- 1Department of Health Sciences and Technology, ETH Zurich, Zurich, Switzerland
- 2Clever.Care AG, Basel, Switzerland
- 3FMH Basel, Basel, Switzerland
- 4Independent Researcher, Tokyo, Japan
- 5Department of Orthodontics and Dentofacial Orthopedics, School of Dentistry, University of Bern, Bern, Switzerland
- 6University Department of Geriatric Medicine FELIX PLATTER, Faculty of Medicine, University of Basel, Basel, Switzerland
The widespread adoption of digital health technologies such as smartphone-based mobile applications, wearable activity trackers and Internet of Things systems has rapidly enabled new opportunities for predictive health monitoring. Leveraging digital health tools to track parameters relevant to human health is particularly important for the older segments of the population as old age is associated with multimorbidity and higher care needs. In order to assess the potential of these digital health technologies to improve health outcomes, it is paramount to investigate which digitally measurable parameters can effectively improve health outcomes among the elderly population. Currently, there is a lack of systematic evidence on this topic due to the inherent heterogeneity of the digital health domain and the lack of clinical validation of both novel prototypes and marketed devices. For this reason, the aim of the current study is to synthesize and systematically analyse which digitally measurable data may be effectively collected through digital health devices to improve health outcomes for older people. Using a modified PICO process and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework, we provide the results of a systematic review and subsequent meta-analysis of digitally measurable predictors of morbidity, hospitalization, and mortality among older adults aged 65 or older. These findings can inform both technology developers and clinicians involved in the design, development and clinical implementation of digital health technologies for elderly citizens.
Introduction
The growing field of digital health attests that digital technologies are increasingly converging with human health and the delivery of healthcare services. In the last decade, the widespread adoption of, among others, smartphone-based mobile applications, wearable activity trackers, and Internet of Things (IoT) systems, have fuelled a socio-technical trend known as the Quantified Self, i.e., the use of digital technology (broadly defined) for self-tracking purposes (1). Tracking parameters relevant to human health, aiming at improving health outcomes (in short, tracking for health) is a primary justification of self-tracking. The first generation of wearable devices and mobile tools could collect data, and provide insights only related to a small portion of human health and physiology, chiefly mobility reports (e.g., daily steps, physical position). Novel applications have expanded their data sources and can now record a broader variety of health-related parameters and underlying processes. This is due to a four-fold technological transformation. First, self-quantification technologies have expanded in variety as to include data sources that previously could only be collected exclusively via medical devices such as heartbeat rate and electroencephalography (2). Second, smartphone-sensing methods have improved in quality and reliability, now permitting fine-grained, continuous and unobtrusive collection of novel health-related data such as sleep patterns and voice records (3). Third, advances in Artificial Intelligence (AI)-driven software, especially deep learning (4), are increasingly allowing to generate insights about human health from digitally measured data. For example, smartphone apps can be used to predict a person's cognitive status from their responses to gamified cognitive tasks such as 3D virtual navigation (5).
Leveraging digital health to track parameters relevant to human health is particularly important for the older segments of the population as old age is associated with multimorbidity (6) and higher care needs. Given the rapid erosion of the old age dependency ratio (reduction in share of working-age people vs. older people) and the often-stated wish of older adults to age in place, these digital technologies can enable novel and more continuous autonomy-preserving tools for health monitoring, prevention and telemedicine. In countries like Italy (34.3%), Switzerland (33.3%), and Germany (32%) this dependency ratio has already shrunk to only three working age people for every person aged 65 and older (7). Personal digital technologies enable continuous and environment-sensitive collection of clinically relevant data which could be used to improve preventative, diagnostic, and therapeutic outcomes. For example, hypertension, systolic, and diastolic blood pressure can be measured by digital sphygmodynamometers and blood pressure monitors. Handheld echo-cardiography can be used for the assessment of a variety of hemodynamic parameters, such as right and left ventricular dimension and function, left ventricular ejection fraction (LVEF), valvulopathies, pulmonary hypertension and arrhythmias. Arrhythmias can also be detected using pulse oximeters, smartwatches, sensors, or contact free electric sensors. ABI can also be measured using portable or digital ABI systems, or automated blood pressure monitors. Diabetes can be measured using a variety of digital blood glucose meters in form of wireless monitors, wearable sensors, or mobile applications. Digital measurements of BMI include digital electronic scales, weight monitors, or smart fat calculators. Respiratory parameters such as respiratory rate, pulmonary ventilation, or oxygen saturation can be measured by pulse oximeters, pressure sensors spirometers, microphones, humidity sensors, accelerometers, or resistive sensors. Finally, physical activity as any other kinematic and cardiovascular factor can be assessed using sensors like patches or necklaces, accelerometers, pedometers, heart rate monitors, or armbands. Balance parameters such as standing, lying, and sitting can be assessed using a variety of sensors, sensitive to capture a wide range of movements in a specific time range. Handgrip strength and muscle strength can be measured using a digital dynamometer. Handgrip strength is also a marker for frailty. Also, a variety of sensors are being used for the diagnosis of fatigue. They are sensitive in detecting circadian variations, electrodermal activity and cardiovascular parameters in fatigue. Furthermore, digital pressure algometers and other devices such as dolorimeters are being used to measure the pressure pain threshold in humans. Finally, for the measurement of fever, new technologies such as wearable thermometers and/or non-contact thermometers have also emerged.
In order to assess the potential of these digital health technologies to improve health outcomes, it is paramount to ground the analysis on solid scientific evidence (8). In particular, it is necessary to investigate which digitally measurable parameters—defined as parameters that are measured or can be measured using personal digital devices—can effectively improve health outcomes among the elderly population. Currently, there is a lack of systematic evidence on this topic due to the inherent heterogeneity of the digital health domain and the lack of clinical validation of both novel prototypes and marketed devices. Our study aims at producing systematic and generalizable knowledge on which digitally measurable data may be effectively collected by future digital health devices to improve health outcomes in certain patient groups. Using a modified PICO process and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework (9), we provide the results of a systematic review and subsequent meta-analysis of digitally measurable predictors of morbidity, hospitalization and mortality among older adults aged 65 or older. These findings can inform both technology developers and clinicians involved in the design, development, and clinical implementation of digital health technologies for elderly citizens.
Methodology
Search Strategy and Study Selection
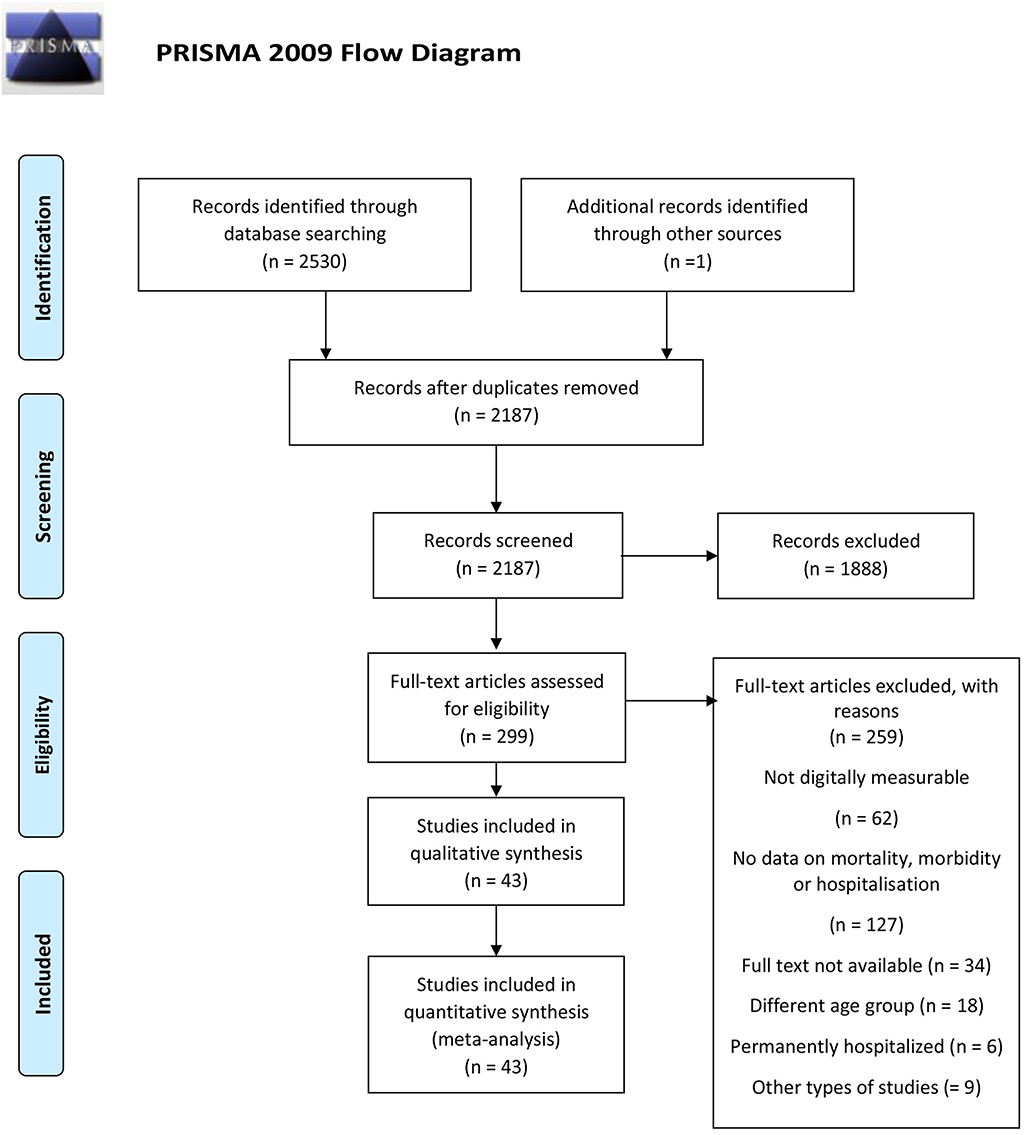
We searched MEDLINE/Pubmed, Embase, Web of Science and PsycInfo on the 30th of March 2020. We searched the databases for eligible peer-reviewed articles on digitally measurable parameters of hospitalization, morbidity, and mortality published in one of the four languages spoken by the authors, namely English, Italian, Greek, or German. After extensive pilot-testing and validation of the search string, we searched the title, abstract, and keywords using a modified PICO process for studies published from 1995 to 2020 (see Annex 1). We set limitations regarding study type excluding secondary studies (e.g., reviews), theoretical studies and studies with no proof of concept. A full description of the search terms is available as Supplementary Material. A total of 4,266 entries were retrieved using this string. The systematic search was performed by the first author (SD) and inspected for validation by the last author (MI). Query logic was adapted to each search database to optimize retrieval. Following the recommendations by (10), the study selection process was conducted and presented using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (http://prisma-statement.org/) as a guide (see Figure 1). The PRISMA study selection process entails four phases: identification, screening, eligibility, and final synthesis.
