- 1Cedars-Sinai Medical Center, Departments of Neurology, Neurosurgery and Biomedical Sciences, Los Angeles, CA, United States
- 2Cedars-Sinai Medical Center, Department of Biomedical Sciences and Smidt Heart Institute, Los Angeles, CA, United States
- 3Nora Eccles Harrison Cardiovascular Research and Training Institute, University of Utah, Salt Lake City, UT, United States
- 4Biostatistics and Informatics Core, Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States
- 5Zilkha Neurogenetic Institute of the Keck School of Medicine, University of South California, Los Angeles, CA, United States
- 6Department of Medicine, Division of Cardiovascular Medicine, University of Utah, Salt Lake City, UT, United States
- 7Department of Pharmacology and Toxicology, University of Utah, Salt Lake City, UT, United States
Background: Neurofilament light chain protein (NfL) and tau are plasma biomarkers of neuronal injury which can be elevated in patients with neurodegenerative diseases. N-terminal pro-brain natriuretic peptide (NT-proBNP) is an established marker of volume status in patients with heart failure (HF) and plasma cBIN1 score (CS) is an emerging biomarker of cardiac muscle health. It is not known if, in HF patients, there is a correlation between cardiac markers and brain injury markers.
Methods: We studied ambulatory HF patients with either preserved and reduced ejection fraction (N = 50 with 25 HFrEF and 25 HFpEF) and age and sex matched healthy controls (N = 50). Plasma NT-proBNP and CS were determined using commercial kits. A bead-based ELISA assay was used to quantify femtomolar concentrations of plasma neuronal markers NfL and total tau.
Results: Plasma levels of NT-proBNP and CS in heart failure patients were significantly higher than those from healthy controls. In both patients with HFrEF and HFpEF, we found independent and direct correlations between the volume status marker NT-proBNP, but not the cardiomyocyte origin muscle health marker CS, with NfL (r = 0.461, p = 0.0007) and tau (r = 0.333, p = 0.0183).
Conclusion: In patients with HF with or without preserved ejection fraction, plasma levels of NfL and tau correlate with volume status rather than muscle health, indicating volume overload-associated neuronal injury.
Introduction
Heart failure (HF) is a growing epidemic currently affecting near six million of Americans and is projected to increase by 46% from 2012–2030 (Virani et al., 2021). One major comorbidity occurring in over 40% of patients with heart failure is brain damage and cognitive impairment, which is associated with poor prognosis yet often overlooked in clinical practice (Villringer and Laufs, 2021). Unfortunately, the relationships between plasma levels of brain injury markers and heart failure markers are not yet known, preventing effective early diagnosis of brain injury in heart failure patients. Neurofilament light chain (NfL), a brain damage marker, is a 68-kDa scaffolding protein component of the neural cytoskeleton that provides structural support for axons and dendrites (Bacioglu et al., 2016). Injury to axons releases NfL into the systemic circulation and plasma levels of NfL reflect the extent of neuronal damage from neurodegenerative etiologies (Bacioglu et al., 2016; Gattringer et al., 2017; Mattsson et al., 2017). Plasma total tau level, meaning tau whether or not it is phosphorylated, is also a known biomarker of general neuronal injury and neurodegeneration with prognostic value of cognitive decline (Mielke et al., 2017).
The etiology of brain injury and cognitive impairment in patients with HF is linked to reduced cardiac output and volume overload with a resultant poor brain perfusion (Villringer and Laufs, 2021). However, the relationships between plasma levels of brain injury markers (NfL, total tau) and markers of clinical heart failure severity and volume status (brain natriuretic peptides) versus markers of cardiac muscle health are not yet known. N-terminal pro-brain natriuretic peptide (NT-proBNP) is a 76-amino acid N-terminal inactive peptide that is released into the systemic circulation in response to volume overload and myocardial wall stress (Sabbah, 2017). NT-proBNP is a well-established biomarker of volume status and a predictor of cardiovascular and all-cause mortality (Haug et al., 1993; Doyama et al., 1998; Sabbah, 2017; Nikolova et al., 2018). On the other hand, cardiac bridging integrator 1 (cBIN1) score (CS), an inverse index of the plasma concentrations of the cardiomyocyte specific membrane scaffolding protein cBIN1, is an emerging heart failure biomarker assaying the intrinsic biochemical health of cardiomyocytes (Xu et al., 2017; Nikolova et al., 2018; Hitzeman et al., 2020). As a marker of intra-cardiomyocyte transverse tubule (t-tubule) membrane microdomains (Xu et al., 2017) that are critical to cardiac inotropy and lusitropy (Hong et al., 2014; Fu et al., 2016; Li et al., 2020; Liu et al., 2020), CS helps determine disease progression and establish prognosis in patients with heart failure (Nikolova et al., 2018; Hitzeman et al., 2020). Meanwhile, different from NT-proBNP that is sensitive to alterations in volume status, CS more accurately measured steady-state cardiomyocyte health in patients with stable HF (Nikolova et al., 2018).
