords: digital therapeutics, dementia, neuropsychiatric symptoms, anxiety, agitation, music, artificial intelligence
Citation: Russo FA, Mallik A, Thomson Z, de Raadt St. James A, Dupuis K and Cohen D (2023) Developing a music-based digital therapeutic to help manage the neuropsychiatric symptoms of dementia. Front. Digit. Health 5:1064115. doi: 10.3389/fdgth.2023.1064115
Received: 10 October 2022; Accepted: 2 January 2023;
Published: 20 January 2023.
Edited by:
Terry D. Ellis, Boston University, United StatesReviewed by:
Joe Wherton, University of Oxford, United Kingdom© 2023 Russo, Mallik, Thomson, de Raadt St. James, Dupuis and Cohen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank A. Russo russo@torontomu.ca
Specialty Section: This article was submitted to Connected Health, a section of the journal Frontiers in Digital Health
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.