In the screening phase, duplicates were removed both automatically using the Endnote tool for duplicate detection and manually based on abstract screening. A total of 343 articles was removed at this stage. The remaining 2,187 entries were screened manually to remove entries whose ineligibility could be detected via abstract assessments. Thousand eight hundred and eighty-eight records were excluded at this stage. Subsequently, full-text screening was performed on the remaining 299 records. Uncertainties and diverging inclusion choices between the two reviewers were discussed among the research team with documented reasons and re-evaluated until a consensus was reached. Studies included in the synthesis had the features described in Table 1.
Data Extraction and Coding
We created three different spreadsheets in Microsoft. Excel, one for each of the outcomes reported. Each spreadsheet included information on the study and outcome characteristics (Supplementary Material). Study characteristics included year of publication, study type, sample size, proportion of male participants, mean age, age range, and population diagnoses. For mortality events, we extracted the digitally measurable predictors, the devices used for these measurements and the duration of follow-up period when mortality was measured. For morbidity events, information included all the digitally measurable predictors, all the digital devices used for these measurements and all the adverse health conditions observed after the investigation period. For hospitalization events, coded information included, apart from the digitally measurable predictors and the used devices, the hospital admission and readmission rates. For the estimation of the outcomes we collected all the hazard ratios (HRs), odds ratios (ORs), and 95% Confidence Intervals (CI) reported for mortality, morbidity, and hospitalization events. In cases where the ORs, HRs, and CIs were not provided as primary data by the studies, we calculated them by extracting for each predictor the number of patients with the outcome and the total number of patients for each predictor assigned to each study group (11). For consistency reasons, crude values were preferred over adjusted. We combined HRs and ORs reported separately across studies per gender, per age groups of older people or per quartile of the same predictor across studies, since the aim was an overall outcome assessment without subgroup differentiations (12–17). We also calculated inverse HRs for specific comparisons (18–21).
Data Analysis
Random-effects meta-analysis was performed using the Knapp-Hartung-Sidik-Jonkman estimator (22, 23). Pooled estimates are presented using odds ratios or hazard ratios. Heterogeneity was assessed using tau2, which defines the variance of the true effects sizes and determines the weight assigned to each of the included studies in the meta-analysis model. In addition, the I2 statistic which describes the magnitude of heterogeneity across studies that is attributable to the true differences of the results rather than chance or sampling error was also examined (23). Heterogeneity can be interpreted as low, when I2 = 0–40%, as moderate, when I2 = 30–60%, as substantial, when I2 = 50–90% and as considerable when I2 = 75–100% (24). Meta-regression was performed to examine whether the results differ based on the diagnosis of the participants. The presence of publication bias was assessed using a funnel plot (23, 25). All analyses were performed using Stata 16.1 (StataCorp, TX, USA).
Risk of Bias
For RCTs we used the revised Cochrane tool (RoB 2) to assess risk of bias in randomized trials. (26) This tool includes seven items that cover six bias domains; (i) selection bias (2 items); (ii) performance bias; (iii) detection bias; (iv) attrition bias; (v) reporting bias; and (vi) other bias. This tool has three grading levels: (i) low, (ii) moderate, and (iii) high risk of bias. The worst grading in individual items define the overall risk of bias for each single study. For the cohort studies we used the Cochrane Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-I tool) (27) and also the Newcastle-Ottawa quality assessment scale (NOS) for Cohort Studies. (28) Main domains for risk of the ROBINS-I bias assessment here are: (i) bias due to confounding; (ii) bias in selection of participants into the study, (iii) bias in classification of interventions, (iv) bias due to deviations from intended interventions, (v) bias due to missing data, (vi) bias in measurement of outcomes, and (vii) bias in selection of the reported result. Grading of this scale includes four levels: (i) low, (ii) moderate, (iii) serious, and (iv) critical. Again, the worst grading in any of these items define the overall risk of bias for every single study. The Ottawa scale consists of nine items that cover three dimensions: (i) patient selection (4 items); (ii) comparability of cohorts (2 items); and (iii) assessment of outcome (three items). A point is assigned to each item that is satisfied by the study. The total score therefore ranges from zero to nine, with higher scores indicating higher quality. A total score ≥7 represents high quality.
Results
A PRISMA flowchart summarizing the article selection process is presented in Figure 1. After the initial database search, 43 studies were considered relevant according to the inclusion criteria and were included in the analysis.