Since neurodegeneration is common in patients with heart failure with volume overload (Vogels et al., 2007a; Vogels et al., 2007b; Sauve et al., 2009; Almeida et al., 2012; Alosco et al., 2013; Athilingam et al., 2013; Huijts et al., 2013; Shimizu et al., 2014; Alosco and Hayes, 2015), in this study, we investigated the relationships among plasma concentrations of NfL with the volume status marker NT-proBNP and the cardiac muscle health marker CS in ambulatory patients with stable chronic heart failure.
Materials and methods
This study was approved by the Cedars-Sinai Medical Center Institutional Review Board and participants gave written informed consent. We utilized plasma samples collected from 50 patients enrolled in a heart failure blood bank repository, of whom 25 had heart failure with preserved ejection fraction (HFpEF) and 25 had heart failure with reduced ejection fraction (HFrEF). Plasma samples from 50 age and sex matched controls were obtained from Innovative Research with full clinical history and informed consent as previously described (Nikolova et al., 2018). In brief, all enrolled control patients are individuals with or without known cardiovascular risk factors (obesity, hypertension, or diabetes, etc.), but without heart failure. In all patients, venous blood was drawn in a K2-EDTA tube, centrifuged at 2,250 g for 20 min at 4°C to separate plasma, which was snap frozen and stored in aliquots at −80°C prior to analysis, and thawed immediately prior to analysis.
Neurological biomarker quantification
Using a bead-based ELISA performed with a fully automated Quanterix Simoa HD-1 Analyzer, we were able to quantify femtomolar concentrations of plasma NfL and total tau (Cat#102153, Quanterix Corporation, Lexington, MA, United States). Simoa HD-1 Analyzer assays are based on the isolation of individual immunocomplexes on paramagnetic beads in femtoliter-sized wells, allowing for a digital readout of each individual bead bound to its target analyte. Within the analyzer, protein concentrations were quantified using highly sensitive bead-based sandwich immunoassay (Neurology-4 PLEX A, Quanterix). The Neurology 4-PLEX A kit contains eight calibrators and two controls per target analyte, sample diluent, beads, detector reagent, streptavidin β-galactosidase and fluorogenic β-galactosidase. All the reagents for this were set up within the Simoa instrument as per the manufacturer’s protocol. In brief, all Simoa calibrators, controls, and reagents were allowed to reach room temperature and the capture bead reagent was vortexed for 1 min immediately prior to performing the assay. Thawed plasma samples were gently vortexed and centrifuged at 12,000 g for 8 min to precipitate particulates, after which 100 µl of plasma was diluted with Quanterix Sample Dilution Buffer to a final volume of 400 µl. During the assay, each sample was mixed with magnetic beads conjugated to capture antibodies, and subsequently mixed with the detector antibody, streptavidin β-galactosidase and fluorogenic β-galactosidase. Each sample was run in duplicate. All samples with a coefficient of variation beyond 20% were repeated with appropriate calibrators and controls.
Quantification for each target (NfL and total tau) was based on a fluorescent readout representing average enzyme per bead. Plasma concentrations were derived from average enzyme per bead values through the generation of an 8-point calibration curve whose accuracy is verified with both a low- and high-concentration recombinant control. This analysis was performed by the Cedars-Sinai Protein and metabolomics Core.
N-terminal pro-brain natriuretic peptide quantification
NT-proBNP concentrations were obtained from the plasma of each of the study patients. Cedars-Sinai Medical Center clinical laboratory sends out the plasma samples to Quest Diagnostics Laboratory to perform the NT-proBNP assay using electrochemiluminescence. NT-proBNP was chosen instead of BNP due to its superior stability and higher mean recovery when obtained from stored frozen plasma (Nowatzke and Cole, 2003; Mueller et al., 2004).
Cardiac bridging integrator 1 score quantification
Plasma CS was obtained by a previously reported ELISA method (Nikolova et al., 2018) using commercially available kits (Sarcotein Diagnostics, Inc.). Detailed CS measurement was obtained following manufacture’s instruction.
Statistical analysis
Statistical analyses were performed using SAS 9.1 for Windows (SAS Institute Inc. Cary, NC, United States) and GraphPad Prism version 9.0 (GraphPad Software, La Jolla, CA, United States). Student’s t-test or Mann-Whitney test was used to test differences between two groups. For log transformed data, one-way analysis of variance followed by uncorrected Fisher’s LSD tests were used for comparisons among three groups. For non-parametric data, a Kruskal–Wallis test followed by uncorrected Dunn’s multiple comparison tests were used for comparison among three groups. Pearson’s Correlations (r) were computed to assess correlations between markers and linear regression modeling was used to test for associations while controlling for patient factors. Data were log-transformed prior to analysis where outliers present, and residuals were inspected to confirm fit. A two-sided p-value<0.05 was considered statistically significant. Data are presented as means with standard deviations (SD) or counts and percentages.
