- 1Population Health and Genomics, University of Dundee, Dundee, United Kingdom
- 2VAMPIRE project, Computing, School of Science and Engineering, University of Dundee, Dundee, United Kingdom
- 3VAMPIRE project, Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom
- 4NHS Tayside; NHS Research Scotland Neuroprogressive Disorders and Dementia Research Network, Ninewells Hospital Dundee; University of Dundee, Dundee, Scotland
- 5Centre for Public Health, Queen’s University Belfast, Belfast, NIR, United Kingdom
Objective: Patients with diabetes have an increased risk of dementia. Improved prediction of dementia is an important goal in developing future prevention strategies. Diabetic retinopathy screening (DRS) photographs may be a convenient source of imaging biomarkers of brain health. We therefore investigated the association of retinal vascular measures (RVMs) from DRS photographs in patients with type 2 diabetes with dementia risk.
Research Design and Methods: RVMs were obtained from 6,111 patients in the GoDARTS bioresource (635 incident cases) using VAMPIRE software. Their association, independent of Apo E4 genotype and clinical parameters, was determined for incident all cause dementia (ACD) and separately Alzheimer's disease (AD) and vascular dementia (VD). We used Cox’s proportional hazards with competing risk of death without dementia. The potential value of RVMs to increase the accuracy of risk prediction was evaluated.
Results: Increased retinal arteriolar fractal dimension associated with increased risk of ACD (csHR 1.17; 1.08–1.26) and AD (HR 1.33; 1.16–1.52), whereas increased venular fractal dimension (FDV) was associated with reduced risk of AD (csHR 0.85; 0.74–0.96). Conversely, FDV was associated with increased risk of VD (csHR 1.22; 1.07–1.40). Wider arteriolar calibre was associated with a reduced risk of ACD (csHR 0.9; 0.83–0.98) and wider venular calibre was associated with a reduced risk of AD (csHR 0.87; 0.78–0.97). Accounting for competing risk did not substantially alter these findings. RVMs significantly increased the accuracy of prediction.
Conclusions: Conventional DRS photographs could enhance stratifying patients with diabetes at increased risk of dementia facilitating the development of future prevention strategies.
Introduction
The retina is an embryological derivative of the brain and can be imaged relatively cheaply and conveniently compared to imaging the brain. There is increasing interest in use of retinal images as a readily accessible source of imaging biomarkers for brain health (1). Such biomarkers could have clinically valuable implications, such as identifying individuals at greater risk of developing dementia for recruitment into dementia prevention trials, or as surrogate outcomes for dementia in such trials. With the failure of multiple clinical trials of potential disease-modifying treatments of dementia, possibly due to pathological changes being too advanced at patient enrolment, there is increasing interest in identifying individuals in the long prodromal phase prior to clinically manifestation of cognitive impairment for targeting prevention strategies (2). A number of studies have indicated links between retinal vascular measures (RVMs) and dementia (reviewed in (2, 3). Most studies to date have been relatively small and case control in design with the possibility of confounding and reverse causation. The Rotterdam study was a large prospective study that considered pathological retinal features such as age related macular degeneration (3) and retinopathy (4) but has only considered measures of retinal vascular calibre in terms of non-pathological RVMs (5).
Patients with type 2 diabetes have an increased risk of dementia compared to healthy individuals (6) and retinal screening with conventional digital photography is widely used to manage the risk of blindness due to diabetic retinopathy. Such diabetic retinopathy screening (DRS) photographs could therefore potentially also provide information about future dementia risk in patients with diabetes. Importantly, the dementias comprise a spectrum of differing and distinct pathoaetiologies dominated by the neurodegenerative features Alzheimer's disease (AD) and vascular dementia (VD) caused by brain injury of vascular origin, such as stroke. It is increasingly acknowledged that there is a pathological interdependence and a continuum between both major subtypes (7). Relatively few studies have compared the association of RVMs with these two major subtypes. We have recently demonstrated the ability to identify incident dementia cases using electronic medical records (EMR) in GoDARTS, a large prospective cohort linked to a genomic bioresource, and to differentiate these into VD and AD (8). In this study we obtained RVMs from the DRS photographs from patients with type 2 diabetes in GoDARTS and assessed the extent to which such measures may be used as predictors of dementia independently of other clinical and genetic risk factors.
Study population and methods
Study population
GoDARTS has been previously described (9). In brief, it comprises a cohort of approximately 8,500 patients who had type 2 diabetes at recruitment and 7,500 individuals of similar age and background without type 2 diabetes, all of whom were recruited from the Tayside region of Scotland over a period of about 10 years commencing in 1997. Comprehensive detailed and continuously accruing EMRs are available for all participants based on an established highly deterministic regional medical information infrastructure. Since recruitment into GoDARTS began, there is now a maximum of over 20 years of follow-up data in the EMR.
