- 1Student Research Committee, School of Public Health, Iran University of Medical Sciences, Tehran, Iran
- 2Student Research committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Student Research Committee, School of Medicine, Arak university of medical sciences, Arak, Iran
- 4School of medicine, Isfahan University of Medical Science, Isfahan, Iran
- 5Student Research Committee, School of Medicine, North Khorasan University of Medical Sciences, Bojnurd, Iran
- 6Student Research Committee, International Campus, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Cancer is among the most life-threatening diseases worldwide. Along with conventional therapies like chemotherapy, surgery, and radiotherapy, alternative treatment approaches such as traditional Chinese medicine have attracted considerable public and scientific interest that could be beneficial for patients diagnosed with cancer. Salvia miltiorrhiza Bunge is greatly beloved for its roots and is extensively applied for various disease therapies, including cancers in traditional Chinese medicine. In this review, we intend to summarize the anti-cancer properties of Cryptotanshinone (CPT), an extract of Danshen (the root of Salvia miltiorrhiza Bunge), on different types of cancer.
Introduction
Salvia miltiorrhiza Bunge, a member of the genus Salvia (Lamiaceae family) and referred to as Chinese sage or red sage, is a popular traditional Chinese medicinal plant (Tan et al., 2004; Zhou et al., 2005; Fang et al., 2008). Its dry root is known as Dansam in Korean, Danshen in Chinese, and Tansen in Japanese (Cheng, 2007). It is a perennial deciduous plant with branched roots, purple flowers, oval leaves, (Ji et al., 2000). and is commonly distributed in northeastern China, Japan, and Korea (Ji et al., 2000; Clebsch, 2003). It has been used in traditional Chinese medicine (TCM) for various conditions such as cerebrovascular disease, cardiovascular disease (Cheng, 2007), hepatitis, apoplexy, immunological disorders (Wang et al., 2014), liver dysfunction (Wasser et al., 1998), renal disease (Ahn et al., 2010), diabetic vascular complications (Cheng, 2007), and also used as a dietary supplement for health improvement (Sung et al., 2015). Moreover, it has anti-inflammatory, antihypertensive, and anti-neurodegenerative properties (Cheng, 2007; Lin and Hsieh, 2010; Zhang et al., 2016). The active components of Danshen (the dry root of the plant) mainly belong to two major groups (Hung et al., 2016). Lipophilic or lipid-soluble compounds (such as Cryptotanshinone (CPT), isoCPT, tanshinone I and IIA, acetyltanshinone IIA, dihydrotanshinone, and miltirone) and hydrophilic phenolic acids or water-soluble phenolic acids compounds (such as salvianolic acids A and B) (Hung et al., 2016). Among the extracts of Danshen, the compounds that have been studied the most are CPT, dihydrotanshinone I, tanshinones I and, IIA (Sung et al., 2015). The extracts of Danshen have antioxidant properties and are helpful for the treatment of several diseases including cancers (Wu and Efferth, 2017). New findings on the anticancer properties of CPT showed that it stops cancer through various mechanisms such as inhibition of cancer cell proliferation, motility and invasion, promotion of cell death in cancer cells, anti-angiogenic activity, anti-lymphangiogenesis, anti-oxidant activity and targeting androgen receptor signaling (Chen et al., 2013).
By 2030, new cases of cancer diagnosed every year around the world is set to increase to 20 million people (Bray, 2014). Although chemotherapy is mostly used for cancer, it also has major side effects such as anemia, hair loss, immunosuppression, etc. (Wu and Efferth, 2017). Natural products derived from TCM are valuable resources of anticancer drugs (Swinney and Anthony, 2011; Newman and Cragg, 2012; Wu and Efferth, 2017) with high therapeutic value and low toxicity or side effects in long-term use (Wu and Efferth, 2017). Therefore, the aim of this study was to evaluate the anticancer effect of CPT and its underlying mechanisms in different types of cancer.
