
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Digit. Health, 30 June 2022
Sec. Connected Health
Volume 4 - 2022 | https://doi.org/10.3389/fdgth.2022.821049
This article is part of the Research TopicDigital Technology for Tobacco Control: Novel Data Collection, Study Designs, and InterventionsView all 9 articles
 Samuel L. Battalio1*
Samuel L. Battalio1* Angela F. Pfammatter1
Angela F. Pfammatter1 Kiarri N. Kershaw1
Kiarri N. Kershaw1 Alexis Hernandez1
Alexis Hernandez1 David E. Conroy2
David E. Conroy2 Bonnie Spring1
Bonnie Spring1Although US tobacco use trends show overall improvement, social disadvantage continues to drive significant disparities. Traditional tobacco cessation interventions and public policy initiatives have failed to equitably benefit socially-disadvantaged populations. Advancements in mobile digital technologies have created new opportunities to develop resource-efficient mobile health (mHealth) interventions that, relative to traditional approaches, have greater reach while still maintaining comparable or greater efficacy. Their potential for affordability, scalability, and efficiency gives mHealth tobacco cessation interventions potential as tools to help redress tobacco use disparities. We discuss our perspectives on the state of the science surrounding mHealth tobacco cessation interventions for use by socially-disadvantaged populations. In doing so, we outline existing models of health disparities and social determinants of health (SDOH) and discuss potential ways that mHealth interventions might be optimized to offset or address the impact of social determinants of tobacco use. Because smokers from socially-disadvantaged backgrounds face multi-level barriers that can dynamically heighten the risks of tobacco use, we discuss cutting-edge mHealth interventions that adapt dynamically based on context. We also consider complications and pitfalls that could emerge when designing, evaluating, and implementing mHealth tobacco cessation interventions for socially-disadvantaged populations. Altogether, this perspective article provides a conceptual foundation for optimizing mHealth tobacco cessation interventions for the socially-disadvantaged populations in greatest need.
Efforts to reduce tobacco use in the US have failed to equitably benefit many socially-disadvantaged populations. Decreased tobacco use over the past two decades is largely because well-resourced, socially-advantaged populations have responded well to public policy efforts and tobacco cessation interventions, reaching endgame levels (<5%) of tobacco use (1, 2). Meanwhile, the prevalence of tobacco use among those who experience significant social disadvantage has either stopped declining or continues to worsen (2). Population-level statistics show significant disparities based on racial and ethnic minority status, poverty, education, and rurality (3–6). Even though quit attempts are common among socially-disadvantaged tobacco users, they rarely succeed, largely because of reliance on non-evidence-based cessation interventions (1, 7, 8). Scalable, effective tobacco cessation interventions with sufficient reach to permeate socially-disadvantaged populations are necessary to redress tobacco use disparities.
Increased recognition of access barriers has led many tobacco experts to turn to digital modalities as a delivery channel for equitable reach. Smartphones, in particular, have become ubiquitous, providing internet connectivity even for those who lack broadband connectivity (9, 10). The ubiquity of smartphones has prompted delivery of evidence-based tobacco cessation interventions to pivot toward mobile health (mHealth) tools. Relatedly, recent best practice guidance encourages state-run programs to leverage digital tools (both web and smartphone) to facilitate mass reach (11). Additionally, because of the barriers it created to in-person health care delivery, the COVID-19 pandemic further fueled the uptake of digitally supported interventions, either as standalone treatment or as a means of supporting remotely-delivered, connected counseling. Given that the pandemic simultaneously ushered in both an exacerbation of existing health disparities and increased use of technology-supported intervention (12–14), it is essential to examine whether the use of digital technologies played any role in worsening disparities.
In this paper, we provide our perspectives on the current state of the science surrounding mHealth interventions to promote health equity in cessation of tobacco use. We will review research on how social determinants of health (SDOH) contribute to tobacco use disparities. Against that backdrop, we propose a conceptual model that can guide the development of mHealth tobacco cessation interventions to address SDOH. In that manner, we hope to ensure that mHealth interventions for tobacco cessation serve the socially disadvantaged populations with greatest need.
It is now accepted that health disparities, including those related to tobacco use, are driven by an interplay of factors that function within and across multiple levels of influence. These factors are commonly referred to as social determinants of health (SDOH). Although widely used, the term SDOH has different meanings based on one's field and scientific background. The term SDOH was originally used as a “catch-all” to designate all health influences that originate and function outside of explicit “medical care.” Even though this usage persists in some sectors today, SDOH are now conceptualized with greater nuance (15, 16).