Results
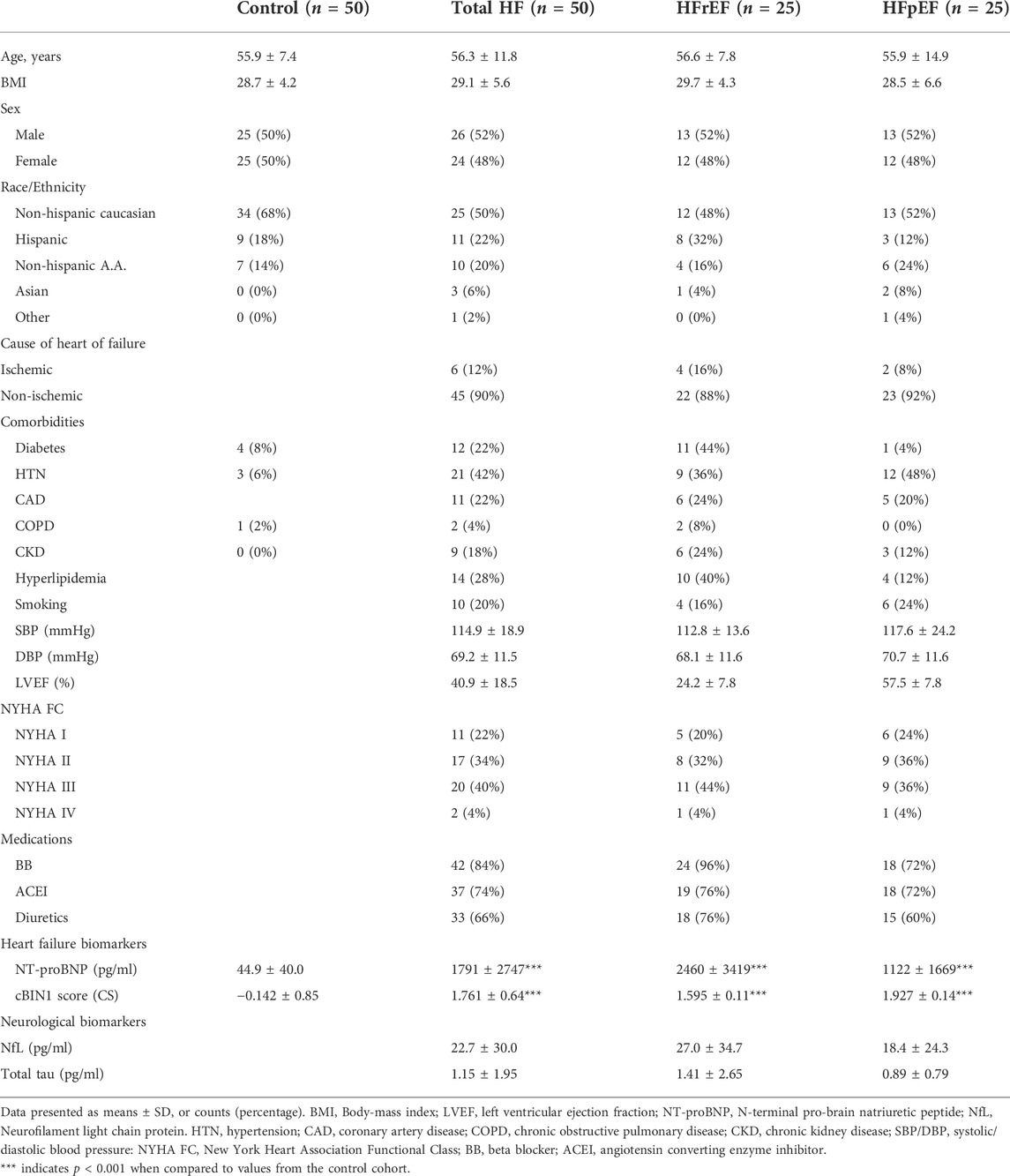
Demographic characteristics and individual patient data for both control cohorts and the 50 patients with heart failure are shown in Table 1. The mean age was 56.3 ± 11.8 years and mean body-mass index (BMI) was 29.1 ± 5.6. There were no significant differences noted across the different race/ethnic categories or by sex. Of the 50 patients enrolled, half of these patients (n = 25) had HFrEF and the other half HFpEF (n = 25). All patients were ambulatory with stable heart failure with similar disease stages between HFrEF and HFpEF cohorts (see Table 1 for disease stages by New York Heart Association Functional Classification—NYHA FC). Compared to age and sex matched healthy controls (n = 50), both heart failure markers CS and NT-proBNP were significantly elevated in patients with HFpEF and HFrEF (Table 1), consistent with previous observations (Nikolova et al., 2018). Patients with HFrEF had significantly higher NT-proBNP concentrations (p = 0.022) and a trend of lower CS levels (p = 0.067) than those with HFpEF (Table 2). Interestingly, HFrEF patients had a trend of increased mean plasma levels of NfL when compared to HFpEF patients (p = 0.068). These data indicate that brain injury markers may be associated with severity of heart failure rather than cardiac muscle status in patients with heart failure.
