- 1Cardiovascular Center Oberallgaeu-Kempten, Clinic Association Allgaeu, Kempten, Germany
- 2Pentracor GmbH, Hennigsdorf, Germany
- 3Diakonissenhospital Flensburg, Flensburg, Germany
- 4Medical Care Center Kempten-Allgaeu, Kempten, Germany
- 5Department of Gastroenterology/Infectiology/Rheumatology, Charité University Medicine Berlin, Berlin, Germany
C-reactive protein (CRP) apheresis may preserve myocardial tissue after acute myocardial infarction with delayed revascularization. Ten consecutive patients with cardiogenic shock were graded using the Society of Cardiovascular Angiography and Interventions shock classification and treated with CRP apheresis. All patients tolerated CRP apheresis well and were discharged in good clinical condition.
1 Introduction
C-reactive protein (CRP) apheresis has emerged as a promising novel intervention to preserve myocardial tissue after acute myocardial infarction (AMI) with delayed revascularization (Ries et al., 2021; Torzewski et al., 2022). CRP may act as a primitive antibody (Torzewski et al., 2022) which labels hypoxic but still recoverable myocardial cells (Sheriff et al., 2021) for FcγR-mediated phagocytosis by macrophages (Manolov et al., 2004). This is achieved by a dramatic rise in circulating CRP levels, from below 5 mg/L (healthy) up to 250 mg/L, within the first 48 h after the incident (Kunkel et al., 2023). Although the increase is highly variable and, among other factors, depends on the extent of cardiac ischemia, CRP levels above 10 mg/L are pathological. CRP apheresis, applied shortly after AMI, interferes with this deleterious process (Pepys et al., 2006) and may reduce myocardial infarct size and improve patient prognosis (Ries et al., 2021). Although CRP apheresis is feasible and safe, and although data from first-in-man case reports (Ries et al., 2018) and pilot trials (Ries et al., 2021) are certainly promising, this therapeutic concept awaits the results of randomized controlled trials (RCTs) to achieve guideline implementation (Torzewski et al., 2022).
In preparation for sophisticated RCTs, the C-reactive protein apheresis in acute myocardial infarction-registry (CAMI-R) investigates the effect of CRP apheresis on all sub-forms of acute coronary syndromes in a daily life. Of these, cardiogenic shock (CS) may be of special interest for the following reasons. First, CRP apheresis in CS has never been reported. In addition, CS is still a disease with a 6%–62% in-hospital mortality, depending on Society of Cardiovascular Angiography and Interventions (SCAI) shock classification (Baran et al., 2019; Hill et al., 2023). Any approach to reducing this mortality may be of particular interest.
Here, we describe the first ten consecutive patients with CS treated by CRP apheresis within CAMI-R.
2 Patients and methods
2.1 Study design
CAMI-R is a prospective, multi-center, non-interventional trial to investigate the effect of CRP apheresis in patients with acute coronary syndromes and highly elevated CRP plasma levels (https://drks.de/search/de/trial/DRKS00017481). Patients with ST-elevation myocardial infarction, non-ST-elevation myocardial infarction and CS were prospectively included. Patients had to provide written informed consent. There were no further exclusion criteria. Patients received treatment in accordance with AHA/ESC guidelines. After acute percutaneous intervention (PCI) and standard care, patients received CRP apheresis. CRP levels as well as other cardiac markers were regularly monitored during the hospital stay. Follow-up included a medical visit at patient discharge and phone calls after 30 days and after 1 year.
2.2 Cardiogenic shock
CS is a disease with various facets. As SCAI has recently developed a standardized language to describe CS severity (Baran et al., 2019), this analysis only relies on the SCAI shock classification and its associated mortality (Hill et al., 2023). Attribution to shock stages A to E was based on clinical symptoms and laboratory parameters assessed in-hospital, starting at patient admission (Hill et al., 2023).
2.3 C-reactive protein apheresis
CRP apheresis was performed with a selective CRP adsorber (PentraSorb® CRP) (Mattecka et al., 2019) by peripheral venous access (Ries et al., 2018; Ries et al., 2021). Patients received 2–4 sessions after PCI and intensive care unit (ICU) admission. First apheresis started 17–65 h after the onset of symptoms, and the second apheresis 24 ± 12 h later. A third or fourth apheresis was performed if, another ∼12 h later, the CRP-concentration exceeded 30 mg/L (Ries et al., 2021). Approximately 7,000 mL of plasma was processed in each apheresis (Ries et al., 2018; Ries et al., 2021).
