
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Drug Discov. , 01 December 2023
Sec. Anti-Infective Agents
Volume 3 - 2023 | https://doi.org/10.3389/fddsv.2023.1304129
As the world adapts to living with SARS-CoV-2, the continuous emergence of new variants has become a primary focus of current studies. In this review, we examined a range of available COVID-19 drugs, including FDA-regulated drugs and those undergoing late-stage clinical trials. Some FDA-regulated drugs, such as Veklury (remdesivir), Olumiant (baricitinib), and Actemra (tocilizumab), have garnered primary clinical status in treatment guidelines, supported by sufficient clinical evidence. Conversely, EUA-authorized therapies, such as some antiviral agents, have demonstrated lower efficacy due to the virus’s constant mutation. We also focused on COVID-19 drugs undergoing late-stage clinical trials, some of which have raised controversy in their administration, such as colchicine and corticosteroids, while others are worth exploring regarding their timing. Several ongoing multi-drug clinical trials are of particular interest, including the “MEDIC-LAUMC” trial that explores drug co-administration, and “ACTIV-2” and “ACTIV-3” trials that compare the effects of different drugs for non-hospitalized and hospitalized patients, respectively. These ongoing clinical trials at a late stage provide essential clinical evidence for future drug authorization and have the potential to provide better drug administration strategies for COVID-19 variants. We look forward to the continued exploration of drug co-administration, comprehensive clinical evidence for treatment, and the investigation of different potential drug utilization.
The COVID-19 pandemic, also known as the coronavirus pandemic, is one of the deadliest outbreaks in history caused by the 2019 global coronavirus pandemic resulting from severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). The virus was first identified in Wuhan, China, in December 2019 during an outbreak. The World Health Organization (WHO) declared the outbreak a public health emergency of international concern on 30 January 2020, and a pandemic on 11 March 2020. As of 10 March 2023, more than 675 million cases of COVID-19 have been confirmed worldwide, resulting in over 6.87 million deaths.
SARS-CoV-2 virus is classified into the B lineage of the β-coronaviruses (β-CoVs). It comprises an enveloped, non-segmented, positive-sense single-stranded RNA virus genome with a length of 29.9 kb and a GC content ranging from 32% to 43% (Rahimi et al., 2021). The genome contains 12 open reading frames (ORFs) that encode 27 proteins, including spike (S) proteins, envelope (E) proteins, membrane (M) proteins, nucleocapsid (N) proteins, and non-structural proteins. The genome of SARS-CoV-2 encodes an enzyme called exonuclease (ExoN) to proofread and correct copies, which is rare among RNA viruses (Scientific American, 2020). Moreover, random mutations and recombination are two main sources of genetic diversity in SARS-CoV-2. Saif Ur Rehman et al. identified nine putative recombination patterns in SARS-CoV-2, with six recombination regions in S gene contributing to the modification of superficial antigenicity to adapt to the mammalian host (Rehman et al., 2020). Mutations in different genomic regions of SARS-CoV-2 are a strategy to improve its reproductive adaptability.
SARS-CoV-2 invades the human body through its special structures, especially the S protein (Scientific American, 2020). It enters the human respiratory system through transmission among different individuals, and its spike protein binds with an ACE2 receptor on the surface of a lung cell. With the help of the virus spike stems, SARS-CoV-2 fuses with the lung cell, allowing its N proteins and RNA to access the lung cell. SARS-CoV-2 RNA initiates the replication process, enabling the package and release of new viruses. These new viruses continue to infect other human cells or overflow into the air.
When infection begins, the human innate immune system and adaptive immune system take corresponding action to protect the human body. The innate immune system shows a quick response within 0–3 days. Inside a lung cell, sensor proteins recognize incoming viruses, and messenger RNA molecules deliver instructions to ribosomes, which promote the assembly of interferon. Subsequently, an infected cell releases interferon protein. They can both alert neighboring cells to create specific protective molecules and recruit cells such as macrophages in the bloodstream that can engulf virus particles. The adaptive immune system’s function is activated by interferon within 6–11 days, including the effects on B-cells and T-cells. After being induced by interferon, B cells produce “neutralizing antibodies” that bind to the virus spike protein and prevent them from binding to a lung cell, while T cells are able to kill viruses and infected cells (new viruses inside them have not been released yet) (Nicholson, 2016).
Nevertheless, SARS-CoV-2 has countermeasures to block the neutralization effect of the immune system. One of the strategies is that the virus spike camouflages itself with sugar chains called glycans, which prevent antibodies from binding to them, therefore preventing the function of the adaptive immune system. In addition, regarding the recognition process by sensor proteins in a lung cell to produce interferon, which is a vital step in the innate immune system, some SARS-CoV-2 proteins are capable of blocking the sensor proteins from acting or interfering with instructions to the ribosome, terminating the formation of interferon.
As the COVID-19 pandemic has evolved, numerous variants of SARS-CoV-2 have emerged worldwide. Researchers have extensively studied five significant SARS-CoV-2 variants, namely, Alpha (B.1.1.7, Q.1-Q.8), Beta (B.1.351, B.1.351.2, B.1.351.3), Gamma (P.1, P.1.1, P.1.2), Lambda (C.37), Delta (B.1.617.2 and AY.1 sublineages), and Omicron (B.1.1.529/BA.1) variants. Yao Jiang et al. suggested that although the Alpha, Beta, and Gamma variants are resistant to neutralization by antibodies gained from primitive SARS-CoV-2 infection, they can still be defended by antibodies induced by vaccines to some extent (Jiang et al., 2021). As for the Delta variant, current vaccines are still capable of preventing Delta variant infection; for instance, people who receive two doses of Pfizer-BioNTech BNT162b2 or AstraZeneca-Oxford ChAdOx1 nCoV-19 vaccines are protected against the Delta variant.
However, the Omicron variant, with the most spike protein mutation sites, only causes mild symptoms, such as muscle soreness, fatigue, cough, fever, etc., under normal circumstances. Shi-Yan Ren emphasized the significance of early careful preventive measures, including vaccination against the Omicron variant, as the existing vaccines are less effective, but boosters should strengthen immunity (Ren et al., 2022).
Nonetheless, in January 2023, a variant of Omicron named CH.1.1 appeared in the United States, a branch of the dominant strain BA.2.75. CH.1.1 has the potential to be more infectious and immune evasive than other subvariants of the novel SARS-CoV-2 and may cause more severe illness. It is still unknown whether the Omicron variant will cause more severe disease and become a “super strain”, or whether it will have stronger transmissibility, virulence, infectivity, or immune responses in the future. In conclusion, vaccination booster is still the most reasonable tool for the existing variants of SARS-CoV-2, and vaccination should be implemented in diverse countries and regions as an effective preventive measure.