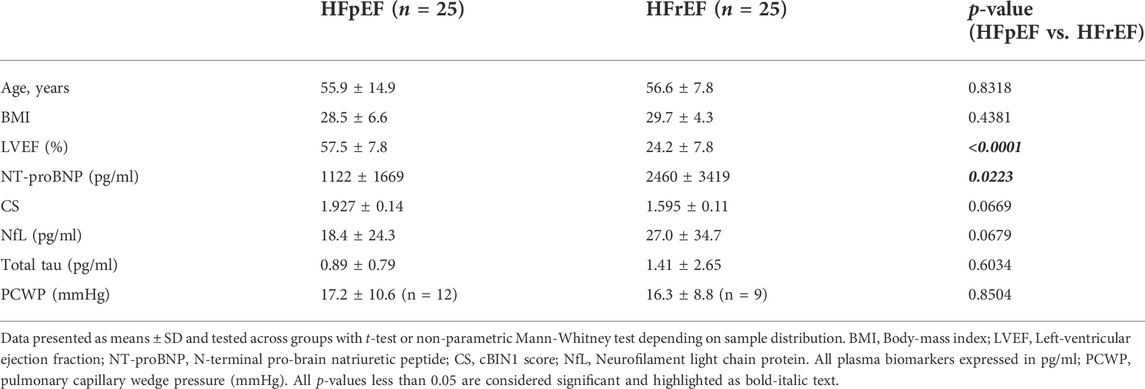
TABLE 2. Comparison of patients with heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF).
We next explored correlations among plasma levels of brain injury markers and heart failure markers. Plasma levels of NfL were positively correlated with total tau (r = 0.395, p = 0.005), validating shared capability of detecting brain injury. Increased plasma concentrations of NfL were also associated with increased age (r = 0.468, p = 0.0006), indicating age-associated neurodegeneration. Interestingly, there was a significant positive correlation between NT-proBNP and NfL or total tau (Figure 1A). Even after controlling for patient sex, age, BMI, and type of heart failure, the significant positive associations remain between NT-proBNP and NfL (p = 0.003) and NT-proBNP and total tau (p = 0.040) in linear regression modeling. When accounting for additional confounding risk factors including hypertension, hyperlipidemia, diabetes, and smoking, the positive association remains between NT-proBNP and NfL (p = 0.005). Furthermore, subgroup analysis in HFrEF (red symbols and lines) and HFpEF (blue symbols and lines) cohorts indicates similar positive correlations between NT-proBNP and NfL and total tau for both HFrEF and HFpEF (Figures 1B,C). On the other hand, analysis between NT-proBNP and CS did not identify a significant correlation between the two heart failure markers (r = −0.2471, p = 0.0836). Different from NT-proBNP, CS did not correlate with NfL (r = 0.01208, p = 0.9337) and total tau (r = −0.137, p = 0.3427) (Figure 2). These data indicate that neuronal injury identified by NfL is more a reflection of volume overload than muscle health.
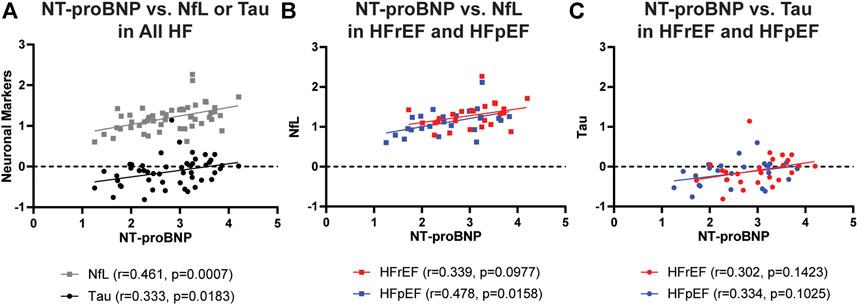
FIGURE 1. Correlations between plasma concentrations of NT-proBNP and plasma neuronal markers NfL (A,B) and total Tau (A,C) in total heart failure cohort (A) or separately in HFrEF (red symbols and lines) and HFpEF (blue symbols and lines) cohorts (B,C). All concentrations in pg/mL are log transformed before analysis of correlation. r, correlation coefficient; NT-proBNP, N-terminal pro-brain natriuretic peptide; NfL, Neurofilament light chain protein.
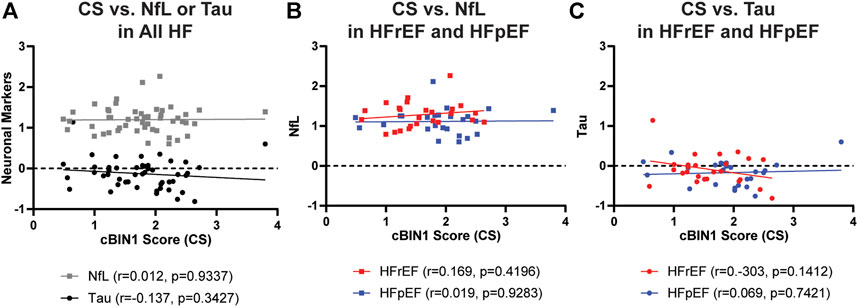
FIGURE 2. Correlations between plasma levels of CS and plasma concentrations of NfL (A,B) and total Tau (A,C) in total HF cohort (A) or separately in HFrEF (red symbols and lines) and HFpEF (blue symbols and lines) cohorts (B,C). All concentrations in pg/ml are log transformed before analysis of correlation. r, correlation coefficient; CS, cBIN1 score; NfL, Neurofilament light chain protein.