Methods
We obtained the digital DRS photographs used for the Scottish national DRS programme for all GoDARTS participants with type 2 diabetes. These photographs comprise macula-centred images with 45-degree field of view, which since the year 2,000 are available as digital images. We selected the earliest available images of the right eye from each patient with acceptable quality for subjecting to measurement. Image quality was defined from DRS programme data that provides information on image gradeability. Images in which the major retinal arcade vessels were blurred, the retinal small vessels were blurred, the retinal nerve fibre layer was not visible, or where there was other technical failure were discarded. This constituted 19% of images. If the right eye image was not technically acceptable, the earliest available image from the left eye was chosen. RVMs were obtained using the Vessel Assessment and Measurement Platform for Images of the Retina (VAMPIRE) software tool (version 3; Universities of Edinburgh and Dundee, UK) which computes morphometric measurements of the retinal vasculature from a large number of retinal fundus camera images efficiently (10). The following six measures were considered for the current analysis: retinal vascular calibre as central retinal arteriole/venule equivalent (CRAE, CRVE); arteriole/venule tortuosity (TORTA/V); and arteriole/venule fractal dimension (FDV/A) which characterises the complexity of the vascular branching pattern. Details of techniques and validation have been reported previously (11–14).
We conducted a retrospective cohort study of the association of this set of RVMs with incident dementia in GoDARTS EMR using our previously described and validated methodology (8); this reference includes a full discussion of the methodology adopted to define dementia events. Among others, this includes hospitalization and death data, prescribing data, adjudication of Alzheimer dementia by EMR, adjudication of vascular dementia and several types of validation data. Cox's proportional hazards was used with entry time being the date of acquisition of the DRS photograph that was measured by VAMPIRE and exit time being the date of first available evidence in the EMR for a diagnosis of dementia, which, as previously, was considered as a surrogate for dementia incidence. Censoring was end of available follow-up EMR data or death without EMR evidence of dementia. In addition to ACD we considered AD and VD separately as defined by our previous analysis. We did not separately evaluate the relatively small numbers of individuals who either could not be adjudicated as having AD or VD or who had a rarer cause of dementia. Apo E4 status defined by rs429358 genotype was included in all analyses and modelled as a codominant co-variable. The following clinical co-variables were also included in all models: sex (females coded as 1, males 0), age at baseline, smoking status (as ever/never smoked), years with diabetes, a history of hospitalisation with major adverse cardiovascular event (previous CVD), body mass index (BMI), systolic blood pressure (SBP), total cholesterol, high density lipoprotein cholesterol (HDL) and glycated haemoglobin. Continuous clinical covariables were evaluated as mean of all available values during a 3-year period prior to date of study entry. Where these values were missing for a particular individual, the value was imputed to the overall cohort mean value. Due to their potential for interdependence, all RVMs and all clinical covariates were included in all models with stepwise backward selection with p = 0.2 as the selection threshold for staying in the final model. To aid meaningful comparisons, all RVMs were z-standardized so hazard ratios (HR) associated with RVMs are all provided per standard deviation (SD) increase.
wAge is the recommended time scale for epidemiological studies (15–17). As age is the major determinant of dementia risk, and RVMs have been previously associated with features of cardiovascular diseases and aging (18), we set the time scale for the analysis as years of age, meaning all models were continuously adjusted for age. We also incorporated a competing risk of death without dementia analysis where risk is expressed as a sub-distribution HR (sdHR). All HR are expressed with their 95% confidence intervals. As this was an incident study, any individuals who had an established dementia diagnosis in their EMR on or prior to the date of study entry were excluded. Finally, to evaluate the potential of RVMs as biomarkers for dementia, we performed receiver operator characteristic (ROC) analyses. In this case, for practicality of comparing area under the curve (AUC) between models, we undertook logistic regression to predict risk of dementia within 10 years. We compared AUC for predicting ACD, VD and AD using clinical variables and ApoE4 genotype alone with AUCs in which RVMs were also added. For further assessment of the potential clinical value of RVMs we determined the Net Reclassification Improvement (NRI) and Integrated Discrimination Improvement (IDI) (19). For NRI we set the risk threshold at 10% and 20% with the motivation being that the 10-year risk of dementia in this population was on average approximately 10%. As has been previously recommended (20), to improve interpretation of the NRI we also determined its sub-components in terms of the NRI for events (NRIe) and non-events (NRIne) separately. STATA 14 was used for all data assembly and analyses. The nriidi package developed by Sundström et al. (21) was used to evaluate IDI and NRI.
Results
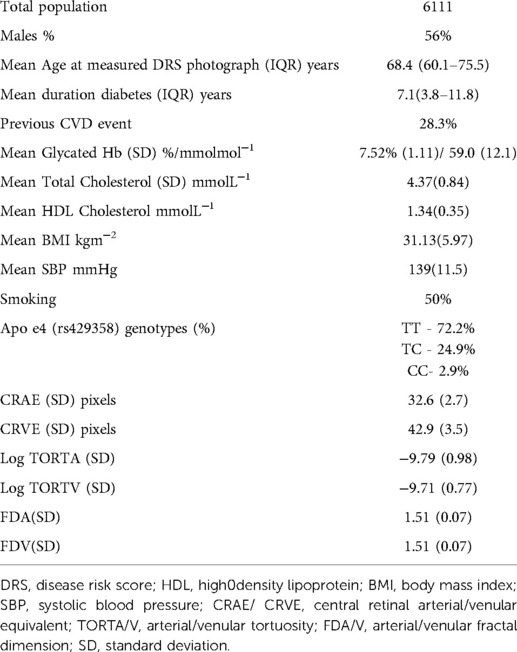
A total of 6,111 patients with type 2 diabetes had RVMs successfully measured and had a full set of data for analysis. The baseline characteristics of this population are provided in Table 1. Supplementary Figure S1 provides a correlation heatmap of all the covariates used in this study. The mean age at study entry was 68.4 years (IQR 60–75.5) with a mean duration of diabetes of 7.1 years (IQR 7.1–11.8).
