- 1Centre for Psychosocial Research in Cancer, Health Sciences, University of Southampton, Southampton, United Kingdom
- 2Patient and Public Contributor, Southampton, United Kingdom
- 3Aston University, College of Health and Life Sciences, School of Optometry, Birmingham, United Kingdom
- 4Department of Gynaecology, St Mary's Hospital, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, United Kingdom
- 5Division of Cancer Science, Faculty of Biology, Medicine and Health, University of Manchester, Oxford Road, Manchester, United Kingdom
- 6College of Medicine and Veterinary Medicine, University of Edinburgh, United Kingdom
- 7Department of Medicine, University of Southampton, Southampton, United Kingdom
Background: People with genetic predispositions to cancer are faced with complex health decisions about managing their risk. Decision aids can support informed, values-based decisions, alongside shared decision-making with a clinician. Whilst diagnoses of genetic predispositions to cancer are increasing, there is no scalable decision aid to support these people. This paper presents an accessible, relevant decision aid template which can be adapted for different predispositions to cancer.
Methods: The decision aid template was co-developed with 12 patients affected by cancer and informed by empirical and theoretical literature. In addition, consultations were conducted with a further 19 people with Lynch syndrome; a specific genetic predisposition to cancer. Clinical stakeholders were consulted regularly. Coulter's framework for decision aid development guided the process, and these activities were complemented by the International Patient Decision Aid Standards, and the latest evidence on communicating risk in decision aids. Programme theory was developed to hypothesise how the decision aid would support decision-making and contextual factors which could influence the process. Guiding principles co-developed with the patient panel described how the decision aid could effectively engage people.
Results: The in-depth co-design process led to the identification of five core components of an accessible decision aid template for people with a genetic predisposition to cancer: defining the decision; a table showing implications of each option; optional further details such as icon arrays to show tailored risk and personal narratives; values clarification activity; and a summary to facilitate discussion with a clinician. Specific guidance was produced describing how to develop each component. The guiding principles identified that the decision aid template needed to promote trust, reduce distress, and be comprehensive, personally relevant and accessible in order to engage people.
Conclusion: Adopting a co-design process helped ensure that the decision aid components were relevant and accessible to the target population. The template could have widespread application through being adapted for different genetic predispositions. The exact content should be co-designed with people from diverse backgrounds with lived experience of the specific predisposition to ensure it is as useful, engaging and relevant as possible.
Introduction
As genetic testing becomes increasingly available and prioritised in mainstream healthcare (1–4) more people are being diagnosed with an increased genetic risk of developing cancer (5). Once diagnosed, their at-risk relatives become eligible for genetic testing too. People with a genetic predisposition to cancer can be faced with an array of complex decisions about their health, including whether and when to have risk-reducing surgery, whether to take medication to reduce risk, and how best to engage in available screening (6–8). Decision aids could be used alongside consultation with healthcare professionals to support these people to make informed, values-based decisions about their options (9). Decision aids have been shown to reduce decisional conflict, improve knowledge and facilitate more realistic expectations about healthcare (9).
However, a systematic literature review identified only six decision aids to support people living with a genetic predisposition to cancer, all of which targeted BRCA carriers (10), demonstrating the need for improved decision support resources for people with other genetic predispositions to cancer. Within the field of genetics, the identification of new variants and rapidly evolving evidence is common (11), suggesting that the development of a decision aid template grounded in users’ preferences and support needs which could be rapidly adapted for different variants could have widespread application. This template would provide a set of core components for the decision aid, as well as guidance about how to write the content. Decision aid templates are also a cost-effective solution given the intensive process of developing new decision aids from scratch (12).
The International Patient Decision Aid Standards (IPDAS) provide guidance regarding best practice for developing decision aid components (13). The IPDAS state that decision aids should be based on evidence, developed using a systematic process, written in plain language, and include information about options, probabilities of outcomes, and values clarification. The Ottawa Patient Decision Aid offers a generic template which meets the IPDAS criteria and allows the decision aid developer to “fill in the gaps” (14). However, this template does not attend to important nuances of how components are worded or presented to ensure acceptability to the target population. Furthermore, the same components may not be appropriate for all decision aids as health decisions and the context in which they are made vary enormously (15–17). There is a need for a template specific enough to ensure clear consistency in the decision aid components, but flexible enough to allow content to be tailored for the specific genetic predisposition.
The template planning and development process was informed by several intervention development approaches. We used tools from the Person-Based Approach, which promotes iterative engagement with the target population to develop in-depth understanding of their beliefs about the health condition and related behaviours, and ensure that the intervention is as engaging and meaningful as possible (18). We also followed Coulter's framework for decision aid development (12) and referred to the updated Medical Research Council (MRC) framework for developing and evaluating complex interventions to ensure that methodological and theoretical considerations were incorporated, such as stakeholder engagement, refining the decision aid template and using programme theory to show the anticipated mechanisms through which the template may support informed decision-making (19).
This paper describes a co-development process to identify the core components and guiding principles for a decision aid template for people living with a genetic predisposition to cancer.
Materials and method
Design
The iterative development of the decision aid template took place from February 2021 to July 2022 and involved multiple cycles of optimisations based on feedback from the target user population and clinical stakeholders. This paper reports the development process using the DEVELOPTOOLS reporting checklist, which specifically focuses on the design of decision aids (20).
Co-development contributors
Patient panel members
Throughout the decision aid development, we worked closely with a patient panel of 12 patients affected by cancer and with an interest in genetics. Some members of our patient panel were invited directly by our panel chair due to a known interest in cancer genetics from previous research projects, some expressed interest to the researchers as they wanted to share their perspective on living with a genetic predisposition, whilst others responded to adverts from patient-led charities such as Lynch Syndrome UK, the National Cancer Research Institute Consumer Forum, and Independent Cancer Patients' Voice who shared a summary of the research via social media or mailouts.
Of the 12 people, four were male, ages ranged from early-20s to mid-50s, eight had a genetic predisposition to cancer, and seven had had cancer.