Materials and Methods
This study summarized current literature about the anticancer properties of CPT. The keywords searched in this study included CPT, cancer, Neoplasm, tumor, melanoma, carcinoma, sarcoma, malignancy, quinoid diterpene, and Salvia miltiorrhiza Bunge. Searches were conducted in online data bases including ISI Web of Science, PubMed, Scopus, and Google scholar. English reported articles in up to 2020 were searched. Then, we reviewed the relevant articles.
Results
Anti-Cancer Effect of CPT Through Regulation of Gene Expression
The anticancer effect of CPT has been investigated in colorectal cancer. Li et al. (2015) suggested that CPT could have an effect on treatment and prevention of colorectal cancer by inhibiting the activation of signal transducer and activator of transcription 3 (Stat3) pathways. Stat3 plays a role in the expression of various genes, which are in relation to inflammation, cell growth, and other cellular processes and could promote several cancer types such as colorectal cancer (Kamran et al., 2013).
The cytotoxic and growth inhibitory effect of Tanshinone I and II extracts from Salvia miltiorrhiza Bunge has been proven on lung carcinoma. Cryptotanshinon has a homologous structure with Tanshinon. Cryptotanshinon acts through different ways to inhibit lung cancer cell growth, as reported by multiple studies (Chen et al., 2014). It significantly decreased the STAT3, and regulated some of the miRNAs, mostly miR-133. Also, a significant reduction in expression of MMP14 gene (matrix metalloproteinase) was reported after the use of CPT. MMP14 plays a crucial role in cell proliferation, migration, apoptosis, and host defense and may mediate the effect of Cryptotanshinon on cellular apoptosis (Wang et al., 2019).
Furthermore, as previous studies have shown, Nrf2 is a transcription factor that is involved in chemoresistance in lung and breast cancer, and its high expression was noted in hepatocellular carcinoma (Zimta et al., 2019). Based on in vitro study conducted by Chen et al. (Xia et al., 2015), CPT could reverse the chemoresistance through downregulation of p-JNK, p-ERK, p-p38, p-Akt, p-STAT3, and other proteins in intracellular pathways of Nrf2. Consequently, could increase the sensitivity of A549 cells to cisplatin [platinum drug, causing cancer cell death (Dasari and Bernard Tchounwou, 2014)]. Also, CPT increased the amount of keap1, which is a down-regulator of Nrf2.
The anti-tumor activity of CPT on breast cancer MCF7 cells has been investigated by two different studies. Zhou et al. (2014) suggested isolated CPT as an important agent to block the growth of MCF7 cells in vivo by up-regulating the phosphorylation of STAT4 and JAK3 that make CD4+ T cells to produce and secrete more perforin, which can restrict tumor growth. Zhang et al. (2015) observed the Enhancing anti-tumor activity of CPT on MCF_7 cells after combining it with intermediate metabolites of arsenic (mainly MMA).
Anti-Cancer Effect of CPT Through Induction of
Kamran et al. (2013) observed that CPT could notably inhibit the growth and viability of SW480, HCT116, and LOVO cell lines by inducing apoptosis and can prevent anchorage-dependent growth on agar.
Ge et al. (2015) Reported anti-tumor impact of CPT on pancreatic cancer BxPC-3 cells’ proliferation, apoptosis, and cellular cycle. CPT could cause upregulation of the levels of cleaved caspase-3, caspase-9, and poly ADP ribose polymerase (pro-apoptotic proteins in BxPC-3 cells), whereas c-myc, survivin, and cyclin D1 levels were downregulated and induced the block of cell cycle at G1-G0 phase. Also, CPT could lower the activities of signal transducer and activator of transcription 3 (STAT3) and some upstream regulatory pathways. While CPT blocked the polyphosphorylation of STAT3 Tyr705 in 30 min, it had a non-noticeable impact on the other proteins, suggesting that CPT directly regulated the signaling pathway of STAT3 in the BxPC-3 cells.