Fifty-nine individuals were excluded as already having a record for dementia in the EMR at the date of acquisition of the retinal photograph. The median follow-up time in the remaining 6,052 individuals was 9.8 years (IQR 5.7–10.6) during which there were 635 incident cases of ACD comprising 327 (51.5%) AD, 218 (34.3%) VD and 90 (14.2%) undeterminable dementia type or other rarer dementia diagnoses. The overall incidence rate was 13 (lower bound 12 upper bound 14) per 1,000 person years. There were 1,844 deaths without evidence dementia in the EMR at or prior to date of death.
Table 2 provides the cause specific-hazard ratios (csHR) for the Cox's regression model and the sub-distribution (sdHR) for the competing risk model for the final set of variables remaining after backward stepwise removal for each of ACD, AD and VD. For ACD we found a significant independent association of increased arteriolar fractal dimension (FDA) with increased risk of incident ACD (csHR 1.17; 1.08–1.26). Accounting for competing risk of death resulted in little change (sdHR 1.16; 1.08–1.25). Furthermore, wider arteriolar calibre (CRAE) was associated with a reduced risk (csHR 0.9; 0.83–0.98) although this was no longer significant when considering competing risk of death (sdHR 0.93; 0.86–1.00).
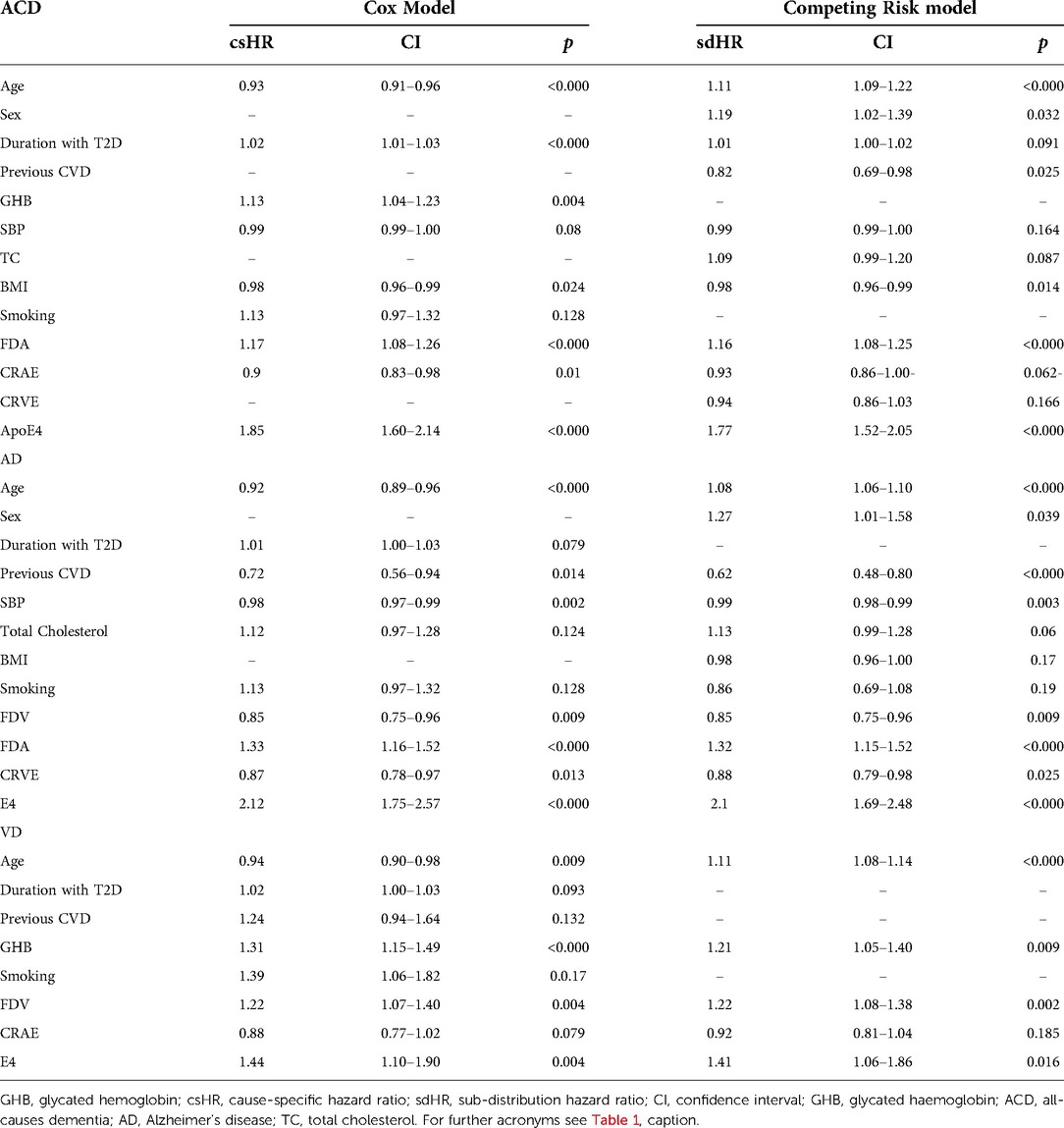
Table 2. Covariates remaining in the Cox and competing risk models after stepwise removal for ACD, AD and VD (- denotes removed covariable).
