- 1Department of Internal Medicine, Penn State Health Holy Spirit Medical Center, Camp Hill, PA, United States
- 2Professor Emeritus of Pharmacology and Neuroscience, Penn State College of Medicine, Hershey, PA, United States
Various literature cited suggests that bismuth may have usefulness against Covid-19 both in vitro and in vivo. During the course of caring for Covid-19 patients we administered bismuth subsalicylate to those who displayed diarrhea and/or gastric complaints. Using relatively conservative criteria, upon retrospective review, we noted marked improvement in oxygen requirements in most of the cases. This improvement was observed even when prior therapy with standard anti-Covid drugs had failed. Our overall impression is that these positive results support a detailed evaluation of bismuth as an adjunct treatment for the treatment of Covid-19.
Introduction
As of 1 July 2022, Covid-19 had infected half a billion people worldwide and over 6.35 million had died. (Dadax. 2022. https://www.worldometers.info/coronavirus/ (Accessed 1 July 2022). Preventative measures include isolation/masking and vaccination whereas treatment options include steroids, vitamins, and drugs such as remdesivir, tocilizumab and baricitinib and monoclonal antibodies. (Covid-19: Management in hospitalized Adults (2022) https://www.uptodate.com/ (Accessed 1 July 2022). In spite of these measures, Covid-19 infection remains a major public health concern and treatments have had limited efficacy: for example, a prominent study (Beigel et al., 2020), showed that while remdesivir appeared to shorten recovery time, the mortality rate was not statistically different from placebo. Another study (Horby et al., 2021) noted that whereas dexamethasone had some efficacy over placebo in reducing mortality rates in intubated patients (ie., 29% vs. 41%) it had no effect for non-ventilated patients over placebo (18% vs. 14%).
The emergence of an inexpensive and efficacious anti-Covid medication with few side effects would be an ideal solution to this conundrum. One potential solution may be bismuth subsalicylate (BiS), a common over-the-counter medication which is widely used for various gastrointestinal symptoms. When taken orally it reacts with hydrochloric acid forming salicylate and bismuth oxychloride and has anti-inflammatory, anti-secretory and anti-microbial activity (Pasricha, 2006). It inhibits two key enzymes (ie, NTPase and RNA helicase) (Yang et al., 2007; Shu et al., 2020) which are necessary for viral replication which might explain its’ efficacy against other viruses including rotavirus, reovirus, noravirus, echovirus, polio and herpes virus (Ward et al., 1985; Waldum et al., 1998; Koo et al., 2010). In vitro studies have also demonstrated bismuth’s anti-Covid effect (Yang et al., 2007). In addition, researchers in Hong Kong (Yuan et al., 2020) noted that bismuth medications had efficacy in the animal model; hamsters who received ranitidine bismuth citrate had a 1000-fold reduction in their Covid-19 viral loads and improvement in their pneumonias. A case in 2020 (Wolf et al., 2020) noted an 85-year-old man with Crohn’s whose Covid symptoms (both URI and diarrhea) improved rapidly after receiving BiS without receiving any other anti-Covid related medications. Finally, preliminary results from a recent study from the University of Louisville noted that Covid patients were noted to experience a decrease in Covid symptoms within 48 h after completing a course of BiS (Yacyshyn et al., 2022).
Materials and methods
In light of the apparent virustatic and/or virucidal effect of bismuth on Covid-19 virus both in vitro and in the animal model, after review of Covid-positive patients who were treated with BiS for their diarrhea, we noted that bismuth containing compounds appeared to have an adjunctive role in the treatment of Covid pneumonia. We herein report eight cases where BiS was administered to control gastric-related complaints (ie, loose stools or diarrhea) in hospitalized patients with Covid pneumonia. Bismuth subsalicylate has long been an over-the-counter drug in the United States and in our review, was used solely to treat diarrhea symptoms in patients hospitalized with Covid-19. Data on each of the cases was extracted and reviewed from the charts independently by two different hospitalists (CK and KN). (Figures 1–8). All patients received BiS, two different manufacturers are used in our institution: Rugby Laboratories, a Division of the Harvard Drug Group, LLC; NDC: 00536-1286-36 or AmerisourceBergen Corporation NDC: 46122-0306-34.