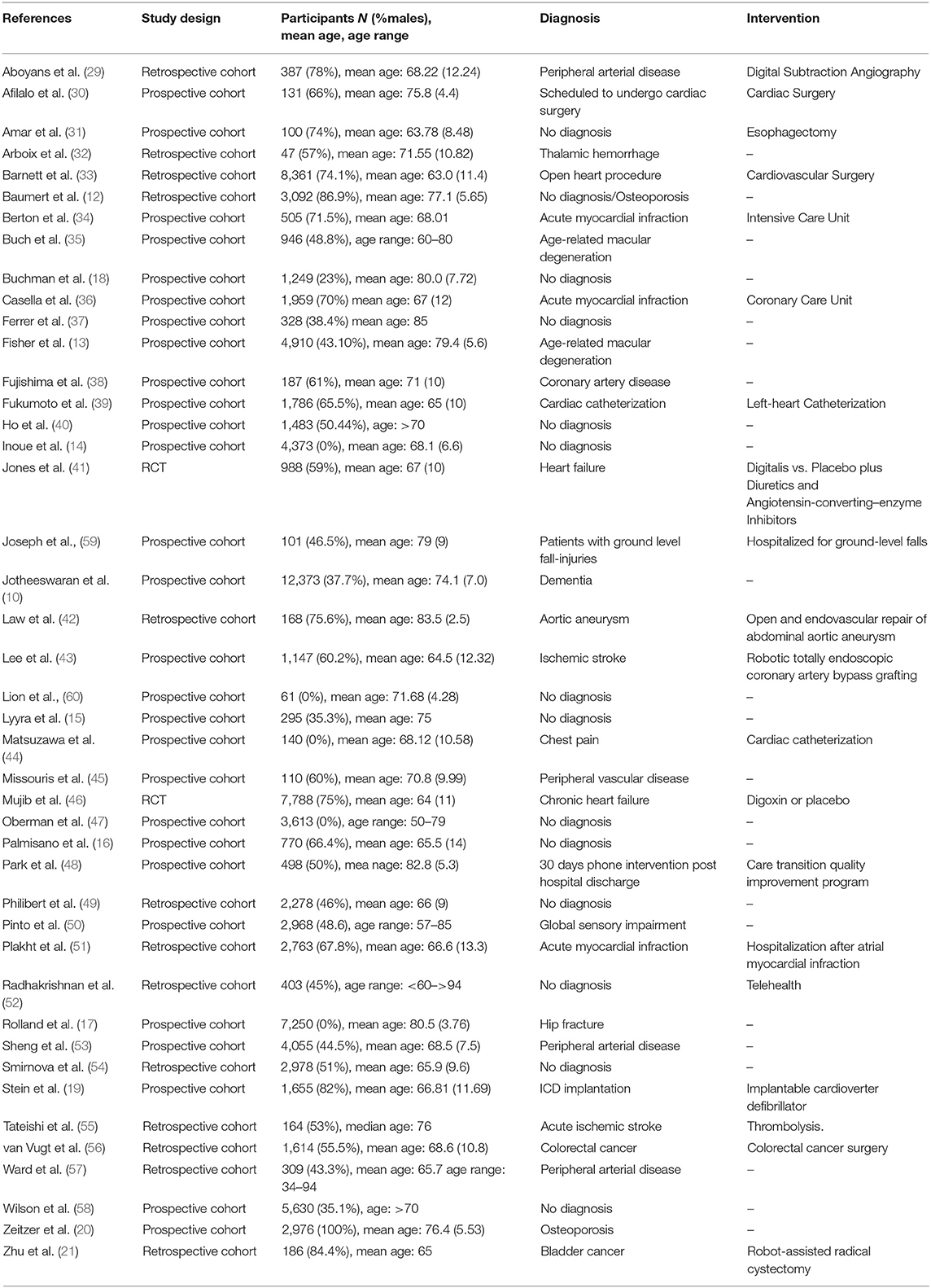
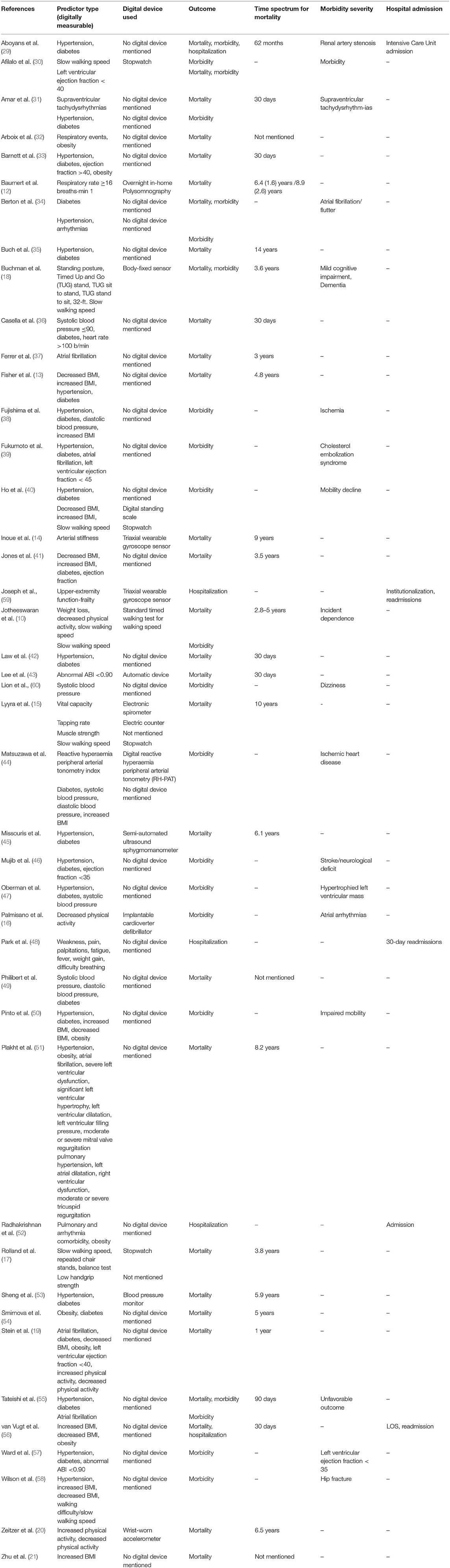
A full description of the included studies is depicted in Tables 2A, 2B. Two of the included studies were RCTs (41, 46), 28 prospective (10, 13–20, 30, 31, 34–40, 43–45, 47, 48, 50, 53, 58–60) and 13 retrospective cohort studies (12, 21, 29, 32, 33, 42, 49, 51, 52, 54–57). The total number of older participants (≥65 years) included in the analysis was 92,994, of whom, 48% (n = 44,461) were males and 52% (n = 48,533) females. Of the 43 studies, 18 studies included cardiovascular patients, 13 studies included patients with other diagnoses, and in 12 studies the participants had no specific diagnosis. Twenty-nine of the included studies reported results about mortality events, 18 about morbidity and five studies reported results about hospitalization and readmissions. Out of these 43 studies, one study (46) analyzed retrospectively data originating from two independent groups and it was included twice in the analysis. Digital measurements were reported in 15 studies for a wide range of physical and physiological functions. Wearable sensors and stopwatches were used for the measurement of walking speed and other kinematic factors, such as balance parameters. Balance parameters such as standing posture and switches between sitting and standing were also measured by body fixed sensors and stopwatches. Also, a wrist-worn accelometer and an implantable defibrillator were used for the assessment of physical activity. A triaxial wearable gyroscope sensor was used for the measurement of the arterial stiffness and frailty among older people. A digital standing scale was used for the measurement of Body Mass Index (BMI) and an inhome polysomnography for the measurement of respiratory rate. Other devices that were used comprised an automatic device for the Ankle Brachial Index (ABI), an electronic spirometry for the vital capacity, an electric counter for the tapping rate and a digital reactive hyperaemia peripheral arterial tonometry (RH-PAT) for the assessment of the reactive hyperaemia peripheral arterial tonometry index. The remaining 28 studies involved measurements that were not collected using personal digital devices but could have been obtained using commercially available digital devices (e.g., hypertension, systolic and diastolic blood pressure, and arrhythmias as they can be measured via, respectively, digital sphygmodynamometers, blood pressure monitors, and pulse oximeters or smartwatches.
Risk of Bias Assessment
Risk of bias assessment was performed independently by two authors (Tables 3–5). Disagreements were solved through discussion and re-evaluation of the differently evaluated points until a consensus was reached. According to RoB and ROBINS-I scales, 17 studies were assessed as being of serious risk of bias, 16 studies were assessed of moderate risk of bias and only 10 studies were assessed as being of low risk of bias. According to the Newcastle Ottawa Scale, only six studies had a total score ≥ 7.
Results
Mortality
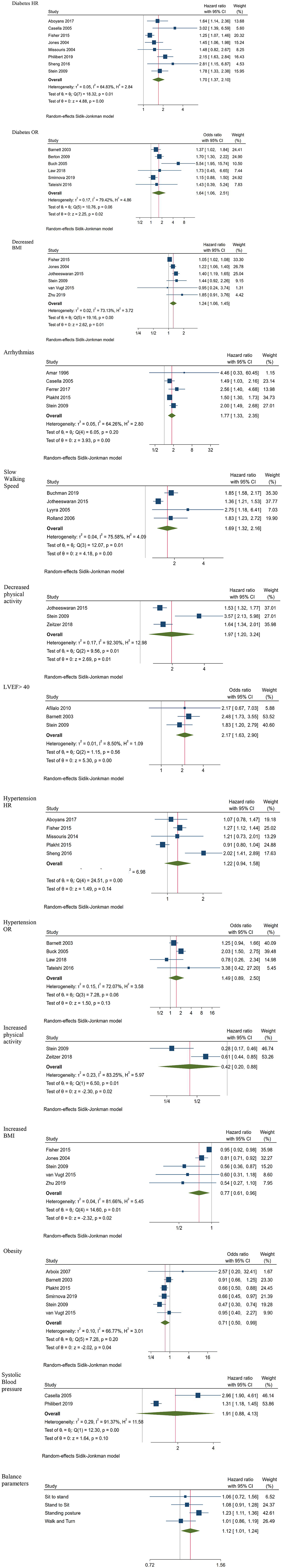
Meta-analysis of all the digitally measurable predictors of mortality identified by the search, indicated six statistically significant predictors (Figure 2). These included diabetes (HR 1.70; CI 1.37, 2.10; 8 studies; OR 1.64; CI 1.06, 2.51; 6 studies), decreased BMI (HR 1.24; CI 1.06, 1.45; 6 studies), arrhythmias (HR 1.77; CI 1.33, 2.35; 5 studies), slow walking speed (HR 1.69; CI 1.32, 2.16; 4 studies), not being physically active (HR 1.97; CI 1.20, 3.24; 3 studies) and LVEF <40 (OR 2.17; CI 1.63, 2.90; 3 studies). Hypertension results were marginally insignificant for mortality (HR 1.22; CI: 0.94, 1.58; 5 studies; OR 1.49; CI: 0.89, 2.50; 4 studies). Being physically active, having increased BMI and obesity were significantly associated with survival (HR 0.42; CI 0.20, 0.88; 2 studies, HR 0.77; CI 0.61, 0.96; 5 studies and OR 0.71; CI 0.50, 0.99; 6 studies, respectively), whereas the results related to systolic blood pressure ≤90 (HR 1.91; CI 0.88, 4.13; 2 studies) were neither for mortality nor for survival statistically significant.
