
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Digit. Health, 06 December 2022
Sec. Human Factors and Digital Health
Volume 4 - 2022 | https://doi.org/10.3389/fdgth.2022.961604
This article is part of the Research TopicDesigning and Evaluating Games for Health and WellbeingView all 9 articles
 Bianca C. Braga1*
Bianca C. Braga1* Alejandra Arrieta2,3
Alejandra Arrieta2,3 Boateng Bannerman4
Boateng Bannerman4 Frank Doyle5
Frank Doyle5 Gloria Folson4
Gloria Folson4 Rohit Gangupantulu5
Rohit Gangupantulu5 Nga Thu Hoang6
Nga Thu Hoang6 Phuong Nam Huynh6
Phuong Nam Huynh6 Bastien Koch3
Bastien Koch3 Peter McCloskey5
Peter McCloskey5 Lan Mai Tran7
Lan Mai Tran7 Trang Huyen T. Tran8
Trang Huyen T. Tran8 Duong Thuy T. Truong8
Duong Thuy T. Truong8 Phuong H. Nguyen3,8
Phuong H. Nguyen3,8 David Hughes5,9
David Hughes5,9 Aulo Gelli3
Aulo Gelli3
Unhealthy diets are a critical global concern while dietary measure methods are time consuming and expensive. There is limited evidence that phone-based interventions can improve nutrition data collection and dietary quality, especially for adolescents in developing countries. We developed an artificial-intelligence-based phone application called Food Recognition Assistance and Nudging Insights (FRANI) to address these problems. FRANI can recognize foods in images, track food consumption, display statistics and use gamified nudges to give positive feedback on healthy food choice. This study protocol describes the design of new pilot studies aimed at measuring the feasibility (acceptability, adherence, and usability) of FRANI and its effects on the quality of food choice of adolescents in Ghana and Vietnam. In each country, 36 adolescents (12–18 years) will be randomly allocated into two groups: The intervention group with the full version of FRANI and the control group with the functionality limited to image recognition and dietary assessment. Participants in both groups will have their food choices tracked for four weeks. The control groups will then switch to the full version of FRANI and both groups will be tracked for a further 2 weeks to assess acceptability, adherence, and usability. Analysis of outcomes will be by intent to treat and differences in outcomes between intervention and control group will use Poisson and odds ratio regression models, accounting for repeated measures at individual levels. If deemed feasible, acceptable and usable, FRANI will address gaps in the literature and advance the nutrition field by potentially improving the quality of food choices of adolescent girls in developing countries. This pilot study will also provide insights on the design of a large randomized controlled trial. The functioning and dissemination of FRANI can be an important step towards highly scalable nutrition data collection and healthier food choices for a population at risk of malnutrition.
The study protocol and the methods and materials were approved by the Institutional Review Board (IRB) of the IFPRI on April 29th, 2020 (registration number #00007490), the Thai Nguyen National Hospital on April 14th, 2020 (protocol code 274/ĐĐĐ-BVTWTN) and the University of Ghana on August 10th, 2020 (Federalwide Assurance FWA 00001824; NMIMR-IRB CPN 078–19/20). The study protocol was registered in the International Standard Randomized Controlled Trial Number (ISRCTN 10681553; https://doi.org/10.1186/ISRCTN10681553) on November 12, 2021.
There are 1.8 billion adolescents in low- and middle-income countries (LMIC) (1). Adolescence is characterized by rapid physical growth, sexual maturation, and increased nutrient requirements (2). Meeting nutrient requirements is essential to optimize biological development, growth, long-term health, and longevity (2–4). Those who complete the physical, mental, social and emotional development of adolescence have better chances to reproduce successfully and upbring their children optimally (2). Investments to end malnutrition during this life stage are a great opportunity to interrupt intergenerational cycles of malnutrition and improve child and maternal health (5, 6).