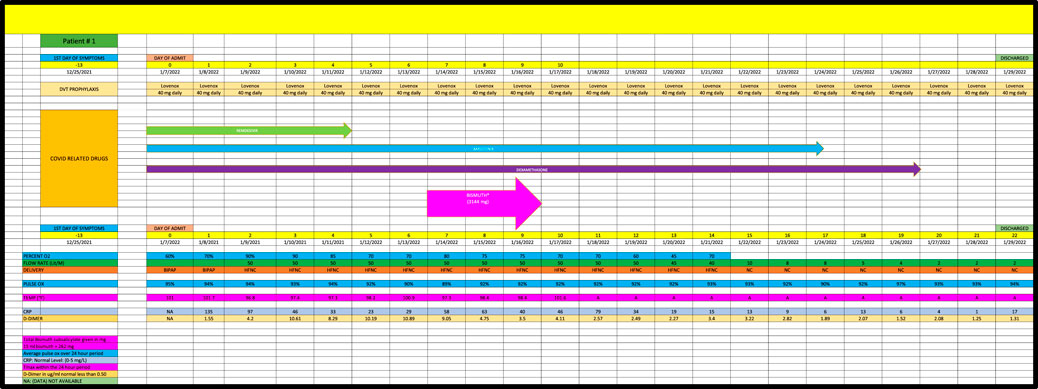
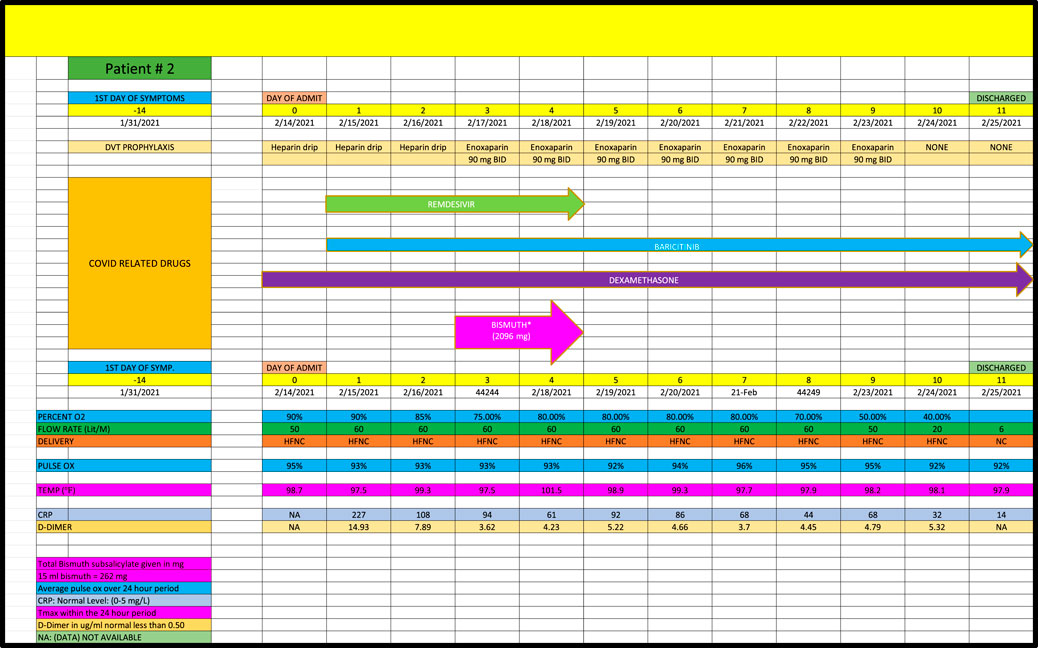
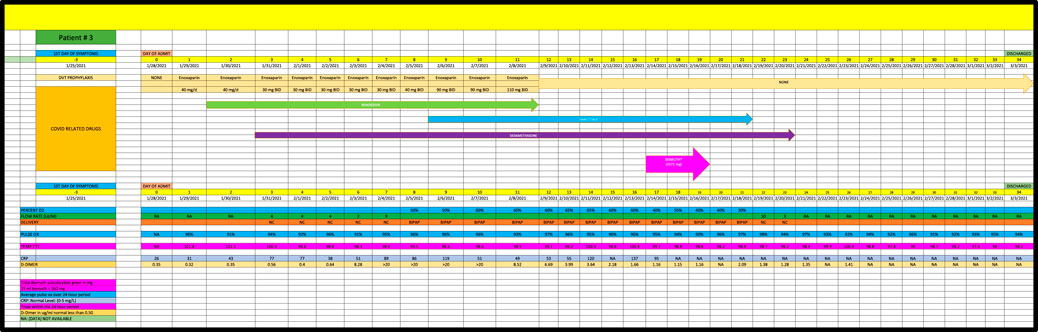
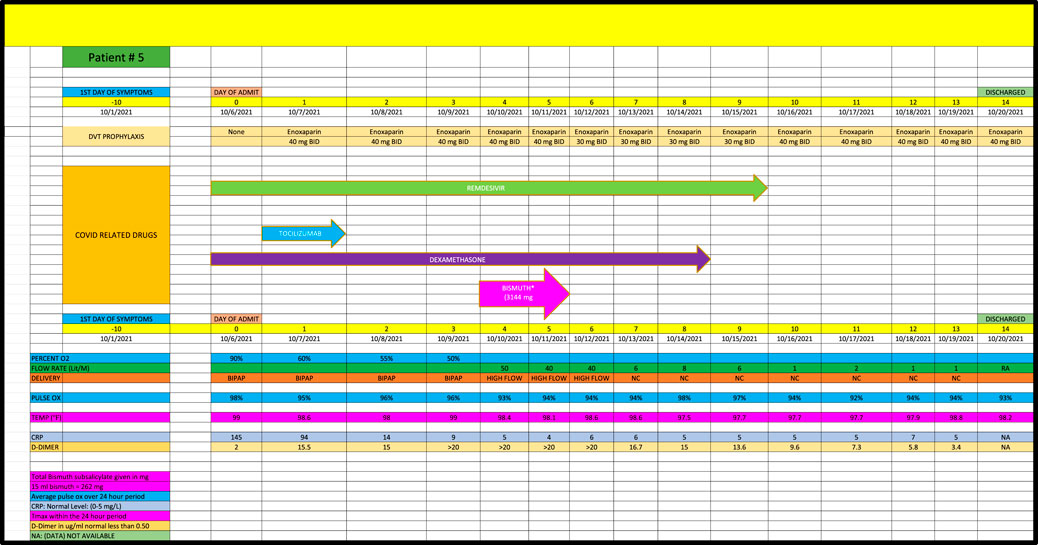
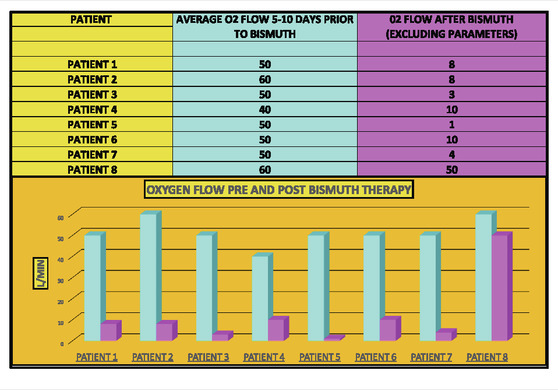
Cases were only included if they had at least 5 days of high-oxygen requirements prior to the dispensation of BiS. Each participant had to receive at least 4 total doses of BiS within a 48-h period of time with a minimum total dose of at least 1096 mg. The efficacy of BiS was graded on an arbitrary scale noted in Table 1; zero (minimal), one (moderate) or two (strong) was assigned to each case based on the response to BiS. Scaling criteria was based on the premise that a positive score required a major reduction in oxygen requirements within days of finishing BiS therapy. Patients who had longer duration of high-oxygen requirements prior to the administration of BiS (ie., spanning 10-45 days) had longer recovery phase criteria. Scaling criteria, although arbitrary, was the best clinical estimate as to what would constitute a minimal, moderate or strong response to therapy. For example, if a patient required high dose oxygen for 5 days prior to the reception of BiS, a positive response would have to be seen within 5 days of the last dose of BiS. Patients who had longer periods of high oxygen requirements prior to administration of BiS (eg, patient #4 who had been ventilated for 6 weeks prior to receiving BiS) had to have a major reduction in oxygen requirements within 10 days of receiving their last dose of BiS. Comprehensive data for each patient