Public involvement contributors
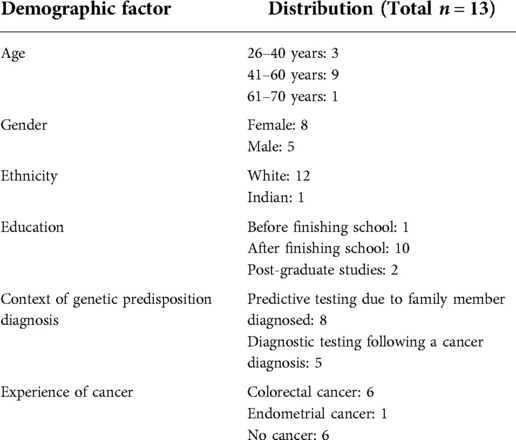
In order to engage with a wider group from our target population, we organised online discussion groups with 19 people with Lynch syndrome; a specific genetic predisposition to cancer. Lynch syndrome is caused by a pathogenic variant in one of five genes, causing increased risks for several cancers including endometrial, ovarian, and colorectal (21). Thirteen of the 19 public involvement contributors with Lynch syndrome completed an optional demographics form after the discussion. This showed a varied distribution in terms of gender and clinical demographics, but most were white and aged between 41 and 60 years, see Table 1.
Clinical/decision aid stakeholders
A world-leading clinician in cancer genetics formed a core part of our research team and inputted to all decisions about the decision aid (DE). In addition, a panel of four stakeholders were recruited to input to the decision aid template development, including gynaecologists with expertise in risk-reducing surgery (EC, NR), a health statistician (PM), and a health psychologist with expertise in developing low literacy decision aids (SS). Stakeholders were invited based on our research team's connections and knowledge of experts in the field.
Co-development procedures
Having risk-reducing surgery to remove the womb and ovaries after diagnosis with Lynch syndrome was chosen as the example decision to inform the decision aid template development. This was chosen because:
a. Diagnosing Lynch syndrome has been identified as a priority by NHS England, as it is one of the most common hereditary cancer predispositions and most people with Lynch syndrome have not been diagnosed (22)
b. Few resources exist for people with Lynch syndrome (10)
c. Whether to have risk-reducing surgery is often a decision faced by people with a genetic predisposition to cancer
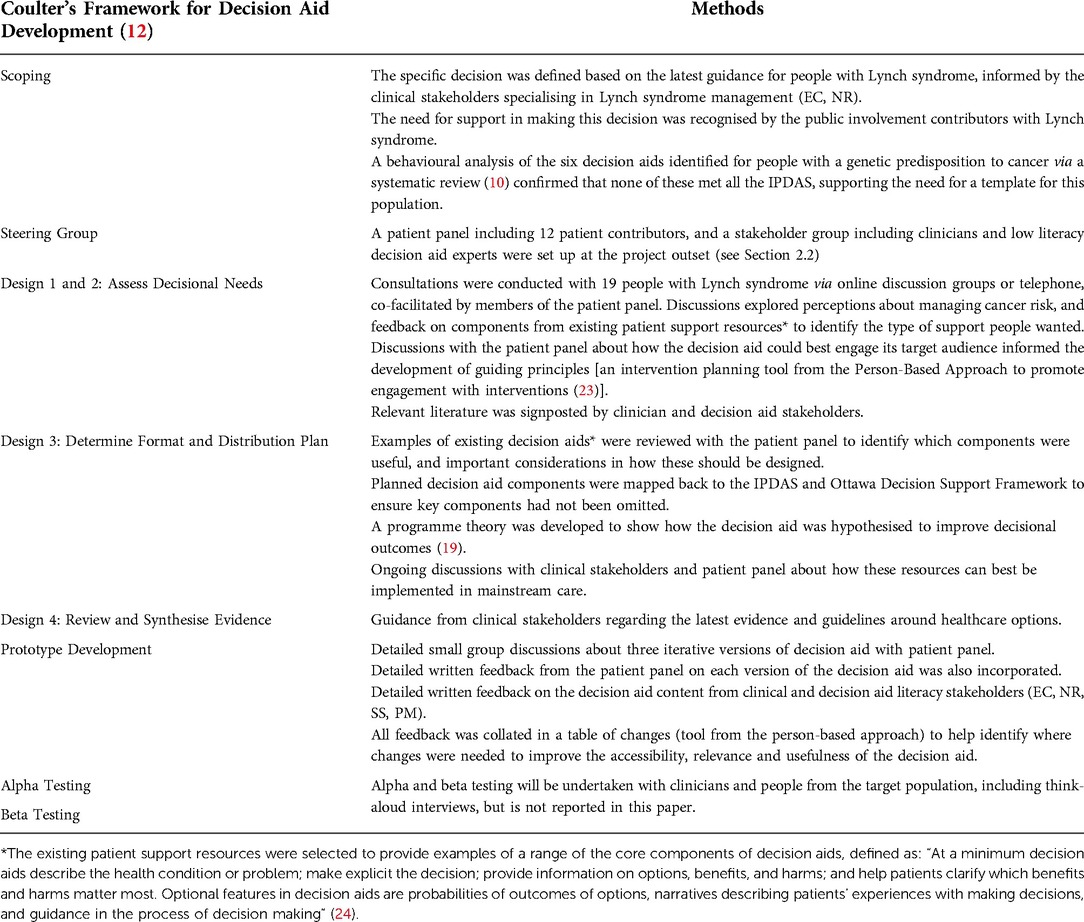
Planning and developing the decision aid template was an iterative process which followed Coulter's framework for decision aid design (12). This is described in Table 2 with reference to how theory, evidence, and public and stakeholder involvement was incorporated throughout. These steps often occurred in parallel.
In order to explore the transferability of the new template, it was then used to develop a decision aid for people considering whether to take aspirin to help manage their genetic predisposition to cancer. Using the core components of the decision aid identified for risk-reducing surgery, we followed a similarly iterative process to plan and optimise the decision aid content, working closely with our patient panel, leading clinical stakeholders specialising in the use of aspirin for managing colorectal cancer risk (JB, KMo, and DC), and a health researcher exploring patients’ and clinicians' perspectives of aspirin for people with Lynch syndrome (KL).
Supplementary material A includes more detail about the engagement methods with the stakeholders.