Most current definitions share two unifying themes. First, modern definitions tend to place greater emphasis on extra-individual rather than intra-individual influences. Second, SDOH are now conceptualized less as a collection of independent influences, and more as factors that function interdependently to influence health across various domains (e.g., biological, behavioral, physical/built environment) and levels (e.g., individual, interpersonal, community) (17).
A substantial body of research links various SDOH with tobacco use through direct and indirect mechanisms. Below, we provide a brief, non-exhaustive overview of particularly well-studied SDOH and the mechanisms by which they are thought to influence tobacco use.
The most substantially researched SDOH are probably those that contribute to individual-level socioeconomic status (SES). The primary SES factors that have been studied include education, income, occupational status, and insurance status (18). Findings indicate that individuals living in poverty smoke for twice as many years as those above the poverty line (19). Moreover, even though the number of quit attempts between those living above vs. below the poverty line are comparable, success at quitting is substantially lower among those of low SES (19, 20).
Increasingly, it is recognized that individual SES may be a proxy for extra-individual influences on tobacco use. Businelle et al. (18) modeled the direct and indirect relationships between individual SES and smoking cessation status. They found that individual SES, measured by combining education, income, insurance status, and employment status, had both direct and indirect effects on smoking cessation status. Indirect effects were mediated partly by extra-individual factors (self-reported neighborhood disadvantage and social support), and partly by intra-individual factors (negative affect and personal agency). To understand extra-individual factors that may share variance with SES, Cambron et al. (21) leveraged ecological momentary assessment to model the relationship between individual SES, social contextual factors (e.g., pro-smoking social contexts, cigarette availability), and smoking lapse. Their finding—that low-SES individuals were more likely to be exposed to pro-smoking social contexts—highlights the fact that individual-level measures of SES may actually encompass variance that is better explained by extra-individual SDOH effects.
Neighborhood environmental and contextual factors have also been shown to play a large role in tobacco use via numerous mechanisms. Upstream neighborhood-level factors in low-SES neighborhoods include greater density of tobacco marketing (22) and tobacco retail outlets (23), which have been directly linked with tobacco use. Ecological momentary assessment and geospatial information systems (GIS) methods show that exposure to point-of-sale tobacco is associated with greater likelihood of same day lapse (24). Moreover, numerous measures of neighborhood-level social disadvantage, including area-level unemployment, low safety, high crime, and low social cohesion, are associated with greater rates of tobacco use and lower rates of tobacco cessation success (25–27). Importantly, these neighborhood-level associations with tobacco use typically remain significant even after controlling for individual-level characteristics (28–31). Although the pathways by which neighborhood social disadvantage affect tobacco use remain unclear, some proposed mechanisms include reduced internal locus of control and elevations in stress and negative affect, each of which has been shown to be an independent driver of tobacco use (32).
Living in a rural area has been established as a longstanding, independent predictor of tobacco use. Longitudinal analyses from the past few decades show that prevalence of tobacco use has declined more rapidly in urban than rural settings (33, 34). Recent estimates suggest that individuals living in rural settings are almost twice as likely as those living in urban areas to smoke cigarettes. Rural residents also have 20% higher lung cancer incidence (35).
Longitudinal analyses suggest that the mechanisms by which rurality influences tobacco use have changed over time. Doogan et al. (34) found that the covariation between rurality and tobacco use during 2007 was statistically explained by differences between urban and rural populations in terms of sociodemographic and psychosocial risk factors (e.g., age, race, education, income, employment status, anxiety, depression, health insurance status). However, in 2014, rurality was directly associated with tobacco use, over and above the effect of those covariates. They posit that this shift may be explained, in part, by differential reach of tobacco control policy efforts, which have preferentially targeted densely-populated, non-rural areas.
Despite being one of the more widely recognized and studied social determinants of tobacco use, problems with operationalizing rurality have posed questions, produced inconsistent findings, and sparked debate. Numerous operational definitions exist, and, as discussed in-depth elsewhere, each carries potential advantages and disadvantages (36–38).