are included in Figures 1–8 which contain data on daily timing and dosing of all Covid related medications, BiS administration, in addition to complete vital signs data including daily oxygen requirements and pulse ox and temperature readings; lab data on CRP (C-Reactive Protein) as well as D-Dimer values were provided if available. Of note, two patients (#3 and #4) transitioned from either BiPAP (ie, Bilevel Positive Airway Pressure) or the ventilator, to lower dose oxygen delivery methods (ie, either simple mask or nasal cannula). In these cases, we estimated that oxygen flow rates for ventilator and/or BiPAP patients to be equivalent to at least 50 L/min of high-flow oxygen (Shoukri, 2021; Beran et al., 2022). Each patient gave informed consent to use his or her medical data for publication; no names or other non-medical information were revealed.
Case reports
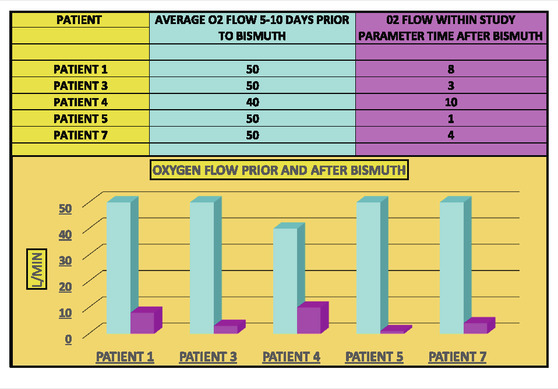
Patient 1 (Case 1) is a 79-year-old man with PMH of hypertension and hyperlipidemia admitted in January 2022 who required high-flow oxygen (ie, 50 L/min) for 10 days prior to the last dose of BiS (Figure 1). During the initial 10 days of treatment, he was given remsedivir, baricitinib and dexamethsone with no effect on his oxygen requirements. Within 7 days of finishing a total of 3144 mg of BiS therapy, he required only 8 L/min of oxygen, consistent with a strong (+2) effect.