For AD we found increasing venular (FDV) and increasing arteriolar (FDA) fractal dimensions associated with a reduced (csHR 0.85; 0.74–0.96) and increased (csHR 1.33; 1.16–1.52) risk respectively. Accounting for competing risks of death did not appreciably change these associations. The ratio of FDA to FDV was also independently significant (1.24; 1.10–1.35). Wider venules indicated by an increased CRVE (0.87; 0.78–0.97) was significantly and independently associated with reduced risk. Again, accounting for competing risk of death did not modify this association.
For VD the associations were markedly different compared to findings for AD. In this case increased FDV alone was associated with increased risk of VD (csHR 1.22, 1.07–1.40). Again this did not change accounting for competing risks of death. There was no indication that the ratio of FDA to FDV was important in this setting. Although CRAE remained in the model indicating a reduced risk it did not achieve significance in either the Cox model or the competing risks model. Measures of tortuosity were dropped from all of the models and therefore did not appear to have an impact on dementia risk in our study.
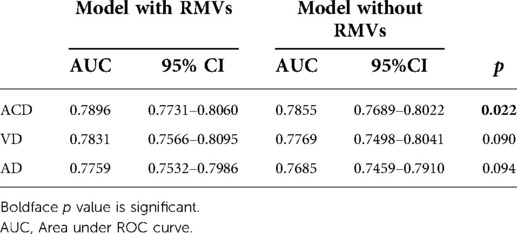
Table 3 provides the results of our ROC analysis. We found that for each of ACD, AD and VD there was a small increase in the AUC when RVMs were included in the model in addition to genetic information (ApoE4 genotype) and clinical covariates compared to the AUC when only considering genetic and clinical covariates. However, this increase only reached significance in the larger ACD population where the AUC using clinical covariates with genetic data alone was 0.7855 and with inclusion of RVMs it was 0.7896, (p = 0.022). Table 4 provides the NRI, its components NRIe and NRIne as well as the IDI metrics for the same comparison. Supplementary Table S1 provides the full NRI tables. Significant increases in both IDI and NRI were found for all models with inclusion of RVMs. However, it can be seen from the negative NRIne for ACD and VD that this was at the expense of an increase in upgrading non-events to an increased risk category.
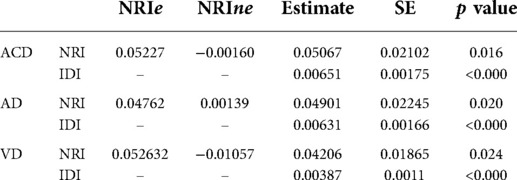
Table 4. Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) for inclusion of RVMs.
Discussion
To the best of knowledge, we have undertaken the largest prospective study to date of the association of three previously established and widely investigated RVMs, namely, venular and arteriolar calibre, tortuosity and fractal dimension with incident dementia. We found that an increase in retinal arteriolar fractal dimension (FDA) and a reduced arteriolar calibre (CRAE) were associated with increased risk of developing ACD with a median of just less than 10 years in the future. Using our previously validated methodology (8) we have separately considered the two major subtypes of dementia, AD and VD. Here we found marked differences in the direction of association of arteriolar and venular fractal dimension (FDV) between these sub-types. Increases in FDA and FDV were independently associated with respectively, increased and decreased risk of AD. On the other hand, for VD, FDA had no evidence of an association whereas increased FDV was associated with increased risk. With respect to retinal vascular calibre, we found that whereas increased CRAE was associated with a reduced risk of VD, although this did not reach significance and was further attenuated when accounting for competing risk of death, wider venules (i.e., CRVE) was associated with a reduced risk of AD. These findings were all independent of a comprehensive range of clinical covariates and also independent of ApoE4 genotype which, other than age, is the strongest known predictor of dementia (22). Measures of vascular tortuosity were not selected in any of the models and this appeared to be mainly due to the simultaneous inclusion of other RVMs in our stepwise models indicating a degree of interdependence and co-linearity as indicated by our correlation matrix. Because RVMs have been associated with a range of vascular-based health outcomes (18), indicating the possibility our findings may be influenced by healthy survivor bias, we undertook a competing risk of death which did not appreciably modify these findings. Finally, we have also demonstrated that RVMs used for this study provide small increments in prediction accuracy for dementia beyond ApoE4 genotype and a broad range of relevant clinical covariates. For the larger study of ACD, the increase in AUC was significant whereas for the smaller sub-studies of AD and VD, although the increases were of a similar magnitude, due to the wider confidence intervals the increase did not achieve significance. A previous case control study has also demonstrated that RVMs can increase the AUC for prediction of AD (23). While this study considered a larger number of RVMs, it did not consider their independence from Apo E4. While evaluation of risk prediction models requires measures of model performance, like the ROC metric, its interpretation is based upon the ability of the predicted probabilities to correctly discriminate between randomly selected diseased and non-diseased subjects. It is not uncommon for the AUC to show only small and non-significant improvements, even for a predictor variable that makes a significant independent contribution. We therefore incorporated previously proposed alternative methods for evaluating the utility of a new marker, especially for cohort studies, that included event-specific reclassification tables and integrated discrimination improvement (24). When using these metrics, we found that RVMs significantly improved risk stratification for each of ACD, AD and VD. This further highlights the concept of the retina potentially containing clinically useful information for predicting future brain health and the potential value of developing further disease-specific imaging biomarkers from retinal images. Importantly, however, in the case of ACD and VD, the positive significant NRI was at the expense of an increased number of non-events being upgraded to a higher risk category. As has previously been indicated (20) when accounting for population prevalence of dementia the NRI value in this case becomes negative with potential implications for use of RVMs for selecting patients for future trials for example.