Factors associated with racial and ethnic background play a significant role in tobacco use and its resulting health consequences. Although not a SDOH, racial and ethnic background is partly a proxy for a variety of the SDOH (e.g., racism, discrimination). Race and ethnicity are also, in and of themselves, independent reflections of all the numerous potential exposures that one's identity encapsulates. Estimates consistently show that cigarette use is most prevalent among American Indian/Alaska Natives (23%), followed by African Americans/Blacks (15%) and Non-Hispanic Whites (15%), Hispanic/Latinx (10%), and non-Hispanic Asians (7%) (39). However, an overarching view of tobacco use trends only tells part of the story. For example, despite having comparable tobacco use rates, those who are non-Hispanic Black are more likely than those who are non-Hispanic White to attempt to quit, and those attempts are less likely to be successful (40).
Multiple SDOH contribute to the association between tobacco use and racial and ethnic background. Experiences of discrimination are associated with greater dependence on tobacco products among multiple racial and ethnic minority populations (41). Additionally, SES-related SDOH tend to explain part of the relationship between racial and ethnic background and tobacco use, but the relationship varies depending on racial and ethnic background. For example, among Mexican Americans, some data indicate that financial strain and insurance status are more influential indicators of capacity to quit smoking than are other SES indicators, such as education and income (42). Similarly, among African Americans, unemployment, at both the individual and neighborhood levels, appears to be a particularly important indicator of smoking cessation capacity (26). Upstream factors to which racial and ethnic minority groups tend to be disproportionally exposed include a higher volume of targeted tobacco advertisements, (43, 44) greater density of tobacco retailers, (45) and weak implementation of tobacco control policies (46, 47).
Although research in mHealth tobacco cessation intervention has grown exponentially, this body of evidence is still in its infancy. A recent systematic review of mHealth tobacco cessation interventions identified 18 trials, of which the majority were identified as pilot or feasibility trials (48). Although several studies had acceptable representation of populations that exhibit tobacco use disparities, few explicitly developed an intervention to redress a disparity or to address SDOH. That said, existing work can provide valuable insights to support developing mHealth tobacco cessation interventions that explicitly advance health equity. So far, this intervention development research has tended to take one of two broad approaches.
In one approach, which we term the SDOH Targeting Approach, researchers select a specific, underrepresented group (usually in a specific socio-geographic context). Then, they leverage models of SDOH to create a customized mHealth intervention package designed to engage individuals who match the demographic and socioenvironmental context of interest. For example, a researcher may take an existing mHealth intervention designed for the general population, and use a SDOH framework [e.g., Cultural Accommodation of Substance Abuse Treatment framework (49)] to adapt the treatment linguistically and culturally for adult Hispanic/Latinx cigarette smokers recruited from an outpatient community health clinic in a rural town. The strength of this approach is that the resulting intervention package is likely to be acceptable and feasible because it is adapted to the target population. The intervention is also likely to retain its efficacy so long as the adaptation has not undone any core components of the validated intervention package.
The other common approach, which we refer to as the Generalist Approach, creates or deploys an intervention designed to meet the broad needs of the overall population of tobacco users. Researchers then pilot test the intervention among socioeconomically and socioculturally diverse samples to examine the intervention's acceptability and feasibility in socially-disadvantaged populations. Assuming that no explicit SDOH-based adaptation is necessary, this approach may result in an intervention package that is feasible, acceptable, and generalizable across a broad variety of subpopulations and contexts.
Along with benefits, each of these approaches has potential for significant drawbacks. For example, the SDOH Targeting Approach commits significant research dollars and resources to develop an intervention that is intentionally designed for a niche context, limiting potential generalizability and reach. Although this degree of specific customization might be necessary, that assumption should be rigorously and empirically tested. By contrast, without adhering to an SDOH framework, a Generalist Approach risks alienating subgroups or underperforming in high-need contexts. The shared risk is that either approach may develop a feasible and acceptable pilot intervention, and then move pre-maturely to evaluate the treatment package in a full-scale randomized controlled trial (RCT). That progression leaves many important questions unanswered about the impact and cost of the intervention's components, their ability to accommodate and address SDOH, and the scalability of the full intervention package.
We propose that these risks can be minimized by creating an intervention development pipeline that integrates community-centered methods, SDOH framework(s), and a translational research framework. This conceptual model is depicted in Figure 1. SDOH frameworks are used to measure multilevel determinants and conceptualize their potential role in intervention. Some examples include the NIMHD Research Framework, (17) the social ecological model, (50) or the Healthy People 2030 model of SDOH (51). The translational framework ensures that researchers iteratively and systematically translate information gathered from SDOH frameworks into equitable intervention packages that contain components that improve health equity and forgo those that do not. Examples of translational research frameworks include the Multiphase Optimization Strategy (MOST), (52). Obesity-Related Behavioral Intervention Trials (ORBIT) model, (53) the experimental medicine approach, (54) or the Medical Research Council (MRC) framework (55). Importantly, we propose that community-engaged methods, such as those used within a Community-Based Participatory Research (CBPR) approach (56) or Citizen Science, (57) are necessary to integrate SDOH frameworks and the translational research framework, grounding each stage of intervention development with voices representing the perspectives of populations of need.