Patient 2 (Case 2) is a 67-year-old with a PMH of diabetes mellitus and a recent deep vein thrombosis admitted in February 2021, who developed acute respiratory failure due to Covid pneumonia who received remdesivir, baricitinib and dexamethasone during the first 5 days of his admission with no change in his high-dose oxygen requirements (Figure 2). After receiving 2096 mg of BiS, his oxygen requirements improved significantly going from 50 L/min to 6 L/min, however, per criteria in Table 1, this was categorized as a minimal response (ie, Grade 0) because the noted decrease in oxygen requirements occurred after the 5-day period of BiS administration. (Note: on the seventh day post-BiS administration, patient’s oxygen requirement had decreased to 6 L/min).
Patient 3 is a 78 year-old African American, admitted in January 2021, with a PMH of obstructive sleep apnea and diabetes mellitus who developed Covid pneumonia (Figure 3). He had no major change in his high oxygen requirements for the first 17 days of his admission while receiving remdesivir, baricitinib and dexamethasone and was given the option of hospice care on Day 17. Patient then received 1592 mg of BiS on Day 17 after developing loose stool and progressed to room air within 5 days after the last day of BiS, consistent with a strong (+2) response.
Patient 4 is a 70-year-old admitted in May 2021, with a PMH of esophageal dysphagia and prostate carcinoma with Covid pneumonia (Figure 4). He remained ventilated for almost 6 weeks despite receiving remdesivir, tocilizumab and dexamethasone; however, he was able to come off the ventilator and progressed to 10 L/min of oxygen within 10 days of receiving 6288 mg of BiS, consistent with a strong response (+2).