We next extracted available pulmonary capillary wedge pressure (PCWP) data obtained from right heart catheterization within 3 months of blood draw. As indicated in Table 2, our results indicate that there were no differences in PCWP levels between HFrEF and HFpEF patients. Correlational analysis further indicates that PCWP significantly correlates with NT-proBNP (r = 0.487, p = 0.0252) and the neuronal markers (NfL, r = 0.456, p = 0.0376; total tau, r = 0.542, p = 0.0111) (Figure 3). Interestingly, subgroup analysis identified that the positive correlations between blood markers and PCWP almost always remain significant in the HFrEF cohort but not necessarily in the HFpEF cohort (Figure 3). While this is a possible result due to fewer data pairs available in the HFpEF group (n = 9 versus n = 12 for HFrEF), these data point out a possible more complex etiology and pathophysiology of neuronal injury in patients with HFpEF.

FIGURE 3. Correlations between PCWP and plasma concentrations of NT-proBNP (A), NfL (B), or total Tau (C). All concentrations in pg/mL are log transformed before analysis of correlation. r, correlation coefficient; PCWP, pulmonary capillary wedge pressure (mmHg); NfL, Neurofilament light chain protein. Black lines: total heart failure; red symbols and lines: HFrEF; blue symbols and lines: HFpEF.
Discussion
In this study, we found a positive correlation between plasma NfL and NT-proBNP concentrations but not CS levels in patients with heart failure. These findings are consistent with prior studies that implicate elevated right atrial pressure in the pathogenesis of neurodegeneration in other populations and suggest that plasma NfL may be a dynamic clinical biomarker that reflects the benefit of reducing volume status (Zuccala et al., 2005; Alosco et al., 2013; Lee et al., 2017). The positive correlation between plasma NT-proBNP and total tau concentrations provides additional support that clinical severity of heart failure may contribute to cerebral damage as tau is a known plasma biomarker of axonal injury and cognitive impairment (Grundke-Iqbal et al., 1986; Zetterberg et al., 2013; Mattsson et al., 2016; Mielke et al., 2017). Furthermore, lack of correlations between NfL or tau and CS further indicates that the hypervolemic status in heart failure, rather than cardiac muscle health, directly correlates with heart failure-associated brain damage. Taken together, these findings suggest that the hypervolemia of HF may contribute to neurological injury, and that plasma NfL may be a dynamic clinical biomarker that reflects the benefit of reducing volume status.
The prevalence of heart failure is expected to increase over the next 20 years as the population ages (Qiu et al., 2006; Yancy et al., 2013; Jefferson et al., 2015; Troncone et al., 2016). Neurodegeneration occurs in up to 80% of patients with heart failure however the exact mechanisms that lead to this remain unclear and there are few treatments (Vogels et al., 2007b; Heidenreich et al., 2011; Mozaffarian et al., 2016), (Packer et al., 2002; Sink et al., 2009; Huijts et al., 2013; Levi Marpillat et al., 2013; Solfrizzi et al., 2013; McMurray et al., 2014). Cerebral embolization due to atrial arrhythmias and cerebral hypoperfusion due to reduced ejection fraction are thought to contribute to neurovascular injury; however, studies show that neurodegeneration is prevalent even in patients who do not have atrial fibrillation or reduced ejection fraction, suggesting that other mechanisms are involved (Suwa and Ito, 2009; Kindermann et al., 2012; Huijts et al., 2013). Our results are consistent with these findings and show that plasma concentrations of biomarkers of neurological injury did not differ significantly between patients with HFrEF and those with HFpEF. Furthermore, in the current cohort studied, plasma levels of NfL and total tau are associated with the left ventricular filling pressure (PCWP), indicating possible contributing mechanism of increased filling pressure and neuronal damage. On the other hand, because NT-proBNP is an established biomarker of volume status, and thus right atrial pressure (Sallach et al., 2009; Nikolova et al., 2018), it is possible that elevated right atrial pressure provokes retrograde cerebral venous congestion and blood-brain barrier injury, thus contributing to neurodegeneration (Lahiri et al., 2017; Lee et al., 2017; Meguro et al., 2017). This putative pathophysiology is analogous to that of cardio-renal syndrome where elevated right atrial pressure is thought to promote kidney injury due to retrograde renal-venous congestion in patients with heart failure (Damman et al., 2009; Mullens et al., 2009).
In summary, we found independent correlations between plasma NT-proBNP and NfL or tau concentrations in patients with heart failure. Strengths of this study include the use of a high-sensitivity immunoassay technique to detect and sequentially measure novel biomarkers of cognitive impairment at femtomolar concentrations in two distinct cohorts of heart failure patients. However, the relatively small sizes of heart failure cohorts and the lack of clinical data on neurological disease status or neuroimaging remain limitations that should be addressed in future studies. Future larger clinical studies are also needed to establish the relationship, over time, between volume status and direct evidence of neurological injury.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Cedars-Sinai Medical Center Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SL, RS, and TTH conceived and designed the study. SL acquired and analyzed data with assistance from JV, MM, JV, TCH, CB, KR, PL, RG, and JF; as well as assistant with statistical analysis from TCH, CB, and TTH. SL, RS, and TTH wrote the manuscript with input from all the authors.
Funding
Funding for this research includes NIH/UCLA CTSI UL1TR001881 (SL), P01 HL112730 (RG), R01HL133286 (TTH), R01HL094414 (RS), R01HL138577 (RS), R01HL159983 and R21AG074593 (RS and TTH).