Every year, around 16 million adolescent girls become mothers in LMIC (7). The absolute number of adolescent pregnancies is increasing, especially in settings of high prevalence of malnutrition, food insecurity and poor dietary quality such as Africa, South Asia and Southeast Asia (2). Both under and overnutrition before and during pregnancy predict altered growth and health of the offspring (8). Pregnant adolescents typically compete for nutrients with the fetus, increasing risk of low birthweight and short birth length (9, 10). The risk of preterm delivery, and poor childhood growth and nutritional status is also higher for neonates of adolescent mothers (11, 12).
Solutions to maternal and child malnutrition in LMIC have to take into account the social and cultural factors that drive adolescent decision making (5, 13, 14). Adolescent neuro-development make them more aware of external influences, preferences and habits of peers and family (15, 16). This increases the desire to have social recognition, status, and autonomy (17–19). Responsibility over food acquisition, preparation, and consumption start in adolescence (6). The sensitivity to external influence coupled with greater power for food choice (20) create a unique opportunity to foster long-term healthy eating (6).
Data collection for adolescent nutrition is fundamental to design effective policies and programs and to assess their progress (5, 6, 13). The Nudging for Good project aims at developing, validating and examining the feasibility of the Food Recognition Assistance and Nudging Insights (FRANI), a phone application that uses artificial intelligence technology to recognize foods in pictures, assess diets and nudge adolescents from Ghana and Vietnam to make healthier food choices (21). First, we made food inventories, prepared and cooked these foods, weighted, annotated and took pictures of them. We linked the pictures to food composition tables, and built models to recognize the images and estimate portion sizes. Finally, we developed the interface according to the preferences showed in focus groups discussions by adolescent girls from Ghana and Vietnam. We validated the FRANI dietary assessment through food image recognition against the gold standards of weigh food records and 24 h recalls. Now, we aim at conducting a randomized controlled pilot study to measure the feasibility of using FRANI and its impact on the quality of food choice.
The FRANI mobile app is designed to improve diets by increasing the consumption of healthy foods and beverages, whilst crowding out the consumption of energy-dense foods. FRANI users can set healthy eating goals – or choose daily dietary goals from a list of simple options based on food groups, such as “have a dark green vegetable” or “eat a whole grain”. Conceptually, this increases motivation and interest in learning about healthy diets, as well as increases awareness of what are healthy and unhealthy foods. Alongside individual-based goals, users can opt-in to team-based goals that may lead to stronger peer support on healthy eating practices and provide social praise for healthier food choices. However, in order to avoid peer-pressure, users can only visualize team progress as a whole and cannot track the individual performance of peers.
FRANI provides feedback based on the information uploaded by users, or on the pictures of foods and beverages they consume. The AI technology is trained on a database of calibrated images with foods and beverages to enable estimation of both the foods and portion sizes in the uploaded pictures. FRANI then coverts the food quantities into nutrients by using food composition tables. The practicality of this technology has the potential to increase the frequency of data points collected on food consumption, addressing the constraint faced by common diet estimation methods with regards to estimating usual intake (22).
The pilot studies are based on two-armed randomized controlled designs to be implemented in Ghana and Vietnam. In each country, 36 adolescents (12–18 years) will be randomly allocated into two groups: The intervention group with the full version of FRANI, including diet records and nudges to encourage healthier food choices. The control group will receive mobile phones with FRANI functionality limited to the dietary assessment. Participants in both groups will have their food choices tracked for a four week period. The control group will then switch to also use FRANI with full functionality and both groups will be tracked for a further 2 weeks to assess acceptability, adherence, and usability. The schedule of the intervention, enrollment and assessment is described in Figure 1. The stylized program impact pathway for the intervention is described in Figure 2 (21).