Based on the SARS-CoV-2 invasion and immune escape mechanism, researchers have designed a series of vaccines and drugs to combat SARS-CoV-2, most of which inhibit rather than kill the virus. Existing drug targets mainly include preventing the virus from entering the cell, encouraging defective viruses, shutting down the virus, and reducing hyperimmune responses. Generally, different therapeutics can block the virus from binding to human cells, prevent virus reproduction, and avoid more severe symptoms (Figure 1). In human lung environment, Tocilizumab disrupts IL-6 signaling, GM-CSF inhibitors suppress tissue injury, and IL-1 inhibitors like Kineret block autoinflammation caused by IL-1. For systemic suppression of inflammatory response, Dexamethasone inhibits pro-inflammatory cytokines, while Baricitinib and other Janus kinase inhibitors block cytokine signaling.

FIGURE 1. The mechanisms and targets of major COVID-19 drugs. Tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, disrupts IL-6 signaling by competitively binding to the soluble IL-6 receptor. Granulocyte-macrophage colony-stimulating factor (GM-CSF) inhibitors reduce tissue injury by suppressing GM-CSF. Interleukin-1 Inhibitors, such as Kineret (anakinra), block the IL-1 receptor (IL-1R), thus preventing autoinflammation caused by IL-1. Monoclonal antibodies, such as Bamlanivimab plus etesevimab, REGEN-COV, Sotrovimab, target the SARS-CoV-2 spike protein to neutralize the virus. High-titer COVID-19 convalescent plasma (CCP) functions similarly since it contains neutralizing antibodies to SARS-CoV-2. Dexamethasone, binding to the glucocorticoid receptor (GCR), inhibits the production of pro-inflammatory cytokines and activates anti-inflammatory cytokines synthesis. Paxlovid, an active 3Cl protease inhibitor, suppresses SARS-CoV-2 protease Mpro, blocking viral replication. Interferons (IFN) activate type-specific cognate cell surface receptors, triggering Janus kinase/signal transducers and activators of transcription (JAK-STAT) signaling for transcriptional activation of IFN-stimulated genes (ISGs) by. Baricitinib, a Janus kinase inhibitor (JAK), inhibits the JAK-STAT signaling pathway utilized by various cytokine. Remdesivir and Molnupiravir act as antiviral agents, inhibiting viral RNA polymerase to block SARS-CoV-2 replication. mAb: monoclonal antibody; CCP: COVID-19 convalescent plasma; IL-6: interleukin-6; sIL-6R: soluble interleukin-6 receptor; gp130: glycoprotein 130; IL-1: interleukin-1; IL-1R: interleukin-1 receptor; GM-CSF: granulocyte-macrophage colony-stimulating factor; ACE-2: angiotensin converting enzyme 2; GCR: glucocorticoid receptor; Mpro: main protease; IFN: interferon; JAK: janus kinase inhibitors; RdRp: RNA dependent RNA polymerases.
The spike protein and ACE2 are considered as ideal drug targets for COVID-19. Bioactive peptides with unique amino acid sequences, such as those that inhibit type II transmembrane serine proteases (TMPRSS2) and the renin-angiotensin-aldosterone system (RAAS) members, have been found to mitigate these targets, according to Khushwant S. Bhullar (Bhullar et al., 2021).
Several EUA-authorized drugs have been developed to target the spike protein of SARS-CoV-2. Bebtelovimab (LY-CoV1404) is a spike protein-neutralizing antibody that binds to the S protein, blocking viral cell fusion. Its structure is stable with high affinity, and its binding site on S protein is not at key mutation points. Amubarvimab/romlusevimab also bind with spike protein, occupying the binding site of ACE2 to spike protein, and have a similar mechanism to Bebtelovimab (Wang et al., 2021). Some existing drugs, such as GT0918, target the ACE2 and spike protein binding mechanism by reducing the production of ACE2, thus inhibiting the entry of SARS-CoV-2 into host cells. GT0918 downregulates the expression of ACE2 and TMPRSS2 at the transcriptional level by inhibiting the function of the androgen receptor (Xiaodan et al., 2022).
Scientists have developed many drugs targeting virus-associated enzymes, with some that can be taken orally, such as Nirmatrelvir with Ritonavir (Paxlovid, a 3C-like protease inhibitor) and Molnupiravir (Lagevrio, targeting RNA-dependent RNA polymerase). Others require intravenous (IV) infusions, such as Remdesivir (Veklury) and Bebtelovimab (Centers For Disease Control and Prevention, 2023). Recently, the Global Health Drug Discovery Institute (GHDDI) has developed oral drugs against coronavirus, including the GDI-4405 series of candidate drugs, which is a small molecule 3-CL (3C-like) protease inhibitor that shows strong antiviral activity against Delta, Omicron, and other mutant strains. Remdesivir (GS-5734), an inhibitor of viral RNA-dependent RNA polymerase, was identified early as a promising therapeutic candidate for COVID-19 due to its ability to inhibit SARS-CoV-2 in vitro (Beigel et al., 2020). VV116 is a derivative of Remdesivir and has significant pharmacokinetic and pharmacodynamic characteristics in vivo, showing remarkable anti-COVID-19 effect (Xie et al., 2021).
In summary, most oral drugs for COVID-19 target virus-associated enzymes, with drugs targeting virus-associated enzymes also making up the majority of drugs already on the market. Here, we focused on focused on existing drugs for COVID-19, including FDA-approved drugs, EUA-authorized products, and drugs undergoing clinical trials, highlighting their properties, mechanisms, target population, and implementation.
Therapies targeting SARS-CoV-2 are typically used for patients in the early stages of COVID-19, while immunosuppressive/anti-inflammatory therapies are usually used for patients in the later stages of COVID-19, which correspond to the infection process of SARS-CoV-2 (National Institutes of Health NIH, 2023a). The initial process of replication and infection of SARS-CoV-2 in the human body causes initial symptoms, and over time, the immune response and inflammation of the human body cause more severe symptoms. Therefore, current approved drugs and therapies authorized under an Emergency Use Authorization (EUA) by the Food and Drug Administration (FDA) for COVID-19 treatment are based on the above principles, which reflect the significance of timing in COVID-19 drug administration. The clinical trials discussed in this article primarily sourced from NIH’s ClinicalTrials.gov website. Our search on ClinicalTrials.gov was refined using filters such as “active, not recruiting”, “Phase 3”, and “Phase 4” to identify relevant COVID-19 drug-related trials. Furthermore, we extracted pertinent details on COVID-19 drugs from the NIH COVID-19 Treatment Guidelines and COVID-19 drugs published on the FDA website. Additionally, the literature and prescription information pertaining to the drugs highlighted in this article have been appropriately cited.