Our findings in relation to retinal vessel calibre and tortuosity seem to be consistent with previous studies conducted with statistical analysis techniques similar to ours, where generally reduced retinal vascular calibres have been found in relation to dementia and findings for vessel tortuosity have been inconsistent (25, 26). In contrast, some previous studies that have measured retinal fractal dimensions separately for both arterioles and venules have found both reduced FDA and FDV being associated with increased risk of Alzheimer's disease (27–30). However, other studies that considered MRI imaging aspects of brain health indicated a less consistent relationship, with opposing associations of FDA and FDV (31, 32) similar to our findings. VD is largely defined by imaging evidence of cerebrovascular disease, often manifesting as lacunar stroke. There have also been conflicting associations of retinal vascular fractal dimensions with cerebrovascular disease for example with increased (33), decreased (34) or no association (35) with overall fractal dimensions. We note that, in this study, we did not investigate associations with each individual parameter, nor tried to cluster retinal parameters into patterns or groups; see Fetit et al. (36) for relevant work from our group.
There are several reasons why our results may differ from previous studies. Firstly, the design of many studies has been case control and of relatively small size, with the inherent challenges with respect to bias and confounding. We have used a large prospective cohort study design considering the association of RVMs many years in advance of a dementia diagnosis. The only other large prospective study we are aware of is the Rotterdam study which to our best knowledge has to date only considered measures of retinal vascular calibre (5). Interestingly however, this study, in contrast to ours, found increased venular calibre was associated with increased dementia risk. Secondly, unlike these previous studies, our study has been conducted entirely in patients with type 2 diabetes. Given the well-established global microvascular disease associated with diabetes, the relationship between retinal RVMs, brain health and dementia outcomes may be different in this patient group. A further potential issue is the fact that in our study we have used EMR to define our dementia cases whereas smaller case control studies tend to have used directly ascertained and assessed Alzheimer's disease cases. Nevertheless, in our previous study we were able to demonstrate that a gene score specific for Alzheimer's disease was strongly associated with AD cases adjudicated by our EMR algorithm, whereas we found no association of this score with VD cases also adjudicated by our algorithm (8). Furthermore, in that study, as well as in the present study, Apo E4 genotype was significantly associated with both AD and VD. Together these are indicators that the pathoaetiological basis of our case definitions in this study are robust. Finally, there are many different algorithm-based approaches to measurement of RVMs and particularly for FD (37) and these may have a profound impact on associations observed in different studies.
A recent study has further highlighted the potential of retinal fundus images as a source of imaging biomarkers to predict disease state and risk (38). In this case a deep learning approach achieved predictions for cardiovascular disease similar to conventional clinical risk screening but using the retina alone. While such an approach may increase the accuracy of prediction, it does not yield features that can be clinically interpreted. However, that study underscored the retinal vasculature as being important in discriminating disease states. In our study the RVMs employed are not necessarily specific to dementia, i.e., have neither been identified as such clinically or pathologically or specifically developed for example through a targeted machine learning approach. Hence, our results suggest that further increases in prediction accuracy may be possible if retinal biomarkers specific to dementia could be identified. This would have important implications for future dementia research aimed at developing therapeutic interventions for reducing dementia risk.