Chang et al. (2018) investigated the corresponding mechanism of CPT action in PGE2-treated HA22T hepatocellular carcinoma cells. The results showed that CPT reduced HA22T cell viability and increased apoptosis in them. It also inhibited PGE2-treated cell migration by enhancing the expression of E-cadherin and GSK3-β and by decreasing the expression of PGE2-induced β-catenin. Along with these results, CPT suppressed the expression of EP2, EP4, and their downstream proteins, including p-PI3K and p-Akt in HA22T cells. Moreover, Zhang et al. (2020) have suggested anticancer effects for CPT in regard to its time- and dose-dependent cytotoxicity on human HCC (Human hepatocellular carcinoma) cells, particularly Huh-7 and HCCLM3 cells (Zhang et al., 2020). It is reported that CPT disrupted intracellular homeostasis, suppressed cell proliferation, and promoted the mitochondrial apoptosis of human HCC cells. This compound also impaired the invasion and migration of HCC cells by suppressing the expression of proteins associated with metastasis (Zhang et al., 2020). All these data suggest that CPT could be used as a new drug to treat human hepatocellular carcinoma (HCC).
The Anti-Cancer Effect of CPT Through Regulation of Signaling Pathways and Other Mechanisms
CPT could also be considered as a potential anticancer agent for gastric cancer. Liu et al. (2016) reported dose-dependent suppression of twelve gastric cancer cell lines viability after treatment with CPT. CPT caused the arrest of cell cycle at the G2/M phase and mitochondrial cell death through the accumulation of reactive oxygen species (ROS), ROS-mediated MAPK (mitogen-activated protein kinase), and AKT signaling pathways. This compound also elevated p-JNK and p-p38 [mediators of intracellular signals which affect cell growth and apoptosis (Taylor et al., 2013)].
Moreover, the effect of Cryptotanshinon on lung cancer both in vivo and ex vivo has been investigated by Chen et al. (2014). Infiltration of CPT to A549 lung cancer cells significantly inhibited cancer cell growth and colonogenic survival. A significant increase in the number of A594 cells in G2/M phase versus G1/S phase showed growth arrest at G2 and mitotic phase. Besides that, In vivo analysis of Xenograft models showed decrease in tumor size and increase in body weight of animals following the use of CPT.
Oxaliplatin is a platinum anti-tumor drug used for the treatment of solid tumors. However, there are some challenges in using it because it induces neuropathic pain (Alian et al., 2012; Balayssac et al., 2015). Lorenzo et al. (Di Cesare Mannelli et al., 2018) showed that CPT could significantly reduce OXA associated pain in a dose-dependent manner. It is presumed that this effect is due to the inhibition of monoacylglycerol lipase (MAGL), followed by an increase in the amount of endocannabinoid. This study also demonstrated that CPT could induce apoptosis in glioblastoma cells selectively by interfering with NFκBp65 expression.
CPT is studied for its ability to perform as an inhibitor of malignant breast cells through different mechanisms. The key role of estrogen receptor-α (ERα) and estrogen in the progression and development of breast cancer is shown in many studies (Platet et al., 2004; Nadji et al., 2005; Germain, 2011). Today ER-positive breast cancer establishes at least 70 percentages of all kinds of breast malignancies (Osborne et al., 2001). These patients have to be administered with some anti-estrogen drugs like Tamoxifen (Klinge, 2001). CPT showed anti-proliferative effects on both ER-positive breast cancer cells in several studies and is suggested as anti-estrogen since it enhanced the sensitivity of ERa + resistant malignant cells by inhibiting ER-mediated AKT/mTOR/IGF-1 pathway and has the ability to bind ERα, causing some effects like Tamoxifen (Pan et al., 2017). CPT also silenced the G protein-coupled ER-mediated PI3K/AKT metabolic pathway in SKBR-3 cell and arrested cell cycle in G1 as well (Klinge, 2001). Moreover, Xiaoman Liu et al. (Liu et al., 2016) demonstrated that CPT inhibited migration and proliferation of ER-negative Bcap37 cells in breast cancer, and they also observed some post-treatment features of apoptotic morphological changes in Bcap37 cells, for instance, fragmentation of DNA and nuclear condensation.