The FDA approves new drug applications (NDAs) under section 505(c) of the FD&C Act and biologics license applications (BLAs) under section 351 of the Public Health Service Act (PHS Act). So far, the FDA has approved three drugs for COVID-19 treatment, including an antiviral drug Veklury (remdesivir), as well as two immune modulators Olumiant (baricitinib) and Actemra (tocilizumab) (Supplementary Table S1).
Actemra (tocilizumab), a monoclonal antibody against interleukin-6 (IL-6) receptor preventing the “cytokine storm”, is used through subcutaneous injection to treat COVID-19. It is approved by the FDA for subjects who are hospitalized, receiving systemic corticosteroids, and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Through meta-analysis, Muhammad Aziz et al. demonstrated that patients who received tocilizumab had better outcomes in terms of mortality (Aziz et al., 2021). That is, tocilizumab has the potential to decrease the mortality rate among COVID-19 patients with severe symptoms. Emna Abidi et al. revealed the timing and efficacy of the drug, concluding that tocilizumab therapy is appropriate for patients with progressive disease and high oxygen requirements rather than patients with mild disease or prolonged invasive mechanical ventilation (Abidi et al., 2022). Besides, they pointed out that concomitant administration of corticosteroids and tocilizumab resulted in further decreases in the 28-day all-cause mortality, as well as a lower likelihood of progression to invasive mechanical ventilation, ECMO, or death at 28 days compared to placebo or standard of care. The results of the RECOVERY and REMAP-CAP trials provide consistent evidence that co-administering tocilizumab and corticosteroids offers hospitalized and high oxygen required COVID-19 patients a modest survival benefit (Abdulakeem et al., 2021; Gordon et al., 2021; Gritti et al., 2021; Gupta and Leaf, 2021). Due to similar effects in improving survival and reducing the duration of organ support, sarilumab may be used as an alternative to tocilizumab (National Institutes of Health NIH, 2023b).
The primary adverse effect of tocilizumab treatment is elevated liver enzyme levels, which is possibly dose-dependent, and additional adverse effects include serious infections (e.g., tuberculosis, bacterial or fungal infections) and bowel perforation (Charan et al., 2021). The dose modifications regarding the management of laboratory abnormalities, including hepatotoxicity, neutropenia, and thrombocytopenia, were updated in the prescribing information of tocilizumab in March 2021. The recommended dose of tocilizumab for adult patients with SSc-ILD (Systemic Sclerosis-Associated Interstitial Lung Disease) is 162 mg given once every week as a subcutaneous injection, which was updated in the prescription in March 2021.
Veklury (remdesivir) and Olumiant (baricitinib) are two drugs that have been approved by the FDA for the treatment of COVID-19. Remdesivir is a nucleotide analog RNA polymerase inhibitor that inhibits viral replication by terminating RNA transcription. It is administered by intravenous injection and is approved for use in adults and pediatric patients (28 days of age and older and weighing at least 3 kg) with positive results of direct SARS-CoV-2 viral testing, who are hospitalized, or not hospitalized and have mild-to-moderate COVID-19 and are at high risk for progression to severe COVID-19, including hospitalization or death.
Remdesivir therapy still requires more exploration and clinical trials. Reports have shown the evidence that remdesivir retains in vitro neutralization activity against the Omicron variant and its subvariants (Takashita et al., 2022a; Takashita et al., 2022b; Vangeel et al., 2022; National Institutes of Health NIH, 2023c). However, the risk of SARS-CoV-2 remdesivir resistance mutations has been demonstrated in some cases, which revealed the necessity of combinatorial therapies (Gandhi et al., 2022).
On the other hand, Olumiant (baricitinib) is a Janus kinase (JAK) inhibitor that modulates downstream inflammatory pathways and inhibits IL-6-induced STAT3 phosphorylation in a dose-dependent manner (McInnes et al., 2019; Stebbing et al., 2020; Zhang et al., 2020). Its antiviral activity has not been confirmed yet. It is approved for use in hospitalized adults who require supplemental oxygen, noninvasive ventilation (NIV), mechanical ventilation, or extracorporeal membrane oxygenation (ECMO), and is also EUA-authorized for the treatment of COVID-19 in certain hospitalized pediatric patients 2 to less than 18 years of age.
Studies have shown that baricitinib plus remdesivir was superior in reducing recovery time and accelerating improvement among COVID-19 patients, notably among those receiving high-flow oxygen or noninvasive ventilation, compared to remdesivir alone (Kalil et al., 2021). This suggests that combinatorial antiviral therapy has the potential to be an eligible option to treat COVID-19 more effectively and prevent the SARS-CoV-2 virus from developing drug resistance.
It is worth noting that the FDA has updated the prescribing information for both remdesivir and baricitinib to ensure that they are administered only in settings where medications to treat severe infusion or hypersensitivity reactions can be accessed immediately. This is important for the safety and wellbeing of patients undergoing treatment with these drugs.
According to therapeutic management recorded in covid19treatmentguidelines.nih.gov, the antiviral agent remdesivir is widely used in both nonhospitalized and hospitalized patients with diverse disease severity, while the two FDA-approved immune modulators, baricitinib and tocilizumab, are used in patients with more severe symptoms (National Institutes of Health NIH, 2023d).
In normal circumstances, for nonhospitalized patients who are at high risk of progressing to severe COVID-19 and hospitalized patients who do not require oxygen supplementation or only require minimal conventional oxygen, remdesivir can be administered alone. However, it should be noted that most patients requiring conventional oxygen are recommended to receive therapy with remdesivir plus dexamethasone. For hospitalized patients who require high-flow nasal cannula (HFNC) oxygen or noninvasive ventilation (NIV) or mechanical ventilation (MV) or extracorporeal membrane oxygenation (ECMO), dexamethasone plus baricitinib or dexamethasone plus tocilizumab should be administered. Pediatric COVID-19 patients require more specific administrations, and more details can be found in covid19treatmentguidelines.nih.gov.
As mentioned above, the role of the antiviral drug and immune modulators in clinical management is aimed at initial symptoms and more severe symptoms, respectively, to precisely treat COVID-19 patients. It is essential to consult healthcare professionals and follow the latest guidelines for the most appropriate management of COVID-19.