2.4 C-reactive protein quantification
The diagnostic laboratories were accredited by European law (Ries et al., 2021), and CRP was quantified with standard procedures. CRP tests remained the same throughout the study. CRP-concentration during apheresis was adjusted by the hematocrit of the same blood sample.
2.5 In-hospital mortality and 30-day follow-up
In-hospital mortality was assessed by a medical visit at patient discharge, 30-day follow-up by phone calls, and patient reports provided by resident cardiologists.
3 Results
3.1 Patients
Patient characteristics are summarized in Table 1. The ten patients were 60 ± 11 years old, all male, and graded using SCAI classification—1*B, 6*C, 1*D, and 2*E (Baran et al., 2019; Hill et al., 2023). Eight patients were resuscitated during CS treatment; one patient required assisted ventilation. Lactate on admission was 2.7 ± 1.4 mmol/L (reference range 0.5–1.6 mmol/L). Acute PCI was performed on all patients, with six culprit lesions in LAD and two in either RCA or RCX, respectively. LVEF (Teichholz/Simpson method), although not routinely quantified in every patient at both time points, was 40.7% ± 10.0% before first apheresis and 46.3% ± 10.5% after final apheresis.
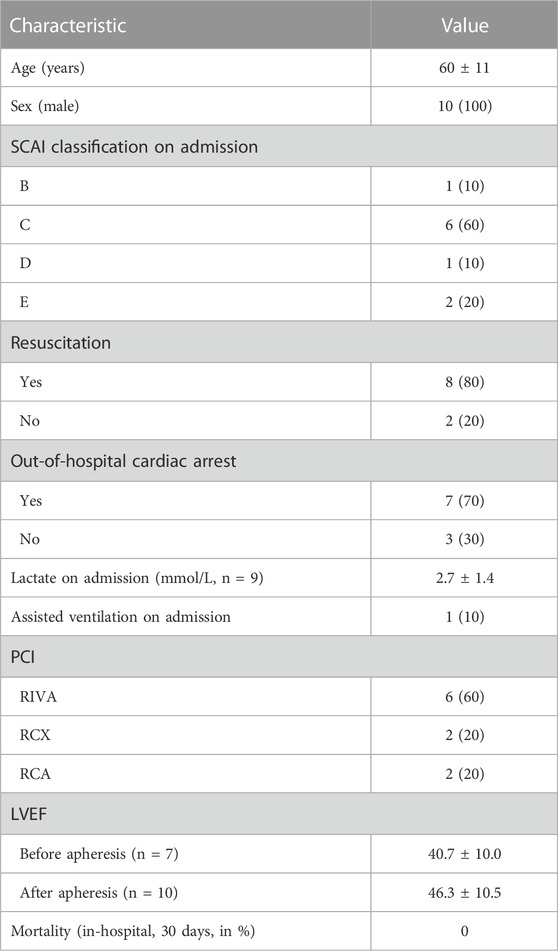
TABLE 1. Patient characteristics. Values are shown as mean ± standard deviation or as total number (portion in %).
3.2 C-reactive protein apheresis
Figure 1 provides data on CRP apheresis. Two patients required four apheresis sessions, two required three apheresis sessions, and six only required two apheresis sessions. A CRP concentration of <30 mg/L was achieved in nine out of the ten patients.