Process for incorporating public and stakeholder perspectives
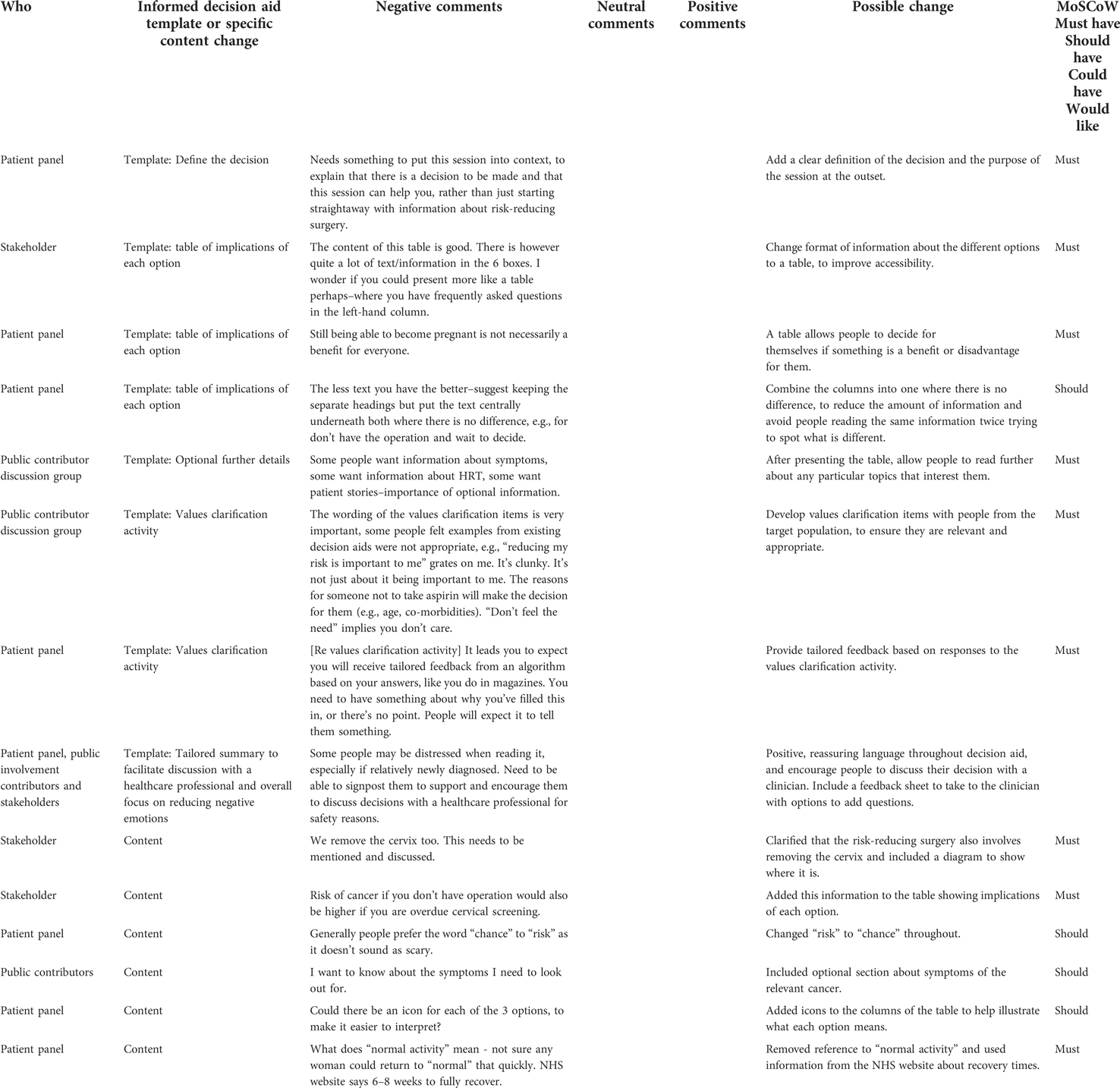
All evidence and feedback from the patient panel, public involvement contributors, and stakeholders was captured in an intervention planning table during the planning phase, and a table of changes during the development phase. This enabled transparent, rigorous recording of the co-development process, and acted as a record of the changes made in each iteration of the decision aid and the rationale behind them (23).
Results
Decision aid template core components
The co-design process identified five components of a decision aid template for supporting people with a genetic predisposition to cancer (©University of Southampton). These were used to develop a decision aid for risk-reducing surgery, and successfully adapted to develop a decision aid for taking aspirin with the same components.
The components are described below with a definition, rationale and example for each. Table 3 shows the sources of evidence informing the inclusion of each component.
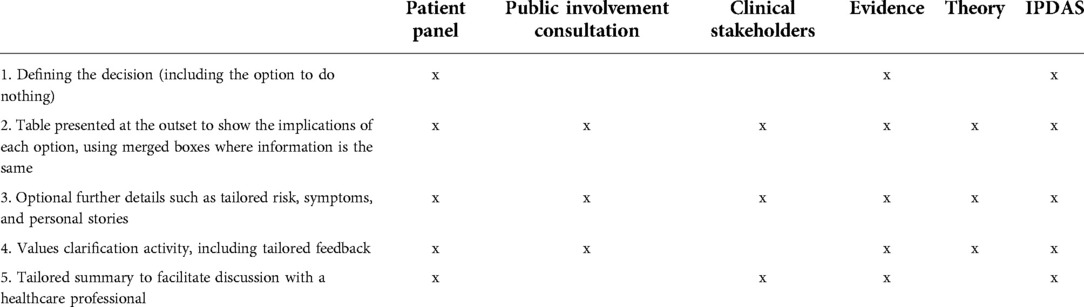
Table 3. Sources of evidence informing the inclusion of each component of the decision aid template (©University of Southampton).
Boxes 1-5 show example content from each component of the prototype decision aid. The content is still being revised in line with feedback from the target population.
1. Defining the decision (including the option to do nothing)
Definition: A clear explanation that there is a decision to be made, which includes the option of doing nothing, and that this decision aid can help you to think about your options.
Rationale: The patient panel indicated that it was important to set the context by letting people know there was a decision to be made, before providing any information. This was seen as particularly relevant for people newly diagnosed with a genetic predisposition, who needed to understand that something about their health has changed and there are now various options available.
This suggestion is consistent with the step in the Ottawa Decision Support Framework of clarifying the decision and inviting participation (14), and the IPDAS criterion of explicitly stating the decision that needs to be considered (13). It was further supported by recommendations from qualitative research exploring perceptions of a decision aid for bowel cancer screening, which suggested that people may be unsure about the purpose of a decision aid and therefore clearly explaining the decision itself at the outset is important (24). Further, in line with IPDAS, the decision aid needed to include the option of doing nothing (13, 25).
Therefore, the decision aid template began by explaining the decision, and how this decision aid can support you (See Box 1).
(2) A table presented at the outset to show the implications of each option, using merged boxes where information is the same
Definition: A table using frequently asked questions with accessible responses for each option. To be included near the start, immediately after the decision is defined.