Perhaps one of the most novel and conceptually promising opportunities to promote health equity enabled by mHealth intervention technologies is the potential for real-time intervention that adapts dynamically to context (e.g., neighborhood stress exposures). Such interventions, termed just-in-time-adaptive-intervention (JITAI), leverage intensive longitudinal data collected by smartphones and wearable sensors to characterize an individual's real-time context, and then deliver an intervention in moments of elevated need or receptivity (58). Recent optimization research has begun to develop JITAI's for tobacco cessation.
In one study, we randomly delivered a digital stress management intervention prompt during minutes when a recently quit smoker was stressed vs. not stressed. The resulting JITAI will be the decision rule that specifies the optimal temporal context (stressed or not stressed) in which the digital intervention should be delivered to maximize protection against smoking relapse (59). We believe that such JITAIs have promise to promote health equity because they can deliver interventions that act downstream on individual-level determinants (e.g., stress management behaviors) to trigger them when needed to address upstream environmental and contextual SDOH (e.g., environmental stress triggers, such as real-time exposure to tobacco retailers or neighborhood physical danger).
To illustrate, we apply our proposed conceptual model to this line of intervention development research aimed at developing a stress management JITAI to protect against smoking relapse. In Figure 2, we propose a non-exhaustive, hypothetical application of our conceptual model using MOST as our guiding translational research framework. MOST integrates insights from engineering, statistics, and behavioral intervention science, for the “development, optimization, and evaluation of behavioral, biobehavioral, and biomedical interventions” (52). Numerous, comprehensive explanations of MOST methodology exist elsewhere [e.g., (60)].
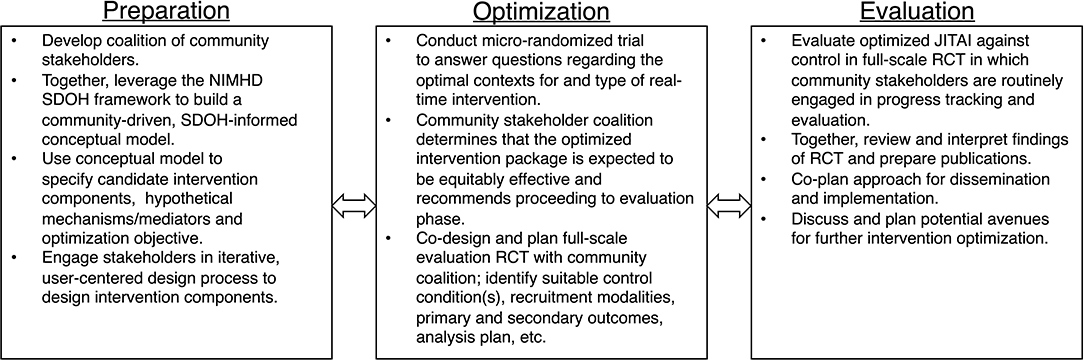
Figure 2. Hypothetical application of proposed conceptual model using the multiphase optimization strategy (MOST).
Multiphase Optimization Strategy is particularly well-equipped to handle the complexity of SDOH and to build interventions that are both effective and scalable enough to tangibly redress tobacco disparities. MOST requires systematic preparation phase work that culminates in measurable objectives and a sound conceptual model to guide subsequent optimization and evaluation. Laying a sound research foundation that links intervention components to SDOH is particularly valuable for developing interventions to promote health equity and eventual uptake. MOST is also specifically used for building interventions that are multicomponent to target different determinants of a risk behavior. To address specific SDOH, researchers may need to systematically add new components, as well as remove or restructure existing components, tasks for which MOST is particularly well-suited.