The Public Health Emergency (PHE) for COVID-19 was declared by the Department of Health and Human Services under the Public Health Service Act and is predicted to expire on 11 May 2023, according to the FDA. Throughout the pandemic, many therapeutic products have been authorized under Emergency Use Authorization (EUA), with some differences (Table 1) in their formulations compared to approved drugs. Despite the upcoming expiration of the COVID-19 PHE, continued study of existing EUA-authorized drugs and potential drugs for COVID-19 treatment is essential. Several therapies authorized under EUA are listed on fda.gov, including monoclonal antibodies targeting SARS-CoV-2, antiviral drugs, immune modulators, sedatives, and renal replacement therapies.
SARS-CoV-2-targeting monoclonal antibodies (mAbs) are laboratory-produced antibodies that target the spike protein of the virus. Currently authorized under EUA are REGEN-COV (casirivimab and imdevimab), Bamlanivimab plus Etesevimab, Bebtelovimab, Sotrovimab, and Evusheld (tixagevimab co-packaged with cilgavimab). However, these therapies may not be effective against SARS-CoV-2 variants, especially the recent Omicron subvariants (National Institutes of Health NIH, 2023e). Due to the high frequency of SARS-CoV-2 mutations, the COVID-19 Treatment Guidelines Panel (the Panel) recommends against the use of SARS-CoV-2-targeting mAbs for antiviral treatment and pre-exposure prophylaxis.
The Panel also recommends against the use of interferon alfa or beta or lambda, as there is insufficient evidence of their safety and efficacy in treating COVID-19. Clinical trials on interferon are small or moderate in size and cannot provide strong evidence of its effectiveness. The current EUA limits the use of COVID-19 Convalescent Plasma (CCP) products that contain high levels of anti-SARS-CoV-2 antibodies, as evidence is still insufficient. Only under certain circumstances, some Panel members use CCP to treat an immunocompromised patient who is not susceptible to available therapies and attempt to obtain high-titer CCP from a vaccinated donor infected by similar SARS-CoV-2 variants with the patient.
FDA-approved Veklury (remdesivir) and EUA-authorized Paxlovid (ritonavir-boosted nirmatrelvir) and Lagevrio (molnupiravir) are rational choices for treating COVID-19. Paxlovid is a combination of nirmatrelvir, a SARS-CoV-2 main protease inhibitor, and ritonavir, an HIV-1 protease inhibitor and CYP3A inhibitor. It received EUA on 22 December 2021, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing who are at high risk of progressing to severe COVID-19 (U.S. Food and Drug Administration, 2023c). Lagevrio capsules contain molnupiravir, a nucleoside analogue that inhibits SARS-CoV-2 replication by viral mutagenesis. It received EUA on 23 December 2021, for the treatment of mild-to-moderate COVID-19 in adults with positive results of direct SARS-CoV-2 viral testing who are at high risk of progressing to severe COVID-19 and for whom alternative COVID-19 treatment options authorized by FDA are not accessible or clinically appropriate (U.S. Food and Drug Administration, 2023b).
The Panel recommends initiating treatment with antiviral drugs, such as Paxlovid and Lagevrio, as soon as possible after the onset of symptoms to prevent patients with mild to moderate COVID-19 from progressing to severe disease. It should be noted that Paxlovid has a higher priority than Lagevrio during drug administration, according to the Panel’s recommendation that considers Lageviro as an alternative therapy when other therapies are not available (National Institutes of Health NIH, 2023f).
Immune modulators play a crucial role in suppressing hyperinflammation in the human body and preventing more severe symptoms of COVID-19. These modulators can be classified into several categories, including corticosteroids, interleukin-6 inhibitors, kinase inhibitors, granulocyte-macrophage colony-stimulating factor (GM-CSF) inhibitors, and interleukin-1 inhibitors (National Institutes of Health NIH, 2023g).
Kineret (anakinra), Olumiant (baricitinib), and Actemra (tocilizumab) are some of the EUA-authorized products that have been used for COVID-19 treatment. However, Kineret is not approved for COVID-19 treatment by the FDA due to insufficient clinical evidence (National Institutes of Health NIH, 2023h). Canakinumab, another interleukin-1 inhibitor, is not recommended for COVID-19 treatment, except in clinical trials.
Olumiant (baricitinib) and Actemra (tocilizumab) are approved by the FDA for the treatment of COVID-19 in pediatric patients 2 to less than 18 years of age requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) (U.S. Food and Drug Administration, 2022b; U.S. Food and Drug Administration, 2022a). Olumiant (baricitinib) is also approved for the same indication for hospitalized adult patients. The use of JAK inhibitors, such as baricitinib and tofacitinib, is recommended by the Panel in combination with dexamethasone in hospitalized patients with evidence of inflammation and increasing oxygen needs (National Institutes of Health NIH, 2023). Actemra (tocilizumab) is recommended for use in hospitalized adults who are receiving systemic corticosteroids and require supplemental oxygen, noninvasive ventilation (NIV), mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Among IL-6 inhibitors, only tocilizumab and sarilumab are recommended for their use in hospitalized adults with COVID-19, while anti-interleukin-6 monoclonal antibodies such as siltuximab are not recommended (National Institutes of Health NIH, 2023b).
Other immune modulators, such as GM-CSF inhibitors and interleukin-1 inhibitors, lack sufficient evidence to prove their efficacy and safety for COVID-19 patients. The Panel does not recommend their use for COVID-19 treatment, except in clinical trials.
The FDA has authorized several drugs and therapies for emergency use in patients with COVID-19, including Propofol-Lipuro 1% injectable emulsion for infusion and continuous renal replacement therapy (CRRT) (U.S. Food and Drug Administration, 2023a). Propofol-Lipuro 1% is a sedative hypnotic drug used to maintain sedation via continuous infusion for patients with COVID-19 requiring mechanical ventilation in an ICU setting (U.S. Food and Drug Administration, 2021). CRRT is a machine treatment that filters and purifies the blood of COVID-19 patients with kidney injury in acute care settings.
Given the association between COVID-19 and thromboembolism (Han et al., 2020; Tang et al., 2020), anticoagulation is recommended for hospitalized, nonpregnant patients with COVID-19 to prevent venous thromboembolism (VTE). Low-molecular-weight heparin (LMWH) is suggested by the National Institute for Health and Care Excellence (NICE) guidelines for young people and adults with COVID-19 who need low-flow oxygen and who do not have an increased bleeding risk. For patients who experience rapid deterioration of pulmonary, cardiac, or neurological function or sudden, localized loss of peripheral perfusion, thromboembolic disease should be evaluated (National Institutes of Health NIH, 2022a).