The description of four balance parameters was based on two studies on standing posture [HR 1.23; CI 1.11, 1.36; (18) and (17)] and sit-to-stand ability [HR 1.06; CI 0.72, 1.56; (18) and (17)] while measurements of the other two variables were reported only in one study (18). Since these multiple balance measurements were originating from the same samples, we did not estimate a common effect size for all the balance parameters to avoid the unit of analysis error (23). In contrast, we created a forest plot visual representation of the outcomes which indicates that the only significant predictor of mortality was pooled standing posture (HR 1.23; CI 1.11, 1.36; 2 studies).
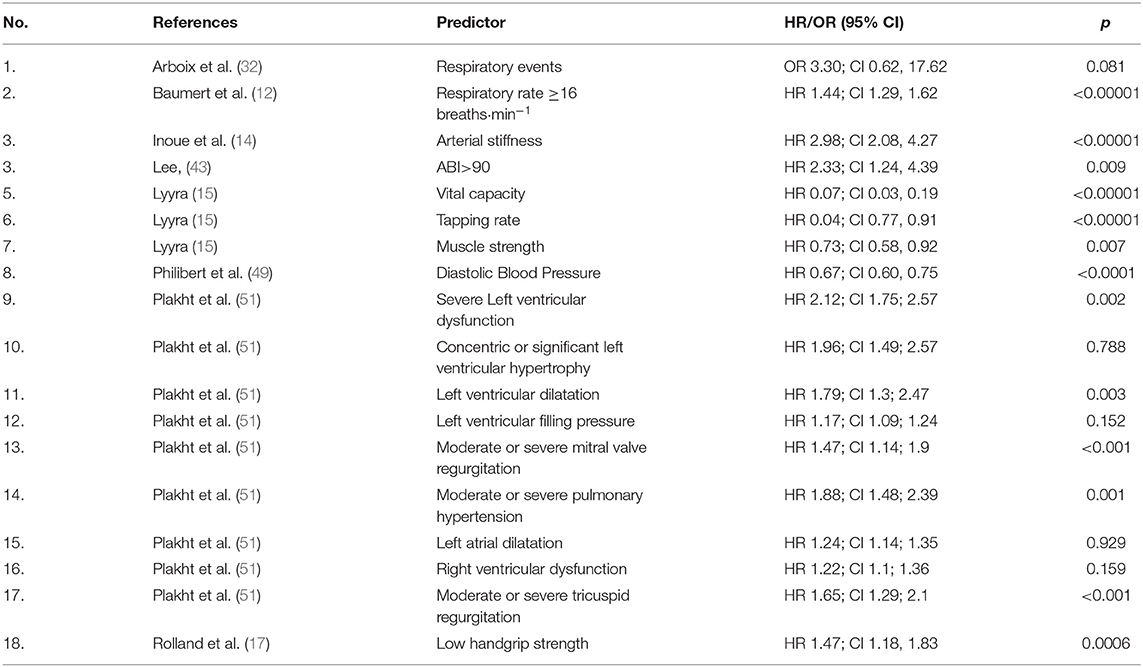
Table 3 summarizes additional predictors of mortality identified only once across the included studies. According to these results, a respiratory rate ≥16 breaths·min−1 (HR 1.44; CI 1.29, 1.62), arterial stiffness (HR 2.98; CI 2.08, 4.27), ABI >90 (HR 2.33; CI 1.24, 4.39) severe left ventricular dysfunction (HR 2.12; CI 1.75; 2.57) significant left ventricular hypertrophy (HR 1.96; CI 1.49; 2.57), left ventricular dilatation (HR 1.79; CI 1.3; 2.47), left ventricular filling pressure (HR 1.17; CI 1.09; 1.24), moderate or severe mitral valve regurgitation (HR 1.47; CI 1.14; 1.9), pulmonary hypertension (HR 1.88; CI 1.48; 2.39), left atrial dilatation (HR 1.24; CI 1.14; 1.35) right ventricular dysfunction (HR 1.22; CI 1.1; 1.36), moderate or severe tricuspid regurgitation (HR 1.65; CI 1.29; 2.1), and low handgrip strength (HR 1.47; CI 1.18, 1.83) were significantly associated to mortality. Vital capacity (HR 0.07; CI 0.03, 0.19), high tapping rate (HR 0.04; CI 0.77, 0.91), and muscle strength (HR 0.73; CI 0.58, 0.92), were significant predictors of longer survival.
Current analysis represents a synthesis of the digitally measurable predictors of mortality. The analysis indicates that a variety of crucial health-related survival parameters, such as hemodynamic, respiratory, kinetic measurements, BMI and diabetes, can be measured and managed remotely. Digital technologies such as blood pressure monitors, pulse oximeters, and sensors for the measurement of heart and respiratory rate, blood glucose meters for diabetes, height-weight monitors for BMI, movement sensors, accelerometers, pedometers for physical activity parameters, dynamometers for muscle strength, spirometers, and hand-held echocardiogram can be efficiently incorporated in routine-care of older people, since they are correlated with survival or mortality, respectively.
Subgroup analyses comparing participants with cardiovascular diseases to those with no cardiovascular diagnoses were performed on three of the statistically significant predictors of mortality. Subgroup analysis for diabetes (HR 1.69; CI 1.43, 2.00 for cardiovascular vs. HR 1.62; CI 0.95, 2.75 for other diagnoses) and decreased BMI (HR 1.24; CI 1.08, 1.42 for cardiovascular patients vs. HR 1.24; CI 0.96, 1.61 for other diagnoses) indicated that diabetes and decreased BMI are significant predictors of mortality only for cardiovascular patients, whereas arrhythmias (HR 1.61; CI 1.35, 1.93 for cardiovascular vs. HR 2.63; CI 1.46, 4.74 for other diagnoses) did not differentiate across diagnoses regarding their association with mortality.
We did not perform a subgroup analysis for slow walking speed, not being physically active and LVEF <40 and since, in the first case none of the studies included cardiovascular patients, and in the two last cases the number of studies included was not sufficient for a subgroup analysis.
Some of the previous analyses were based on a small number of studies and the instability of these results should be considered.
The 95% Confidence Intervals of the included studies are very narrow, and although estimates are close to each other suggesting homogeneity, the I2 is relatively high (23, 24). Non-significant heterogeneity tests for Hypertension in ORs (I2 = 59, p = 0.06) and systolic blood pressure (I2 = 92, p = 0.11) possibly occurred due to low power, since the number of studies included in these analyses was small (23).
Morbidity
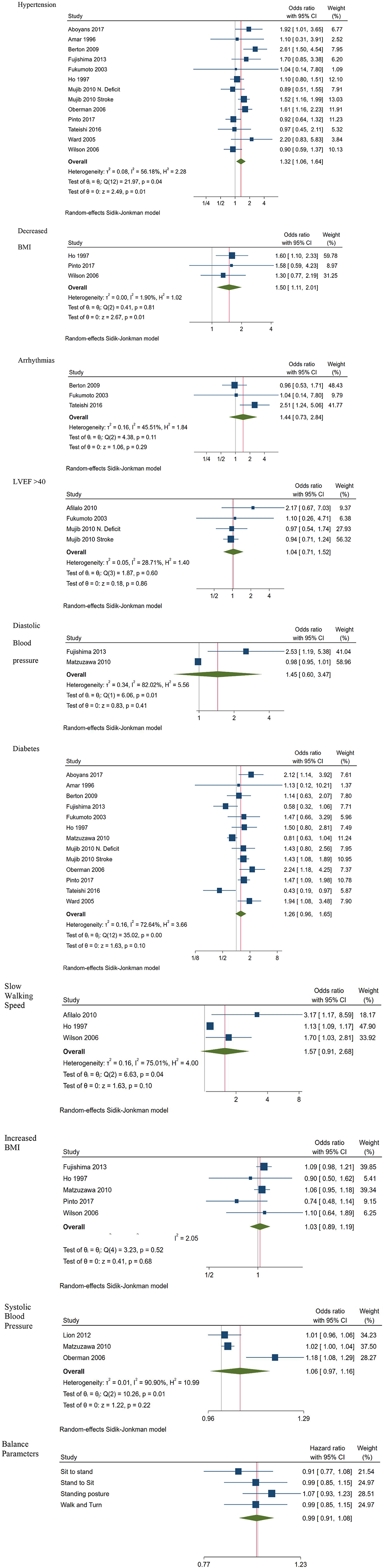
Predictors of morbidity are depicted in Figure 3. Hypertension (OR 1.32; CI 1.06, 1.64; 13 studies), and decreased BMI (OR 1.50; CI 1.11, 2.01; 3 studies) were identified as significant predictors of morbidity. Meta-analysis of outcomes reporting results for arrhythmias (OR 1.44; CI 0.73, 2.84; 3 studies), LVEF <45 (OR 1.04; CI 0.71, 1.52; 4 studies) and diastolic blood pressure (OR 1.45; CI 0.60, 3.47; 2 studies) did not provide any statistically significant result, whereas results regarding diabetes (OR; CI 1.26 0.96, 1.65; 13 studies), slow walking speed (OR 1.57; CI 0.91, 2.68; 3 studies) increased BMI (OR 1.03; CI 0.89, 1.19; 5 studies) and systolic blood pressure (OR 1.06; CI 0.97, 1.16; 3 studies) were marginally insignificant.