In our hypothetical intervention development example depicted in Figure 2, we highlight specific activities that use community-based participatory research to infuse consideration of SDOH into preparation, optimization, and evaluation of the intervention. For example, during the preparation phase, researchers form a coalition of community stakeholders and together leverage the NIMHD SDOH framework to build an SDOH-informed conceptual model for optimizing the stress management JITAI to address upstream SDOH. From the foundation of this conceptual model, researchers and community stakeholders identify candidate components (e.g., real-time stress management Apps, triggered based on proximity to high crime zones and tobacco retailers), hypothetical mechanisms and the optimization objective. Researchers then engage community stakeholders in user-centered design of the intervention, ensuring the intervention is appropriate for the intended population. Next, during optimization, researchers conduct a micro-randomized trial (MRT) to answer questions regarding the optimal context(s) and form of real-time intervention. Based on findings from the MRT, the community coalition determines whether the optimized intervention package is expected to be equitably effective and, if so, recommends proceeding to evaluation. Researchers and the community coalition then co-design a full-scale evaluation RCT (e.g., identify suitable comparator condition(s), plan logistics such as recruitment and analytic plan). Finally, in the evaluation stage, the team evaluates the optimized JITAI using the co-designed RCT. Together, the team also reviews and interprets findings, prepares publications, and co-designs the approach to dissemination and implementation. Simultaneously, based on the continual optimization principle, the team discusses avenues to continue improving the intervention.
In this article, we provided a current perspective on the science surrounding mHealth interventions to promote equitable intervention for tobacco cessation. We provided a non-exhaustive overview of how SDOH contribute to tobacco use disparities. Against that backdrop, we reviewed current mHealth tobacco cessation intervention research, highlighting that much of this work is in the early intervention development phase (e.g., formative design, pilot trials), and pointing out important considerations to ensure that intervention development pipeline outputs interventions that tangibly work to redress tobacco use disparities. We propose a conceptual model for developing interventions that uses community-centered methods to systematically integrate SDOH framework(s) with a suitable translational research framework. We close by suggesting several benefits of using MOST as a translational research framework, including capacity to integrate SDOH framework(s) and community-centered methods, and adept handling of multicomponent interventions. We also elevate the JITAI for its potential to support intervention that dynamically accommodates upstream, contextual SDOH exposures.
Despite burgeoning research support for mHealth in tobacco cessation, from the lens of health equity, many limitations may yet be uncovered. Given the relative novelty of mHealth, we may not necessarily know at this stage whether mHealth creates additional barriers or complications to intervening on traditionally marginalized tobacco users. Although mHealth technologies have become increasingly ubiquitous, questions remain regarding their acceptability and feasibility across society's most socially-disadvantaged populations. Indeed, numerous barriers may disproportionately impact socially-disadvantaged populations, including limited technology and internet accessibility, low digital literacy, and linguistic barriers (61, 62). For these reasons, as we continue to uncover opportunities and challenges posed by implementing mHealth for tobacco cessation, it is essential that researchers approach intervention development systematically, in partnership with the community, and through the lens of SDOH.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
SB led the conceptualization, drafting, and editing of this manuscript. AP, KK, AH, DC, and BS assisted with conceptualization, drafting, and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fiore MC, Baker TB. Treating smokers in the health care setting. N Engl J Med. (2011) 365:1222–31. doi: 10.1056/NEJMcp1101512
2. Kingsbury JH, D'Silva J, O'Gara E, Parks MJ, Boyle RG. How much progress have we made? Trends in disparities in tobacco use. Prev Chronic Dis. (2020) 17:E107. doi: 10.5888/pcd17.200090
3. Benowitz N, Blum A, Braithwaite R, Castro F. Tobacco Use Among US Racial/Ethnic Minority Groups-African Americans, American Indians and Alaska natives, Asian Americans and Pacific islanders, and Hispanics: A Report of the Surgeon General. (1998).
4. Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. Natl Vital Stat Rep Jun. (2016) 65:1–122.
5. Schoenborn CA, Adams PF, Peregoy JA. Health behaviors of adults: United States, 2008–2010. Vital Health Stat 10. (2013) 257:1–184.
6. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults—United States, 2019. MMWR Morbid Mortal Wkly Rep. (2020) 69:1736. doi: 10.15585/mmwr.mm6946a4
7. DiGiulio A, Jump Z, Babb S, Schecter A, Williams KA, Yembra D, et al. State medicaid coverage for tobacco cessation treatments and barriers to accessing treatments-United States, 2008–2018. MMWR Morbid Mortal Wkly Rep. (2020) 69:155–60. doi: 10.15585/mmwr.mm6906a2
8. DiGiulio A, Jump Z, Yu A, Babb S, Schecter A, Williams KA, et al. State Medicaid coverage for tobacco cessation treatments and barriers to accessing treatments—United States, 2015–2017. MMWR Morbid Mortal Wkly Rep. (2018) 67:390. doi: 10.15585/mmwr.mm6713a3
9. Hooper MW, Carpenter KM, Salmon EE. Web-based tobacco cessation interventions and digital inequality across US racial/ethnic groups. Ethn Dis. (2019) 29:495. doi: 10.18865/ed.29.3.495
10. Businelle MS. The potential of mHealth for tobacco dependence treatment: domestic and international examples from NCI's smokefree.gov initiative. Nicotine Tob Res. (2014) 16:1033. doi: 10.1093/ntr/ntu071
11. Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs-−2014. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health (2014).