Another suggested mechanism is the effects of CPT on silencing BCRP (breast cancer resistance protein) expression in breast cells, reported by Wenting ni et al. (Ni et al., 2020). BCRP plays the main role in causing resistance in malignant cells of breast (Nakanishi and Ross, 2012). They also observed CPT is effective in inhibiting the dimerization of BCRP and consequently performing anti-tumor activities in breast cancer by reversing resistance based on BCRP.
Moreover, some research investigated the effects of modified forms of CPT with enhanced anti-tumor activity and reduced side effects. Recent nanoparticles coloaded with CPT and Silibinin (SLB) were able to block metastasis of lung in 4T1 cells of breast cancer in mice models, reported by Ying Liu et al. (Liu et al., 2020). The architecture of these nanoparticles increased CPT and SLB penetration, oral bioavailability, and they also had the ability to enhance cellular uptake of CPT and SLB. Modified CPT, named KYZ3, with more anti-tumor activity than CPT and less effect on normal breast cells like MCF10 cells, were formed by Zhang et al. (2018). Inhibition of STAT3 phosphorylation was also observed after testing KYZ3. Results showed the ability of KYZ3 in reducing the rate of MMP-9, which was directly balanced by STAT3, and with lessening levels of MMP-9, the rate of TNBC cell metastasis also decreased.
As reported by Wang et al. (2017), CPT can act in a dose-dependent manner to prevent migration and proliferation of TSCC (tongue squamous cell carcinoma) cells and trigger apoptosis in them by suppressing the expression of p-STAT3, Bcl-2, CDK2, Snail, and MMP2 and inducing the expression of E-cadherin, P53, P21, and β-catenin. Furthermore, the combined treatment of paclitaxel and CPT has been found to have similar effects to CPT through inhibiting the STAT3 signaling pathway (anticancer potential of Cryptotanshinone is summarized in Figure 1).
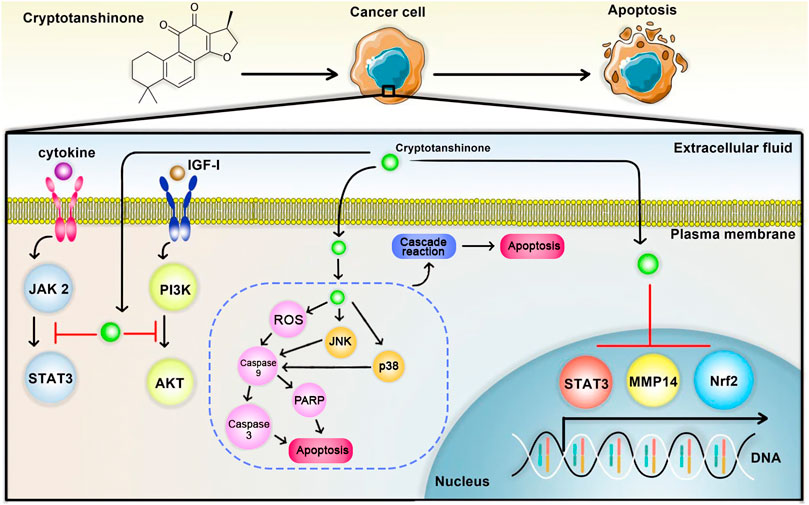
FIGURE 1. The antitumor effect of Cryptotanshinone. Cryptotanshinone can induce the apoptosis of cancer cells by inhibiting PI3K/AKT, STAT3 signaling pathways, as well as transcription of MMP14, STAT3, and Nrf2 genes. The arrow represents the upregulation, and the T area represents the downregulation.