Microvascular integrity is emerging as an important aspect of the neurodegenerative process and brain health (39). The fractal dimension of a complex branching pattern, such as the retinal vascular tree, can be considered as a measure of how complex the pattern is and how it fills in the surrounding space (40); it can be therefore regarded as a measure of the extent of tissue vascularisation and, by implication, its vascular health and resilience. Retinal vascular fractal dimensions may be important indicators of susceptibility to neurodegenerative disease (41). In this context it is interesting to speculate how our findings of the relative balance between FDV and FDA predispose to one type of disease compared to another. Intra-brain vascular dysregulation is an early pathological event during AD development (42) and the role of venular networks in the perivascular clearance of Aβ is emerging as potentially important (43). Our finding of an increased ratio FDV to FDA being associated with increased future AD risk together with a significantly reduced venular calibre may indicate an underlying suboptimal venular network with a predisposition to impaired Aβ clearance by the venular network. The extent to which these findings indicate an inherent developmental or genetic predisposition or are influenced by other environmental exposures cannot be determined from this work, although the independence of the findings from clinical and genetic covariates perhaps indicates an endogenous predisposition. As the number of genomic studies of retinal vascular parameters increase (44) this be approached with genomic instruments in future studies.
Conclusions
In our large prospective study in patients with type 2 diabetes we have found that RVMs predict future incidence of dementia independently of a wide range of clinical and genetic risk factors. This indicates the possibility that inter-individual developmental or acquired differences in microvascular parameters within the retina can be measured and used to predict susceptibility in patients with diabetes. Further development of such an approach may in future be used in developing strategies for dementia prevention.
Data availability statement
'The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation
Ethics statement
The studies involving human participants were reviewed and approved by Tayside Medical Ethics Committee 053/04. The patients/participants provided their written informed consent to participate in this study.
Author contributions
A.S.F.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. ASFD performed all the analyses and drafted the paper. AN and YH contributed to data extraction from GoDARTS clinical datasets and preparation VAMPIRE data for analysis. ET and TM provided the VAMPIRE software and contributed to finalising the manuscript. PC, GPL and GJM provided clinical expertise and contributed to editing and advising on the manuscript content and discussion. All authors contributed to the article and approved the submitted version.
Funding
The GoDARTS bioresource was funded largely by Wellcome Trust (072960/Z/ 03/Z, 084726/Z/08/Z, 084727/Z/08/Z, 085475/Z/08/Z, and 085475/B/08/Z) and as part of the European Union IMI-SUMMIT program (SUrro- gate markers for Micro- and Macro-vascular hard endpoints for Innovative diabetes Tools from the Innovative Medicines Initiative) (www.imi-summit.eu). Retinal measures were funded by the Engineering and Physical Sciences Research Council (EP/M005976/1) as well as by Medical Research Council (MR/K003364/1, Gareth McKay). This research was also funded by the National Institute for Health Research (NIHR) project reference 16/136/102 using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK Department of Health and Social Care
Acknowledgments
The authors acknowledge all the GoDARTS participants and all personnel at the University of Dundee who have assisted in the development and maintenance of the GoDARTS bioresource. We also acknowledge the Health Informatics Centre at the University of Dundee for the provision of anonymised clinical data from NHS Tayside the original data owner.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2022.945276/full#supplementary-material.
References
1. London A, Benhar I, Schwartz M. The retina as a window to the brain - from eye research to CNS disorders. Nat Rev Neurol. (2013) 9(1):44–53.doi: 10.1038/nrneurol.2012.227
2. Mehta D, Jackson R, Paul G, Shi J, Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? Expert Opin Investig Drugs. (2017) 26(6):735–9. doi: 10.1080/13543784.2017.1323868
3. Klaver CC, Ott A, Hofman A, Assink JJ, Breteler MM, de Jong PT. Is age-related maculopathy associated with Alzheimer’s disease ? Am J Epidemiol. (1999) 150(9):963–8. doi: 10.1093/oxfordjournals.aje.a010105
4. Schrijvers EMC, Buitendijk GHS, Ikram MK, Koudstaal PJ, Hofman A, Vingerling JR, et al. Retinopathy and risk of dementia: the rotterdam study. Neurology. (2012) 79(4):365–70. doi: 10.1212/WNL.0b013e318260cd7e
5. de Jong F, Schrijvers E, Ikram M, Koudstaal P, De Jong P, Hofman A, et al. Retinal vascular caliber and risk of dementia. Neurology. (2011) 76(9):816–21. doi: 10.1212/WNL.0b013e31820e7baa
6. Xue M, Xu W, Ou Y-N, Cao X-P, Tan M-S, Tan L, et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. (2019) 55:100944. doi: 10.1016/j.arr.2019.100944
7. Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. (2016) [cited 2019 Jan 3] 131(5):659–85. doi: 10.1007/s00401-016-1571-z
8. Doney ASF, Bonney W, Jefferson E, Walesby KE, Bittern R, Trucco E, et al. Investigating the relationship between type 2 diabetes and dementia using electronic medical records in the GoDARTS bioresource. Diabetes Care. (2019) [cited 2019 Oct 7] 42(10):1973–80. doi: 10.2337/dc19-0380
9. Hébert HL, Shepherd B, Milburn K, Veluchamy A, Meng W, Carr F, et al. Cohort profile: genetics of diabetes audit and research in tayside Scotland (GoDARTS). Int J Epidemiol. (2017) (2018) 47(2):380–1. Available from: http://academic.oup.com/ije/article/doi/10.1093/ije/dyx140/4107246/Cohort-Profile-Genetics-of-Diabetes-Audit-and.