12. Merianos AL, Fevrier B, Mahabee-Gittens EM. Telemedicine for tobacco cessation and prevention to combat COVID-19 morbidity and mortality in rural areas. Front Public Health. (2021) 8:598905. doi: 10.3389/fpubh.2020.598905
13. Bhaskar S, Rastogi A, Menon KV, Kunheri B, Balakrishnan S, Howick J. Call for action to address equity and justice divide during COVID-19. Front Psychiatry. (2020) 11:559905. doi: 10.3389/fpsyt.2020.559905
14. Bhaskar S, Nurtazina A, Mittoo S, Banach M, Weissert R. Editorial: telemedicine during and beyond COVID-19. Front Public Health. (2021) 9:662617. doi: 10.3389/fpubh.2021.662617
15. Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. (2011) 32:381–8. doi: 10.1146/annurev-publhealth-031210-101218
16. Braveman P, Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. (2014) 129 Suppl 2(Suppl 2):19–31. doi: 10.1177/00333549141291S206
17. National Insitute on Minority Health Health Disparities. NIMHD Research Framework. Available online at: https://www.nimhd.nih.gov/about/overview/research-framework.html (accessed June 1, 2022).
18. Businelle MS, Kendzor DE, Reitzel LR, Costello TJ, Cofta-Woerpel L, Li Y, et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol. (2010) 29:262. doi: 10.1037/a0019285
19. Siahpush M, Singh GK, Jones PR, Timsina LR. Racial/ethnic and socioeconomic variations in duration of smoking: results from 2003, 2006, and 2007 tobacco use supplement of the current population survey. J Public Health. (2009) 32:210–8. doi: 10.1093/pubmed/fdp104
20. Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: national longitudinal mortality study. Cancer Causes Control. (2009) 20:417–35. doi: 10.1007/s10552-008-9256-0
21. Cambron C, Hopkins P, Burningham C, Lam C, Cinciripini P, Wetter DW. Socioeconomic status, mindfulness, and momentary associations between stress and smoking lapse during a quit attempt. Drug Alcohol Depend. (2020) 209:107840. doi: 10.1016/j.drugalcdep.2020.107840
22. Lee JGL, Henriksen L, Rose SW, Moreland-Russell S, Ribisl KM. A systematic review of neighborhood disparities in point-of-sale tobacco marketing. Am J Public Health. (2015) 105:e8–18. doi: 10.2105/AJPH.2015.302777
23. Cantrell J, Anesetti-Rothermel A, Pearson JL, Xiao H, Vallone D, Kirchner TR. The impact of the tobacco retail outlet environment on adult cessation and differences by neighborhood poverty. Addiction. (2015) 110:152–61. doi: 10.1111/add.12718
24. Kirchner TR, Cantrell J, Anesetti-Rothermel A, Ganz O, Vallone DM, Abrams DB. Geospatial exposure to point-of-sale tobacco: real-time craving and smoking-cessation outcomes. Am J Prev Med. (2013) 45:379–85. doi: 10.1016/j.amepre.2013.05.016
25. Patterson F, Seravalli L, Hanlon A, Nelson DB. Neighborhood safety as a correlate of tobacco use in a sample of urban, pregnant women. Addict Behav. (2012) 37:1132–7. doi: 10.1016/j.addbeh.2012.05.011
26. Kendzor DE, Reitzel LR, Mazas CA, Cofta-Woerpel LM, Cao Y, Ji L, et al. Individual- and area-level unemployment influence smoking cessation among African Americans participating in a randomized clinical trial. Soc Sci Med (1982). (2012) 74:1394–1401. doi: 10.1016/j.socscimed.2012.01.013
27. Wheeler DC, Do EK, Hayes RB, Fugate-Laus K, Fallavollita WL, Hughes C, et al. Neighborhood disadvantage and tobacco retail outlet and vape shop outlet rates. Int J Environ Res Public Health. (2020) 17:2864. doi: 10.3390/ijerph17082864
28. Shareck M, Ellaway A. Neighborhood crime and smoking: the role of objective and perceived crime measures. BMC Public Health. (2011) 11:930. doi: 10.1186/1471-2458-11-930
29. Rachele JN, Wood L, Nathan A, Giskes K, Turrell G. Neighborhood disadvantage and smoking: examining the role of neighborhood-level psychosocial characteristics. Health Place. (2016) 40:98–105. doi: 10.1016/j.healthplace.2016.04.012