Conclusion
Based on research outcomes, the cytotoxic and growth inhibitory effect of CPT, on cancer patients cannot be ignored, since cytotoxicity is key inhibitor of cancer cells proliferation. Indeed, CPT with restrictive activities arranges G2/M arrest, apoptosis and inhibition of STAT signaling pathway cellular movement which can be explained by inhibition of NFκB. Although current researches indicate its therapeutic effect, performing new investigations to discover the biosynthetic pathways, specific reaction sequences, as well as other underlying mechanisms is necessary.
Author Contributions
Study concept and design: ND. Acquisition of data: MN, AG, HM, ER. Drafting of manuscript: MN, AG, HM, ER, FN, AA. Critical revision of the manuscript for important intellectual content: ND. Study supervision: ND.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fddsv.2022.815017/full#supplementary-material
References
Ahn, Y.-M., Kim, S. K., Lee, S.-H., Ahn, S.-Y., Kang, S. W., Chung, J.-H., et al. (2010). Renoprotective Effect of Tanshinone IIA, an Active Component ofSalvia Miltiorrhiza, on Rats with Chronic Kidney Disease. Phytother. Res. 24 (12), 1886–1892. doi:10.1002/ptr.3347
Alian, O. M., Azmi, A. S., and Mohammad, R. M. (2012). Network Insights on Oxaliplatin Anti-cancer Mechanisms. Clin. Transl Med. 1 (1), 26. doi:10.1186/2001-1326-1-26
Balayssac, D., Ferrier, J., Pereira, B., Gillet, B., Petorin, C., Vein, J., et al. (2015). Prevention of Oxaliplatin-Induced Peripheral Neuropathy by a Polyamine-Reduced Diet--NEUROXAPOL: Protocol of a Prospective, Randomised, Controlled, Single-Blind and Monocentric Trial. BMJ Open 5 (4), e007479. doi:10.1136/bmjopen-2014-007479
Bray, F. (2014). Transitions in Human Development and the Global Cancer burden. World cancer report, 34.
Chang, J. H.-M., Lin, C.-H., Shibu, M. A., Chou, Y.-C., Liu, J.-Y., Chou, Y.-H., et al. (2018). Cryptotanshinone (Dsh-003) from Salvia Miltiorrhiza Bunge Inhibits Prostaglandin E2-Induced Survival and Invasion Effects in HA22T Hepatocellular Carcinoma Cells. Environ. Toxicol. 33 (12), 1254–1260. doi:10.1002/tox.22633
Chen, L., Wang, H.-J., Xie, W., Yao, Y., Zhang, Y.-S., and Wang, H. (2014). Cryptotanshinone Inhibits Lung Tumorigenesis and Induces Apoptosis in Cancer Cells In Vitro and In Vivo. Mol. Med. Rep. 9 (6), 2447–2452. doi:10.3892/mmr.2014.2093
Chen, W., Lu, Y., Chen, G., and Huang, S. (2013). Molecular Evidence of Cryptotanshinone for Treatment and Prevention of Human Cancer. Acamc 13 (7), 979–987. doi:10.2174/18715206113139990115
Cheng, T. O. (2007). Cardiovascular Effects of Danshen. Int. J. Cardiol. 121 (1), 9–22. doi:10.1016/j.ijcard.2007.01.004
Dasari, S., and Bernard Tchounwou, P. (2014). Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 740, 364–378. doi:10.1016/j.ejphar.2014.07.025
Di Cesare Mannelli, L., Piccolo, M., Maione, F., Ferraro, M. G., Irace, C., De Feo, V., et al. (2018). Tanshinones from Salvia Miltiorrhiza Bunge Revert Chemotherapy-Induced Neuropathic Pain and Reduce Glioblastoma Cells Malignancy. Biomed. Pharmacother. 105, 1042–1049. doi:10.1016/j.biopha.2018.06.047