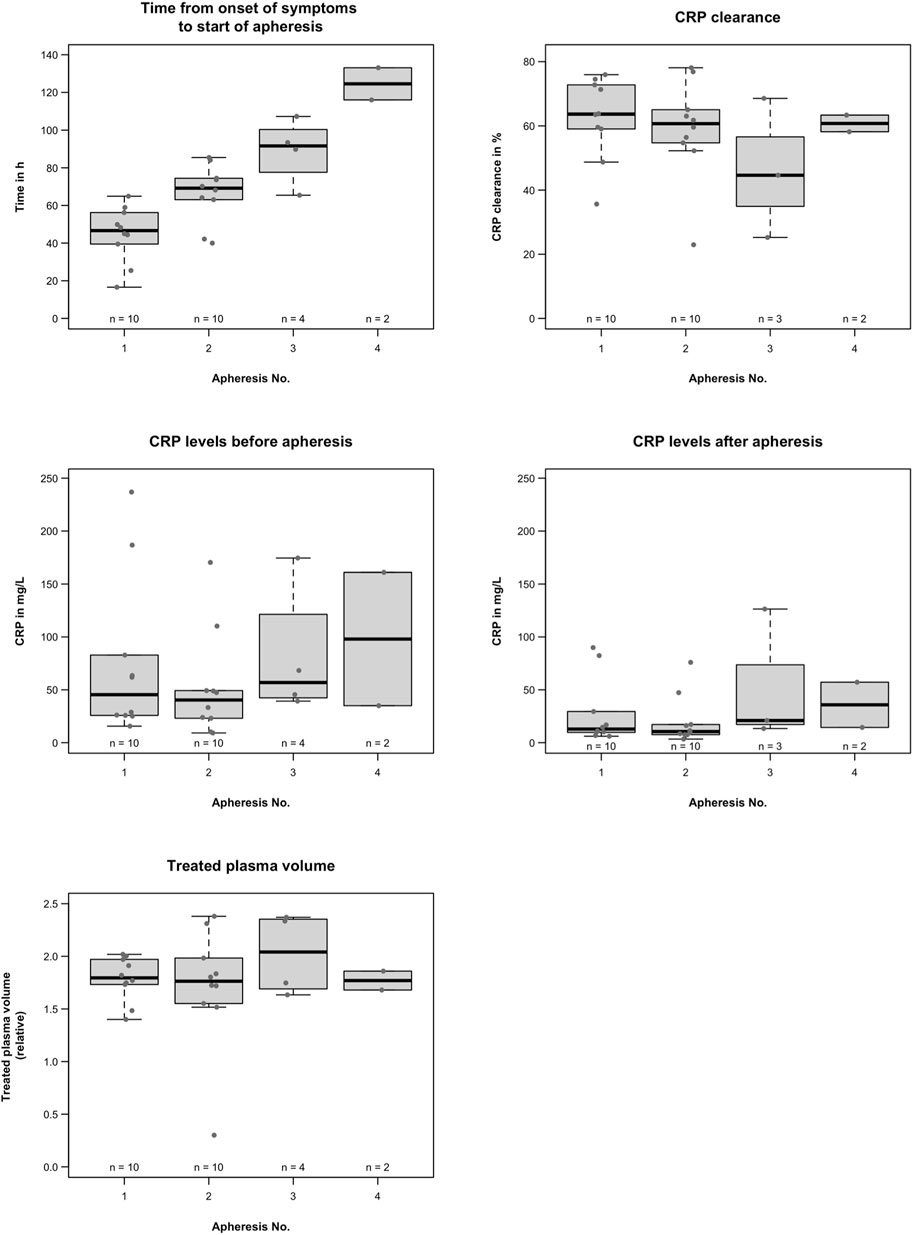
FIGURE 1. C-reactive protein apheresis; time of the first CRP apheresis was variable, probably due to inter-patient variability of CRP synthesis in CS or to logistical reasons in the participating hospitals. CRP levels were also variable depending on the extent of cardiac damage. CRP clearance ranged from 23% to 78%, depending on blood flow and treated plasma volume, apheresis time intervals, and individual CRP synthesis rates. Overall, apheresis procedures effectively reduced CRP without side effects.
The time of the first CRP apheresis was variable, probably due to the inter-patient variability of CRP synthesis in CS or to logistical reasons in the participating hospitals. The first CRP apheresis started at a mean of 44.9 ± 14.8 h after the onset of symptoms, the second started at a mean of 66.5 ± 15.3 h, the third at a mean of 89.0 ± 17.4 h, and the fourth CRP apheresis at a mean of 124.5 ± 12.1 h after the onset of symptoms.
CRP levels were also variable. The first CRP apheresis started at a median of 45.4 (15.6–236.9) mg/L, the second at a median of 40.3 (9.2–170.3) mg/L, the third at a mean 81.9 ± 63.0 mg/L, and fourth CRP apheresis at a mean 98.0 ± 89.1 mg/L. All patients tolerated CRP apheresis well without side effects.
3.3 Clinical outcomes
3.3.1 In-hospital mortality
A medical visit at patient discharge revealed no in-hospital mortality (Figure 1). None of the ten patients had neurological complications, and all were discharged in good clinical condition.
3.3.2 30-day follow-up
Phone calls revealed no 30-day mortality (Figure 1). One-year follow-up has already been completed for nine out of ten patients. One patient died approximately 8 months after the acute event. The tenth patient is still alive but has not completed follow-up yet.