30. Mayne SL, Auchincloss AH, Moore KA, Michael YL, Tabb LP, Echeverria SE, et al. Cross-sectional and longitudinal associations of neighborhood social environment and smoking behavior: the multiethnic study of atherosclerosis. J Epidemiol Community Health. (2017) 71:396–403. doi: 10.1136/jech-2016-207990
31. Ma P, Businelle MS, Balis DS, Kendzor DE. The influence of perceived neighborhood disorder on smoking cessation among urban safety net hospital patients. Drug Alcohol Depend. (2015) 156:157–61. doi: 10.1016/j.drugalcdep.2015.09.004
32. Reitzel LR, Lahoti S, Li Y, Cao Y, Wetter DW, Waters AJ, et al. Neighborhood vigilance, health locus of control, and smoking abstinence. Am J Health Behav. (2013) 37:334–41. doi: 10.5993/AJHB.37.3.6
33. Talbot JA, Williamson ME, Pearson KB, Lenardson J, Ziller E, Jimenez F, et al. Advancing Tobacco Prevention and Control in Rural America. Washington, DC: National Network of Public Health Institutes (2019).
34. Doogan NJ, Roberts ME, Wewers ME, Stanton CA, Keith DR, Gaalema DE, et al. A growing geographic disparity: rural and urban cigarette smoking trends in the United States. Prev Med. (2017) 104:79–85. doi: 10.1016/j.ypmed.2017.03.011
35. Doescher MP, Jackson JE, Jerant A, Gary Hart L. Prevalence and trends in smoking: a national rural study. J Rural Health. (2006) 22:112–8. doi: 10.1111/j.1748-0361.2006.00018.x
36. Hall SA, Kaufman JS, Ricketts TC. Defining urban and rural areas in U. S epidemiologic studies. J Urban Health. (2006) 83:162–75. doi: 10.1007/s11524-005-9016-3
37. Bennett KJ, Borders TF, Holmes GM, Kozhimannil KB, Ziller E. What is rural? Challenges and implications of definitions that inadequately encompass rural people and places. Health Affairs. (2019) 38:1985–92. doi: 10.1377/hlthaff.2019.00910
38. Newby H. The sociology of agriculture: toward a new rural sociology. Ann Rev Sociol. (1983) 9:67–81. doi: 10.1146/annurev.so.09.080183.000435
39. Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. (2019) 68:1013–9. doi: 10.15585/mmwr.mm6845a2
40. Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. (2017) 65:1457–64. doi: 10.15585/mmwr.mm6552a1
41. Kendzor DE, Businelle MS, Reitzel LR, Rios DM, Scheuermann TS, Pulvers K, et al. Everyday discrimination is associated with nicotine dependence among African American, Latino, and White smokers. Nicotine Tob Res. (2014) 16:633–40. doi: 10.1093/ntr/ntt198
42. Vinci C, Guo L, Spears CA, Li L, Correa-Fernández V, Etcheverry PE, et al. Socioeconomic indicators as predictors of smoking cessation among Spanish-Speaking Mexican Americans. Ethn Health. (2019) 24:841–53. doi: 10.1080/13557858.2017.1373074
43. Primack BA, Bost JE, Land SR, Fine MJ. Volume of tobacco advertising in African American markets: systematic review and meta-analysis. Public Health Rep. (2007) 122:607–15. doi: 10.1177/003335490712200508
44. Assari S. Association of educational attainment and race/ethnicity with exposure to tobacco advertisement among US young adults. JAMA Netw Open. (2020) 3:e1919393. doi: 10.1001/jamanetworkopen.2019.19393
45. Reid RJ, Peterson NA, Lowe JB, Hughey J. Tobacco outlet density and smoking prevalence: does racial concentration matter? Drugs: Educ Prev Policy. (2005) 12:233–8. doi: 10.1080/09687630500035485
46. Cwalina SN, Ihenacho U, Barker J, Smiley SL, Pentz MA, Wipfli H. Advancing racial equity and social justice for black communities in US tobacco control policy. Tob Control. (2021). doi: 10.1136/tobaccocontrol-2021-056704
47. Tauras JA. Differential impact of state tobacco control policies among race and ethnic groups. Addiction. (2007) 102:95–103. doi: 10.1111/j.1360-0443.2007.01960.x