Fang, Z.-y., Lin, R., Yuan, B.-x., Yang, G.-D., Liu, Y., and Zhang, H. (2008). Tanshinone IIA Downregulates the CD40 Expression and Decreases MMP-2 Activity on Atherosclerosis Induced by High Fatty Diet in Rabbit. J. ethnopharmacology 115 (2), 217–222. doi:10.1016/j.jep.2007.09.025
Ge, Y., Yang, B., Chen, Z., and Cheng, R. (2015). Cryptotanshinone Suppresses the Proliferation and Induces the Apoptosis of Pancreatic Cancer Cells via the STAT3 Signaling Pathway. Mol. Med. Rep. 12 (5), 7782–7788. doi:10.3892/mmr.2015.4379
Germain, D. (2011). Estrogen Carcinogenesis in Breast Cancer. Endocrinol. Metab. Clin. North Americavii 40 (3), 473–484. doi:10.1016/j.ecl.2011.05.009
Hung, Y.-C., Pan, T.-L., and Hu, W.-L. (2016). Roles of Reactive Oxygen Species in Anticancer Therapy with Salvia Miltiorrhiza Bunge. Oxidative Medicine and Cellular Longevity. doi:10.1155/2016/5293284
Ji, X. Y., Tan, B. K., and Zhu, Y. Z. (2000). Salvia Miltiorrhiza and Ischemic Diseases. Acta Pharmacol. Sin 21 (12), 1089–1094.
Kamran, M. Z., Patil, P., and Gude, R. P. (2013). Role of STAT3 in Cancer Metastasis and Translational Advances. Biomed. Res. Int. 2013, 421821. doi:10.1155/2013/421821
Klinge, C. M. (2001). Estrogen Receptor Interaction with Estrogen Response Elements. Nucleic Acids Res. 29 (14), 2905–2919. doi:10.1093/nar/29.14.2905
Li, W., Saud, S. M., Young, M. R., Colburn, N. H., and Hua, B. (2015). Cryptotanshinone, a Stat3 Inhibitor, Suppresses Colorectal Cancer Proliferation and Growth In Vitro. Mol. Cel Biochem 406 (1-2), 63–73. doi:10.1007/s11010-015-2424-0
Lin, T.-H., and Hsieh, C.-L. (2010). Pharmacological Effects of Salvia Miltiorrhiza (Danshen) on Cerebral Infarction. Chin. Med. 5 (1), 22. doi:10.1186/1749-8546-5-22
Liu, X., Pan, L., Liang, J., Li, J., and Wu, S. (2016). Cryptotanshinone Inhibits Proliferation and Induces Apoptosis via Mitochondria-Derived Reactive Oxygen Species Involving FOXO1 in Estrogen Receptor-Negative Breast Cancer Bcap37 Cells. RSC Adv. 6 (27), 22232–22243. doi:10.1039/c5ra22523j
Liu, Y., Xie, X., Hou, X., Shen, J., Shi, J., Chen, H., et al. (2020). Functional Oral Nanoparticles for Delivering Silibinin and Cryptotanshinone against Breast Cancer Lung Metastasis. J. Nanobiotechnology 18 (1), 83–15. doi:10.1186/s12951-020-00638-x
Nadji, M., Gomez-Fernandez, C., Ganjei-Azar, P., and Morales, A. R. (2005). Immunohistochemistry of Estrogen and Progesterone Receptors Reconsidered. Am. J. Clin. Pathol. 123 (1), 21–27. doi:10.1309/4wv79n2ghj3x1841
Nakanishi, T., and Ross, D. D. (2012). Breast Cancer Resistance Protein (BCRP/ABCG2): its Role in Multidrug Resistance and Regulation of its Gene Expression. Chin. J. Cancer 31 (2), 73–99. doi:10.5732/cjc.011.10320
Newman, D. J., and Cragg, G. M. (2012). Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 75 (3), 311–335. doi:10.1021/np200906s
Ni, W., Fan, H., Zheng, X., Xu, F., Wu, Y., Li, X., et al. (2020). Cryptotanshinone Breaks ERα-dependent And-independent BCRP Dimerization to Reverse the Multidrug Resistance in Breast Cancer.