Patient 5 is a 38-year-old African-American female admitted in October 2021, with a PMH of hypothyroidism who remained on high dose oxygen (ie, 50 L/min) flow for the first 5 days of her admission despite receiving remdesivir, tocilizumab and dexamethasone. (Figure 5). She received a total of 3144 mg of BiS and progressed to an oxygen requirement of only 1 L/min within 5 days of her last dose of BiS, consistent with a strong (2+) response.
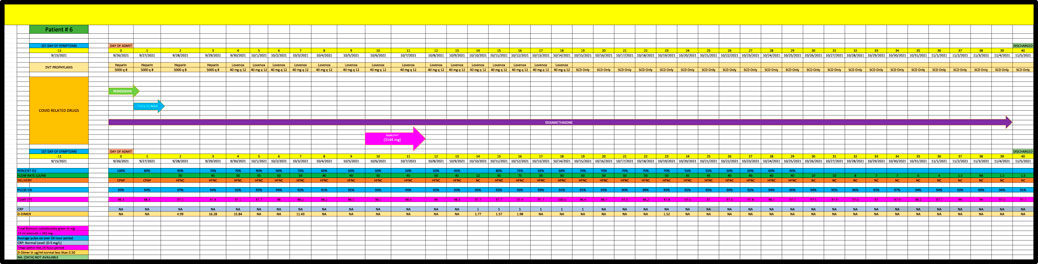
Patient 6 is a 57-year-old man admitted in September 2021, with a PMH of coronary heart disease and hypertension (Figure 6) who remained on high dose oxygen (ie, 50 L/min) flow for the first 5 days of his admission despite receiving remdesivir, tocilizumab and dexamethasone. He received 3144 mg of BiS and eventually progressed down to 1.5 L/Min of oxygen but received a score of 0 (minimal change) since he remained on high flow oxygen for more than 7 days after receiving his last dose of BiS.
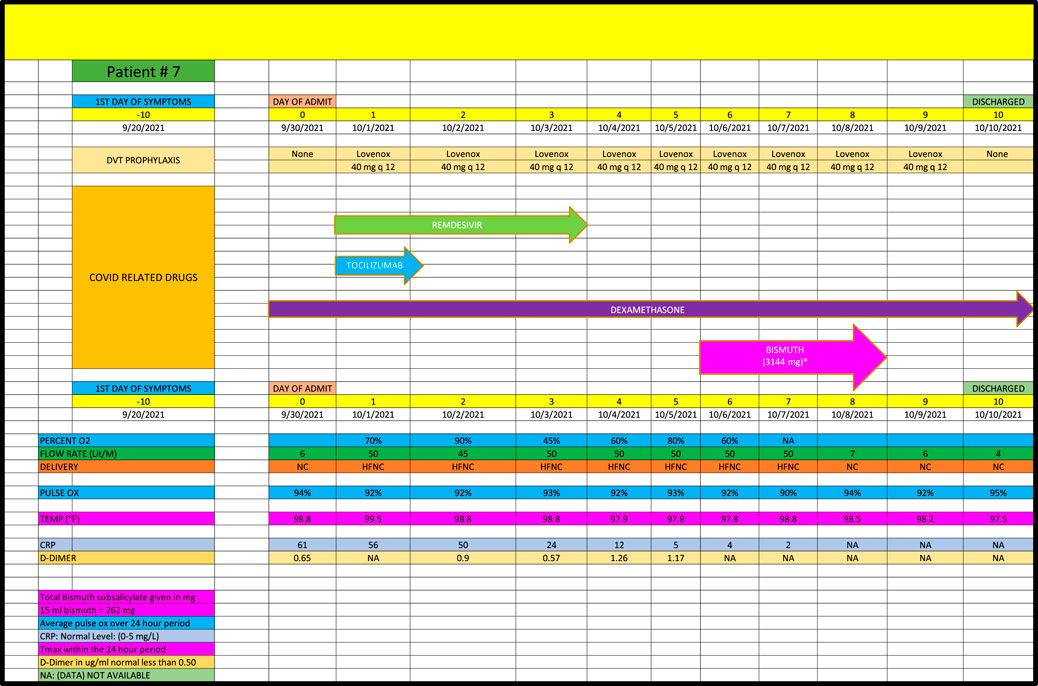
Patient 7 is a 50-year-old man admitted in September 2021, with a PMH of asthma (Figure 7) who remained on high dose oxygen (ie, 50 L/min) for the first 8 days of his admission despite receiving remdesivir, tocilizumab and dexamethasone. He progressed down to 4 L/min of oxygen within 2 days of receiving a total of 3144 mg of BiS consistent with a strong (+2) response.