The use of certain miscellaneous drugs such as colchicine, fluvoxamine, ivermectin, intravenous immunoglobulin, and metformin is not recommended for COVID-19 treatment (National Institutes of Health NIH, 2023). While the use of supplements like vitamin C, vitamin D, and zinc for COVID-19 treatment lacks sufficient evidence, several clinical trials for supplements are being conducted (National Institutes of Health NIH, 2022b). Concomitant medication for underlying medical conditions in COVID-19 patients should not be discontinued (National Institutes of Health NIH, 2021), and drug-drug interactions and potential adverse effects should be evaluated before administering concomitant medication for COVID-19 patients.
FDA-approved drugs and EUA-authorized drugs can meet the therapeutic needs of most COVID-19 patients. Antiviral agents are used for nonhospitalized patients with mild to moderate COVID-19 to prevent progression to more severe COVID-19. Immune modulators are used for hospitalized patients to deal with hyperimmunity, and prophylactic doses of heparin are given to prevent thromboembolism. Combination therapies and drug-drug interactions require continuous clinical exploration due to the constant mutation of SARS-CoV-2. The use of various supplements, COVID-19 drug administration for patients with underlying diseases, pediatric patients, and pregnant patients also requires more exploration and confirmation through clinical trials.
In summary, Table 2 provides a brief classification and recommendation of COVID-19 drugs. The ongoing phase III and IV clinical trials of COVID-19 drugs are reviewed in the next part, and their therapeutic potential and future trends in drug administration for the treatment of COVID-19 are analyzed.
We examined 31 clinical trials related to COVID-19, focusing on drugs or therapies currently in phase 3 or 4. Of these trials, 6 involve multiple drugs, while the remainder focus on a single drug or therapy. The classification, target population, phase of clinical trial and authorization status of them are summarized in Table 3. More detailed information is also summarized in the Supplementary Table S2.
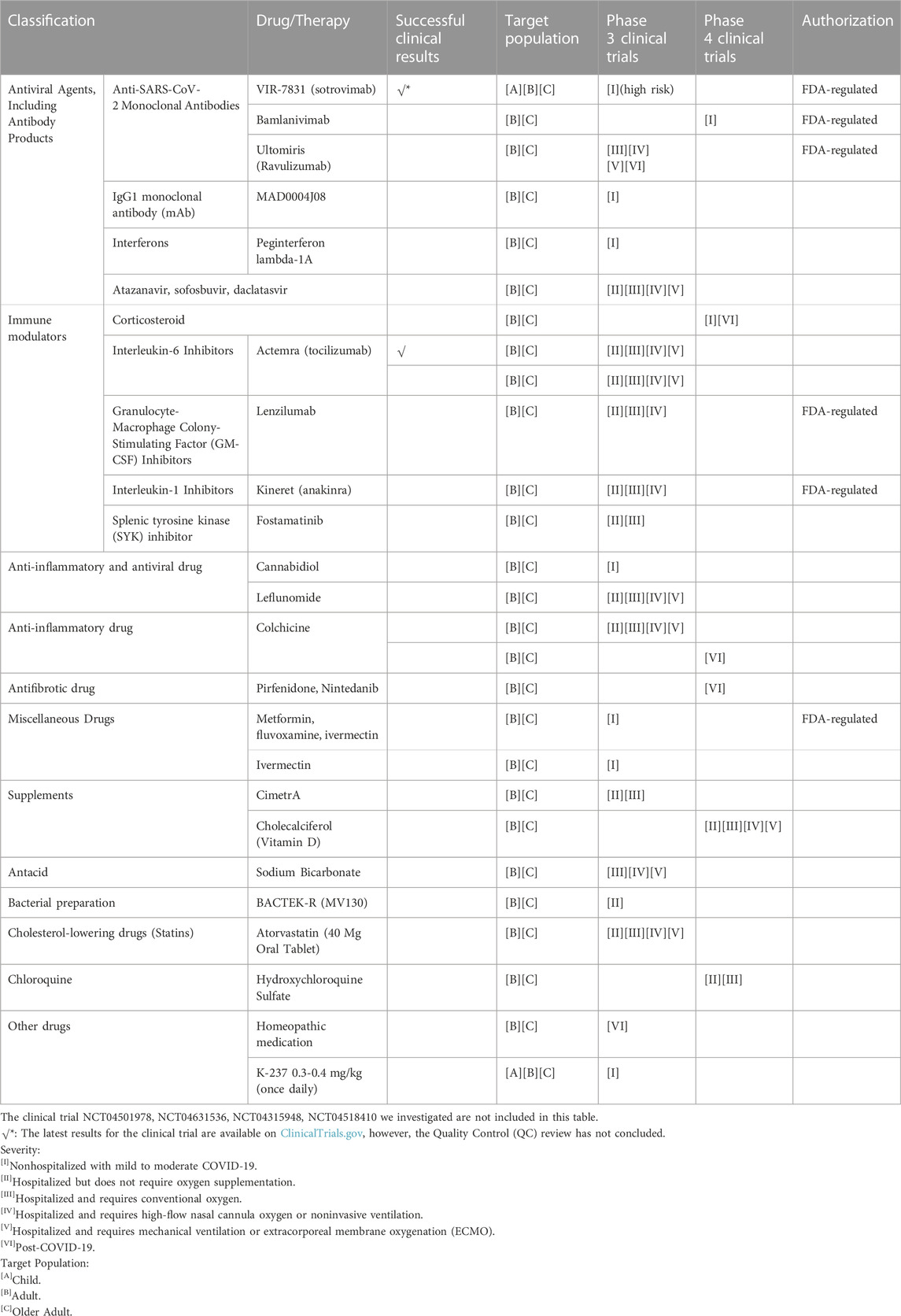
TABLE 3. The classification, target population, phase of clinical trial and authorization status of COVID-19 drugs undergoing late stage clinical trials.
We first examined FDA-regulated drugs in these clinical trials. Although some drugs received an Emergency Use Authorization (EUA), such as antiviral drugs, they may not be effective against SARS-CoV-2 variants. Several drugs in the clinical trials we examined are regulated by the FDA, including 3 anti-SARS-CoV-2 monoclonal antibodies (VIR-7831 (sotrovimab), Bamlanivimab, Ultomiris (Ravulizumab)), a granulocyte-macrophage colony-stimulating factor (GM-CSF) inhibitor Lenzilumab, an interleukin-1 inhibitor Anakinra, and metformin, fluvoxamine, ivermectin.