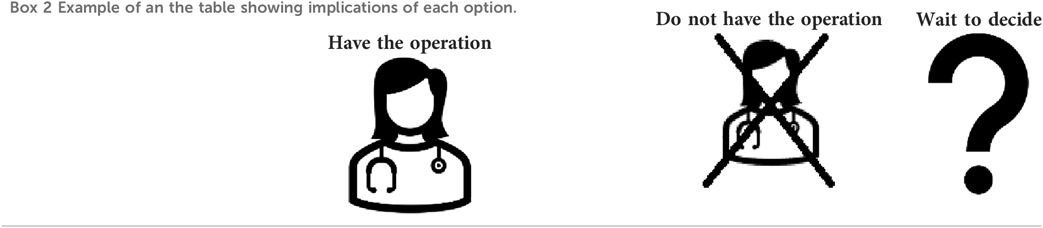
Rationale: Presenting the implications or consequences of each option in a parallel format rather than sequentially is in line with the IPDAS checklist to give a balanced presentation of information to avoid giving one option precedence (25). The template initially aimed to achieve this with a table to enable comparison of the “positive and negative features of the available options” (13). However, feedback from the patient panel indicated that framing the outcomes as positive or negative did not allow for individual differences in how people might perceive them, with some outcomes (such as still being able to get pregnant) possibly a benefit for some people but a disadvantage for others. Furthermore, feedback from an accessibility specialist (SS) suggested that the amount of information listed as benefits and disadvantages for each option made it difficult to compare the consequences. Instead, SS suggested using an a table format which presents the answers to frequently asked questions for each option, to enable easier, rapid comparison of related outcomes (26). This change was implemented, and the patient panel agreed it was both more accessible and more appropriate. Avoiding framing outcomes as either benefits or disadvantages and allowing people to decide for themselves presents an alternative way of implementing the IPDAS criterion of showing positive and negative features of each option (25).
The table was further simplified following feedback from the patient panel that where two options had the same response to a question, these boxes should be merged to save people reading the same information twice and trying to detect if there is a small difference or not. This is in line with guidance to reduce cognitive load as we only have limited capacity to process information about different options simultaneously (25), and it provides a novel approach to achieving this (See Box 2).
(3) Optional further details such as tailored risk, symptoms, and personal stories
Definition: The defining feature of this component is that it is optional, as some people may not want to read further information. The content of the further information may depend on the exact decision being made, but standard components included in this template are: tailored risk information, symptoms of the health condition, other people's stories, and specific further details relating to the decision in question such as possible side effects/outcomes (e.g., menopause) of taking a certain decision (See Box 3).
Rationale: In early versions of the decision aid, users were tunnelled through information to support decision-making before they could access the table. However, the patient panel and public involvement consultations confirmed that people would like the essential information upfront as not everyone wants to read additional details. Therefore, the table was presented at the start of the decision aid, with the chance to read further supporting information afterwards if people were interested. This is in line with fuzzy-trace theory which suggests presenting key information first as people often make decisions based on a gist or feeling rather a rational consideration of all information (26, 27). It is also in line with IPDAS criterion to “highlight essential content, with the option for patients to explore more comprehensive information they find salient” (25).
Specific details about the lessons learned in how to present the optional components are included below:
Tailored risk:
• The literature recommends including a comparison group (e.g., general population) to help people interpret their risk statistics (28, 29), and the patient panel agreed this was important to facilitate understanding but encouraged presentation of this side-by-side with the at-risk population to enable easy comparison. This is consistent with evidence about effective risk communication (30)
• In line with recommendations for transparency, information was clearly provided about the time span over which a risk applied, e.g., life time vs. over the next ten years (28). The patient panel agreed this was important in order to ensure that people were not misled about the impact a decision could have.
• Whilst the evidence suggests framing risks both positively and negatively to avoid influencing people (29), our patient panel preferred less written information about risks. This is in line with another user-focused study which found that presenting risk information in multiple written formats could be confusing for people (31).
Personal stories: Personal stories have been defined as “stories, testimonials, or anecdotes that provide illustrative examples of the experiences of others that are relevant to the decision at hand” (32) and can be particularly liked by people with lower health literacy (33). Initial feedback from the public involvement consultations suggested that some contributors found personal stories engaging, therefore they were included in the decision aid template despite mixed evidence about their effectiveness (32).
The personal stories focused on the process of decision-making rather than the outcomes of decisions people had made, to minimise the risk of influencing people's choices (32, 34). The patient panel reported liking these personal stories, and their comments indicated that the stories may reassure people as well as increase engagement, suggesting that just seeing that someone else has been through the process of making the same decision can be comforting for people. We recommended including one story to describe each available option for a decision, to ensure the options were represented equitably in line with IPDAS (13).
(4) Values clarification activity, including tailored feedback
Definition: Items co-developed with target population to explore relevant personal values, with an algorithm to calculate personal feedback.
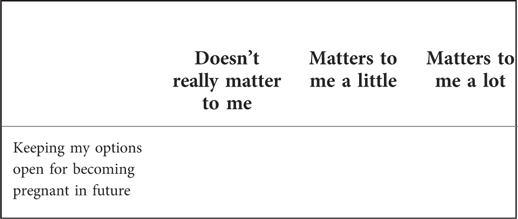
Rationale: The inclusion of values clarification methods to encourage decision-making in line with personal values is well established (20, 35), but this development process showed the importance of the exact wording of the values themselves. Patient panel and public involvement contributor feedback on values clarification activities adapted from existing decision aids indicated that the items could be seen as inappropriate or insensitive, which reduced their perceived value. For example, an item asking “I feel that taking action to lower my chance of getting bowel cancer is very important/slightly important/not important to me” was seen as inappropriate because it implies that someone at increased risk of cancer might not care about reducing their risk, and does not acknowledge that some people may not be able to take action due to other factors, such as age or co-morbidities. Therefore the identification of values to include in the values-based activity was directly informed by the issues raised when talking about the different options during our public involvement consultations, to ensure they were relevant to our specific population (36) (See Box 4).
Feedback from our patient panel also revealed that a tailored summary about which option people were leaning towards was an expected output from this kind of “quiz”, and were disappointed when they were not rewarded in this way for doing the values clarification activity. Therefore, the decision aid template was adapted to use an algorithm to calculate to what extent someone is in favour of an option based on their values. This contrasts slightly with the preference for the table showing implications of each option to avoid imposing assumptions about what is a benefit for people, but is in line with evidence that people want to explicitly see how their values map on to the options available (20).
(5) Tailored summary to facilitate discussion with a healthcare professional
Definition: A tailored summary of patients’ preferences according to their values clarification activity, with explicit encouragement to bring a copy to discuss at the next appointment with a relevant healthcare professional. An open-text box for the user to add any questions for the healthcare professional, and links to relevant supporting material to help them consider what they might want to ask (See Box 5).