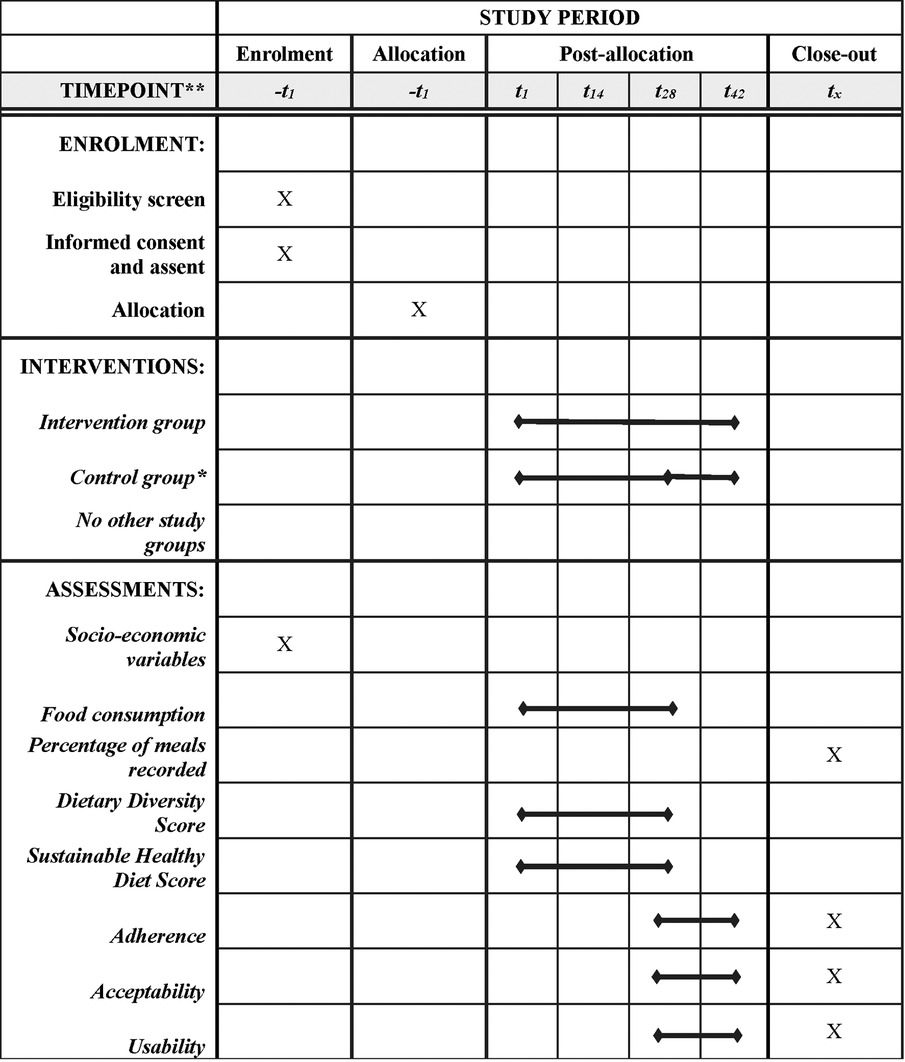
Figure 1. Adapted SPIRIT diagram (29). Schedule of enrolment, interventions, and assessments of the two-armed randomized controlled pilot studies in Ghana and Vietnam. *The participants in the control group will use FRANI with limited functionality to track their food choices for 28 days (4 weeks). After that, the control group will switch to also use FRANI with full functionality for the last two weeks of the post-allocation period. The bullet in t28 indicates that the control group switched for the full version app.