Figure 3 also provides a visualization of the contribution of each of the four balance parameters to morbidity, based on the combined outcomes of two independent groups originating from the same study (18). Results indicate that none of them was associated to dementia and/or mild cognitive impairment or to being healthy.
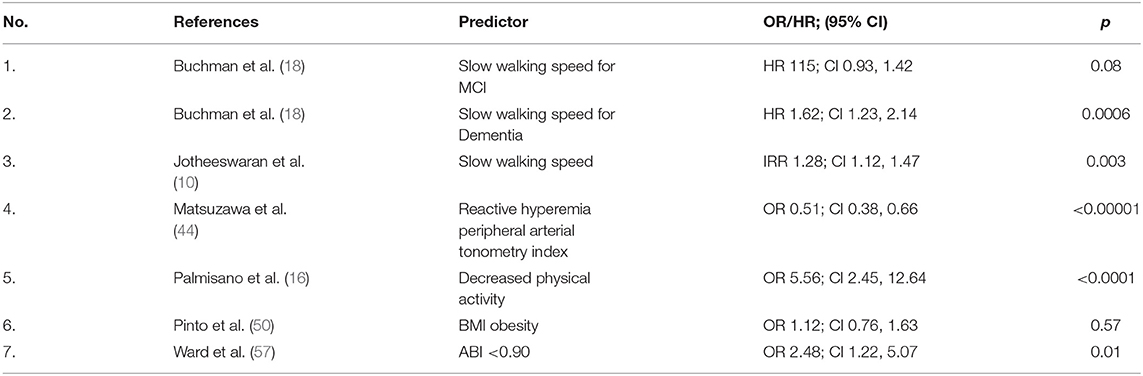
Other statistically important predictors of morbidity identified only once through the literature search (Table 4), were slow walking speed reported as HR for dementia (HR 1.62; 1.23, 2.14) and as incidence rate ratio IRR (IRR 1.28; 1.12, 1.47). Other statistically important predictors of morbidity were decreased physical activity (OR 5.56; CI 2.45, 12.64) and an abnormal ABI <90 (OR 2.48; CI 1.22, 5.07).
Subgroup analysis indicated that hypertension was a significant predictor of morbidity for cardiovascular patients, compared to people with other diagnoses (OR 1.55; CI 1.19, 2.00 vs. OR 1.12; CI 0.88, 1.43), while diabetes was a significant predictor of morbidity only for non-cardiovascular patients (OR 1.23; CI 0.95, 1.59; for cardiovascular vs. OR 1.57; CI 1.22, 2.00 for other diagnoses).
Heterogeneity was moderate for hypertension (I2 = 45, p = 0.006) and diabetes (I2 = 47, p = 0.004), and no heterogeneity was evident for decreased BMI studies (I2 = 0 p = 0.006). The small number of studies included in the remaining analyses, could account for the non-significant heterogeneity values, indicating limiting power for estimating the true effect (23).
Hospitalization
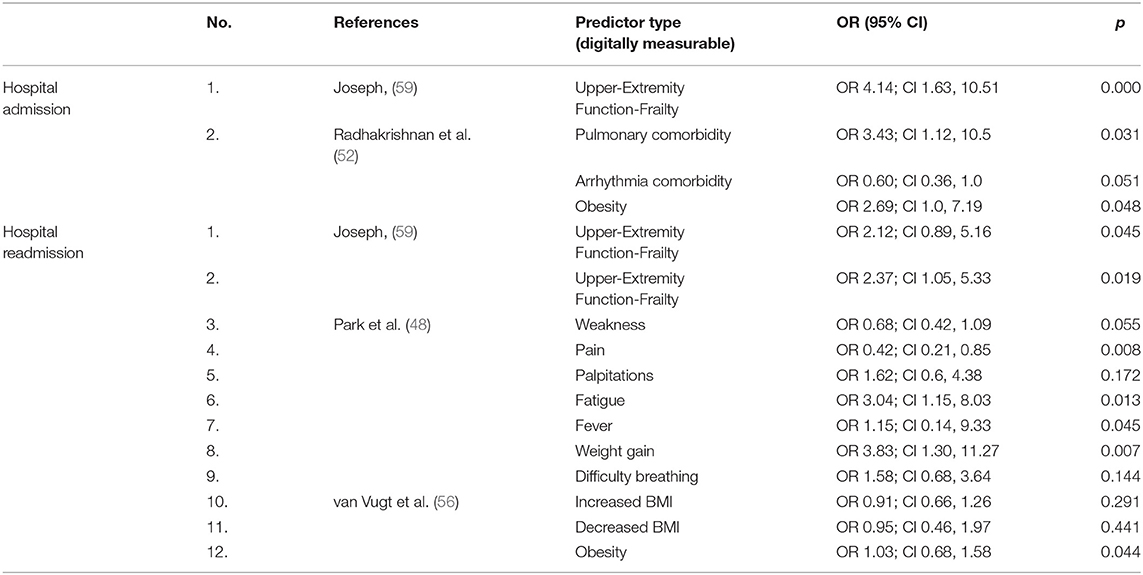
Two studies reported results about predictors of hospitalization, three about predictors of hospital readmission and one study provided a ratio for the Intensive Care Unit (ICU) admission. Identified predictors of the included studies are presented in Table 5. The odds for hospitalization were higher for people with ground-level fall injuries diagnosed as frail compared to those who were not diagnosed as frail (OR 4.14; CI 1.63, 10.51). Obesity (OR 2.69; CI 1.0, 7.19) and pulmonary problems (OR 3.43; CI 1.12, 10.5) were significant predictors of hospitalization for people with colorectal cancer. Frailty (OR 2.37; CI 1.05, 5.33) was reported as a significant predictor of 60-day readmission for people with fall injuries, while fatigue (OR 3.04; CI 1.15, 8.03) and weight gain (OR 3.83; CI 1.30, 11.27) were reported as significant predictors of 30-day readmissions for people with a history of hospitalization followed for 30 days after the last hospital admission. Finally, supraventricular tachydysrhythmias seem to be an important predictor of ICU admission (OR 18.9; CI 4.59, 77.87) for people that have undergone esophageal operation. Although hospitalization outcomes did not provide us with an adequate number of studies to proceed to an analysis with multiple predictors, we succeeded however to find associations between additional technologies and health management of older people. These technologies include digital dynamometers for the assessment of frailty and weakness in older people, sensors, sensitive in identifying fatigue symptoms and, digital pressure algometers and dolorimeters for the measurement of pain.