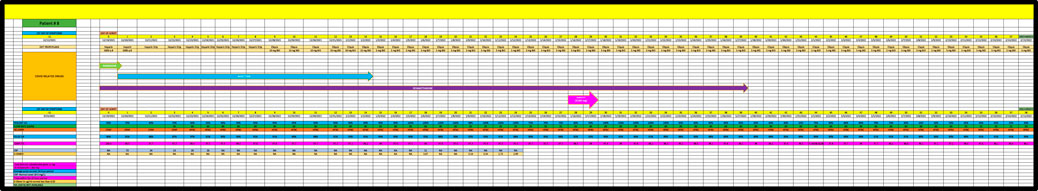
Patient 8 is a 61-year-old admitted in December 2021, with PMH of COPD who developed acute respiratory failure due to Covid pneumonia and received remdesivir, baricitinib and dexamethasone but remained on high flow (ie, 60 L/min of oxygen) until day 28 of his admission at which time BiS was started; he received a total of 3144 mg of BiS (Figure 8). He eventually reduced his oxygen requirements to 50 L/min on day 58 and received a score of 0 since his response was well after the last day of BiS administration.
Results
Overall, we noted that five out of eight Covid pneumonia patients (ie, 62.5%) had a strong response to BiS treatment as per criteria (+2) (Table 2). In addition, seven of eight patients who received BiS got dramatically better (ie, 87.5%) (Table 3) although two of the cases got better outside the time period criteria noted in Table 1. No side effects of BiS treatment were observed.
Discussion
We acknowledge that a short series of case reports rarely provides definitive information of drug efficacy, mechanism of action or pharmacokinetic/pharmacodynamic properties. Nevertheless, consistent and unexpected clinical outcomes are noteworthy and raise questions worthy of answers. Herein, we describe the outcomes of eight diverse cases of Covid-19 pneumonia where patients were treated with common Covid drugs including corticosteroids, remdesivir, baricitinib and tocilizumab. A common denominator in these patients was the short-term use of BiS for gastric complaints/symptoms. Even when the prognosis was guarded, some of the patients showed a relatively rapid rebound and clinically improved lung function.
Bismuth’s apparent efficacy may be partly due to its’ inhibitory effect on viral enzymes. Bismuth salts inhibit two key enzymes (ie, NTPase and RNA helicase) which are necessary for viral replication (Shu et al., 2020). The mechanism of action of bismuth in the post-acute phase is unclear, however, the dramatic response of most of the cases, often weeks after contracting Covid-19, raises the question of whether the Covid-19 virus is still alive in lung cells even after a patient has developed antibodies. There appears to be support for this: (Zhao et al., 2020): noted that 100% of hospitalized patients developed antibodies to Covid by Day 15, while viral RNA detectability from upper respiratory samples remained positive in many patients weeks after a positive antibody response. They noted that critically ill patients may take a long time to clear Covid from their respiratory tracts: “It should be noted that the risings of antibodies were not always accompanied by RNA clearance, particularly in the 3 critical patients. This finding suggested that antibodies may not be sufficient to clear the virus” (Zhao et al., 2020). Support for this phenomenon exists with other viruses (eg, herpes) which are known to live in the body for years despite positive antibody responses (eg, Ebstein Barr and Varicella viruses). We do not know if Covid-19 virus could still be living in lung cells long after the formation of antibodies, but the dramatic response in our cases who often responded even weeks after contracting Covid pneumonia, raises this possibility.