However, the three antibodies may not be effective against highly mutated SARS-CoV-2. For the two immune modulators Lenzilumab and Anakinra, there is insufficient evidence to recommend for or against their use. Most of the clinical trials are still ongoing, with no study results posted on ClinicalTrials.gov. Therefore, although these drugs were authorized for emergency use early in the COVID-19 pandemic, they are not preferred in most COVID-19 cases, unless in countries or regions lacking more effective antiviral drugs (Remdesivir) and immune modulators (tocilizumab, sarilumab, baricitinib, tofacitinib). Late-stage clinical trials of these drugs will be a vital basis for subsequent changes in drug authorizations as the COVID-19 pandemic evolves.
Furthermore, more attention can be drawn to the drugs listed in the COVID-19 treatment guidelines recommended by the Panel, which are currently undergoing late-stage clinical trials (National Institutes of Health NIH, 2023d). The interleukin-6 inhibitor Actemra (tocilizumab) and corticosteroids, which are strongly recommended for COVID-19 treatment, are among these drugs. Tocilizumab is undergoing phase 3 clinical trials with successful results posted on ClinicalTrials.gov. It has been proven to be effective and safe when used with standard of care (SOC) in hospitalized participants with COVID-19 pneumonia. Corticosteroids are immune modulators recommended by the Panel for hospitalized COVID-19 patients requiring oxygen supplementation or with more severe symptoms. They can be coadministered with remdesivir or oral baricitinib or intravenous tocilizumab.
Initially, the use of corticosteroids lacked clinical evidence, and some studies suggested they may cause lung damage (Russell et al., 2020). However, subsequent clinical trials provided supportive evidence for their use, making them recommended by the Panel (National Institutes of Health NIH, 2023). There is no evidence to object to the view that corticosteroids have no survival benefit for patients who did not require supplemental oxygen. Therefore, they are not administered in most mild or moderate cases. An ongoing phase 4 clinical trial with 752 participants is studying the timing of early-corticosteroid administration among patients with mild or moderate COVID-19 (National Institutes of Health NIH, 2020a). The trial is expected to be completed on 15 March 2023, and the results may provide evidence to support the view that early-corticosteroid administration for immunosuppression can reduce the rate of hospitalization or oxygen supplementation among mild or moderate COVID-19 patients.
Out of the late stage clinical trials we have focused on, four trials involved testing multiple drugs. The National Institute of Allergy and Infectious Diseases (NIAID) conducted a clinical trial called “ACTIV-2: A Study for Outpatients with COVID-19″that focused on 4044 COVID-19-positive patients who did not require hospitalization (National Institutes of Health NIH, 2020b). Multiple drugs from different companies were utilized in this trial to test their efficacy and safety. The results of the trial, which were submitted to ClinicalTrials.gov on 7 April 2023, are expected to provide evidence for the prevention of COVID-19 disease progression and transmission in the community.
NIAID also conducted another clinical trial, “ACTIV-3: Therapeutics for Inpatients with COVID-19 (TICO)”, which recruited 2,753 hospitalized patients (National Institutes of Health NIH, 2020c). The study evaluated several drugs, including LY3819253, VIR-7831, BRII-196/BRII-198, AZD7442, MP0420, and PF-07304814, in addition to the standard of care (SOC) drug Remdesivir. Participants were treated with either a study drug plus SOC or a placebo plus SOC. “Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy)” evaluated the safety and efficacy of possible therapeutic agents in hospitalized adult COVID-19 patients (2,416 patients) (National Institutes of Health NIH, 2020d). Most of the treatment arms in the study have been discontinued, except for AZD7442. This decision was based on the analysis review by both DSMC/DSMB, the Solidarity Executive Group, and the DisCoVeRy steering committee. The current protocol is blinded and placebo-controlled during the investigation of AZD7442, making it impossible to set new trial arms or perform efficacy or futility analyses.
In addition, a clinical trial called “Managing Endothelial Dysfunction in COVID-19: A Randomized Controlled Trial at LAUMC (MEDIC-LAUMC)” evaluated the endothelial treatment protocol in patients already on optimal medical therapy for the treatment of COVID-19 (National Institutes of Health NIH, 2020e). The trial involved a drug co-administration protocol on a small scale of patients (42 participants), including atorvastatin, nicorandil, l-arginine, folic acid, and nebivolol. However, based on the results of the clinical trial, the investigators claimed that no clinically significant improvement in outcome was observed and trials targeting endothelial should not be recommended (Matli et al., 2022).
Unlike clinical trials that focus on the effects of one or two drugs on a medical condition and involve only one company, multi-drug clinical trials are promising as they can efficiently and simultaneously test the effects of diverse therapies and provide comprehensive COVID-19 treatment recommendations. For example, ACTIV-2 provides guidance on the prevention of disease progression and transmission in COVID-19-positive patients. Currently, only one clinical trial on drug co-administration is ongoing. Due to the emergence of SARS-CoV-2 variants with stronger transmissibility and infectivity, more studies on drug co-administration or horizontal comparison of different therapies are urgently needed to provide more effective treatment guidance.
Previous clinical trials had some limitations, such as small recruitment scales, open-label, and contradictions with other clinical trials, which did not provide sufficient evidence to confirm the efficacy and safety of certain COVID-19 drugs. Many COVID-19 drugs listed on the COVID-19 Treatment Guidelines lack sufficient evidence to recommend for or against their use. Moreover, different types of drugs should be administered at different stages of COVID-19, diverse ages of patients, and various regions or communities. Thus, comparisons among drugs and breakthroughs on drug co-administration are required under current circumstances. In conclusion, it is essential to focus on ongoing late stage clinical trials on COVID-19 drugs, especially for potential drugs
with insufficient evidence (such as corticosteroids and drugs shown in “ACTIV-3”).
In this article, our focus was on FDA-regulated COVID-19 drugs and drugs currently undergoing late-stage clinical trials. According to the COVID-19 Treatment Guidelines, Remdesivir, an antiviral agent, is used for patients with mild or moderate symptoms or requiring conventional oxygen, while two immune modulators, tocilizumab and baricitinib, are used for patients with more severe symptoms. These FDA-approved drugs align with the principle that therapies targeting SARS-CoV-2 are typically used in the early stages of COVID-19, while immunosuppressive/anti-inflammatory therapies are used in the later stages of the disease.