Acknowledgments
We would like to acknowledge the Cedars-Sinai Proteomics and Metabolomic Core.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almeida, O. P., Garrido, G. J., Beer, C., Lautenschlager, N. T., Arnolda, L., and Flicker, L. (2012). Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur. Heart J. 33 (14), 1769–1776. doi:10.1093/eurheartj/ehr467
Alosco, M. L., Brickman, A. M., Spitznagel, M. B., Garcia, S. L., Narkhede, A., Griffith, E. Y., et al. (2013). Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest. Heart Fail. 19 (4), E29–E34. doi:10.1111/chf.12025
Alosco, M. L., and Hayes, S. M. (2015). Structural brain alterations in heart failure: A review of the literature and implications for risk of alzheimer's disease. Heart fail. Rev. 20 (5), 561–571. doi:10.1007/s10741-015-9488-5
Athilingam, P., D'Aoust, R. F., Miller, L., and Chen, L. (2013). Cognitive profile in persons with systolic and diastolic heart failure. Congest. Heart Fail. 19 (1), 44–50. doi:10.1111/chf.12001
Bacioglu, M., Maia, L. F., Preische, O., Schelle, J., Apel, A., Kaeser, S. A., et al. (2016). Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 91 (2), 494–496. doi:10.1016/j.neuron.2016.07.007
Damman, K., van Deursen, V. M., Navis, G., Voors, A. A., van Veldhuisen, D. J., and Hillege, H. L. (2009). Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 53 (7), 582–588. doi:10.1016/j.jacc.2008.08.080
Doyama, K., Fukumoto, M., Takemura, G., Tanaka, M., Oda, T., Hasegawa, K., et al. (1998). Expression and distribution of brain natriuretic peptide in human right atria. J. Am. Coll. Cardiol. 32 (7), 1832–1838. doi:10.1016/s0735-1097(98)00494-x
Fu, Y., Shaw, S. A., Naami, R., Vuong, C. L., Basheer, W. A., Guo, X., et al. (2016). Isoproterenol promotes rapid ryanodine receptor movement to bridging integrator 1 (BIN1)-organized dyads. Circulation 133 (4), 388–397. doi:10.1161/CIRCULATIONAHA.115.018535
Gattringer, T., Pinter, D., Enzinger, C., Seifert-Held, T., Kneihsl, M., Fandler, S., et al. (2017). Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 89 (20), 2108–2114. doi:10.1212/WNL.0000000000004645
Grundke-Iqbal, I., Iqbal, K., Tung, Y. C., Quinlan, M., Wisniewski, H. M., and Binder, L. I. (1986). Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U. S. A. 83 (13), 4913–4917. doi:10.1073/pnas.83.13.4913
Haug, C., Metzele, A., Kochs, M., Hombach, V., Grunert, A., and Grunert, A. (1993). Plasma brain natriuretic peptide and atrial natriuretic peptide concentrations correlate with left ventricular end-diastolic pressure. Clin. Cardiol. 16 (7), 553–557. doi:10.1002/clc.4960160708
Heidenreich, P. A., Trogdon, J. G., Khavjou, O. A., Butler, J., Dracup, K., Ezekowitz, M. D., et al. (2011). Forecasting the future of cardiovascular disease in the United States: A policy statement from the American heart association. Circulation 123 (8), 933–944. doi:10.1161/CIR.0b013e31820a55f5
Hitzeman, T. C., Xie, Y., Zadikany, R. H., Nikolova, A. P., Baum, R., Caldaruse, A. M., et al. (2020). cBIN1 score (CS) identifies ambulatory HFrEF patients and predicts cardiovascular events. Front. Physiol. 11, 503. doi:10.3389/fphys.2020.00503
Hong, T., Yang, H., Zhang, S. S., Cho, H. C., Kalashnikova, M., Sun, B., et al. (2014). Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat. Med. 20 (6), 624–632. doi:10.1038/nm.3543
Huijts, M., van Oostenbrugge, R. J., Duits, A., Burkard, T., Muzzarelli, S., Maeder, M. T., et al. (2013). Cognitive impairment in heart failure: Results from the trial of intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) randomized trial. Eur. J. Heart Fail. 15 (6), 699–707. doi:10.1093/eurjhf/hft020
Jefferson, A. L., Beiser, A. S., Himali, J. J., Seshadri, S., O'Donnell, C. J., Manning, W. J., et al. (2015). Low cardiac index is associated with incident dementia and alzheimer disease: The framingham heart study. Circulation 131 (15), 1333–1339. doi:10.1161/CIRCULATIONAHA.114.012438
Kindermann, I., Fischer, D., Karbach, J., Link, A., Walenta, K., Barth, C., et al. (2012). Cognitive function in patients with decompensated heart failure: The cognitive impairment in heart failure (CogImpair-HF) study. Eur. J. Heart Fail. 14 (4), 404–413. doi:10.1093/eurjhf/hfs015
Lahiri, S., Schlick, K. H., Padrick, M. M., Rinsky, B., Gonzalez, N., Jones, H., et al. (2017). Cerebral pulsatility index is elevated in patients with elevated right atrial pressure. J. Neuroimaging 28, 95–98. doi:10.1111/jon.12456