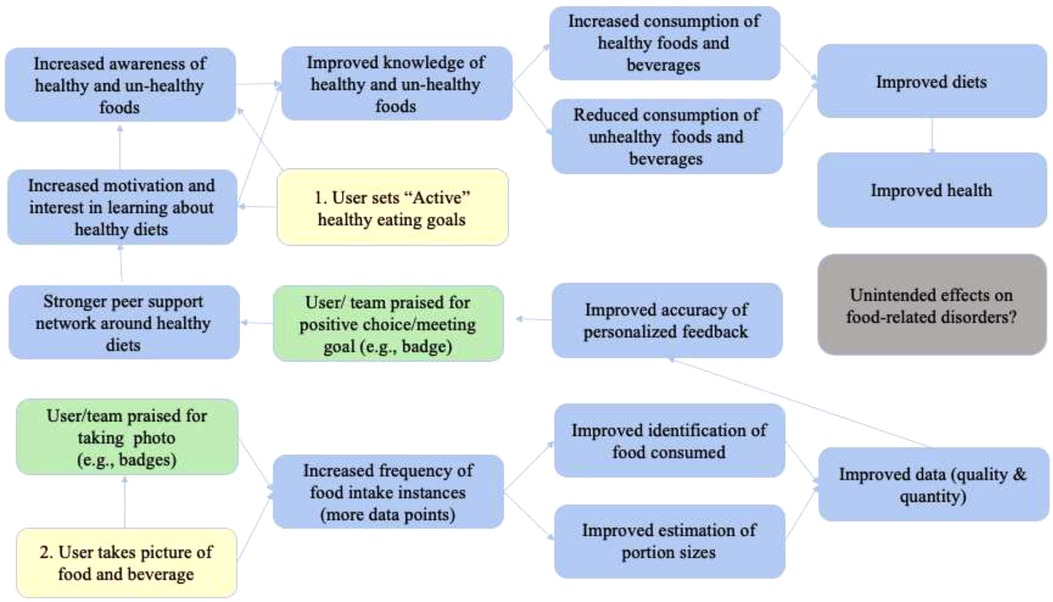
Figure 2. The stylized program impact pathways for the FRANI. (Source: Adapted from (21)) Notes: Yellow represents the two steps users have to follow to have their dietary consumption tracked and to be nudged to make healthier food choices. Green boxes show nudges to influence the use of the FRANI and healthy food choices. Blue boxes represent what is expected to improve with the FRANI use. Gray is for a possible unintended consequence of the FRANI use that should be fixed in future versions of the app.
The main differences in the application versions for the intervention and control groups are described in Table 1. Briefly, both the intervention and control versions of FRANI recognize food images, assess the quality of the user's diet, and receive notifications with reminders to take pictures. However, only the treatment group can set food consumption goals, see the home screen, scores and statistics about dietary quality, receive badges and receive a daily report summarizing everything they ate in that day.
The options for setting food consumption goals were based on the EAT-Lancet Diet (23) and the Dietary Guidelines of Vietnam (24). These FRANI goals can be individual- or team-oriented, and involve five main food groups: (1) grains and starchy staples; (2) legumes, nuts and seeds; (3) fruits and vegetables; (4) dairies; (5) animal-source foods (excluding dairy). In addition to setting food consumption goals, participants from the intervention group be nudged with game elements. These nudges include: (1) tracking progress towards healthy-eating goals; (2) giving personalized feedback on food consumption; (3) reinforcing healthy food consumption; (4) tracking progress in the quality of food consumption with statistics; (5) participating in goals shared with other users (team-based goals); (6) sharing positive feedback with peers. Participants in the intervention group will be able to choose from team-based goals on a weekly basis. They will receive information about their team's overall performance but in order to avoid excessive peer pressure they will not be able to see each individual team member's performance.
The study protocol and the methods and materials were approved by the Institutional Review Board (IRB) of the IFPRI on April 29th, 2020 (registration number #00007490), the Thai Nguyen National Hospital on April 14th, 2020 (protocol code 274/ĐĐĐ-BVTWTN) and the University of Ghana on August 10th, 2020 (Federalwide Assurance FWA 00001824; NMIMR-IRB CPN 078–19/20). The study protocol was registered in the International Standard Randomized Controlled Trial Number (ISRCTN 10681553; https://doi.org/10.1186/ISRCTN10681553) on November 12, 2021. Participants will be instructed to report any problem occurring during the study to the country-lead researchers, which will be in direct contact with them. Although risk from using FRANI is minimal, any adverse effect will be discussed by the team, and reported to the IRB of the IFPRI and to the ethics boards of correspondent countries. All procedures were and will be in accordance with the Declaration of Helsinki.
The FRANI pilot test is targeted to adolescent girls from 12 to 18 years old living Accra, Ghana and Thai Nguyen, Vietnam. These sites were selected based on relevance of the dietary quality concern and proximity to research partners.