If further studies were to corroborate our case-reports, it might benefit people around the world who often have no access to expensive medications such as remdesivir or tocilizumab, monoclonal antibodies or the vaccine; for example, as of September 2021 only 1% of people from low-income countries had received even one dose of the vaccine [https://www.kff.org/coronavirus-covid-19/press-release/disparities-in-global-vaccination-progress-are-large-and-growing-with-low-income-countries-and-those-in-africa-lagging-behind/ (Accessed 6 September 2021)]. We also note that all of our patients received less than 9 doses of BiS: whether bismuth medications would have a more pronounced effect if given over longer periods of time is not known. In addition, all of our patients received BiS after several days/weeks of being hospitalized and requiring high-dose oxygen flows. Whether BiS could have even greater efficacy had it been given earlier in our patient’s stay or even prophylactically are questions that merit further study. In addition, very little BiS is absorbed from the gastrointestinal tract when given orally. Patients who were given oral doses of ranitidine bismuth citrate 800 mg twice daily only absorbed 0.5% of the dose with a peak plasma concentration did not exceed 19 ng/ml (Koch et al., 1996). If higher absorption rates could be achieved, as noted in a recent study when BiS was combined with N-acetyl cysteine (Wang et al., 2021) it potentially could increase BiS’s efficacy.
Bismuth is available in many forms such as bismuth subsalicylate, bismuth citrate, bismuth subcitrate and bismuth subgallate, many of which are widely available around the world and often over-the counter. While bismuth may alter the absorption of other medications there are few risks of taking a short course although theoretically BiS could increase the risk of bleeding slightly due to the salicylate effect. However, taking high doses of bismuth for short or extended periods of time may cause severe renal or neurological problems (Cengiz et al., 2005; Borbinha et al., 2019).
Conclusion
During the care of hospitalized Covid-19 patients we administered bismuth subsalicylate to those patients having diarrhea or other gastric issues. Upon retrospective review, we noticed that these patients became less dependent on supplemental oxygen. Using arbitrary yet conservative criteria, we reviewed these cases and herein report the results. All patients showed at least some improvement in their oxygen needs and several showed marked improvement. Noteworthy, none had any side effects from the bismuth, and the improvement came after the patients had already been treated with common Covid drugs. Based on these observations and literature relating to bismuth and Covid we recommend a detailed clinical evaluation of bismuth’s potential role as an adjunct treatment for Covid 19.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Each patient gave informed consent to use his or her medical data for publication; no names or other non-medical information were revealed.
Author contributions
CK gathered initial information on case reports; both CK and KN reviewed all clinical data. CK, KN, and WS all contributed to writing, analyzing and reviewing this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al. (2020). Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 383, 1813–1826. doi:10.1056/NEJMoa2007764
Beran, A., Srour, O., Malhas, S. E., Mhanna, M., Ayesh, H., Sajdeya, O., et al. (2022). High-flow nasal cannula oxygen versus non-invasive ventilation in subjects with COVID-19: A systematic review and meta-analysis of comparative studies. Respir. Care. Respcare., 09987. doi:10.4187/respcare.09987
Borbinha, C., Serrazina, F., Salavisa, M., and Viana-Baptista, M. (2019). Bismuth encephalopathy- a rare complication of long-standing use of bismuth subsalicylate. BMC Neurol. 19, 212. doi:10.1186/s12883-019-1437-9
Cengiz, N., Uslu, Y., Gök, F., and Anarat, A. (2005). Acute renal failure after overdose of colloidal bismuth subcitrate. Pediatr. Nephrol. 20, 1355–1358. doi:10.1007/s00467-005-1993-7
Horby, P., Lim, W. S., Emberson, J. R., Mafham, M., Bell, J. L., Linsell, L., et al. (2021). Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. Overseas. Ed. 384, 693–704. doi:10.1056/NEJMoa2021436
Koch, K. M., Kerr, B. M., Gooding, A. E., and Davis, I. M. (1996). Pharmacokinetics of bismuth and ranitidine following multiple doses of ranitidine bismuth citrate. Br. J. Clin. Pharmacol. 42, 207–211. doi:10.1046/j.1365-2125.1996.39310.x
Koo, H. L., Ajami, N., Atmar, R. L., and DuPont, H. L. (2010). Noroviruses: The leading cause of gastroenteritis worldwide. Discov. Med. 10 (50), 61–70. PMID: 20670600; PMCID: PMC3150746.