Lee, W. J., Jung, K. H., Ryu, Y. J., Kim, J. M., Lee, S. T., Chu, K., et al. (2017). Association of cardiac hemodynamic factors with severity of white matter hyperintensities in chronic valvular heart disease. JAMA Neurol. 75, 80–87. doi:10.1001/jamaneurol.2017.2853
Levi Marpillat, N., Macquin-Mavier, I., Tropeano, A. I., Bachoud-Levi, A. C., and Maison, P. (2013). Antihypertensive classes, cognitive decline and incidence of dementia: A network meta-analysis. J. Hypertens. 31 (6), 1073–1082. doi:10.1097/HJH.0b013e3283603f53
Li, J., Agvanian, S., Zhou, K., Shaw, R. M., and Hong, T. (2020). Exogenous cardiac bridging integrator 1 benefits mouse hearts with pre-existing pressure overload-induced heart failure. Front. Physiol. 11, 708. doi:10.3389/fphys.2020.00708
Liu, Y., Zhou, K., Li, J., Agvanian, S., Caldaruse, A. M., Shaw, S., et al. (2020). In mice subjected to chronic stress, exogenous cBIN1 preserves calcium-handling machinery and cardiac function. JACC. Basic Transl. Sci. 5 (6), 561–578. doi:10.1016/j.jacbts.2020.03.006
Mattsson, N., Andreasson, U., Zetterberg, H., and Blennow, K.Alzheimer’s Disease Neuroimaging Initiative (2017). Association of plasma neurofilament light with neurodegeneration in patients with alzheimer disease. JAMA Neurol. 74 (5), 557–566. doi:10.1001/jamaneurol.2016.6117
Mattsson, N., Zetterberg, H., Janelidze, S., Insel, P. S., Andreasson, U., Stomrud, E., et al. (2016). Plasma tau in Alzheimer disease. Neurology 87 (17), 1827–1835. doi:10.1212/WNL.0000000000003246
McMurray, J. J., Packer, M., Desai, A. S., Gong, J., Lefkowitz, M. P., Rizkala, A. R., et al. (2014). Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371 (11), 993–1004. doi:10.1056/NEJMoa1409077
Meguro, T., Meguro, Y., and Kunieda, T. (2017). Atrophy of the parahippocampal gyrus is prominent in heart failure patients without dementia. Esc. Heart Fail. 4 (4), 632–640. doi:10.1002/ehf2.12192
Mielke, M. M., Hagen, C. E., Wennberg, A. M. V., Airey, D. C., Savica, R., Knopman, D. S., et al. (2017). Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the mayo clinic study on aging. JAMA Neurol. 74 (9), 1073–1080. doi:10.1001/jamaneurol.2017.1359
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2016). Heart disease and stroke statistics-2016 update: A report from the American heart association. Circulation 133 (4), e38–e360. doi:10.1161/CIR.0000000000000350
Mueller, T., Gegenhuber, A., Dieplinger, B., Poelz, W., and Haltmayer, M. (2004). Long-term stability of endogenous B-type natriuretic peptide (BNP) and amino terminal proBNP (NT-proBNP) in frozen plasma samples. Clin. Chem. Lab. Med. 42 (8), 942–944. doi:10.1515/CCLM.2004.153
Mullens, W., Abrahams, Z., Francis, G. S., Sokos, G., Taylor, D. O., Starling, R. C., et al. (2009). Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 53 (7), 589–596. doi:10.1016/j.jacc.2008.05.068
Nikolova, A. P., Hitzeman, T. C., Baum, R., Caldaruse, A. M., Agvanian, S., Xie, Y., et al. (2018). Association of a novel diagnostic biomarker, the plasma cardiac bridging integrator 1 score, with heart failure with preserved ejection fraction and cardiovascular hospitalization. JAMA Cardiol. 3, 1206–1210. doi:10.1001/jamacardio.2018.3539
Nowatzke, W. L., and Cole, T. G. (2003). Stability of N-terminal pro-brain natriuretic peptide after storage frozen for one year and after multiple freeze-thaw cycles. Clin. Chem. 49 (9), 1560–1562. doi:10.1373/49.9.1560
Packer, M., Califf, R. M., Konstam, M. A., Krum, H., McMurray, J. J., Rouleau, J. L., et al. (2002). Comparison of omapatrilat and enalapril in patients with chronic heart failure: The omapatrilat versus enalapril randomized trial of utility in reducing events (OVERTURE). Circulation 106 (8), 920–926. doi:10.1161/01.cir.0000029801.86489.50
Qiu, C., Winblad, B., Marengoni, A., Klarin, I., Fastbom, J., and Fratiglioni, L. (2006). Heart failure and risk of dementia and alzheimer disease: A population-based cohort study. Arch. Intern. Med. 166 (9), 1003–1008. doi:10.1001/archinte.166.9.1003
Sabbah, H. N. (2017). Pathophysiology of acute heart failure syndrome: A knowledge gap. Heart fail. Rev. 22 (6), 621–639. doi:10.1007/s10741-017-9651-2