A total of 72 adolescents girls aged between 12 and 18 years will be randomly selected from two schools, one in each city, which staff has a good working relationship with our team. Those who assented for participation and which parents consented will receive a smartphone pre-configured with the FRANI app. Participants will be free to discontinue study participation at any time. Candidates will be excluded from the study if they do not provide assent or their parents do not provide consent to participate.
A computer-generated random number will randomize participants in the intervention and control groups at a 1:1 ratio for each country separately. The allocation sequence will be generated and the enrollment and assignment will be done by the senior researchers of the Vietnamese and Ghanaian teams. Although a similar description of FRANI will be presented for intervention and control groups in the process of informed assent and consent, it will not be possible to blind who is receiving feedback from FRANI and who is not. The data will be collected by the application without assessors.
The feasibility of FRANI will be assessed by measuring outcomes related to adherence, acceptability, and usability as summarized in Table 2 (25, 26). According to the World Health Organization (WHO), adherence is “the extent to which a person's behavior – taking medication, following a diet, or executing lifestyle changes, corresponds with agreed recommendations from a health care provider” (27). Although there is no consensus in the literature on the best measure for adherence to eHealth technology interventions (25, 26), maximum benefit from the intervention as defined by creators should be considered (28). We define the sample adherent to FRANI if 70% or more of participants-days upload complete dietary recalls in the last two weeks of the pilot study. Acceptability summarizes likeability and satisfaction with FRANI, while usability summarizes what affects the use of the FRANI. Acceptability and usability are measured according to answers from structured questionnaires described in Table 3. If participants grade the acceptability or usability of FRANI as 30 or less points in their respective structured questionnaires, the app will be considered accepted or usable.
Based on the analysis of the program theory, the effects of the FRANI and gamified nudges on the dietary quality of adolescent girls will be assessed on a set of secondary outcomes including the percentage of meals recorded and the quality of food choices. The quality of food choices will be measured according to the Dietary Diversity Score (DDS) and the Sustainable Healthy Diet Score (SHDS). Adherence, percentage of meals recorded, the DDS and the SHDS will be measured using data captured by FRANI. Although the secondary outcome measures will be based on intervention and control group comparisons, the primary measures will not.
The data for outcomes will be recorded on FRANI and uploaded to a data collection platform at real time during the intervention period. Data collection will happen automatically once the participants took pictures of the foods they ate and confirmed their recognition. Since the image recognition technology from FRANI has been shown to be promising in the validation study (not published yet), the automatization of the data entry process may minimize mistakes if compared to other forms of food consumption data collection such as 24 h recalls and food frequency questionnaires. Baseline variables such as parents occupation, household assets and school performance will be collected with questionnaires. The data will be stored in protected clouds at the Pennsylvania State University.
The sample size was determined by the number of smartphones and data packages that could be supported by budget and is consistent with other pilot studies in the literature. The effect size of the treatment on the DDS and the SHDS on the first four weeks of the study will be used to calculate the sample size of a larger experiment.
The data from the pilot studies from Ghana and Vietnam will be analyzed and reported separately, though a pooled analysis will also be undertaken once both pilots have been completed. The analysis will be by intent to treat, and we do not expect to have loss of follow-up as this is a small scale pilot. Descriptive statistics and balance tables will be calculated for all variables and will be presented as medians or means with standard deviations. Differences in the outcomes between intervention and control groups will be estimated by multi-level Poisson regression models with the DDS and the SHDS as outcomes, a dummy variable for the treatment, a continuous time variable for the days and an interaction of treatment and time, and random effects at the individual level to account for the repeated measures. We will also undertake exploratory analysis for each food group of the DDS and the SHDS separately using odds ratio regressions. Socio-demographic variables that were not balanced after randomization will also be included in the robustness analysis.
A p-value < 0.05 will be considered statistically significant and p-value < 0.1 will be considered marginally significant. We will assess the need to screen outliers values greater than three standard deviations and will analyze the sensitivity of the treatment effect to the outliers.