Box 5. Example of an excerpt from the tailored summary to facilitate discussion with a healthcare professional
Rationale: The IPDAS recommend providing an output to facilitate shared decision making with a healthcare professional, such as a summary of users' preferences or values, key knowledge gained, or outstanding decisional needs e.g., by prompting the user to think about questions they might want to discuss with their healthcare professional (37). Interestingly, there is limited evidence for the effectiveness of this component in terms of improving informed decision-making, but this is due to a lack of research specifically addressing this question rather than refuting evidence (37). The Winton Centre's research in developing accessible genetic test reports similarly emphasises the importance of “actionability” or clear guidance as to what happens next (31).
The patient panel and public contributors agreed that it was very important that the decision aid was positioned as a tool to be used alongside conversations with a healthcare professional, rather than to reach a decision entirely independently. This was for safety reasons to ensure that all health-related decisions are made in consultation with appropriate specialists as part of shared decision-making. The tailored summary was seen as an important component to reinforce this by explicitly encouraging users to discuss their summary with a healthcare professional. The panel and stakeholders also suggested the type of healthcare professional referred to by the decision aid needs to be appropriate for discussing the decision in question, in order to avoid frustrating experiences of being referred around the healthcare system.
Table 4 provides an excerpt from the table of changes which shows how input from the patient panel, public contributors and stakeholders informed the development of the template, as well as some examples of more specific optimisations to the content. Decisions about the template were largely guided by the patient panel, public contributors and stakeholders specialising in accessible decision aids, whilst clinical stakeholders provided guidance on transparent, accurate information.
Guiding principles
Discussions with our patient panel and the public involvement consultations informed the co-development of guiding principles, see Table 5. These guiding principles complemented the specific structure outlined above by defining how decision aids for this population can best be designed to promote engagement. Key design objectives included promoting trust, reducing distress, being comprehensive, personally relevant and accessible.

Table 5. Guiding principles for engaging target population in decision aid, co-developed with patient panel.
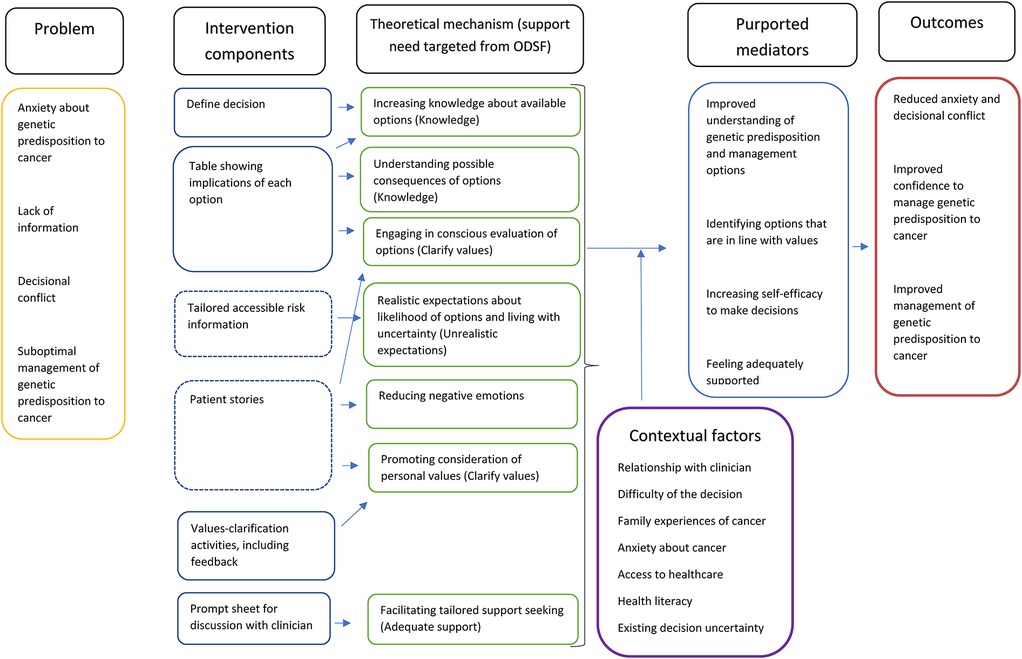
Programme theory
The programme theory to show the mechanisms through which the decision aid template would support decision-making was developed bottom-up, informed by our discussions with the patient panel and public involvement consultations as well as evidence around shared decision-making processes (38). The mechanisms were subsequently mapped onto theory using the model of decision-making outlined by the Ottawa Decision Support Framework which draws on a range of decision-making theories and outlines four modifiable support needs that can reduce decisional conflict: knowledge, realistic expectations, clear values and adequate support (14), see Figure 1. While most of the decision aid mechanisms mapped on to the modifiable factors outlined by the Ottawa Decision Support Framework, negative emotions (such as distress) are described by the framework as a symptom of decisional conflict but not something that can be modified. However, this programme theory included managing negative emotions as a key modifiable mechanism.
In Figure 1, solid lines around intervention components indicate these are core components of the decision aid template, while dotted lines indicate they are optional components which users can choose to view if they are interested.
Adaptation of the template for taking aspirin
The iterative process of adapting the template for another decisional context supported that the five components remained appropriate and relevant, the content could be easily adapted, and no new components were needed. However, it was still essential to work iteratively with relevant clinical specialists and our patient panel to co-design the specific decision aid content, with important optimisations including ensuring the items for the values clarification activity were salient and useful, and determining which optional further details people might want, such as clear communication about the current evidence for the benefit of taking aspirin.
Discussion
This paper presents the first co-developed decision aid template for people to use independently to promote values-based decision making. The template has been named “PredispDA™” (Predisposition Decision Aid template, ©University of Southampton), and can be used to facilitate rapid development of decision aids for different predispositions. Our process shows how the selection and ordering of relevant components and specific content was driven by the user population and subsequently mapped onto theory and evidence rather than being deductively driven. This enabled important adjustments to the template to promote its acceptability to the target population, whilst still meeting all criteria from the IPDAS. Similarly, despite including comparable components to the Ottawa decision aid template, this template uses language and ordering which were user-led to increase acceptability to the target population.