Limitations
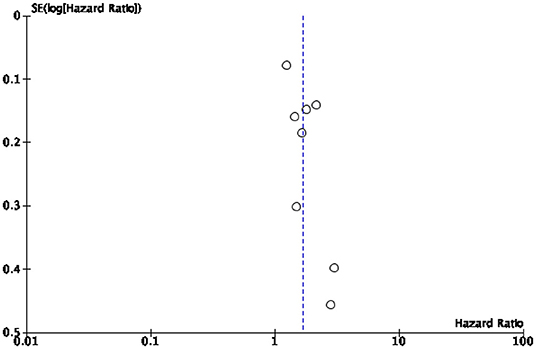
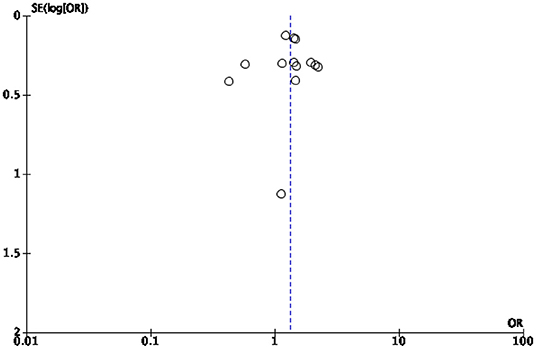
This study presents several limitations mostly due to the high heterogeneity of the study population. A first limitation is the relatively small number of studies included in the synthesis given the large number of variables examined. Some of our analyses were based on a small (<5) number of studies, which is typically considered the minimal threshold for random-effects meta-analyses to maintain to maintain statistical power. In particular, the quantification of hospital admissions could not be continued because the meta-analysis showed that too few studies shed light on the topic of “hospitalizations” to quantify them in a statistically significant way. Secondly, no review protocol was published prior to the start of our analysis. Thirdly, we included in the synthesis only studies written in one of the languages spoken by the research team. This limitation had no effect on the final synthesis as all retrieved studies were written in English. Finally, some of the studies we analyzed appeared to be subject to a risk of bias. To minimize this risk, we implemented several bias assessments, especially a risk of bias and publication bias assessment. The results indicate that most studies under review (39) were assessed as being of high and moderate risk of bias, while 10 studies were assessed as having a low risk of bias. Publication bias assessment was conducted to assess small study effects via funnel plot (61, 62). In case of publication bias, the results of smaller studies are spread widely, due to lower precision, and asymmetrically around the average estimate compared to the results of larger studies. This asymmetry is suggestive of missing studies. In the absence of publication bias, individual study results are more evenly distributed around the pooled estimate (23, 62, 63). However, caution should be exercised when interpreting funnel plots especially when the number of included studies is smaller than 10 (25). In our cases, the funnel plots for diabetes related mortality and morbidity (Figures 4, 5, respectively) are hard to interpret.
In spite of these limitations, our study provided a systematic synthesis of digital measurements that can be predictive of mortality, morbidity, and hospitalizations among older adults. Our findings identify a number of digitally measurable physiological parameters that can serve as proxies for the worsening of an older person's health. This is information is critical to evaluate the current promises and challenges of digital health technologies in the care and health promising of older people, especially in the context of telemedicine and assisted living. Furthermore, this information can inform evidence-based decision making in the context of digital health and gerontechnology.
Discussion
Our results identified the following predictors of mortality: diabetes, decreased BMI, arrhythmias, slower walking speed, and insufficient physical activity. Hypertension, diabetes and decreased BMI were also identified as significant predictors of morbidity, while frailty, pulmonary comorbidity, obesity, pain, fatigue, and fever were identified as significant predictors of hospital admission or readmission. Overall, our results show that personal digital health technologies that can adequately measure the above parameters have the potential to improve health outcomes for older people. This investigation is a prerequisite for the design, development, and deployment of personal digital health technologies that can effectively measure the most informative parameters and thereby leverage that information to enhance health outcomes within the older population segment. Our analysis indicates that a variety of health parameters, such as hemodynamic, respiratory, kinetic parameters, BMI, and diabetes, which are potentially collectable using personal digital technologies can be effectively used to predict and improve the health outcomes of older people aged 65 or older. Further, digital technologies such as blood pressure monitors, pulse oximeters and sensors for the measurement of heart and respiratory rate, blood glucose meters for diabetes, height-weight monitors for BMI, movement sensors, accelerometers, pedometers for physical activity parameters, dynamometers for muscle strength, spirometers and hand-held echocardiogram can be efficiently incorporated in routine-care of older people, since they are correlated with survival or mortality, respectively. All the digitally measurable predictors of morbidity pertained to parameters that can be managed remotely using personal digital health technology. Our results suggest that the incorporation of blood pressure monitors, of blood glucose monitors, of digital height-weight monitors, of movement sensors and stopwatches aiming to measure physical activity and gait speed as well as the incorporation of hand-held echocardiogram in routine care of older people can efficiently contribute to health maintenance and to the protection from adverse health conditions. Since the purpose of the current research was to provide a synthesis of the new technologies that can be used to measure risk factors of morbidity, we did not distinguish morbid conditions regarding their pathogenesis.
These results are consistent with previous studies that revealed positive correlations between specific technologies and health outcomes. For example, the use of remote digital arrhythmia monitoring has been observed to have an impact on medical care regarding hospitalization rates and effects on morbidity and mortality (64, 65). The systematic and meta-analytic nature of our study, however, allows contextualizing this evidence against a broader technological and medical context, comparing different data sources and thereby achieving more solid and generalizable knowledge. Some of the associations revealed by our study may appear prima facie counter-intuitive. One of them is the fact that obesity is positively associated with survival (OR 0.70; CI 0.56, 0.87; 6 studies) in older adults. However, this so-called “obesity paradox” appears to be well-known. Among others, Abramowitz et al. (66). report that numerous studies over the past two decades have shown a body-mass index (BMI) in the normal range is associated with the lowest risk of death. Other large cohort studies in various populations have reached different conclusions, demonstrating a survival benefit for overweight or even obesity, which has been interpreted by many as a causal relationship (66). Although obesity has been associated with a higher risk for cardiovascular and peripheral diseases and also for different types of cancer, previous studies have found that, in cases of acute decompensation or chronic hypertensive disease, type 2 diabetes, chronic kidney disease, or metastatic cancer, obese people in the older population segments tend to live longer (67–69), suggesting that obesity-induced health outcomes depend on variables such as age (68). Although for younger patients obesity is a risk factor for a higher mortality, in older patients it can become protective due to greater reserve for the fight against a disease. In the elderly, recent studies indicate that obesity is associated with a lower mortality risk (70, 71). These findings could be possibly explained by the fact that many previous studies were retrospective analyses which did not examine obesity as primary outcome and did not control for potential confounders that could influence the outcome, such as the presence of specific chronic conditions (69, 72). Further, current data are compatible with the view that not obesity but BMI changes are the primary factor which requires continuous monitoring in the old age as losing weight with age is generally associated with worse outcomes. Nonetheless, the possibility of this “obesity paradox” continues to be debated in the literature and is of great public health importance, not least because of the message communicated to the public (66). Another counter-intuitive result is that hypertension does not appear to be a significant predictor of mortality of people aged 65+. However, the little effect of blood pressure values on mortality risk is not surprising. As part of their treatment for stroke and CHD, many of the individuals were under treatment with agents to decrease triglyceride or lipid levels. It is possible that inclusion of categorical diagnostic information for hypertension and lipid treatment could have improved the prediction model. Unfortunately, these data are not currently available to us. However, we will note that hypertension exerts its deadly effects through CHD and stroke, so it is possible that some if not most of all the variance with respect to death are being captured by those variables (49).
Conclusions
Our meta-analysis has systematically reviewed and compared 43 studies. Our results identified the following predictors of mortality for people aged 65 years or older: diabetes, reduced BMI, arrhythmias, slower walking speed, and insufficient physical activity. Hypertension, diabetes and decreased BMI were also identified as significant predictors of morbidity. Overall, our results show that digital health technologies that can adequately measure the above parameters have the potential to improve health outcomes for older people. This information is essential to develop digital health technologies for older people that could improve their overall health and well-being.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
SD developed the methodology, collected and analyzed the data, and drafted the manuscript. ARa conceived of the study, reviewed the data, and contributed to the manuscript. CH and MW reviewed the data and contributed to the manuscript. ARu obtained the funding, conceived of the study, and contributed to the manuscript. AS and NP analyzed the data and contributed to the manuscript. RK contributed to the study design and to the manuscript. MI obtained funding, conceived of the study, developed the methodology, reviewed the data, and drafted the manuscript. All authors approve the final version of this manuscript.
Funding
This study was funded by the Swiss Innovation Agency (Innosuisse) under project number 40158.1 INNO-ICT.