Pasricha, P. J. (2006). “Treatment of disorders of bowel motility and water flux; antiemetics: Agents used in biliary and pancreatic disease” in Goodman & Gilmans The Pharmacological Basis of Therapeutics. 11th ed. (New York, NY: McGraw-Hill), 983–1008.
Shoukri, A. M. (2021). High flow nasal cannula oxygen and non-invasive mechanical ventilation in management of COVID-19 patients with acute respiratory failure: A retrospective observational study. Egypt. J. Bronchol. 15, 17. doi:10.1186/s43168-021-00063-0
Shu, T., Huang, M., Wu, D., Ren, Y., Zhang, X., Han, Y., et al. (2020). SARS-Coronavirus-2 Nsp13 possesses NTPase and RNA helicase activities that can Be inhibited by bismuth salts. Virol. Sin. 35 (3), 321–329. doi:10.1007/s12250-020-00242-1
Waldum, H. D., Nordbø, S. A., and Bevanger, L. S. (1998). A bismuth salt inhibits herpes simplex virus. Microb. Ecol. Health Dis. 10, 13–15. doi:10.1080/089106098435395
Wang, R., Chan, J. F., Wang, S., Li, H., Zhao, J., Ip, T., et al. (2021). Orally administered bismuth drug together with N-acetyl cysteine as a broad-spectrum anti-coronavirus cocktail therapy. Chem. Sci. 13 (8), 2238–2248. doi:10.1039/d1sc04515f
Ward, R. L., Sander, D. S., and Knowlton, D. R. (1985). In vitro activities of bismuth salts against rotaviruses and other enteric viruses. Antimicrob. Agents Chemother. 27 (3), 306–308. doi:10.1128/AAC.27.3.306
Wolf, D. C., Wolf, C. H., and Rubin, D. T. (2020). Temporal improvement of a COVID-19-positive crohn's disease patient treated with bismuth subsalicylate. Am. J. Gastroenterol. 115 (8), 1298. doi:10.14309/ajg.0000000000000725
Yacyshyn, M. B., Collins, J., Chua, M., Siegwald, A., Yacyshyn, S., Briones-Pryor, V., et al. (2022). Feasibility study of Bismuth Subsalicylate (BSS) as an addition to standard of care for COVID-19 therapy. Curr. Ther. Res. Clin. Exp. 96, 100667. doi:10.1016/j.curtheres.2022.100667
Yang, N., Tanner, J. A., Wang, Z., Huang, J. D., Zheng, B. J., Zhu, N., et al. (2007). Inhibition of SARS coronavirus helicase by bismuth complexes. Chem. Commun. (42), 4413. doi:10.1039/b709515e
Yuan, S., Wang, R., Chan, J. F., Jinxia, A., Cheng, T., Ka-Heng, K., et al. (2020). Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat. Microbiol. 5, 1439–1448. doi:10.1038/s41564-020-00802-x
Keywords: COVID, bismuth subsalicylate, pneumonia, oxygen, remdesivir
Citation: Kahlenborn C, Severs WB and Nawab K (2022) Bismuth subsalicylate as potential treatment for Covid-19 pneumonia: A case series report. Front. Drug. Discov. 2:962988. doi: 10.3389/fddsv.2022.962988
Received: 07 June 2022; Accepted: 08 July 2022;
Published: 31 August 2022.
Edited by:
Bruno Villoutreix, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Cesar Augusto Roque-Borda, São Paulo State University, BrazilYing Hong Li, Chongqing University of Posts and Telecommunications, China
Copyright © 2022 Kahlenborn, Severs and Nawab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chris Kahlenborn, Q2thaGxlbmJvcm5AZ21haWwuY29t
 Chris Kahlenborn
Chris Kahlenborn Walter B. Severs
Walter B. Severs Khalid Nawab
Khalid Nawab