Sallach, J. A., Tang, W. H., Borowski, A. G., Tong, W., Porter, T., Martin, M. G., et al. (2009). Right atrial volume index in chronic systolic heart failure and prognosis. JACC. Cardiovasc. Imaging 2 (5), 527–534. doi:10.1016/j.jcmg.2009.01.012
Sauve, M. J., Lewis, W. R., Blankenbiller, M., Rickabaugh, B., and Pressler, S. J. (2009). Cognitive impairments in chronic heart failure: A case controlled study. J. Card. Fail. 15 (1), 1–10. doi:10.1016/j.cardfail.2008.08.007
Shimizu, A., Sakurai, T., Mitsui, T., Miyagi, M., Nomoto, K., Kokubo, M., et al. (2014). Left ventricular diastolic dysfunction is associated with cerebral white matter lesions (leukoaraiosis) in elderly patients without ischemic heart disease and stroke. Geriatr. Gerontol. Int. 14 (2), 71–76. doi:10.1111/ggi.12261
Sink, K. M., Leng, X., Williamson, J., Kritchevsky, S. B., Yaffe, K., Kuller, L., et al. (2009). Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the cardiovascular health study. Arch. Intern. Med. 169 (13), 1195–1202. doi:10.1001/archinternmed.2009.175
Solfrizzi, V., Scafato, E., Frisardi, V., Seripa, D., Logroscino, G., Kehoe, P. G., et al. (2013). Angiotensin-converting enzyme inhibitors and incidence of mild cognitive impairment. The Italian Longitudinal Study on Aging. Age 35 (2), 441–453. doi:10.1007/s11357-011-9360-z
Suwa, M., and Ito, T. (2009). Correlation between cognitive impairment and left ventricular diastolic dysfunction in patients with cardiovascular diseases. Int. J. Cardiol. 136 (3), 351–354. doi:10.1016/j.ijcard.2008.04.099
Troncone, L., Luciani, M., Coggins, M., Wilker, E. H., Ho, C. Y., Codispoti, K. E., et al. (2016). Aβ amyloid pathology affects the hearts of Patients With Alzheimer's disease: Mind the heart. J. Am. Coll. Cardiol. 68 (22), 2395–2407. doi:10.1016/j.jacc.2016.08.073
Villringer, A., and Laufs, U. (2021). Heart failure, cognition, and brain damage. Eur. Heart J. 42 (16), 1579–1581. doi:10.1093/eurheartj/ehab061
Virani, S. S., Alonso, A., Aparicio, H. J., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., et al. (2021). Heart disease and stroke statistics-2021 update: A report from the American heart association. Circulation 143 (8), e254–e743. doi:10.1161/CIR.0000000000000950
Vogels, R. L., Scheltens, P., Schroeder-Tanka, J. M., and Weinstein, H. C. (2007). Cognitive impairment in heart failure: A systematic review of the literature. Eur. J. Heart Fail. 9 (5), 440–449. doi:10.1016/j.ejheart.2006.11.001
Vogels, R. L., van der Flier, W. M., van Harten, B., Gouw, A. A., Scheltens, P., Schroeder-Tanka, J. M., et al. (2007). Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur. J. Heart Fail. 9 (10), 1003–1009. doi:10.1016/j.ejheart.2007.07.006
Xu, B., Fu, Y., Liu, Y., Agvanian, S., Wirka, R. C., Baum, R., et al. (2017). The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles. PLoS Biol. 15 (8), e2002354. doi:10.1371/journal.pbio.2002354
Yancy, C. W., Jessup, M., Bozkurt, B., Butler, J., Casey, D. E., Drazner, M. H., et al. (2013). ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 62 (16), e147–e239. doi:10.1016/j.jacc.2013.05.019
Zetterberg, H., Wilson, D., Andreasson, U., Minthon, L., Blennow, K., Randall, J., et al. (2013). Plasma tau levels in Alzheimer's disease. Alzheimers Res. Ther. 5 (2), 9. doi:10.1186/alzrt163
Keywords: neurofilament light chain (NfL), total tau, heart failure, volume status, cBIN1 score
Citation: Lahiri S, Mastali M, Van Eyk JE, Hitzeman TC, Bresee C, Raedschelders K, Lyden PD, Gottlieb RA, Fang JC, Shaw RM and Hong TT (2022) Plasma brain injury markers are associated with volume status but not muscle health in heart failure patients. Front. Drug. Discov. 2:1042737. doi: 10.3389/fddsv.2022.1042737
Received: 12 September 2022; Accepted: 06 October 2022;
Published: 18 October 2022.
Edited by:
Charles C. Hong, University of Maryland, United StatesReviewed by:
Mark J. Ranek, Johns Hopkins University, United StatesEmmanouil Tampakakis, Johns Hopkins University, United States
Copyright © 2022 Lahiri, Mastali, Van Eyk, Hitzeman, Bresee, Raedschelders, Lyden, Gottlieb, Fang, Shaw and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shouri LahiriLFNob3VyaS5sYWhpcmlAY3NtYy5lZHU=TingTing Hong, dGluZ3RpbmcuaG9uZ0BwaGFybS51dGFoLmVkdQ==
 Shouri Lahiri
Shouri Lahiri Mitra Mastali2
Mitra Mastali2 Tara C. Hitzeman
Tara C. Hitzeman Catherine Bresee
Catherine Bresee Roberta A. Gottlieb
Roberta A. Gottlieb Robin M. Shaw
Robin M. Shaw Ting Ting Hong
Ting Ting Hong