4 Discussion
CS mortality is high (Baran et al., 2019; Hill et al., 2023). As CS is a volatile clinical condition characterized by changing symptoms during the clinical course, precise definition is of utmost importance. Recently, SCAI has defined shock classes A–E relying on physical findings, laboratory findings, and hemodynamics during the in-hospital stay (Baran et al., 2019). SCAI shock classification correlates with mortality risk and provides an adequate basis for scientifically evaluating new treatment options (Hill et al., 2023).
Here, we grade patients using SCAI shock classification and evaluate mortality at patient discharge and after 30 days. In addition to guideline-adopted standard treatment with PCI and ICU care, the patients were treated with CRP apheresis within CAMI-R.
Elevation of CRP plasma levels after AMI has an inverse correlation with prognosis (Dimitrijević et al., 2006; Stumpf et al., 2017; Ries et al., 2021; Świątkiewicz et al., 2021). This has also been shown specifically in cardiogenic shock patients (Kunkel et al., 2023). CRP obviously plays a significant role in damaging hypoxic cardiomyocytes (Manolov et al., 2004; Sheriff et al., 2021). CRP apheresis interferes with this deleterious process and may preserve LVEF by reducing the deleterious concentration of CRP (Ries et al., 2021; Tiller et al., 2021; Torzewski et al., 2022). This may explain the positive effects of CRP apheresis in the open-label, non-randomized (and therefore inconclusive) CAMI-1 trial (Ries et al., 2021). Recently, the German Federal Joint Committee has approved the potential of CRP apheresis in AMI and will even provide funding for a RCT that may pave the way to guideline implementation (CRP-Apherese bei akutem Herzvorderwandinfarkt (§137eSGBV) - Gemeinsamer Bundesausschuss (g-ba.de).
This case series is hypothesis-generating—no more, no less. CRP apheresis in CS seems feasible and safe. Some 70% of cardiac arrests were out-of-hospital. This fits well with the expected range of 50%–80% out-of-hospital cardiac arrests described in the USA (and Germany) (Virani et al., 2021). A 30-day mortality of 0% and a good neurological outcome in ten consecutive CS patients with SCAI shock classifications B–E are remarkable, but far from conclusive—the results may still be accidental. However, given its 6%–62% in-hospital mortality (Hill et al., 2023), any potential new CS treatment may be of considerable importance. Consequently, CRP apheresis in CS should be carefully evaluated in future clinical trials, predominantly in a RCT.
5 Learning objectives
1) To understand the role of inflammation/CRP in CS.
2) To understand the potential role of CRP apheresis in AMI and CS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JT: conceptualization, supervision, writing–original draft, writing–review and editing, and investigation. SM: formal analysis, writing–review and editing, and data curation. WR: writing–review and editing and investigation. CG: writing–review and editing and investigation. FH: writing–review and editing and investigation. JF: writing–review and editing and investigation. AS: conceptualization, funding acquisition, supervision, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Pentracor GmbH provided limited support for patient inclusion in the CAMI-1 registry. Patient treatment is covered by the German healthcare system.
Conflict of interest
Authors SM and AS were employed by Pentracor GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRP, C-reactive protein; AMI, acute myocardial infarction; RCT, randomized controlled trial; CS, cardiogenic shock; SCAI, Society of Cardiovascular Angiography and Interventions; ICU, intensive care unit; LVEF, left ventricular function.