The data collected will be de-identified and stored on the computers of the institutions involved. Random numbers will be generated and linked to each participant, and they will be referred to exclusively by that participant code. The document linking both numbers will be destroyed once results of the study were published.
The project is based on an interdisciplinary collaboration between the IFPRI, the Pennsylvania State University in partnership with the FAO, the University of Ghana, the Thai Nguyen National Hospital, the Thai Nguyen University of Medicine and Pharmacy, and the National Institute of Nutrition (NIN) of Vietnam. The principal investigator at IFPRI is responsible for coordinating the study, while the research teams based in Ghana and Vietnam recruit potential participants, send participants the informed assent and their parents the informed consent, answer questions and attend requests, and distribute the smartphones with the FRANI to eligible participants. The local research teams will also monitor participants throughout the study period, and report problems to the whole team. Because we do not anticipate negative outcomes for the participants, we have not established formal stopping rules nor an auditing conduct. The research team agreed on the final version of the pilot protocol. Weekly meetings are planned to report progress, verify deadlines, and manage the project. Any modifications in the protocol such as eligibility criteria, outcomes and analyses will be communicated to IFPRI and in-country IRBs when needed. The engineering team, based at the Pennsylvania State University, is responsible for data storage and the maintenance of FRANI. Members of all teams will be responsible for different parts of the results dissemination through journal articles, conferences and presentations.
This protocol describes a randomized controlled pilot study that will measure adherence, acceptability, and usability and determine the impact of an AI technology-based app in the quality of food choice of adolescent girls from Ghana and Vietnam. There is limited evidence on intervention studies that used technology to change eating habits (21). The FRANI technology has the potential to replace expensive and time-consuming questionnaires, and simplify and improve the precision of food consumption data collection. The pilot study will address gaps in the literature and advance the nutrition field by providing evidence on adherence, acceptability and usability of technology-based nutrition interventions, and by impacting the quality of food choice of adolescent girls in developing countries.
The pilot test of the FRANI is an important first step towards better quality, highly scalable data collection in nutrition and healthier food choices for a population at risk of malnutrition. The success of this intervention will depend on the use of the FRANI and the change in intake of nutritious and diverse foods. If the pilot and validation the FRANI technology are successful, the next steps in the development process include a larger randomized control trial to examine if technology-based nutrition intervention works for a more extended period of time.
Methods and materials were approved by the Institutional Review Boards of the International Food Policy Research Institute (protocol code #00007490), the Thai Nguyen National Hospital (protocol code 274/ĐĐĐ-BVTWTN) and the University of Ghana (Federalwide Assurance FWA 00001824; NMIMR-IRB CPN 078–19/20). The study requires assent of participants and consent from their parents. All procedures were and will be in accordance with the Declaration of Helsinki. The study protocol was registered in the International Standard Randomized Controlled Trial Number (ISRCTN 10681553; https://doi.org/10.1186/ISRCTN10681553). The dissemination of the results will be done in scientific journals and conferences.
This study was implemented in a partnership between the International Food Policy Research Institute, Pennsylvania State University/Food and Agricultural Organization, the University of Ghana, the National Institute of Nutrition and the Thai Nguyen National Hospital in Vietnam. AG & DH led the research and development; BCB led the pilot test design and wrote the protocol study; AA, BB, BK, DTT, GF, LMT, NTH, PM, PHN, PNH, RG, THT provided inputs in the pilot test design. FD, PM, RG developed the software for the intervention. All authors contributed to the article and approved the submitted version.