Osborne, C. K., Schiff, R., Fuqua, S. A., and Shou, J. (2001). Estrogen Receptor: Current Understanding of its Activation and Modulation. Clin. Cancer Res. 7 (12), 4338s–4412s.
Pan, Y., Shi, J., Ni, W., Liu, Y., Wang, S., Wang, X., et al. (2017). Cryptotanshinone Inhibition of Mammalian Target of Rapamycin Pathway Is Dependent on Oestrogen Receptor Alpha in Breast Cancer. J. Cel. Mol. Med. 21 (9), 2129–2139. doi:10.1111/jcmm.13135
Platet, N., Cathiard, A. M., Gleizes, M., and Garcia, M. (2004). Estrogens and Their Receptors in Breast Cancer Progression: a Dual Role in Cancer Proliferation and Invasion. Crit. Rev. oncology/hematology 51 (1), 55–67. doi:10.1016/j.critrevonc.2004.02.001
Sung, B., Chung, H. S., Kim, M., Kang, Y. J., Kim, D. H., Hwang, S. Y., et al. (2015). Cytotoxic Effects of Solvent-Extracted Active Components of Salvia Miltiorrhiza Bunge on Human Cancer Cell Lines. Exp. Ther. Med. 9 (4), 1421–1428. doi:10.3892/etm.2015.2252
Swinney, D. C., and Anthony, J. (2011). How Were New Medicines Discovered. Nat. Rev. Drug Discov. 10 (7), 507–519. doi:10.1038/nrd3480
Tan, B. K., Bay, B.-H., and Zhu, Y.-Z. (2004). Novel Compounds from Natural Products in the New Millennium: Potential and Challenges. World Scientific.
Taylor, C. A., Zheng, Q., Liu, Z., and Thompson, J. E. (2013). Role of P38 and JNK MAPK Signaling Pathways and Tumor Suppressor P53 on Induction of Apoptosis in Response to Ad-eIF5A1 in A549 Lung Cancer Cells. Mol. Cancer 12 (1), 35. doi:10.1186/1476-4598-12-35
Wang, H., Zhang, Y., Zhang, Y., Liu, W., and Wang, J. (2019). Cryptotanshinone Inhibits Lung Cancer Invasion via microRNA-133a/matrix Metalloproteinase 14 Regulation. Oncol. Lett. 18 (3), 2554–2559. doi:10.3892/ol.2019.10580
Wang, N., Yang, J., Lu, J., Qiao, Q., Wu, T., Du, X., et al. (2014). A Polysaccharide from Salvia Miltiorrhiza Bunge Improves Immune Function in Gastric Cancer Rats. Carbohydr. Polym. 111, 47–55. doi:10.1016/j.carbpol.2014.04.061
Wang, Y., Lu, H.-l., Liu, Y.-d., Yang, L.-y., Jiang, Q.-k., Zhu, X.-j., et al. (2017). Cryptotanshinone Sensitizes Antitumor Effect of Paclitaxel on Tongue Squamous Cell Carcinoma Growth by Inhibiting the JAK/STAT3 Signaling Pathway. Biomed. Pharmacother. 95, 1388–1396. doi:10.1016/j.biopha.2017.09.062
Wasser, S., Ho, J. M. S., Ang, H. K., and Tan, C. E. L. (1998). Salvia Miltiorrhiza Reduces Experimentally-Induced Hepatic Fibrosis in Rats. J. Hepatol. 29 (5), 760–771. doi:10.1016/s0168-8278(98)80257-2
Wu, C.-F., and Efferth, T. (2017). “Anticancer Activity of Salvia Miltiorrhiza and its Secondary Metabolites,” in Salvia Biotechnology (Springer), 179–207. doi:10.1007/978-3-319-73900-7_5