COVID-19 Drugs such as Ivermectin, Canakinumab, and Colchicine are recommended against their use. And COVID-19 drugs such as hydroxychloroquine, chloroquine, and Fluvoxamine lack sufficient evidence to support their efficacy. Therefore, their administration in practical healthcare should be cautiously executed, and they should not be used when safer alternatives are available. Notably, some of them are still tested in ongoing clinical trials and co-administered with other drugs.
It is interesting to note that ongoing clinical trials have the potential to provide evidence for new drug authorizations in the future. For example, Colchicine, although not recommended by the Panel, is still being studied in two clinical trials for the treatment of hospitalized patients with COVID-19 and for preventing post-COVID-19 pulmonary fibrosis, respectively. Novel applications of familiar drugs may lead to changes in their subsequent authorization. Additionally, corticosteroids, a controversial therapy, are currently used in patients with severe symptoms but may have the potential to be used in patients with mild or moderate symptoms. A phase 4 clinical trial is underway to investigate this possibility (National Institutes of Health NIH, 2020a). The future use of these drugs remains a topic of debate.
BF: Conceptualization, Investigation, Writing–original draft, Writing–review and editing. KF: Conceptualization, Investigation, Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fddsv.2023.1304129/full#supplementary-material
Abdulakeem, A., Adams, T., Ahmad, S., Abbas, M., Abbasi, S., Abbass, H., et al. (2021). Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet (British Ed. 397 (10285), 1637–1645. doi:10.1016/S0140-6736(21)00676-0
Abidi, E., El, N. W., Alefishat, E., Rahman, N., Petroianu, G. A., El-Lababidi, R., et al. (2022). Tocilizumab and COVID-19: timing of administration and efficacy. Front. Pharmacol. 13, 825749. doi:10.3389/fphar.2022.825749
Aziz, M., Haghbin, H., Abu, S. E., Nawras, Y., Fatima, R., Sharma, S., et al. (2021). Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J. Med. Virol. 93 (3), 1620–1630. doi:10.1002/jmv.26509
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al. (2020). Remdesivir for the treatment of covid-19-final report. N. Engl. J. Med. 383 (19), 1813–1826. doi:10.1056/NEJMoa2007764
Bhullar, K. S., Drews, S. J., and Wu, J. (2021). Translating bioactive peptides for COVID-19 therapy. Eur. J. Pharmacol. 890, 173661. doi:10.1016/j.ejphar.2020.173661
Centers For Disease Control and Prevention (2023). COVID-19 treatments and medications. https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html (Accessed June 16, 2023).
Charan, J., Dutta, S., Kaur, R., Bhardwaj, P., Sharma, P., Ambwani, S., et al. (2021). Tocilizumab in COVID-19: a study of adverse drug events reported in the WHO database. Expert Opin. Drug Saf. 20 (9), 1125–1136. doi:10.1080/14740338.2021.1946513
Gandhi, S., Klein, J., Robertson, A., Peña-Hernández, M. A., Lin, M. J., Roychoudhury, P., et al. (2022). De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report. Nat. Commun. 13 (1), 1547. doi:10.1038/s41467-022-29104-y
Gordon, A. C., Mouncey, P. R., Al-Beidh, F., Rowan, K. M., Nichol, A. D., Arabi, Y. M., et al. (2021). Interleukin-6 receptor antagonists in critically ill patients with covid-19. N. Engl. J. Med. 384 (16), 1491–1502. doi:10.1056/NEJMoa2100433
Gritti, G., Raimondi, F., Bottazzi, B., Ripamonti, D., Riva, I., Landi, F., et al. (2021). Siltuximab downregulates interleukin-8 and pentraxin 3 to improve ventilatory status and survival in severe COVID-19. Leukemia 35 (9), 2710–2714. doi:10.1038/s41375-021-01299-x
Gupta, S., and Leaf, D. E. (2021). Tocilizumab in COVID-19: some clarity amid controversy. Lancet 397 (10285), 1599–1601. doi:10.1016/S0140-6736(21)00712-1
Han, H., Yang, L., Liu, R., Liu, F., Wu, K. L., Li, J., et al. (2020). Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 58 (7), 1116–1120. doi:10.1515/cclm-2020-0188
Jiang, Y., Wu, Q., Song, P., and You, C. (2021). The variation of SARS-CoV-2 and advanced research on current vaccines. Front. Med. (Lausanne) 8, 806641. doi:10.3389/fmed.2021.806641
Kalil, A. C., Patterson, T. F., Mehta, A. K., Tomashek, K. M., Wolfe, C. R., Ghazaryan, V., et al. (2021). Baricitinib plus remdesivir for hospitalized adults with covid-19. N. Engl. J. Med. 384 (9), 795–807. doi:10.1056/NEJMoa2031994
Matli, K., Al Kotob, A., Jamaleddine, W., Al Osta, S., Salameh, P., Tabbikha, R., et al. (2022). Managing endothelial dysfunction in COVID-19 with statins, beta blockers, nicorandil, and oral supplements: a pilot, double-blind, placebo-controlled, randomized clinical trial. Clin. Transl. Sci. 15 (10), 2323–2330. doi:10.1111/cts.13369
McInnes, I. B., Byers, N. L., Higgs, R. E., Lee, J., Macias, W. L., Na, S., et al. (2019). Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res. Ther. 21 (1), 183. doi:10.1186/s13075-019-1964-1
National Institutes of Health (NIH) (2021). Concomitant medications _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/concomitant-medications/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023i). Janus kinase inhibitors _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/janus-kinase-inhibitors/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023j). Miscellaneous drugs summary recommendations _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/miscellaneous-drugs/summary-recommendations/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023). Table_ systemic corticosteroids clinical data _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/tables/systemic-corticosteroids-data/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2020a). Timing of corticosteroids in COVID-19, II. https://clinicaltrials.gov/ct2/show/NCT04530409?recrs=d&cond=COVID-19&phase=23&draw=3 (Accessed June 16, 2023).
National Institutes of Health (NIH) (2020b). ACTIV-2_ A study for Outpatients with COVID-19 - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/study/NCT04518410?recrs=d&cond=COVID-19&phase=23&draw=2 (Accessed June 16, 2023).
National Institutes of Health (NIH) (2020c). ACTIV-3_ therapeutics for Inpatients with COVID-19 - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04501978?recrs=d&cond=COVID-19&phase=23&draw=3 (Accessed June 16, 2023).
National Institutes of Health (NIH) (2020d). Trial of treatments for COVID-19 in hospitalized adults - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04315948?recrs=d&cond=COVID-19&phase=23&draw=2 (Accessed June 16, 2023).