References
Baran, D. A., Grines, C. L., Bailey, S., Burkhoff, D., Hall, S. A., Henry, T. D., et al. (2019). SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American college of cardiology (ACC), the American heart association (AHA), the society of critical care medicine (SCCM), and the society of thoracic surgeons (STS) in april 2019. Catheter Cardiovasc Interv. 94, 29–37. doi:10.1002/ccd.28329
Dimitrijević, O., Stojcevski, B. D., Ignjatović, S., and Singh, N. M. (2006). Serial measurements of C-reactive protein after acute myocardial infarction in predicting one-year outcome. Int. Heart J. 47, 833–842. doi:10.1536/ihj.47.833
Hill, K. L., Rustin, M. A., Asche, M. A., Bennett, C. E., Patel, P. C., and Jentzer, J. C. (2023). Cardiogenic shock classification and associated mortality risk. Mayo Clin. Proc. 98, 771–783. doi:10.1016/j.mayocp.2022.12.007
Kunkel, J. B., Josiassen, J., Helgestad, O. K. L., Schmidt, H., Holmvang, L., Jensen, L. O., et al. (2023). Inflammatory response by 48 h after admission and mortality in patients with acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. Acute Cardiovasc Care 12, 306–314. doi:10.1093/ehjacc/zuad018
Manolov, D. E., Rocker, C., Hombach, V., Nienhaus, G. U., and Torzewski, J. (2004). Ultrasensitive confocal fluorescence microscopy of C-reactive protein interacting with FcgammaRIIa. Arterioscler. Thromb. Vasc. Biol. 24, 2372–2377. doi:10.1161/01.ATV.0000147407.17137.02
Mattecka, S., Brunner, P., Hähnel, B., Kunze, R., Vogt, B., and Sheriff, A. (2019). PentraSorb C-reactive protein: characterization of the selective C-reactive protein adsorber resin. Ther. Apher. Dialysis 23, 474–481. doi:10.1111/1744-9987.12796
Pepys, M. B., Hirschfield, G. M., Tennent, G. A., Gallimore, J. R., Kahan, M. C., Bellotti, V., et al. (2006). Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 440, 1217–1221. doi:10.1038/nature04672
Ries, W., Sheriff, A., Heigl, F., Zimmermann, O., Garlichs, C. D., and Torzewski, J. (2018). First in man": case report of selective C-reactive protein apheresis in a patient with acute ST segment elevation myocardial infarction. Case Rep. Cardiol. 2018, 4767105. doi:10.1155/2018/4767105
Ries, W., Torzewski, J., Heigl, F., Pfluecke, C., Kelle, S., Darius, H., et al. (2021). C-reactive protein apheresis as anti-inflammatory therapy in acute myocardial infarction: results of the CAMI-1 study. Front. Cardiovasc Med. 8, 8. doi:10.3389/fcvm.2021.591714
Sheriff, A., Kayser, S., Brunner, P., and Vogt, B. (2021). C-reactive protein triggers cell death in ischemic cells. Front. Immunol. 12, 12. doi:10.3389/fimmu.2021.630430
Stumpf, C., Sheriff, A., Zimmermann, S., Schaefauer, L., Schlundt, C., Raaz, D., et al. (2017). C-reactive protein levels predict systolic heart failure and outcome in patients with first ST-elevation myocardial infarction treated with coronary angioplasty. Arch. Med. Sci. 13, 1086–1093. doi:10.5114/aoms.2017.69327
Świątkiewicz, I., Magielski, P., and Kubica, J. (2021). C-reactive protein as a risk marker for post-infarct heart failure over a multi-year period. Int. J. Mol. Sci. 22, 3169. doi:10.3390/ijms22063169
Tiller, C., Reindl, M., Holzknecht, M., Lechner, I., Simma, F., Schwaiger, J., et al. (2021). High sensitivity C-reactive protein is associated with worse infarct healing after revascularized ST-elevation myocardial infarction. Int. J. Cardiol. 328, 191–196. doi:10.1016/j.ijcard.2020.12.006
Torzewski, J., Brunner, P., Ries, W., Garlichs, C. D., Kayser, S., Heigl, F., et al. (2022). Targeting C-reactive protein by selective apheresis in humans: pros and cons. J. Clin. Med. 11, 1771. doi:10.3390/jcm11071771
Keywords: CRP, CRP apheresis, cardiogenic shock, SCAI shock classification, anti-inflammatory therapy
Citation: Torzewski J, Mattecka S, Ries W, Garlichs CD, Heigl F, Fiedler J and Sheriff A (2023) Case report: C-reactive protein apheresis in cardiogenic shock: case series from the C-reactive protein apheresis in acute myocardial infarction-registry. Front. Drug Discov. 3:1286710. doi: 10.3389/fddsv.2023.1286710
Received: 31 August 2023; Accepted: 17 November 2023;
Published: 11 December 2023.
Edited by:
Rajeev K. Tyagi, Institute of Microbial Technology (CSIR), IndiaReviewed by:
Werner J. Geldenhuys, West Virginia University, United StatesPraveen Sharma, All India Institute of Medical Sciences Jodhpur, India
Copyright © 2023 Torzewski, Mattecka, Ries, Garlichs, Heigl, Fiedler and Sheriff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Torzewski, amFuLnRvcnpld3NraUBrbGluaWt2ZXJidW5kLWFsbGdhZXUuZGU=
 J. Torzewski
J. Torzewski S. Mattecka
S. Mattecka W. Ries3
W. Ries3 F. Heigl
F. Heigl A. Sheriff
A. Sheriff