10. Perez-Rovira A, MacGillivray T, Trucco E, Chin KS, Zutis K, Lupascu C, et al. VAMPIRE: Vessel assessment and measurement platform for images of the REtina. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society [Internet]. IEEE (2011) [cited 2018 Jan 13]. p. 3391–4. Available from: http://ieeexplore.ieee.org/document/6090918/
11. Lupascu C, Tegolo D, Trucco E. Accurate estimation of retinal vessel width using bagged decision trees and an extended multiresolution Hermite model. Med Image Anal. (2013) 17(8):1164–80. doi: 10.1016/j.media.2013.07.006
12. Mookiah MRK, Hogg S, MacGillivray TJ, E Trucco on behalf of the INSPIRED consortium. On the quantitative effects of compression of retinal fundus images on morphometric vascular measurements in VAMPIRE. Comput Methods Programs Biomed. (2021) 202:105969. doi: 10.1016/j.cmpb.2021.105969
13. Pellegrini E, Robertson G, MacGillivray TJ, van Hemert J, Houston G, Trucco E. A Graph Cut Approach to Artery/Vein Classification in Ultra-Widefield Scanning Laser Ophthalmoscopy. IEEE Trans on Medical Imaging. (2018) 37(2):516–26. doi: 10.1109/TMI.2017.2762963
14. Giachetti A, Ballerini L. E trucco: accurate and reliable segmentation of the optic disc in digital fundus images. SPIE J Med Imaging. (2014) 1(2). doi: 10.1117/1.JMI.1.2.024001
15. Cologne J, Hsu W-L, Abbott RD, Ohishi W, Grant EJ, Fujiwara S, et al. Proportional hazard regression in epidemiological follow-up studies: an intuitive consideration of primary time scale. Epidemiology. (2021) 23(4):565–73. doi: 10.1097/EDE.0b013e318253e418
16. Hurley AM. A reference relative time-scale as an alternative to chronological age for cohorts with long follow-up. Emerg Themes Epidem. (2015) 12–8. doi: 10.1186/s12982-015-0043-6
17. Thiébaut ACM, Bénichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med. (2004) 23(24):3803–20. doi: 10.1002/sim.2098
18. MacGillivray TJ, Trucco E, Cameron JR, Dhillon B, Houston JG, van Beek EJRR. Retinal imaging as a source of biomarkers for diagnosis, characterization and prognosis of chronic illness or long-term conditions. Br J Radiol. (2014) [cited 2018 Jan 13] 87(1040):20130832. doi: 10.1259/bjr.20130832
19. Pencina MJ, D’Agostino RB, Pencina KM, Janssens ACJW, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. (2012) 176(6):473–81. doi: 10.1093/aje/kws207
20. Kerr KF, Wang Z, Janes H, Mcclelland RL, Pepe MS. Net reclassification: critical review. Epidemiol. (2015) 25(1):114–21. doi: 10.1097/EDE.0000000000000018
21. Byberg L, Gedeborg R, Michae K. Useful tests of usefulness of new risk factors : tools for assessing reclassification and discrimination. Scand J Public Health. (2011) 39(4):439–41. doi: 10.1177/1403494810396556
22. Michaelson DM. APOE Ε4: the most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimer’s Dement. (2014) [cited 2019 Jan 7] 10(6):861–8. doi: 10.1016/j.jalz.2014.06.015
23. Cheung CYL, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimer’s Dement. (2014) 10(2):135–42. doi: 10.1016/j.jalz.2013.06.009
24. Pencina MJ, D’Agostino Sr RB, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker : from area under the ROC curve to reclassification and beyond. Stat Med. (2008) 27(2):157–72. doi: 10.1002/sim.2929
25. Wu H, Wang C, Chen C, Xu X, Zhu Y, Sang A, et al. Association between retinal vascular geometric changes and cognitive impairment: a systematic review and meta-analysis. J Clin Neurol. (2020) 16(1):19–28. doi: 10.3988/jcn.2020.16.1.19
26. McGrory S, Cameron JR, Pellegrini E, Warren C, Doubal FN, Deary IJ, et al. The application of retinal fundus camera imaging in dementia: a systematic review. Alzheimer’s Dement (Amst). (2017) (2016) 6:91–107. doi: 10.1016/j.dadm.2016.11.001
27. Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry. (2013) [cited 2019 Jan 17] 3(2):e233. doi: 10.1038/tp.2012.150
28. Cheung CY, Ong S, Ikram MK, Ong YT, Chen CP, Venketasubramanian N, et al. Retinal vascular fractal dimension is associated with cognitive dysfunction. J Stroke Cerebrovasc Dis. (2014) [cited 2019 Jan 17] 23(1):43–50. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.002
29. Williams MA, McGowan AJ, Cardwell CR, Cheung CY, Craig D, Passmore P, et al. Retinal microvascular network attenuation in Alzheimer’s disease. Alzheimer’s Dement Diagnosis. Assess Dis Monit. (2015) 1(2):229–35. doi: 10.1016/j.dadm.2015.04.001