Xia, C., Bai, X., Hou, X., Gou, X., Wang, Y., Zeng, H., et al. (2015). Cryptotanshinone Reverses Cisplatin Resistance of Human Lung Carcinoma A549 Cells through Down-Regulating Nrf2 Pathway. Cell Physiol Biochem 37 (2), 816–824. doi:10.1159/000430398
Zhang, Q., Wang, L., Gan, C., Yu, Y., Li, Y., Deng, Y., et al. (2020). Cryptotanshinone Induces Apoptosis and Inhibits Migration and Invasion in Human Hepatocellular Carcinoma Cells In Vitro. Nat. Product. Commun. 15 (1), 1934578X19899570. doi:10.1177/1934578x19899570
Zhang, W., Yu, W., Cai, G., Zhu, J., Zhang, C., Li, S., et al. (2018). A New Synthetic Derivative of Cryptotanshinone KYZ3 as STAT3 Inhibitor for Triple-Negative Breast Cancer Therapy. Cell Death Dis 9 (11), 1098–1111. doi:10.1038/s41419-018-1139-z
Zhang, X.-Z., Qian, S.-S., Zhang, Y.-J., and Wang, R.-Q. (2016). Salvia Miltiorrhiza: A Source for Anti-alzheimer's Disease Drugs. Pharm. Biol. 54 (1), 18–24. doi:10.3109/13880209.2015.1027408
Zhang, Y. F., Zhang, M., Huang, X. L., Fu, Y. J., Jiang, Y. H., Bao, L. L., et al. (2015). The Combination of Arsenic and Cryptotanshinone Induces Apoptosis through Induction of Endoplasmic Reticulum Stress-Reactive Oxygen Species in Breast Cancer Cells. Metallomics 7 (1), 165–173. doi:10.1039/c4mt00263f
Zhou, J., Xu, X.-Z., Hu, Y.-R., Hu, A.-R., Zhu, C.-L., and Gao, G.-S. (2014). Cryptotanshinone Induces Inhibition of Breast Tumor Growth by Cytotoxic CD4+ T Cells through the JAK2/STAT4/Perforin Pathway. Asian Pac. J. Cancer Prev. 15 (6), 2439–2445. doi:10.7314/apjcp.2014.15.6.2439
Zhou, L., Zuo, Z., and Chow, M. S. S. (2005). Danshen: an Overview of its Chemistry, Pharmacology, Pharmacokinetics, and Clinical Use. J. Clin. Pharmacol. 45 (12), 1345–1359. doi:10.1177/0091270005282630
Keywords: cryptotanshinone, cancer, Salvia miltiorrhiza bunge, Chinese medicine, herbal medicine
Citation: Naziri M, Ghafari A, Mehrabi H, Ramezannezhad E, Nazari F, Ansari A, Nikzad F and Deravi N (2022) A Mini-Review of the Anticancer Properties of Cryptotanshinone: A Quinoid Diterpene Extracted From the Root of Salvia miotiorrhiza Bunge. Front. Drug. Discov. 2:815017. doi: 10.3389/fddsv.2022.815017
Received: 14 November 2021; Accepted: 05 January 2022;
Published: 10 March 2022.
Edited by:
Sigrid A. Langhans, Alfred I. duPont Hospital for Children, United StatesReviewed by:
Xuelin Zhou, Capital Medical University, ChinaCopyright © 2022 Naziri, Ghafari, Mehrabi, Ramezannezhad, Nazari, Ansari, Nikzad and Deravi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niloofar Deravi, bmlsb29mYXJkZXJhdmlAc2JtdS5hYy5pcg==
 Mahdyieh Naziri1
Mahdyieh Naziri1 Arezoo Ghafari
Arezoo Ghafari Niloofar Deravi
Niloofar Deravi