National Institutes of Health (NIH) (2020e). Managing endothelial dysfunction in COVID-19 _ A randomized controlled trial at LAUMC - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04631536?recrs=d&cond=COVID-19&phase=23&draw=3 (Accessed June 16, 2023).
National Institutes of Health (NIH) (2022a). Antithrombotic therapy _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2022b). Supplements summary recommendations _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/supplements/summary-recommendations/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023a). Clinical management of adults summary _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/clinical-management-of-adults-summary/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023b). Interleukin-6 inhibitors _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/interleukin-6-inhibitors/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023c). Remdesivir _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/remdesivir/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023d). Management _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/management/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023e). Anti-SARS-CoV-2 monoclonal antibodies _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/tables/variants-and-susceptibility-to-mabs/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023f). Molnupiravir _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/molnupiravir/ (Accessed June 16, 2023).
National Institutes of Health (NIH) (2023g). Immunomodulators summary recommendations _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/summary-recommendations/(Accessed June 16, 2023).
National Institutes of Health (NIH) (2023h). Interleukin-1 inhibitors _ COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/interleukin-1-inhibitors/(Accessed June 16, 2023).
Nicholson, L. B. (2016). The immune system. Essays Biochem. 60 (3), 275–301. doi:10.1042/EBC20160017
Rahimi, A., Mirzazadeh, A., and Tavakolpour, S. (2021). Genetics and genomics of SARS-CoV-2: a review of the literature with the special focus on genetic diversity and SARS-CoV-2 genome detection. Genomics 113 (1), 1221–1232. doi:10.1016/j.ygeno.2020.09.059
Rehman, S. U., Shafique, L., Ihsan, A., and Liu, Q. (2020). Evolutionary trajectory for the emergence of novel coronavirus SARS-CoV-2. Pathogens 9 (3), 240. doi:10.3390/pathogens9030240
Ren, S. Y., Wang, W. B., Gao, R. D., and Zhou, A. M. (2022). Omicron variant (B.1.1.529) of SARS-CoV-2: mutation, infectivity, transmission, and vaccine resistance. World J. Clin. Cases 10 (1), 1–11. doi:10.12998/wjcc.v10.i1.1
Russell, C. D., Millar, J. E., and Baillie, J. K. (2020). Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395 (10223), 473–475. doi:10.1016/S0140-6736(20)30317-2
Scientific American, (2020). Inside the coronavirus. Editor M. Fischetti https://www.scientificamerican.com/interactive/inside-the-coronavirus/?utm_source=newsletter&utm_medium=email&utm_campaign=today-in-science&utm_content=link&utm_term=2020-06-23_featured-this-week&spMailingID=67190029&spUserID=MTI0NzgzODY5OTU5S0&spJobID=1903751641&spReportId=MTkwMzc1MTY0MQS2 (Accessed June 16, 2023).
Stebbing, J., Phelan, A., Griffin, I., Tucker, C., Oechsle, O., Smith, D., et al. (2020). COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 20 (4), 400–402. doi:10.1016/S1473-3099(20)30132-8
Takashita, E., Kinoshita, N., Yamayoshi, S., Sakai-Tagawa, Y., Fujisaki, S., Ito, M., et al. (2022a). Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N. Engl. J. Med. 386 (15), 1475–1477. doi:10.1056/NEJMc2201933
Takashita, E., Yamayoshi, S., Simon, V., van Bakel, H., Sordillo, E. M., Pekosz, A., et al. (2022b). Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N. Engl. J. Med. 387 (5), 468–470. doi:10.1056/NEJMc2207519
Tang, N., Bai, H., Chen, X., Gong, J., Li, D., and Sun, Z. (2020). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 18 (5), 1094–1099. doi:10.1111/jth.14817
U.S. Food and Drug Administration (2021). BBraun propofol 1 percent LOA March 12 (clean). https://www.fda.gov/media/146680/download (Accessed June 16, 2023).
U.S. Food and Drug Administration (2022a). Baricitinib EUA letter of authorization 10272022. https://www.fda.gov/media/143822/download (Accessed June 16, 2023).
U.S. Food and Drug Administration (2022b). Actemra LOA 122122. https://www.fda.gov/media/150319/download (Accessed June 16, 2023).
U.S. Food and Drug Administration (2023a). Coronavirus (COVID-19) _ drugs _ FDA. https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (Accessed June 16, 2023).
U.S. Food and Drug Administration (2023b). EUA 108 merck Lagevrio LOA (02012023)_0. https://www.fda.gov/media/155053/download (Accessed June 16, 2023).
U.S. Food and Drug Administration (2023c). EUA-105-Pfizer-Paxlovid-LOA-05252023. https://www.fda.gov/media/155049/download (Accessed June 16, 2023).
Vangeel, L., Chiu, W., De Jonghe, S., Maes, P., Slechten, B., Raymenants, J., et al. (2022). Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 198, 105252. doi:10.1016/j.antiviral.2022.105252
Wang, R., Zhang, Q., Ge, J., Ren, W., Zhang, R., Lan, J., et al. (2021). Analysis of SARS-CoV-2 variant mutations reveals neutralization escape mechanisms and the ability to use ACE2 receptors from additional species. Immunity 54 (7), 1611–1621. doi:10.1016/j.immuni.2021.06.003
Xiaodan, H., Honghua, Y., Ao, W., Cong, L., Qianxiang, Z., Liandong, M., et al. (2022). Inhibitory effects of GT0918 on acute lung injury and the molecular mechanisms of anti-inflammatory response. bioRxiv, 2022–2026. doi:10.1101/2022.06.29.498191
Xie, Y., Yin, W., Zhang, Y., Shang, W., Wang, Z., Luan, X., et al. (2021). Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2. Cell Res. 31 (11), 1212–1214. doi:10.1038/s41422-021-00570-1
Keywords: COVID-19, SARS-CoV-2, drug administration, COVID-19 clinical trials, FDA regulated drugs
Citation: Feng B and Fu K (2023) Latest development of approved COVID-19 drugs and COVID-19 drugs undergoing late stage clinical trials. Front. Drug Discov. 3:1304129. doi: 10.3389/fddsv.2023.1304129
Received: 28 September 2023; Accepted: 21 November 2023;
Published: 01 December 2023.
Edited by:
Danilo Ciccone Miguel, State University of Campinas, BrazilReviewed by:
Marco A. Loza-Mejía, Universidad La Salle, MexicoCopyright © 2023 Feng and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingru Feng, YmYzM0ByaWNlLmVkdQ==; Kai Fu, a2FpZnVAdWNsYS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.