Conflict of Interest
ARa, ARu, and MW were employed by Clever.Care AG. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This study was funded by Innosuisse- Swiss Innovation Agency, Grant Number: 40158.1 INNO-ICT. This funding scheme is purposively designed to facilitate and promote collaboration between academia and private companies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2020.602093/full#supplementary-material
References
2. Ienca M, Haselager P, and Emanuel EJ. Brain leaks and consumer neurotechnology. Nat Biotechnol. (2018) 36:805–10. doi: 10.1038/nbt.4240
3. Harari GM, Lane ND, Wang R, Crosier BS, Campbell AT, and Gosling SD. Using smartphones to collect behavioral data in psychological science: opportunities, practical considerations, and challenges. Perspect Psychol Sci. (2016) 11:838–54. doi: 10.1177/1745691616650285
4. LeCun Y, Bengio Y, and Hinton G. Deep learning. Nature. (2015) 521:436–44. doi: 10.1038/nature14539
5. Morgan J, Gaming for dementia research: a quest to save the brain. Lancet Neurol. (2016) 15:1313. doi: 10.1016/S1474-4422(16)30123-5
6. Banerjee S, Multimorbidity-older adults need health care that can count past one. Lancet. (2015) 385:587–9. doi: 10.1016/S0140-6736(14)61596-8
8. Vayena E, and Ienca M. Digital medicine and ethics: rooting for evidence. Am J Bioethics. (2018) 18:49–51. doi: 10.1080/15265161.2018.1498955
9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
10. Jotheeswaran AT, Bryce R, Prina M, Acosta D, Ferri CP, Guerra M, et al. Frailty and the prediction of dependence and mortality in low- and middle-income countries: a 10/66 population-based cohort study. BMC Med. (2015) 13:4. doi: 10.1186/s12916-015-0378-4
11. Higgins JPT, Li T, and Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Cochrane (2020). Available online at: www.training.cochrane.org/handbook
12. Baumert M, Linz D, Stone K, McEvoy RD, Cummings S, Redline S, et al. Mean nocturnal respiratory rate predicts cardiovascular and all-cause mortality in community-dwelling older men and women. Eur Respir J. (2019) 54:1802175. doi: 10.1183/13993003.02175-2018
13. Fisher DE, Jonasson F, Eiriksdottir G, Sigurdsson S, Klein R, Launer LJ, et al. Age-related macular degeneration and mortality in community-dwelling elders the age, gene/environment susceptibility reykjavik study. Ophthalmology. (2015) 122:382–90. doi: 10.1016/j.ophtha.2014.08.006
14. Inoue N, Kawakami H, Yamamoto H, Ito C, Fujiwara S, Sasaki H, et al. Second derivative of the finger photoplethysmogram and cardiovascular mortality in middle-aged and elderly Japanese women. Hypertens Res. (2017) 40:207–11. doi: 10.1038/hr.2016.123
15. Lyyra TM, Leskinen E, and Heikkinen E. A cohort study found good respiratory, sensory and motor functions decreased mortality risk in older people. J Clin Epidemiol. (2005) 58:509–16. doi: 10.1016/j.jclinepi.2004.08.015
16. Palmisano P, Guerra F, Ammendola E, Ziacchi M, Luigi Pisanò EC, Dell'Era G, et al. Physical activity measured by implanted devices predicts atrial arrhythmias and patient outcome: results of IMPLANTED (Italian multicentre observational registry on patients with implantable devices remotely monitored). J Am Heart Assoc. (2018) 7:e008146. doi: 10.1161/JAHA.117.008146
17. Rolland Y, Lauwers-Cances V, Cesari M, Vellas B, Pahor M, and Grandjean H. Physical performance measures as predictors of mortality in a cohort of community-dwelling older French women. Eur J Epidemiol. (2006) 21:113–22. doi: 10.1007/s10654-005-5458-x
18. Buchman AS, Dawe RJ, Leurgans SE, Curran TA, Truty T, Yu L, et al. Different combinations of mobility metrics derived from a wearable sensor are associated with distinct health outcomes in older adults. J Gerontol A Biol Sci Med Sci. (2019) 75:1176–83. doi: 10.1093/gerona/glz160
19. Stein KM, Mittal S, Gilliam FR, Gilligan DM, Zhong Q, Kraus SM, et al. Predictors of early mortality in implantable cardioverter-defibrillator recipients. Europace. (2009) 11:734–40. doi: 10.1093/europace/eup055
20. Zeitzer JM, Blackwell T, Hoffman AR, Cummings S, Ancoli-Israel S, Stone K, et al. Osteoporotic fractures men mr, daily patterns of accelerometer activity predict changes in sleep, cognition, and mortality in older men. J Gerontol Ser A-Biol Sci Med Sci. (2018) 73:682–7. doi: 10.1093/gerona/glw250
21. Zhu ZW, Wang XJ, Wang JG, Wang SZ, Fan YF, Fu TL, et al. Preoperative predictors of early death risk in bladder cancer patients treated with robotassisted radical cystectomy. Cancer Med. (2019) 8:3447–52. doi: 10.1002/cam4.2237
22. Sidik K, and Jonkman JN. A simple confidence interval for meta-analysis. Stat Med. (2002) 21:3153–9. doi: 10.1002/sim.1262
23. Borenstein M, Hedges LV, Higgins JP, and Rothstein HR. Introduction to Meta-Analysis. Cambridge: John Wiley & Sons (2011).
24. Higgins JP, Thompson SG, Deeks JJ, and Altman DG. Cambridge: Measuring inconsistency in metaanalyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
25. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cambridge: John Wiley & Sons (2019). doi: 10.1002/9781119536604
26. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
27. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in nonrandomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
28. Wells GA, Tugwell P, O'Connell D, Welch V, Peterson J, Shea B, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses (2015).
29. Aboyans V, Desormais I, Magne J, Morange G, Mohty D, and Lacroix P. Renal artery stenosis in patients with peripheral artery disease: prevalence, risk factors and long-term prognosis. Eur J Vasc Endovasc Surg. (2017) 53:380–5. doi: 10.1016/j.ejvs.2016.10.029
30. Afilalo J, Eisenberg MJ, Morin J-F, Bergman H, Monette J, Noiseux N, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. (2010) 56:1668–76. doi: 10.1016/j.jacc.2010.06.039
31. Amar D, Burt ME, Bains MS, and Leung DHY. Symptomatic tachydysrhythmias after esophagectomy: Incidence and outcome measures. Ann Thorac Surg. (1996) 61:1506–9. doi: 10.1016/0003-4975(96)00111-7
32. Arboix A, Rodríguez-Aguilar R, Oliveres M, Comes E, García-Eroles L, and Massons J. Thalamic haemorrhage vs internal capsule-basal ganglia haemorrhage: clinical profile and predictors of in-hospital mortality. BMC Neurol. (2007) 7:7. doi: 10.1186/1471-2377-7-32
33. Barnett SD, Halpin LS, Speir AM, Albus RA, Akl BF, Massimiano PS, et al. Postoperative complications among octogenarians after cardiovascular surgery. Ann Thorac Surg. (2003) 76:726–31. doi: 10.1016/S0003-4975(03)00676-3
34. Berton G, Cordiano R, Cucchini F, Cavuto F, Pellegrinet M, and Palatini P. Atrial fibrillation during acute myocardial infarction: association with all-cause mortality and sudden death after 7-year of follow-up. Int J Clin Practice. (2009) 63:712–21. doi: 10.1111/j.1742-1241.2009.02023.x
35. Buch H, T. Vinding, la Cour M, Jensen GB, Prause JU, Nielsen NV. Age-related maculopathy: a risk indicator for poorer survival in women - The Copenhagen city eye study. Ophthalmology. (2005) 112:305–12. doi: 10.1016/j.ophtha.2004.08.025
36. Casella G, Savonitto S, Chiarella F, Gonzini L, Di Chiara A, Bolognese L, et al. Clinical characteristics and outcome of diabetic patients with acute myocardial infarction. Data from the BLITZ-1 study. Ital Heart J. (2005) 6:374–83.