48. Chu K-H, Matheny SJ, Escobar-Viera CG, Wessel C, Notier AE, Davis EM. Smartphone health apps for tobacco cessation: a systematic review. Addict Behav. (2021) 112:106616. doi: 10.1016/j.addbeh.2020.106616
49. Burrow-Sanchez JJ, Martinez CR, Hops H, Wrona M. Cultural accommodation of substance abuse treatment for Latino adolescents. J Ethn Subst Abuse. (2011) 10:202–25. doi: 10.1080/15332640.2011.600194
50. Stokols D. Translating social ecological theory into guidelines for community health promotion. Am J Health Promot. (1996) 10:282–98. doi: 10.4278/0890-1171-10.4.282
51. U.S. Department of Health and Human Services OoDPaHP. Healthy People 2030. Available online at: https://health.gov/healthypeople/objectives-and-data/social-determinants-health (accessed June 1, 2022).
52. Collins LM. Conceptual introduction to the multiphase optimization strategy (MOST). Optimization of Behavioral, Biobehavioral, and Biomedical Interventions. Springer International Publishing (2018). p. 1–34. doi: 10.1007/978-3-319-72206-1_1
53. Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, et al. From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. (2015) 34:971–82. doi: 10.1037/hea0000161
54. Riddle M Science of Behavior Change Working G. News from the NIH: using an experimental medicine approach to facilitate translational research. Transl Behav Med. (2015) 5:486–8. doi: 10.1007/s13142-015-0333-0
55. Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. (2021) 374:n2061. doi: 10.1136/bmj.n2061
56. Viswanathan M, Ammerman A, Eng E, Garlehner G, Lohr KN, Griffith D, et al. Community-based participatory research: assessing the evidence: summary. AHRQ evidence report summaries. (2004). doi: 10.1037/e439622005-001
57. Bonney R, Cooper CB, Dickinson J, Kelling S, Phillips T, Rosenberg KV, et al. Citizen science: a developing tool for expanding science knowledge and scientific literacy. BioScience. (2009) 59:977–84. doi: 10.1525/bio.2009.59.11.9
58. Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, et al. Just-in-time adaptive interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann Behav Med. (2018) 52:446–62. doi: 10.1007/s12160-016-9830-8
59. Battalio SL, Conroy DE, Dempsey W, Liao P, Menictas M, Murphy S, et al. Sense2Stop: a micro-randomized trial using wearable sensors to optimize a just-in-time-adaptive stress management intervention for smoking relapse prevention. Contemp Clin Trials. (2021) 109:106534. doi: 10.1016/j.cct.2021.106534
60. Guastaferro K, Strayhorn JC, Collins LM. The multiphase optimization strategy (MOST) in child maltreatment prevention research. J Child Fam Stud. (2021) 30:2481–91. doi: 10.1007/s10826-021-02062-7
61. Smith B, Magnani JW. New technologies, new disparities: the intersection of electronic health and digital health literacy. Int J Cardiol. (2019) 292:280–2. doi: 10.1016/j.ijcard.2019.05.066
Keywords: disparities (health, mHealth (mobile health), tobacco and tobacco product, cessation, disparities (health racial)
Citation: Battalio SL, Pfammatter AF, Kershaw KN, Hernandez A, Conroy DE and Spring B (2022) Mobile Health Tobacco Cessation Interventions to Promote Health Equity: Current Perspectives. Front. Digit. Health 4:821049. doi: 10.3389/fdgth.2022.821049
Received: 23 November 2021; Accepted: 06 June 2022;
Published: 30 June 2022.
Edited by:
David W. Wetter, The University of Utah, United StatesReviewed by:
Sonu M. M. Bhaskar, Liverpool Hospital & South West Sydney Local Health District (SWSLHD), AustraliaCopyright © 2022 Battalio, Pfammatter, Kershaw, Hernandez, Conroy and Spring. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel L. Battalio, c2FtdWVsLmJhdHRhbGlvQG5vcnRod2VzdGVybi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.