This research was supported by a Foundation Botnar (info@foundationbotnar.org) grant (REG 19–018) and by the Consortium of International Agricultural Research Centers (CGIAR) program on Agriculture, Nutrition and Health (A4NH) (France). BCB is a Ph.D. candidate supported by the Friedman Nutrition and Citizenship Fellowship from the Friedman School of Nutrition Science and Policy at Tufts University. The sponsors were not involved in the study design, in the collection, analysis and interpretation of data, or on the writing of the report and its submission to publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Das Gupta M, Engelman R, Levy J, Luchsinger G, Tom M, James R. (2014). The power of 1.8 billion: Adolescents, youth and the transformation of the future. https://www.unfpa.org/sites/default/files/pub-pdf/EN-SWOP14-Report_FINAL-web.pdf
2. Norris SA, Frongillo EA, Black MM, Dong Y, Fall C, Lampl M, et al. Nutrition in adolescent growth and development. The Lancet. (2022) 399(10320):172–84. doi: 10.1016/S0140-6736(21)01590-7
3. Depauw E, Oxley D. Toddlers, teenagers, and terminal heights: the importance of puberty for Male adult stature, flanders, 1800–76. Econ Hist Rev. (2019) 72(3):925–52. doi: 10.1111/ehr.12745
4. Falconi A, Gemmill A, Dahl RE, Catalano R. Adolescent experience predicts longevity: evidence from historical epidemiology. J Dev Orig Health Dis. (2014) 5(3):171–7. doi: 10.1017/S2040174414000105
5. Patton GC, Sawyer SM, Santelli JS, Ross DA, Afifi R, Allen NB, et al. Our future: a lancet commission on adolescent health and wellbeing. The Lancet. (2016) 387(10036):2423–78. doi: 10.1016/S0140-6736(16)00579-1
6. Hargreaves D, Mates E, Menon P, Alderman H, Devakumar D, Fawzi W, et al. Strategies and interventions for healthy adolescent growth, nutrition, and development. The Lancet. (2022) 399(10320):198–210. doi: 10.1016/S0140-6736(21)01593-2
7. Singer K, Lumeng CN. The initiation of metabolic inflammation in childhood obesity. J Clin Invest. (2017) 127(1):65–73. doi: 10.1172/JCI88882
8. Aurino E. Do boys eat better than girls in India? Longitudinal evidence on dietary diversity and food consumption disparities among children and adolescents. Econ & Hum Biol. (2017) 25:99–111. doi: 10.1016/j.ehb.2016.10.007
9. Scholl TO, Hediger ML. A review of the epidemiology of nutrition and adolescent pregnancy: maternal growth during pregnancy and its effect on the fetus. J Am Coll Nutr. (1993) 12(2):101–7. doi: 10.1080/07315724.1993.10718289
10. Hsu JW, Thame MM, Gibson R, Baker TM, Tang GJ, Chacko SK, et al. Unlike pregnant adult women, pregnant adolescent girls cannot maintain glycine flux during late pregnancy because of decreased synthesis from serine. Br J Nutr. (2016) 115(5):759–63. doi: 10.1017/S0007114515005279
11. Fall CHD, Osmond C, Haazen DS, Sachdev HS, Victora C, Martorell R, et al. Disadvantages of having an adolescent mother. The Lancet Global Health. (2016) 4(11):e787–8. doi: 10.1016/S2214-109X(16)30263-7
12. Fall CHD, Sachdev HS, Osmond C, Restrepo-Mendez MC, Victora C, Martorell R, et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low-income and middle-income countries (COHORTS collaboration). The Lancet Glob Health. (2015) 3(7):e366–77. doi: 10.1016/S2214-109X(15)00038-8
13. Patton GC, Coffey C, Cappa C, Currie D, Riley L, Gore F, et al. Health of the world's Adolescents: a synthesis of internationally comparable data. The Lancet. (2012) 379(9826):1665–75. doi: 10.1016/S0140-6736(12)60203-7
14. Dennison CM, Shepherd R. Adolescent food choice: an application of the theory of planned behaviour. J Hum Nutr Diet. (1995) 8(1):9–23. doi: 10.1111/j.1365-277X.1995.tb00292.x
15. Tremblay L, Lariviere M. The influence of puberty onset, body mass Index, and pressure to be thin on disordered eating behaviors in children and adolescents. Eat Behav. (2009) 10(2):75–83. doi: 10.1016/j.eatbeh.2008.12.001