30. Jung NY, Han JC, Ong YT, lui CC, Chen CP, Wong TY, et al. Retinal microvasculature changes in amyloid-negative subcortical vascular cognitive impairment compared to amyloid-positive Alzheimer’s disease. J Neurol Sci. (2019) 396:94–101. doi: 10.1016/j.jns.2018.10.025
31. Crystal HA, Holman S, Lui YW, Baird AE, Yu H, Klein R, et al. Association of the fractal dimension of retinal arteries and veins with quantitative brain MRI measures in HIV-infected and uninfected women. PLoS One. (2016) 11(5):1–11. doi: 10.1371/journal.pone.0154858
32. Hilal S, Ong YT, Cheung CY, Tan CS, Venketasubramanian N, Niessen WJ, et al. Microvascular network alterations in retina of subjects with cerebral small vessel disease. Neurosci Lett. (2014) 577:95–100.doi: 10.1016/j.neulet.2014.06.024
33. Cheung N, Liew G, Lindley RI, Liu EY, Wang JJ, Hand P, et al. Retinal fractals and acute lacunar stroke. Ann Neurol. (2010) 68(1):107–11. doi: 10.1002/ana.22011
34. Doubal FN, MacGillivray TJ, Patton N, Dhillon B, Dennis MS, Wardlaw JM. Fractal analysis of retinal vessels suggests that a distinct vasculopathy causes lacunar stroke. Neurology. (2010) 74(14):1102–7. doi: 10.1212/WNL.0b013e3181d7d8b4
35. Cheung CYL, Tay WT, Ikram MK, Ong YT, De Silva DA, Chow KY, et al. Retinal microvascular changes and risk of stroke: the Singapore Malay eye study. Stroke. (2013) 44(9):2402–8. doi: 10.1161/STROKEAHA.113.001738
36. Fetit AE, Doney AS, Hogg S, Wang R, MacGillivray T, Joanna M, Wardlaw JM, et al. A computational approach to multimodal risk stratification for cardiovascular disease in patients with type 2 diabetes incorporating retinal, genomic and clinical features. Sci Rep. (2019) 9:3591. doi: 10.1038/s41598-019-40403-1
37. Huang F, Dashtbozorg B, Zhang J, Bekkers E, Abbasi-Sureshjani S, Berendschot TTJM, et al. Reliability of using retinal vascular fractal dimension as a biomarker in the diabetic retinopathy detection. J Ophthalmol. (2016) 2016:6259047. doi: 10.1155/2016/6259047
38. Poplin R, Varadarajan A V, Blumer K, Liu Y, McConnell M V, Corrado GS, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. (2018) 2(3):158–64. doi: 10.1038/s41551-018-0195-0
39. Erdener ŞE, Dalkara T. Small vessels are a big problem in neurodegeneration and neuroprotection. Front Neurol. (2019) 10:1–20. doi: 10.3389/fneur.2019.00889
40. Patton N, Aslam TM, MacGillivray T, Deary IJ, Dhillon B, Eikelboom RH, et al. Retinal image analysis: concepts, applications and potential. Prog Retin Eye Res. (2006) 25(1):99–127. doi: 10.1016/j.preteyeres.2005.07.001
41. Lemmens S, Devulder A, Van Keer K, Bierkens J, De Boever P, Stalmans I. Systematic review on fractal dimension of the retinal vasculature in neurodegeneration and stroke: assessment of a potential biomarker. Front Neurosci. (2020) 14. doi: 10.3389/fnins.2020.00016
42. Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC, Weiner MW, et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. (2016) 7:11934. doi: 10.1038/ncomms11934
43. Morrone CD, Bishay J, McLaurin J. Potential role of venular amyloid in Alzheimer’s disease pathogenesis. Int J Mol Sci. (2020) 21(6):1–18. doi: 10.3390/ijms21061985
Keywords: retinal biomarkers, dementia risk prediction, VAMPIRE retinal image software, diabetes, dementia
Citation: Doney ASF, Nar A, Huang Y, Trucco E, MacGillivray T, Connelly P, Leese GP and McKay GJ (2022) Retinal vascular measures from diabetes retinal screening photographs and risk of incident dementia in type 2 diabetes: A GoDARTS study. Front. Digit. Health 4:945276. doi: 10.3389/fdgth.2022.945276
Received: 16 May 2022; Accepted: 10 August 2022;
Published: 31 August 2022.
Edited by:
Giovanni Ometto, City University of London, United KingdomReviewed by:
Rupsa Bhattacharjee, Medical Center, University of California, United StatesRyo Kawasaki, Osaka University, Japan
© 2022 Doney, Nar, Huang, Trucco, MacGillivray, Connelly, Leese and McKay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander S.F. Doney YS5kb25leUBkdW5kZWUuYWMudWs=
Specialty Section: This article was submitted to Personalized Medicine, a section of the journal Frontiers in Digital Health
 Alexander S. F. Doney1*
Alexander S. F. Doney1* Emanuele Trucco
Emanuele Trucco