Building on IPDAS
Whilst this decision aid met all the IPDAS criteria, it was not guided solely by the IPDAS in terms of content. For example, a large section of the IPDAS focus on presenting probabilities, but this is only an optional component of the decision aid template for those who want to see it. Meanwhile the format and positioning of the table showing implications of each option to clearly present the outcomes of different options was a critical component of the decision aid template development which underwent several iterations based on feedback from the target population and stakeholders, but the IPDAS are quite open about how this is achieved only stating “The decision support technology makes it possible to compare the positive and negative features of the available options and shows the negative and positive features of options with equal detail (for example using similar fonts, order, and display of statistical information)” (13). The guidance provided in this paper about how to develop and present the core components of the decision aid template helps expand on the IPDAS by taking the target population's preferences into account.
Personal stories
Personal stories are not included in the IPDAS due to the lack of clear evidence for any benefit to decision-making outcomes (32) and are not part of the Ottawa decision aid template, but they were liked by people during initial discussions and perceived to be reassuring and engaging. Therefore, narratives focusing on the decision-making process as opposed to the outcomes were an optional component of this template for users to read if they are interested. Guided by the patient panel, images were chosen to represent the person narrating each story to make the story seem more realistic, which influences the impact of the narrative (39). However, there is a risk that whichever narrative the user feels the strongest connection with may be more likely to influence their decision-making process, and images might exacerbate this situation by triggering feelings of similarity or difference in terms of narrators’ ethnic, gender or cultural identity (34). Real-time interviews with a wider group of people about their perspectives of these personal stories will be used to better understand how they could facilitate or hinder informed decision-making. If the images appear to influence people, more neutral illustrations created by an artist could be used which may not elicit such strong feelings of identity.
In addition to outcome and process narratives, a third type of narrative has been termed experience narratives, which are theorised to offer powerful insights to help people gain a more realistic understanding of what it would be like to follow a certain option, and possibly increase resilience (34). This could be an effective way to implement the IPDAS recommendation of helping people imagine the psychological, physical and social effects of each option (13), but, as with outcome narratives, could risk influencing people and introducing bias. This decision aid template avoided incorporating outcomes or experiences into the personal stories, and instead used them as an opportunity to model values-informed decision-making and reassure people. Considering the mechanism through which the stories were theorised to support people, alongside users' preferences, was useful for ensuring the content was consistent with its purpose.
Values clarification methods
The mode of feedback from the values clarification activity was regarded as important to optimise its practical application to decision-making, with users expecting to see tailored feedback generated from their responses to the values items. Feedback on values clarification activities was not covered by the IPDAS, which only state that ‘The decision support technology asks patients to think about which positive and negative features of the options matter most to them (13), while the Ottawa Patient Decision Aid template suggests people select for themselves which option best aligns with their values (14). However, a recent review of values clarification methods does suggest that ‘multicriteria decision analysis’ be used in decision aids (20), which is defined as “The user is asked to directly indicate the extent to which a decision attribute or outcome matters to them or how good or bad they deem it to be. These values are then used in a model that calculates alignment between what matters to the user and the available decision options”. This suggests that both evidence and user preferences support the incorporation of personalised feedback on values clarification to enhance the usefulness of these methods in decision aids.
However, while attempts have been made to guide decision aid developers in which methods for clarifying values are most effective (20), less attention has been paid to the importance of the wording of the values themselves. In this case, detailed discussion with the target population was essential to ensure that items for values clarification were perceived as genuinely useful, relevant and as recognising the complexities of people's situations, while generically worded items such as “How important is it to you to reduce your risk of cancer?” (14) were seen as irrelevant and irritating which could reduce engagement with these activities.
Strengths and limitations
The co-development process enabled detailed written and verbal input from a dedicated patient panel with an in-depth understanding of the decision aid's purpose and the development process. This was complemented by consultations with a wider group of public involvement contributors living with the genetic predisposition in question, which enhanced understanding of the specific needs and barriers to decision-making for the target population. However, self-reported demographics indicated that most of these public involvement contributors were white and aged between 30 and 60 years. Further work is needed to ensure the decision aid template is appropriate and supportive for people from different ethnic groups and ages, and we will begin to address this by purposively seeking diversity during our think-aloud interviews in the next phase of development. Participants in these interviews will also be asked if they are happy to complete a self-reported health literacy questionnaire, to enable exploration of the extent to which people with lower health literacy have inputted to the development of the decision aid template. However, we recommend that further work with underserved groups including people under 30 and over 70 years, different ethnic communities, LGBTQ+communities, socio-economically disadvantaged people, and people with learning disabilities or physical disabilities will be important to explore how the inclusivity of the template components could be improved. In addition, while public involvement suggests the decision aid template is highly acceptable for the target population, further work is needed using validated psychometric scales to explore the impact of this decision aid template on outcomes.
Conclusions
A decision aid template called PredispDA™ (Predisposition Decision Aid) has been co-developed with people from the target population to provide key components and supporting guidance for anyone wishing to produce decision aids for people living with genetic predispositions. The template was developed through a robust and rigorous process which incorporated best practice guidance alongside in-depth co-development activities with the target population to produce a relevant and accessible template. This template has already been successfully adapted for another decisional context with close input from public involvement contributors and clinical stakeholders, supporting its potential to be further adapted for other genetic predispositions. The guiding principles help to ensure that adaptations to the content will remain engaging and appropriate for the target population, whilst the programme theory provides transparency about how the decision aid is theorised to work which should help developers ensure adaptations are consistent with hypothesised underlying mechanisms.
Given the importance of the target population's input to the co-development of this template, we believe that any adaptations would need close collaboration with people with lived experience of the genetic predisposition in question. The ADAPT guidance for adapting interventions for different contexts emphasises the importance of involving relevant stakeholders, and could be used to help guide this process (40).
While this template has provided a guide to developing key components of a decision aid, a challenge remains in how to provide and fund sustainable open-source software which can be readily updated to facilitate rapid development of digital decision aids for different conditions (41).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Patient Reference Panel
Caroline Dale, Sue Duncombe, Rochelle Gold, Sonia Patton, Warren Rook, Richard Stephens, Lesley Turner, Frankie Vale, Helen White, Ivan Woodward, Steve Worrall, Julie Young.