16. Contento IR, Williams SS, Michela JL, Franklin AB. Understanding the food choice process of adolescents in the context of family and friends. J Adolesc Health. (2006) 38(5):575–82. doi: 10.1016/j.jadohealth.2005.05.025
17. Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. (2004) 1021(1):1–22. doi: 10.1196/annals.1308.001
18. Peper JS, Koolschijn PCMP, Crone EA. Development of risk taking: contributions from adolescent testosterone and the orbito-frontal Cortex. J Cogn. (2013) 25(12):2141–50. doi: 10.1162/jocn_a_00445
19. Cardoos SL, Ballonoff Suleiman A, Johnson M, van den Bos W, Hinshaw SP, Dahl RE. Social status strategy in early adolescent girls: testosterone and value-based decision making. Psychoneuroendocrinol. (2017) 81:14–21. doi: 10.1016/j.psyneuen.2017.03.013
20. Neufeld LM, Andrade EB, Ballonoff Suleiman A, Barker M, Beal T, Blum LS, et al. Food choice in transition: adolescent autonomy, agency, and the food environment. The Lancet. (2022) 399(10320):185–97. doi: 10.1016/S0140-6736(21)01687-1
21. Braga BC, Aberman N-L, Arrieta A, Bannerman B, Burns A, Folson G, et al. (n.d.). Design of a Mobile phone-based artificial intelligence (AI) application to assess dietary intake and provide nudges to improve healthy eating choices. IFPRI Discussion Paper. 37 doi: 10.1162/jocn_a_00445
22. Willet W. (n.d.). Harvard Willett Food Frequency Questionnaire. SNAP Education Connection. Retrieved May 6, 2020, from https://snaped.fns.usda.gov/library/materials/harvard-willett-food-frequency-questionnaire
23. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the anthropocene: the EAT–lancet commission on healthy diets from sustainable food systems. The Lancet. (2019) 393:46. doi: 10.1162/jocn_a_00445
24. Food-Based Dietary Guideline of Vietnam. (n.d.). Food and Agriculture Organization of the United Nations. Retrieved February 17, 2021, from http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/vietnam/en/
25. Knuppel A, Papier K, Key TJ, Travis RC. EAT-Lancet score and major health outcomes: the EPIC-Oxford study. The Lancet. (2019) 394(10194):213–4. doi: 10.1016/S0140-6736(19)31236-X
26. Food and Agriculture Organization. (2016). Minimum dietary diversity for women—a guide to measurement. Rome: FAO, 82.
27. Sabate E. Adherence to long term therapies: Evidence for action. Geneva: World Health Organization (2003).
28. Sieverink F, Kelders SM, van Gemert-Pijnen JE. Clarifying the concept of adherence to eHealth technology: systematic review on when usage becomes adherence. J Med Internet Res. (2017) 19(12):e402. doi: 10.2196/jmir.8578
Keywords: dietary assessment method, behavior change app, artificial intelligence - AI, food choice, digital food environment
Citation: Braga BC, Arrieta A, Bannerman B, Doyle F, Folson G, Gangupantulu R, Hoang NT, Huynh PN, Koch B, McCloskey P, Tran LM, Tran THT, Truong DTT, Nguyen PH, Hughes D and Gelli A (2022) Measuring adherence, acceptability and likability of an artificial-intelligence-based, gamified phone application to improve the quality of dietary choices of adolescents in Ghana and Vietnam: Protocol of a randomized controlled pilot test. Front. Digit. Health 4:961604. doi: 10.3389/fdgth.2022.961604
Received: 4 June 2022; Accepted: 24 October 2022;
Published: 6 December 2022.
Edited by:
Sunny Jung Kim, Virginia Commonwealth University, United StatesReviewed by:
Kavita Chauhan, Penn Centre for Social Norms and Behavioral Dynamics, India© 2022 Braga, Arrieta, Bannerman, Doyle, Folson, Gangupantulu, Hoang, Huynh, Koch, McCloskey, Tran, Tran, Truong, Nguyen, Hughes and Gelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bianca C. Braga Y3VyaS5iaWFuY2FAdHVmdHMuZWR1
Specialty Section: This article was submitted to Human Factors and Digital Health, a section of the journal Frontiers in Digital Health
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.