37. Ferrer A, Formiga F, Sanz H, Almeda J, and Padros G. Multimorbidity as specific disease combinations, an important predictor factor for mortality in octogenarians: the Octabaix study. Clin Interv Aging. (2017) 12:223–31. doi: 10.2147/CIA.S123173
38. Fujishima S, Murakami N, Haga Y, Nyuta E, Nakate Y, Ishihara S, et al. Low diastolic blood pressure was one of the independent predictors of ischemialike findings of electrocardiogram in patients who underwent coronary angiography. J Cardiol. (2013) 62:230–5. doi: 10.1016/j.jjcc.2013.05.005
39. Fukumoto Y, Tsutsui H, Tsuchihashi M, Masumoto A, and Takeshita A. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol. (2003) 42:211–6. doi: 10.1016/S0735-1097(03)00579-5
40. Ho SC, Woo J, Yuen YK, Sham A, and Chan SG. Predictors of mobility decline: the Hong Kong old-old study. J Gerontol Ser A Biol Sci Med Sci. (1997) 52:M356–62. doi: 10.1093/gerona/52A.6.M356
41. Jones RC, Francis GS, and Lauer MS. Predictors of mortality in patients with heart failure and preserved systolic function in the Digitalis Investigation Group trial. J Am Coll Cardiol. (2004) 44:1025–9. doi: 10.1016/j.jacc.2004.05.077
42. Law Y, Chan YC, and Cheng SW. Predictors of early operative mortality and long-term survival in octogenarians undergoing open and endovascular repair of abdominal aortic aneurysm. Asian J Surg. (2018) 41:490–7. doi: 10.1016/j.asjsur.2017.09.004
43. Lee JD, Bonaros N, Hong PT, Kofler M, Srivastava M, Herr DL, et al. Factors influencing hospital length of stay after robotic totally endoscopic coronary artery bypass grafting. Ann Thorac Surg. (2013) 95:813–9. doi: 10.1016/j.athoracsur.2012.10.087
44. Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. (2010) 55:1688–96. doi: 10.1016/j.jacc.2009.10.073
45. Missouris CG, Kalaitzidis RG, Kerry SM, and Cappuccio FP. Predictors of mortality in patients with peripheral vascular disease. A prospective follow-up study. Br J Diab Vasc Dis. (2004) 4:196–200. doi: 10.1177/14746514040040030901
46. Mujib M, Giamouzis G, Agha SA, Aban I, Sathiakumar N, Ekundayo OJ, et al. Epidemiology of stroke in chronic heart failure patients with normal sinus rhythm: findings from the DIG stroke sub-study. Int J Cardiol. (2010) 144:389–93. doi: 10.1016/j.ijcard.2009.04.035
47. Oberman A, Prineas RJ, Larson JC, LaCroix A, and Lasser NL. Prevalence and determinants of electrocardiographic left ventricular hypertrophy among a multiethnic population of postmenopausal women (The women's health initiative). Am J Cardiol. (2006) 97:512–9. doi: 10.1016/j.amjcard.2005.08.071
48. Park J, Hain DJ, Tappen R, Diaz S, and Ouslander JG. Factors associated with 30-day hospital readmissions among participants in a care transition quality improvement program. J Soc Soc Work Res. (2012) 3:308–28. doi: 10.5243/jsswr.2012.19
49. Philibert RA, Dogan MV, Mills JA, and Long JD. AHRR methylation is a significant predictor of mortality risk in framingham heart study. J Insur Med. (2019) 48:79–89. doi: 10.17849/insm-48-1-1-11.1
50. Pinto JM, Wroblewski KE, Huisingh-Scheetz M, Correia C, Lopez KJ, Chen RC, et al. Global sensory impairment predicts morbidity and mortality in older US adults. J Am Geriatr Soc. (2017) 65:2587–95. doi: 10.1111/jgs.15031
51. Plakht Y, Shiyovich A, and Gilutz H. Predictors of long-term (10-year) mortality postmyocardial infarction: age-related differences. Soroka Acute Myocardial Infarction (SAMI) Project. J Cardiol. (2015) 65:216–23. doi: 10.1016/j.jjcc.2014.06.001
52. Radhakrishnan K, Jacelon CS, Bigelow C, Roche J, Marquard J, and Bowles KH. Use of a homecare electronic health record to find associations between patient characteristics and rehospitalizations in patients with heart failure using telehealth. J Telemed Telecare. (2013) 19:107–12. doi: 10.1258/jtt.2012.120509
53. Sheng CS, Li Y, Huang QF, Kang YY, Li FK, and Wang JG. Pulse waves in the lower extremities as a diagnostic tool of peripheral arterial disease and predictor of mortality in elderly Chinese. Hypertension. (2016) 67:527–34. doi: 10.1161/HYPERTENSIONAHA.115.06666
54. Smirnova E, Leroux A, Cao Q, Tabacu L, Zipunnikov V, Crainiceanu C, et al. The predictive performance of objective measures of physical activity derived from accelerometry data for 5-year all-cause mortality in older adults: NHANES 2003-2006. J Gerontol A Biol Sci Med Sci. (2019) 75:1779–85. doi: 10.1093/gerona/glz193
55. Tateishi Y, Hamabe J, Kanamoto T, Nakaoka K, Morofuji Y, Horie N, et al. Subacute lesion volume as a potential prognostic biomarker for acute ischemic stroke after intravenous thrombolysis. J Neurol Sci. (2016) 369:77–81. doi: 10.1016/j.jns.2016.08.006
56. van Vugt JL, Cakir H, Kornmann VN, Doodeman HJ, Stoot JH, Boerma D, et al. The new Body Mass Index as a predictor of postoperative complications in elective colorectal cancer surgery. Clin Nutr. (2015) 34:700–4. doi: 10.1016/j.clnu.2014.08.006
57. Ward RP, Min JK, McDonough KM, and Lang RM. High prevalence of important cardiac findings in patients with peripheral arterial disease referred for echocardiography. J Am Soc Echocardiogr. (2005) 18:844–9. doi: 10.1016/j.echo.2005.01.004
58. Wilson RT, Chase GA, Chrischilles EA, and Wallace RB. Hip fracture risk among community-dwelling elderly people in the United States: a prospective study of physical, cognitive, socioeconomic indicators. Am J Publ Health. (2006) 96:1210–8. doi: 10.2105/AJPH.2005.077479
59. Joseph B, Toosizadeh N, Jokar TO, Heusser MR, Mohler J, and Najafi B. Upper-extremity function predicts adverse health outcomes among older adults hospitalized for ground- level falls. Gerontology. (2017) 63:299–307. doi: 10.1159/000453593
60. Lion A, Spada RS, Bosser G, Gauchard GC, Anello G, Bosco P, et al. Biological determinants of postural disorders in elderly women. Int J Neurosci. (2013) 123:24–30. doi: 10.3109/00207454.2012.722570
61. Hussain R, Hassali MA, Patel M, and Babar ZUD. Publication Bias, Encyclopedia of Pharmacy Practice and Clinical Pharmacy. Oxford: Elsevier (2019).
62. Song F, Hooper L, and Loke Y. Publication bias: what is it? How do we measure it? How do we avoid it? Open Access J Clin Trials. (2013) 2013:71–81. doi: 10.2147/OAJCT.S34419
64. Akar JG, Bao H, Jones PW, Wang Y, Varosy PD, Masoudi FA, et al.-Normand LT, Curtis JP. Use of remote monitoring is associated with lower risk of adverse outcomes among patients with implanted cardiac defibrillators. Circulation. (2015) 8:1173–80. doi: 10.1161/CIRCEP.114.003030
65. Shinbane JS, and Saxon LA. Digital monitoring and care: virtual medicine. Trends Cardiovasc Med. (2016) 26:722–30. doi: 10.1016/j.tcm.2016.05.007
66. Abramowitz MK, Hall CB, Amodu A, Sharma D, Androga L, and Hawkins M. Muscle mass, BMI, and mortality among adults in the United States: a population-based cohort study. PLoS ONE. (2018) 13:e0194697. doi: 10.1371/journal.pone.0194697
67. Tsang NM, Pai PC, Chuang CC, Chuang WC, Tseng CK, Chang KP, et al. Overweight and obesity predict better overall survival rates in cancer patients with distant metastases. Cancer Med. (2016) 5:665–75. doi: 10.1002/cam4.634
68. Amundson DE, Djurkovic S, and Matwiyoff GN. The obesity paradox. Crit Care Clin. (2010) 26:583–96. doi: 10.1016/j.ccc.2010.06.004
69. Ades PA, and Savage PD. The obesity paradox: perception vs knowledge. In: Mayo Clinic Proceedings. Rochester: Mayo Foundation (2010). doi: 10.4065/mcp.2009.0777
70. Oreopoulos A, Kalantar-Zadeh K, Sharma AM, and Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med. (2009) 25:643–59. doi: 10.1016/j.cger.2009.07.005
71. Dorner TE, and Rieder A. Obesity paradox in elderly patients with cardiovascular diseases. Int J Cardiol. (2012) 155:56–65. doi: 10.1016/j.ijcard.2011.01.076
Keywords: digital health (eHealth), systematic (literature) review, meta-analysis, predictor, hospitalization-, mortality, elderly
Citation: Daniolou S, Rapp A, Haase C, Ruppert A, Wittwer M, Scoccia Pappagallo A, Pandis N, Kressig RW and Ienca M (2021) Digital Predictors of Morbidity, Hospitalization, and Mortality Among Older Adults: A Systematic Review and Meta-Analysis. Front. Digit. Health 2:602093. doi: 10.3389/fdgth.2020.602093
Received: 26 September 2020; Accepted: 17 December 2020;
Published: 04 February 2021.
Edited by:
Constantinos S. Pattichis, University of Cyprus, CyprusReviewed by:
Christiane Hartmann, University Hospital Rostock, GermanyManuel Ottaviano, Polytechnic University of Madrid, Spain
Ryan Patrick William Kenny, Newcastle University, United Kingdom
Copyright © 2021 Daniolou, Rapp, Haase, Ruppert, Wittwer, Scoccia Pappagallo, Pandis, Kressig and Ienca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcello Ienca, bWFyY2VsbG8uaWVuY2EmI3gwMDA0MDtoZXN0LmV0aHouY2g=
 Sofia Daniolou
Sofia Daniolou Andreas Rapp2
Andreas Rapp2 Nikolaos Pandis
Nikolaos Pandis Reto W. Kressig
Reto W. Kressig Marcello Ienca
Marcello Ienca