International Lynch Decision Aid Stakeholders
Munaza Ahmed, Lyndsy Ambler, Antonis Antoniou, Stephanie Archer, Ruth Armstrong, Elizabeth Bancroft, Kristine Barlow-Stewart, Elizabeth Barnett, Marion Bartlett, Julian Barwell, Hilary Bekker, Dany Bell, Cheryl Berlin, Matilda Bradford, John Burn, Sarah Cable, Dharmisha Chauhan, Ruth Cleaver, Elizabeth Coad, Gayatri Connolly, Gillian Crawford, Emma Crosbie, Victoria Cuthill, Tabib Dahir, Karina Dahl Steffensen, Eleanor Davies, Glyn Elwyn, Mary Jane Esplen, D Gareth Evans, Pia Fabricius, Andrea Forman, Kaisa Fritzell, Claire Giffney, Joana Gomes, Rebecca Hall, Helen Hanson, Menna Hawkins, Deborah Holliday, Roberta Horgan, Karen Hurley, Margaret James, Ros Jewell, Sarah John, Siobhan John, Victoria Kiesel, Anna Koziel, Anjana Kulkarni, Fiona Lalloo, Helen Liggett, Aela Limbu, Kate Lippiett, Anne Lowry, Manami Matsukawa, Tracie Miles, Shakira Milton, Pål Møller, Kevin Monahan, Laura Monje-Garcia, Alex Murray, Jennie Murray, Kai-Ren Ong, Anbu Paramasivam, Alison Pope, Sarah Pugh, Gabriel Recchia, Nicola Reents, Peter Risby, Neil Ryan, Sibel Saya, Raza Sayyed, Salma Schickh, Lucy Side, Sian Smith, Tracy Smith, Dawn Stacey, Eriko Takamine, Katrina Tatton-Brown, Helle Vendel Petersen, Robert Volk, Jennifer Wiggins, Lisa Wilde, Jennet Williams, Catherine Willis, Elizabeth Winchester, Kristi Withington, Emma Woodward, Alice Youngs.
Author contributions
Engaged in public involvement activities: KM, KK, LT, the CanGene CanVar patient reference panel Developed the decision aid template: All authors Drafted the manuscript: KM Reviewed the manuscript and approved the content: All authors Met authorship criteria: All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Cancer Research UK [grant no. CG1296/A27223]. EJC is supported by a National Institute for Health and Care Research (NIHR) Advanced Fellowship (NIHR300650) and the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007).
Acknowledgments
We would like to thank each of our patient panel members for their detailed and thoughtful input to the decision aid content: Caroline Dale, Sue Duncombe, Rochelle Gold, Sonia Patton, Warren Rook, Richard Stephens, Lesley Turner, Frankie Vale, Helen White, Ivan Woodward, Steve Worrall, and Julie Young. We would also like to thank our clinical stakeholders whose work has contributed to the decision aid components: Pal Moller and Gabriel Recchia, and our clinical stakeholders who have advised on the content of the aspirin session (which is still being refined): Sir John Burn, Dharmisha Chauhan, Kevin Monahan, and our health researcher specialising in decision-making: Kelly Lloyd. Your input has been invaluable.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2022.1039701/full#supplementary-material.
References
1. Percival N, George A, Gyertson J, Hamill M, Fernandes A, Davies E, et al. The integration of BRCA testing into oncology clinics. Br J Nurs. (2016) 25(12):690–4. doi: 10.12968/bjon.2016.25.12.690
4. Bokkers K, Vlaming M, Engelhardt EG, Zweemer RP, van Oort IM, Kiemeney L, et al. The feasibility of implementing mainstream germline genetic testing in routine cancer care-a systematic review. Cancers. (2022) 14(4):1059. doi: 10.3390/cancers14041059
5. George A, Kaye S, Banerjee S. Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nat Rev Clin Oncol. (2017) 14(5):284–96. doi: 10.1038/nrclinonc.2016.191
6. Segerer R, Peschel C, Kämmerer U, Häussler S, Wöckel A, Segerer S. Factors impacting on decision-making towards prophylactic surgeries in BRCA mutation carriers and women with familial predisposition. Breast Care. (2020) 15(3):253–9. doi: 10.1159/000503370
7. Lloyd K, Foy R, Hall L, Ziegler L, Green S, Haider Z, et al. Barriers and facilitators to using aspirin for preventive therapy: a qualitative study exploring the views and experiences of people with Lynch syndrome and healthcare providers. (2022).
8. Watkins KE, Way CY, Fiander JJ, Meadus RJ, Esplen MJ, Green JS, et al. Lynch syndrome: barriers to and facilitators of screening and disease management. Hered Cancer Clin Pract. (2011) 9(1):8. doi: 10.1186/1897-4287-9-8
9. Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. (2017) 4(4):Cd001431. doi: 10.1002/14651858.CD001431.pub5
10. Kohut K, Morton K, Turner L, Shepherd J, Fenerty V, Woods L, et al. Using patient decision aids to inform decisions about cancer susceptibility genetic testing and risk management: A narrative review of impact and experience. (2021).
11. Taylor A, Brady AF, Frayling IM, Hanson H, Tischkowitz M, Turnbull C, et al. Consensus for genes to be included on cancer panel tests offered by UK genetics services: guidelines of the UK cancer genetics group. J Med Genet. (2018) 55(6):372–7. doi: 10.1136/jmedgenet-2017-105188
12. Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. (2013) 13(Suppl 2):S2. doi: 10.1186/1472-6947-13-S2-S2
13. Joseph-Williams N, Newcombe R, Politi M, Durand M-A, Sivell S, Stacey D, et al. Toward minimum standards for certifying patient decision aids: a modified delphi consensus process. Med Decis Making. (2013) 34(6):699–710. doi: 10.1177/0272989X13501721
14. Ottawa Patient Decision Aids Research Group OHRI. The Ottawa decision support framework (ODSF). (2020) (Available at: https://decisionaid.ohri.ca/docs/develop/ODSF.pdf).
15. Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them—a scoping review. Implement Sci. (2018) 13(1):40. doi: 10.1186/s13012-018-0731-z
16. Trevena L. Commentary on history of IPDAS. Med Decis Making. (2021) 41(7):734–5. doi: 10.1177/0272989X211033274
17. Gillies K, Campbell MK. Development and evaluation of decision aids for people considering taking part in a clinical trial: a conceptual framework. Trials. (2019) 20(1):401. doi: 10.1186/s13063-019-3489-y
18. Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res. (2015) 17(1):e30. doi: 10.2196/jmir.4055
19. Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of medical research council guidance. Br Med J. (2021) 374:n2061. doi: 10.1136/bmj.n2061
20. Witteman HO, Ndjaboue R, Vaisson G, Dansokho SC, Arnold B, Bridges JFP, et al. Clarifying values: an updated and expanded systematic review and meta-analysis. Med Decis Making. (2021) 41(7):801–20. doi: 10.1177/0272989X211037946
21. Tiwari AK, Roy HK, Lynch HT. Lynch syndrome in the 21st century: clinical perspectives. Int J Med. (2015) 109(3):151–8. doi: 10.1093/qjmed/hcv137
23. Bradbury K, Morton K, Grist R, Band R, Van Woezik A, Connelly D, et al. Using the person-based approach to optimise a digital intervention for the management of hypertension. PLoS One. (2018) 13(5):e0196868. doi: 10.1371/journal.pone.0196868
24. Smith SK, Kearney P, Trevena L, Barratt A, Nutbeam D, McCaffery KJ. Informed choice in bowel cancer screening: a qualitative study to explore how adults with lower education use decision aids. Health Expect. (2014) 17(4):511–22. doi: 10.1111/j.1369-7625.2012.00780.x
25. Martin RW, Brogård Andersen S, O’Brien MA, Bravo P, Hoffmann T, Olling K, et al. Providing balanced information about options in patient decision aids: an update from the international patient decision aid standards. Med Decis Making. (2021) 41(7):780–800. doi: 10.1177/0272989X211021397
26. Elwyn G, Lloyd A, Joseph-Williams N, Cording E, Thomson R, Durand MA, et al. Option grids: shared decision making made easier. Patient Educ Couns. (2013) 90(2):207–12. doi: 10.1016/j.pec.2012.06.036
27. Reyna VF. A theory of medical decision making and health: fuzzy trace theory. Med Decis Making. (2008) 28(6):850–65. doi: 10.1177/0272989X08327066
28. Bonner C, Trevena LJ, Gaissmaier W, Han PKJ, Okan Y, Ozanne E, et al. Current best practice for presenting probabilities in patient decision aids: fundamental principles. Med Decis Making. (2021) 41(7):821–33. doi: 10.1177/0272989X21996328
29. Trevena LJ, Bonner C, Okan Y, Peters E, Gaissmaier W, Han PKJ, et al. Current challenges when using numbers in patient decision aids: advanced concepts. Med Decis Making. (2021) 41(7):834–47. doi: 10.1177/0272989X21996342
30. Recchia G, Lawrence ACE, Freeman ALJ. Investigating the presentation of uncertainty in an icon array: a randomized trial. PEC Innovation. (2022) 1:100003. doi: 10.1016/j.pecinn.2021.100003
31. Recchia G, Chiappi A, Chandratillake G, Raymond L, Freeman ALJ. Creating genetic reports that are understood by nonspecialists: a case study. Genet Med. (2020) 22(2):353–61. doi: 10.1038/s41436-019-0649-0
32. Shaffer VA, Brodney S, Gavaruzzi T, Zisman-Ilani Y, Munro S, Smith SK, et al. Do personal stories make patient decision aids more effective? an update from the international patient decision aids standards. Med Decis Making. (2021) 41(7):897–906. doi: 10.1177/0272989X211011100
33. Bekker HL, Winterbottom AE, Butow P, Dillard AJ, Feldman-Stewart D, Fowler FJ, et al. Do personal stories make patient decision aids more effective? a critical review of theory and evidence. BMC Med Inform Decis Mak. (2013) 13(2):S9. doi: 10.1186/1472-6947-13-S2-S9
34. Shaffer VA, Focella ES, Hathaway A, Scherer LD, Zikmund-Fisher BJ. On the usefulness of narratives: an interdisciplinary review and theoretical model. Ann Behav Med. (2018) 52(5):429–42. doi: 10.1093/abm/kax008
35. Elwyn G, O'Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international delphi consensus process. Br Med J. (2006) 333(7565):417. doi: 10.1136/bmj.38926.629329.AE
36. Hackert MQN, Ankersmid JW, Engels N, Prick JCM, Teerenstra S, Siesling S, et al. Effectiveness and implementation of SHared decision-making supported by OUTcome information among patients with breast cancer, stroke and advanced kidney disease: SHOUT study protocol of multiple interrupted time series. BMJ Open. (2022) 12(8):e055324. doi: 10.1136/bmjopen-2021-055324
37. Rahn AC, Jull J, Boland L, Finderup J, Loiselle MC, Smith M, et al. Guidance and/or decision coaching with patient decision aids: scoping reviews to inform the international patient decision aid standards (IPDAS). Med Decis Making. (2021) 41(7):938–53. doi: 10.1177/0272989X21997330
38. Waldron T, Carr T, McMullen L, Westhorp G, Duncan V, Neufeld S-M, et al. Development of a program theory for shared decision-making: a realist synthesis. BMC Health Serv Res. (2020) 20(1):59. doi: 10.1186/s12913-019-4649-1
39. van Laer T, de Ruyter K, Visconti LM, Wetzels M. The extended transportation-imagery model: a meta-analysis of the antecedents and consequences of Consumers’ narrative transportation. J Consum Res. (2013) 40(5):797–817. doi: 10.1086/673383
40. Moore G, Campbell M, Copeland L, Craig P, Movsisyan A, Hoddinott P, et al. Adapting interventions to new contexts—the ADAPT guidance. Br Med J. (2021) 374:n1679. doi: 10.1136/bmj.n1679
Keywords: decision aid (DA), template, genetic predisposition, cancer, patient and public involvement (PPI), person-based approach
Citation: Morton K, Kohut K, Turner L, Smith S, Crosbie EJ, Ryan N, Grimmett C, Eccles DM, Foster C and The CanGene CanVar Patient Reference Panel, The International Lynch Decision Aid Stakeholder (LDAS) Panel (2022) Person-based co-design of a decision aid template for people with a genetic predisposition to cancer. Front. Digit. Health 4:1039701. doi: 10.3389/fdgth.2022.1039701
Received: 8 September 2022; Accepted: 28 October 2022;
Published: 23 November 2022.
Edited by:
Tania Estapé, Independent Researcher, Barcelona, SpainReviewed by:
Allan Spigelman, University of New South Wales, AustraliaXia Jing, Clemson University, United States
© 2022 Morton, Kohut, Turner, Smith, Crosbie, Ryan, Grimmett, Eccles, Foster and The CanGene CanVar Patient Reference Panel, The International Lynch Decision Aid Stakeholder (LDAS) Panel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate Morton ay5zLm1vcnRvbkBzb3Rvbi5hYy51aw==
Specialty Section: This article was submitted to Connected Health, a section of the journal Frontiers in Digital Health
 Kate Morton
Kate Morton Kelly Kohut
Kelly Kohut Lesley Turner2
Lesley Turner2 Chloe Grimmett
Chloe Grimmett Diana M. Eccles
Diana M. Eccles Claire Foster
Claire Foster